Abstract
Bisphenol A (BPA) and diclofenac (DCF) are among the most prevalent micropollutants in aquatic environments, with concentrations reaching up to several hundred µg/L. These compounds pose significant risks to biodiversity and environmental health, necessitating the development of effective removal methods. However, both BPA and DCF can be resistant to conventional treatment technologies, highlighting the need for innovative approaches. Electrochemical oxidation (EO) has emerged as a promising solution. In this study, we assessed the effectiveness of EO using boron-doped diamond (BDD) anodes to remove BPA and DCF from two types of treated wastewater (TWW-W and TWW-D) and landfill leachate (LL). The evaluation included an analysis of the removal efficiency of BPA and DCF and the identification of transformation products generated during the process. Additionally, the feasibility of the EO-BDD process to remove ammonium nitrogen (N-NH4+) and organic compounds present in these environmental matrices was investigated. The EO-BDD treatment achieved remarkable removal efficiencies, reducing BPA and DCF concentrations by over 96% in LL and TWW-W. Transformation product analyses identified four intermediates formed from parent compounds during the oxidation process. Furthermore, the EO-BDD process effectively removed both chemical oxygen demand (COD) and ammonium nitrogen from LL, although weaker results were observed for TWWs. These findings underscore the potential of the EO-BDD process as an effective method for the removal of BPA and DCF from challenging matrices, such as wastewater containing micropollutants. It also shows promise as a complementary technology for enhancing current conventional wastewater treatment methods, especially biological degradation.
1. Introduction
Organic micropollutants (MPs) include a broad spectrum of compounds, like pharmaceutical and personal care products, pesticides, industrial chemicals, steroid hormones, and microplastics [1]. The widespread and persistent presence of micropollutants has led to significant adverse effects on urban water systems worldwide and on human and animal health. Currently, their concentration in the aquatic environment is measured at about a few ng/L to even a few mg/L [2]. Here, special attention should be given to wastewater treatment plants (WWTPs) as these facilities receive micropollutants with wastewater, which can subsequently pass through the treatment processes and enter the environment. Thus, this study focuses on two commonly occurring micropollutants in wastewater: bisphenol A (BPA) and diclofenac (DCF).
Bisphenols, classified as organic micropollutants, represent a specific group of chemicals associated with plastic production [3]. Bisphenols have been widely used for decades and can be determined in epoxy resins, polycarbonates, flame retardants, and medical equipment [4]; however, they are added as endogenous chemicals that, for the most part, are not chemically bonded to the polymer, thus easily migrating to food and water systems [5]. The release of BPA primarily occurs due to the hydrolysis of carbonate linkages under high temperatures and neutral to alkaline pH conditions [6]. According to the literature, wastewater effluents contain BPA in concentrations of up to 100 μg/L [7]. However, in some countries, the reported concentrations are higher, even in surface water. For example, Chou et al. [8] reported concentrations of BPA in the range of 0.01 to 725 μg/L in several river water samples in Taiwan. The risk of human exposure to BPA is not insignificant [2,6]. Bisphenol A is classified as an endocrine-disrupting chemical (EDC), causing neurotoxic effects, mimicking or blocking receptors, altering hormone concentrations and gene expression, and affecting metabolism [9].
Diclofenac is a common non-steroidal anti-inflammatory drug (NSAID), which can be administered topically or orally and is almost fully metabolised in the human body. Less than 1% of the orally administered dose of DCF is excreted unmetabolised, and the majority undergoes phase II metabolism [10]. The annual consumption of DCF for both human and veterinary purposes is estimated to be around 940 tons globally [11]. Consequently, DCF has been widely detected in municipal wastewater treatment influents and effluents, as well as in surface and groundwater, with an average concentration of 1.2 μg/L [12]. Diclofenac elimination in wastewater treatment plants varies from 22% to 75% in conventional activated sludge processes. However, DCF is still among the most frequently detected micropollutants. The removal of DCF is primarily attributed to sorption onto sludge, and its effective degradation remains low [11]. Consequently, alternative methods are being explored to eliminate this substance from aquatic environments.
In light of the new Directive of the European Parliament and Council on urban wastewater treatment (EU-2022/0345(COD)) [13], which mandates the monitoring and removal of selected micropollutants (MPs), including diclofenac (DCF), wastewater treatment plants must adopt efficient technologies for MP removal. Currently, various methods are employed for this purpose, including adsorption [14], membrane separation [15], Fenton-like processes [16], sonolysis [17], photolysis, and photoelectrocatalytic degradation [18]. Among these, electrochemical oxidation (EO) is increasingly gaining attention due to its high efficiency. Previous studies have demonstrated the effectiveness of EO in removing persistent pollutants such as polyfluorinated alkyl substances (PFASs) and carbamazepine from wastewater [19,20]. Electrochemical oxidation operates through two primary mechanisms: (I) direct EO, where organic compounds are oxidised via direct electron transfer (DET) between the anode surface and the adsorbed contaminant molecules without the involvement of chemical intermediates, and (II) indirect EO, where strong oxidants such as hydroxyl radicals, reactive chlorine species (RCS), hydrogen peroxide, and persulfate ions are generated in situ on the anode surface, facilitating the degradation of compounds in the bulk phase [12]. The efficiency of the EO process heavily depends on the material and structure of the anode. A strong interaction between micropollutants and the anode surface can lead to electrode fouling caused by the adsorption of oxidised micropollutant transformation products. This fouling reduces the anode’s sensitivity and efficiency. Boron-doped diamond (BDD) electrodes are increasingly favoured to mitigate this issue due to their exceptional resistance to fouling compared to glassy carbon electrodes. This resistance arises from their non-polar nature (when they are hydrogen-terminated) and minimal π–π electron interactions [21]. BDD electrodes offer numerous advantages, including electrical conductivity that ranges from semiconducting to metallic, high chemical resistance, and exceptional stability. These properties enable BDD electrodes to function effectively in highly aggressive environments, achieving superior degradation efficiencies compared to electrodes containing critical raw materials [22].
A crucial electrochemical parameter for any electrode is its potential window, which defines the range of potentials within which water does not decompose into hydrogen and hydroxide ions. Standard microcrystalline diamond electrodes exhibit an extensive electrochemical window, spanning from −1.25 V to +2.3 V relative to the standard hydrogen electrode (SHE) [23,24,25]. This broad potential window allows for the near-complete mineralisation of organic matter on BDD anodes with very high current efficiency. This study presents a novel investigation into the BDD-driven EO process using electrodes with varying boron doping levels, specifically targeting the removal of two prevalent micropollutants—bisphenol A and diclofenac—from real-world water matrices. Unlike previous studies, this research focuses on the effect of boron doping on the degradation of BPA and DCF in treated wastewater from two different wastewater treatment plants and landfill leachate, providing a more realistic assessment of EO performance in complex environmental conditions. Furthermore, this work aims to lower the potential formation of unwanted degradation transformation products, a key concern in advanced oxidation processes, by analysing and identifying the transformation products of the micropollutants as the effect of the boron doping of the electrode. Through this comprehensive approach, this study not only enhances our understanding of the EO-BDD process but also supports the objectives of the new European Union Wastewater Directive, which calls for the effective removal of micropollutants from wastewater.
2. Materials and Methods
2.1. Environmental Sample Source
Landfill leachate (LL) samples were collected from the Municipal Waste Plant “Eko Dolina” sp. z o.o. in Łężyce. Additionally, treated wastewater samples were obtained from two locations: the Readaptation Center of the Association Solidarni “PLUS” EKO “School of Life” in Wandzino (TWW-W) and the Group Wastewater Treatment Plant “Debogórze,” operated by PEWIK Gdynia (TWW-D). The physical and chemical parameters of the collected samples are presented in Table 1.

Table 1.
Physical and chemical characteristics of environmental samples. Abbreviations: COD—chemical oxygen demand; BOD—biochemical oxygen demand; TSSs—total suspended solids; MSSs—mineral suspended solids; VSSs—volatile suspended solids; TN—total nitrogen; and TP—total phosphorus.
2.2. Reagents and Solutions
For the EO experiment, analytical-grade bisphenol A (C15H16O2) was obtained from Sigma-Aldrich (St. Louis, MO, USA) (97%), while diclofenac (C14H11Cl2NO2) was acquired from Olfen 75 SR (including 75 mg/pill). For chromatographic analysis, analytical standards of BPA (≥99%) and DCF (≥98.5%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemical reagents, such as potassium ferricyanide(III), sodium sulfate, and phosphoric acid, were reagent grade (≥99%) and sourced from Sigma-Aldrich (St. Louis, MO, USA). A 100 mM phosphate buffer solution (prepared by dissolving 8.7331 g of K2HPO4 and 125 µL of 85% H3PO4 in a 500 mL volumetric flask with purified water) was selected over Cl− and SO42− electrolytes due to reduced interference in indirect BPA and DCF oxidation.
2.3. Boron-Doped Diamond (BBD) Electrode
Boron-doped diamond (BDD) films were prepared on 50-mm diameter niobium substrates using a microwave plasma-assisted chemical vapour deposition (MWPECVD) system (SEKI Technotron AX5400S, Tokyo, Japan). Before deposition, the substrates underwent sandblasting, followed by cleaning in acetone and isopropanol in an ultrasonic bath and seeding with a water-based diamond slurry via sonication. During deposition, the microwave power was maintained at 1300 W, with a total pressure of 50 Torr, and the substrate holder temperature was set to 700 °C. A gas mixture of H2, CH4, and B2H6 was used for BDD growth. The flow rates of B2H6 and CH4 were adjusted to achieve two boron doping levels at ~1.5 × 1017 at/cm3 and 3 × 1021 at/cm3 (referred to as 0.5 k and 10 k, respectively). Our previous studies have extensively reported the surface morphology and electrochemical properties of the BDD electrodes [20,26].
2.4. Electrochemical Oxidation (EO) Setup
Electrochemical experiments were conducted using a custom-designed setup comprising a 400-mL undivided electrolytic cell, a cooling bath, and a magnetic stirrer (Electrochemical Stirrer, ES24, Wigo, Poland). The cell featured a boron-doped diamond electrode as the anode and a stainless-steel mesh as the cathode. The anode’s geometric surface area was 10.5 cm2, with an anode–cathode distance maintained at approximately 2.5 cm. A schematic representation of the electrochemical oxidation setup is shown in Figure 1. All electrochemical oxidation tests were performed under galvanostatic conditions. The experiments were designed to elucidate the degradation pathways of bisphenol A (BPA) and diclofenac (DCF) while evaluating the efficiency of concentration reduction during electrolysis using newly developed electrode materials. In these experiments, the electrochemical oxidation of BPA and DCF was carried out in landfill leachate and two types of treated wastewater using a BDD electrode. The BDD electrode was fabricated via microwave plasma-enhanced chemical vapour deposition (MWPECVD) employing boron-to-carbon gas phase concentrations of 0.5k ppm and 10k ppm. For the oxidation process, BPA and DCF in TWW-W and TWW-D were sampled at 2 h and 6 h intervals, while LL underwent EO for 4 and 8 h. Untreated solutions (0 h) were used as references. A 50 mL sample was taken for micropollutant concentration analysis, while 20 mL was for ammonium and COD determination. The test was conducted in triplicate. The current density was optimised previously by Pierpaoli et al. [20] to achieve maximum oxidation rates, set at j = 120 mA/cm2 for LL and 25 mA/cm2 for both TWW-W and TWW-D. Further experimental details can be found in Pierpaoli et al. [20].
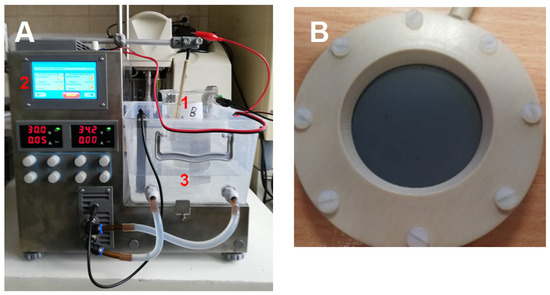
Figure 1.
Set for electrolytic oxidation of micropollutants. (A): electrolyser with thermostatic bath (1—electrochemical reactor, 2—power supply, and 3—electromagnetic stirrer) and (B): holder including BDD anode.
2.5. Analysis of COD and N-NH4+
Determination of ammonium nitrogen (N-NH4+) and chemical oxygen demand (COD) was performed using the spectrophotometric method with Hach Lange cuvette tests (methodology compliant with APHA 2005) [27].
2.6. BPA and DCF Analysis Concentration and Transformation Product Determination
The concentrations of the selected micropollutants in the experiments were determined using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry and electrospray ionisation (UHPLC-MS/MS; Shimadzu Nexera X2, LC-MS 8040, Kioto, Japonia). Sample preparation was performed via solid-phase extraction (SPE) using Strata-X cartridges (Phenomenex, 200 mg/3 mL, 33 µm, Torrance, CA, USA). Chromatographic separation was carried out on a Phenomenex Luna Omega Polar column (100 × 2.1 mm; 1.6 μm; C18; 100 Å, Torrance, CA, USA). The mobile phase comprised MS-grade water (phase A) and acetonitrile (phase B), controlled by pumps A and B. A gradient elution program was applied, starting with 80% A and 20% B at 0 min, ramping to 20% A and 80% B by 8 min, reverting to 80% A and 20% B at 8.1 min, and holding until 10.5 min. The column temperature was maintained at 35 °C, with the autosampler set to 15 °C. The flow rate was 0.4 mL/min. The UHPLC-ESI-MS method was used to identify electrochemical oxidation transformation products of BPA and DCF, focusing on ionisable molecules. The qualitative analysis employed the scanning mode (SCAN, m/z range: 100–520) to detect transformation products. Subsequently, Single Ion Monitoring (SIM) in negative ionisation mode was applied to monitor increases or decreases in identified transformation products. Quantification of BPA and DCF concentrations was performed using Multiple Reaction Monitoring (MRM) in negative ionisation mode with the following transitions: BPA: [m/z]: 226.9 > 211.8 (20 V) and 226.9 > 133.0 (21 V) and DCF: [m/z]: 293.9 > 250.0 (11 V) and 293.9 > 214.0 (21 V). The limits of quantification (LOQs) were 0.40 µg/L for BPA and 0.08 µg/L for DCF. The MS parameters were optimised as follows: nebulising gas (N2) flow rate of 3 L/min, drying gas (N2) flow rate of 8 L/min, desolvation temperature of 250 °C, and heat block temperature of 400 °C. All analytes were measured using Single Ion Monitoring (SIM) in the negative ionisation mode.
3. Results and Discussion
3.1. Electrochemical Removal of Ammonium and Organic Compounds
Organic carbon and ammonium nitrogen are the main components that wastewater treatment plants are obligated to remove from wastewater. This also applies to the treatment of landfill leachate. While biological processes effectively remove both ammonium nitrogen and COD compounds, the new European Union wastewater directive mandates lower maximum concentrations of these substances in treated wastewater, necessitating the use of additional removal methods. Table 2 presents the results of COD and N-NH4+ removal in the electrochemical oxidation process on BDD anodes. The results indicate that the removal of both COD and N-NH4+ was most effective in landfill leachate. This outcome may be associated with the presence of other compounds in landfill leachate that can generate free radicals. According to Comninellis’ model [28,29,30], the oxidation of organic matter on BDD electrodes is mainly due to hydroxyl radical (•OH) formation. However, in a complex matrix such as landfill leachate or treated wastewater, the BDD anode may also generate strong oxidants (radicals) from salts utilising anions such as sulfates, carbonates, and chlorides [31]. Considering the chloride ion content in the examined environmental matrices, only landfill leachate, in which chloride ions have a concentration of 2620–2760 mg/L, may have active chlorine (HOCl, OCl−, and Cl2), which also plays a significant role in the EO of contaminants. Ding et al. [32] and Lacasa et al. [33] have described their significant impact on removing N-NH4+ from wastewater. Detailed mechanisms of the electrooxidation of ammonium compounds in the presence of chloride ions are presented in Figure 2. Furthermore, strong oxidants such as sulfate radicals (SO4•−) may also be generated using the BDD anode in the reaction of hydroxyl radicals with HSO4− and undissociated H2SO4 [31]. However, the role of sulfate radicals in mineralising organic matter in environmental matrices remains largely unknown.

Table 2.
Effectiveness of COD and N-NH4+ removal from different environmental matrices in the EO-BDD process at two boron doping levels (0.5 k and 10 k).
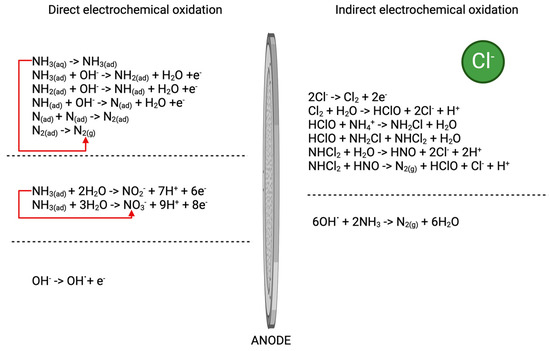
Figure 2.
Reactions showing direct and indirect electrochemical oxidation of ammonium compounds in the presence of chloride ions [34,35].
For all the environmental matrices studied, the reduction in ammonium nitrogen was consistently lower than that of COD. Additionally, variations in the boron doping levels of diamond electrodes showed minimal impact on the efficiency of ammonium removal. This finding aligns with the results presented by Pierpaoli et al. [20], which highlighted that current density plays a predominant role in influencing the process, whereas boron doping has a lower effect. They also observed that the most significant reduction in ammonium nitrogen occurs within the first hour of the EO-BDD process. This observation is consistent with the findings of Ghazouani et al. [36] and Zhou et al. [37], who noted a similar pattern using BDD electrodes in treated wastewater matrices, where ammonium nitrogen removal was most effective during the initial hour, followed by stabilisation. Moreover, Fudala-Ksiazek et al. [38] reported that during the EO of landfill leachate, ammonia adsorbed on the electrodes reacts with water to form nitrate ions, as illustrated in Figure 2. Interestingly, during the EO-BDD process for TWW-D samples, an increase in ammonium nitrogen concentration was observed. This phenomenon is likely attributed to the release of ammonium nitrogen from organic compounds during the EO process. Moreover, with an initial ammonium nitrogen concentration of 0.103 mg/L (see Table 1), even minor fluctuations in its concentration caused by the decomposition of organic matter can significantly impact the removal efficiency.
In summary, the EO-BDD process demonstrates high efficiency in removing organic compounds from complex environmental matrices such as wastewater and landfill leachate, while the efficiency of ammonium nitrogen removal is closely related to the type of environmental matrix and its chemical composition.
3.2. Removal Efficiency of BPA and DIC from Different Environmental Matrices
Electrochemical oxidation with BDD achieved a BPA reduction rate of over 99.8% in the landfill leachate matrix, demonstrating exceptionally high process efficiency after just 4 h of treatment (Table 3). The analysis of BPA reduction in treated wastewater indicates that higher removal efficiency of BPA is achieved with a lower load of other organic substances (measured by COD in TWW-W—312 mg/L and TWW-D—34 mg/L) as well as with a lower initial concentration of BPA. The highest BPA reduction rate (>96.64%) in treated wastewater was achieved after only 2 h of treatment for the matrix with a lower organic load (COD). Moreover, similar to COD and N-NH4+ reduction, the highest reduction level of BPA was observed at the beginning of the EO-BDD process, and negligible differences were observed between anodes with different levels of boron doping. The results obtained for LL and TWW-D are comparable to those reported by del Rosario Salas-Sandoval et al. [39], where the BDD anode achieved 95% BPA removal after 60 min of electrolysis. The broader oxygen overpotential window of the BDD anode enhances the generation and stabilisation of oxidising species, such as physisorbed hydroxyl radicals produced during water electrolysis [40,41]. Additionally, sulfate radicals can be electrogenerated through the direct oxidation of sulfate, contributing to BPA degradation [42]. Comparing our results to those obtained through other oxidation methods, one can observe the advantage of EO-BDD, especially in terms of efficiency. Zorzo et al. [3] analysed the efficiency of BPA removal in the UV–solar/H2O2 process in different pH conditions. In this process, the main reason for BPA degradation in a system based on •OH attack is the direct photolysis of H2O2 [43]. The results obtained by Zorzo et al. [3] show that the removal efficiency of BPA is highly dependent on pH, reaching the best results in acidic pH, while neutral and alkaline pH presented significantly lower efficiencies. In neutral (pH 7.0) solutions, BPA degradation rates decreased by 62.3–34.6%, while in alkaline (pH 9.0 and 11.0) solutions, the reduction rates reached 41.0–26.2%, and 58.2–31%, respectively. This reduction can be attributed to the lower production of •OH radicals and increased hydroperoxide (HO•2) production; however, HO•2 dissociates into H2O2, which has weaker oxidative properties [43,44]. Comparatively, in the results presented in this study, the pH of the environmental matrix was close to neutral or slightly alkaline (see Table 1), and the efficiencies obtained were close to 100%, which indicates less dependence on the pH of the environment.

Table 3.
BPA removal efficiency [%] in the EO process using boron-doped diamond electrodes from landfill leachate and treated wastewater in fortified samples.
In the analysis of DCF reduction in the landfill leachate matrix, a very high process efficiency of >99.23% was achieved after only 4 h of treatment (Table 4). In contrast, when analysing the efficiency of DCF removal in the treated wastewater matrix, it is evident that higher removal efficiency is achieved in matrices with a lower load of organic substances (see Table 1). The highest DCF reduction (96.64%) was achieved after only 2 h of treatment in TWW-D. The obtained efficiencies for removing DCF from LL and TWW-D are similar to those reported by Iovino et al. [12], who used electrodes made of a platinum layer placed on an inert titanium support (Ti/Pt). The authors compared the DCF removal efficiency in the EO process in the presence of three different electrolytes (NaCl, NaClO4, or NaNO3). In their study, the highest DCF removal (>98%) was achieved in the presence of NaCl (50 mA/cm2). In this case, the presence of chlorine is essential because the efficiency of DCF removal can be improved by generating reactive chlorine species at the anode during the EO process [45]. The effectiveness of this mechanism in supporting DCF removal was further corroborated by Liu et al. [46]. This generation of reactive chlorine species can be especially significant in high-salinity environments, such as landfill leachate (see Table 1).

Table 4.
BPA removal efficiency [%] in the EO process using boron-doped diamond electrodes from landfill leachate and treated wastewater in spiked samples.
The EO effect can be enhanced by applying a higher current density. This may be particularly important for environmental matrices with high concentrations of substances that facilitate the generation of additional reactive species, such as chlorine or sulfate ions [47,48]. Additionally, this can be significant when using active anodes, such as BDD, which allow for the generation of large quantities of reactive species [49]. This study applied a current density of 100 mA/cm2 for landfill leachate, while 25 mA/cm2 was used for treated wastewater. The results indicate that for both LL and TWW-D, the applied current density was sufficient to remove all the investigated micropollutants effectively. However, for TWW-W, a higher current density could have been applied. Nevertheless, there is a risk that using higher current densities might not yield any additional benefits due to the occurrence of side reactions, such as oxygen evolution and/or mass transfer limitations [50,51,52].
3.3. Detection of Transformation Products
The EO-BDD process may result in the incomplete mineralisation of the primary compound, sometimes leading to the formation of transformation products that are more toxic than the original contaminant [12,53]. Moreover, the type and quantity of intermediates produced during treatment depend on the operational conditions applied [54]. Therefore, identifying these transformation products is crucial for determining the specific intermediates formed during EO processing and for assessing their potential toxicity.
In this study, the identification of transformation products generated during the electrochemical treatment of phosphate buffer (PB) using a boron-doped diamond electrode was performed after spiking the solutions with bisphenol A (Table 5) and diclofenac (Table 6). Initially, samples were analysed using UHPLC-MS/MS to identify the transformation products formed during the EO-BDD process, providing insights into subsequent electrochemical tests. Simultaneously, degradation products from electrolysis were determined using a single mass spectrometer in Single Ion Monitoring (SIM) mode, following an initial SCAN for precursor ions. Samples were collected at the start of (0 h) and after EO-BDD treatment (at 2 h and 6 h). This approach enabled the characterisation of degradation mechanisms specific to the direct EO process while excluding matrix effects such as active chlorine or sulfate ions.

Table 5.
List of BPA degradation products of registered samples fortified with bisphenol A in SIM (negative ionisation) mode and area fields (10 µL injection volume). The initial concentration of BPA in PB = 184 mg/L.

Table 6.
List of diclofenac sodium (DCF-Na) degradation products recorded in a matrix of phosphate buffer and effluent treated in SIM (negative ionisation) mode, along with area fields (5 µL injection volume). The initial concentration of DCF in PB = 600 mg/L.
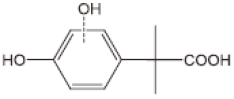
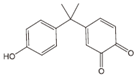
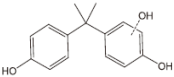
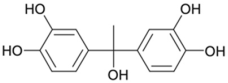
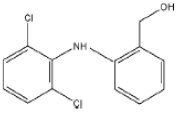
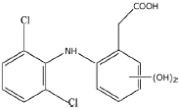
As a result of the EO-BDD process, four BPA degradation products were identified, which are listed in Table 5. Moreover, the peak areas are given in the table to describe changes in the generation of these transformation products during the EO-BDD process. In the case of BPA results, the degradation products obtained are similar to those presented in a previous study [55,56,57]. Bakr et al. [58] presented the degradation pathway of BPA under electrooxidation using a multi-wall carbon nanotube-based anode. The authors showed that the degradation of BPA starts with the formation of hydroxylated derivatives due to an attack by superoxides on the aromatic rings, such as m/z 234, which was also detected in our experiment (PBA_3). BPA_3 can be further hydroxylated to PBA_2 (m/z 242) or isopropylidene bridge cleavage can occur, leading to the formation of one-ring hydroxylated phenolic derivatives at m/z 195 (BPA_1). Kondrakov et al. [59] identified structures of BPA_4 under a photocatalytic process using TiO2 as a catalyst. However, the authors’ proposed degradation pathway pointed out that BPA_4 is generated from free OH radical’s suppression of m/z 261 (4,4′-ethane-1,1-diyl-dicatechol), which was not detected in our study; therefore, its generation in the EO-BDD process should be different. Generally, the peak areas were increased along with the EO time in both 0.5k BDD and 10k BDD, while higher peak areas (apart from BPA_2) were detected for 0.5k BDD after 6 h of the EO-BDD process. del Rosario Salas-Sandoval et al. [39] analysed the degradation of BPA using EO-BDD based on total organic carbon and their results suggested that during the EO process, transformation products are generated that can be resistant toward the oxidation treatment process; thus, EO-BDD of BPA requires a longer treatment time. Interestingly, BPA_1 for the 0.5k BDD anode was not detected until after 6 h of the process, while for the 10k BDD anode, it was discovered as early as 2 h of the process, and later, a decrease in the peak area was observed after 6 h of the process. This result may suggest that the product appears later on the 0.5 k BDD anode, indicating slower reaction processes. Moreover, the decrease in the BPA_1 peak area after 6 h on the 10k BDD anode may suggest that the intermediate product (BPA_1) undergoes further transformations at later stages of the process, such as degradation or mineralisation.
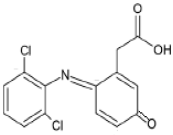
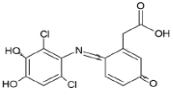
A total of four transformation products of DCF were detected during the EO-BDD process in the PB matrix, which were labelled as DCF_1, DCF_2, DCF_3, and DCF_4. According to Jewell et al., [60] DCF_3 is a primary transformation product; however, the authors reported that this intermediate product was formed under microbial activity during wastewater treatment. On the other hand, the same transformation products were detected during the EO process by Iovino et al. [12]. Hydroxylated derivatives, such as DCF_3, are formed either through the direct oxidation of DCF or by hydroxyl radicals (OH•) generated at the anode [61,62]. These radicals preferentially attack the ortho and para positions of aromatic rings, driven by the high electron density associated with electron-donating groups such as -OH or -NH2 [63]. This aligns with the findings of Pérez-Estrada et al. [64], who reported that DCF is initially targeted by hydroxyl radicals to produce a monohydroxylated derivative (DCF_3), which subsequently undergoes further oxidation to form the intermediate 2,5-iminoquinone-DCF (DCF_2). DCF_4 increased by 32 mass units in relation to DCF_2, which corresponded to an addition of oxygen. The same pathway of transformation of DCF_2 to DCF_4 was proposed by Lu et al., [65] under microbial activity. On the other hand, the intermediate product with m/z 341 was not generated during the EO process conducted by Iovino et al. [12]. As a result of the EO-BDD process, a transformation product labelled DCF_1 (m/z = 267) was detected. Consistent with the findings of Faber et al. [66], this compound is likely formed through decarboxylation reactions of DCF, involving the loss of its polar carboxylic moiety [53]. Based on this assumption, the molecular structures were proposed by leveraging the conclusions reported by Agüera et al. [67]. Furthermore, Iovino et al. [12] noted that during the EO process, diclofenac (DIC) could be transformed into 2,6-dichloroaniline, a common transformation product resulting from the cleavage of the C–N bond in DIC during oxidative treatments [68]. However, in this study, 2,6-dichloroaniline was not identified among the transformation products of DCF oxidation. This finding underscores the variation in DCF degradation pathways depending on the specific EO method employed. Additionally, the EO-BDD process conducted in more chemically complex matrices may result in the formation of other intermediates. These potential intermediates warrant further investigation in future studies.
4. Conclusions
Our results show that the EO-BDD process is suitable for removing organic compounds from complex environmental matrices such as treated wastewater or landfill leachate, achieving removal efficiencies exceeding 90%. Moreover, the EO-BDD process removed both micropollutants, BPA and DCF, with over 99% and 96% efficiency from LL and TWW-D, respectively. However, for TWW-W, the efficiencies were significantly lower and did not exceed 80%, even after 6 h of processing. The use of HPLC-MS enabled the identification of intermediate products from the transformation of BPA and DCF formed during the EO-BDD process. Conducting studies on the identification of transformation products in the PB matrix allowed for an understanding of the mechanisms of degradation product generation resulting directly from the EO-BDD process, excluding additional chemical processes related to electrooxidation. These results indicate the applicability of EO-BDD as an advanced oxidation process for a quaternary wastewater treatment stage. This would help to mitigate the negative environmental consequences of the presence of micropollutants in municipal and industrial wastewater effluents and help to meet the new EU Directive requirements (proposal for 2022/0345(COD) starting from 1 March 2024). Nonetheless, in further stages of research, additional tests should be conducted on more complex matrices to better understand the environmental consequences of transformation product generation.
Author Contributions
Conceptualisation, F.G. and M.S.; methodology, M.P., R.B., A.Ł. and S.F.-K.; validation, F.G., S.Ż., D.Z. and M.S.; formal analysis, F.G.; investigation, F.G. and M.S.; data curation, M.P., R.B., A.Ł. and S.F.-K.; writing—original draft preparation, F.G.; writing—review and editing, F.G., W.A.; visualisation, F.G.; supervision, M.P., R.B., A.Ł. and S.F.-K.; and project administration, R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the project Regional Fund for Environmental Protection and Water Management in Gdansk Poland (RX-15/13/2017) and by the Gdańsk University of Technology: the DEC-20/1/2022/IDUB/I3b/Ag grant under the Argentum Program ‘Excellence Initiative—Research University’.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Krzeminski, K.; Tomei, M.C.; Karaolia, P.; Langenhoff, L.; Almeida, C.M.A.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial pharmaceuticals in the aquatic environment—Occurrence and environmental implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef] [PubMed]
- Zorzo, C.F.; Inticher, J.J.; Borba, F.H.; Cabrera, L.C.; Dugatto, J.S.; Baroni, S.; Bergamasco, R. Oxidative degradation and mineralisation of the endocrine-disrupting chemical bisphenol A by an eco-friendly system based on UV-solar/H2O2 with reduction of genotoxicity and cytotoxicity levels. Sci. Total Environ. 2021, 770, 145296. [Google Scholar] [CrossRef] [PubMed]
- Ghahremani, M.H.; Ghazi-Khansari, M.; Farsi, Z.; Yazdanfar, N.; Jahanbakhsh, M.; Sadighara, P. Bisphenol A in dairy products: Amount, potential risks, and various analytical methods—A systematic review. Food Chem. X 2024, 21, 101142. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Steinmetz, R. Xenoestrogens: The emerging story of bisphenol A. Trends Endocrinol. Metab. 1998, 9, 124–128. [Google Scholar] [CrossRef]
- Petrie, B.; Lopardo, L.; Proctor, K.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Assessment of bisphenol-A in the urban water cycle. Sci. Total Environ. 2019, 650, 900–907. [Google Scholar] [CrossRef]
- Chou, P.-H.; Lin, Y.-L.; Liu, T.-C.; Chen, K.-Y. Exploring potential contributors to endocrine disrupting activities in Taiwan’s surface waters using yeast assays and chemical analysis. Chemosphere 2015, 138, 814–820. [Google Scholar] [CrossRef]
- Presunto, M.; Mariana, M.; Lorigo, M.; Cairrao, E. The effects of bisphenol A on human male infertility: A review of current epidemiological studies. Int. J. Mol. Sci. 2023, 24, 12417. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plants—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and its transformation products: Environmental occurrence and toxicity—A review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Iovino, P.; Lavorgna, M.; Orlo, E.; Russo, C.; De Felice, B.; Campolattano, N.; Musmarra, D. An integrated approach for the assessment of the electrochemical oxidation of diclofenac: By-product identification, microbiological and eco-genotoxicological evaluation. Sci. Total Environ. 2024, 909, 168511. [Google Scholar] [CrossRef] [PubMed]
- Proposal for a Directive of the European Parliament and of the Council Concerning Urban Wastewater Treatment 2024. Available online: https://data.consilium.europa.eu/doc/document/ST-7108-2024-INIT/en/pdf (accessed on 21 November 2024).
- Yin, Y.; Shi, M.; Ren, Y.; Wang, S.; Hua, M.; Lu, J.; Zhang, W.; Lv, L. Wrinkle structure on multifunctional MOFs to facilitate PPCPs adsorption in wastewater. Chem. Eng. J. 2020, 387, 124196. [Google Scholar] [CrossRef]
- Huang, Z.; Gong, B.; Huang, C.-P.; Pan, S.-Y.; Wu, P.; Dang, Z.; Chiang, P.-C. Performance evaluation of integrated adsorption-nanofiltration system for emerging compounds removal: Exemplified by caffeine, diclofenac, and octylphenol. J. Environ. Manag. 2019, 231, 121–128. [Google Scholar] [CrossRef]
- Hong, M.; Wang, Y.; Lu, G. UV-Fenton degradation of diclofenac, sulpiride, sulfamethoxazole, and sulfisomidine: Degradation mechanisms, transformation products, toxicity evolution, and effect of real water matrix. Chemosphere 2020, 258, 127351. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Botero-Coy, A.M.; Martínez-Pachón, D.; Moncayo-Lasso, A.; Ibañez, M.; Hernández, F.; Torres-Palma, R.A. Degradation of seventeen contaminants of emerging concern in municipal wastewater effluents by sonochemical advanced oxidation processes. Water Res. 2019, 154, 349–360. [Google Scholar] [CrossRef]
- Sigcha-Pallo, C.; Peralta-Hernández, J.M.; Alulema-Pullupaxi, P.; Carrera, P.; Fernández, L.; Pozo, P.; Espinoza-Montero, P.J. Photoelectrocatalytic degradation of diclofenac with a boron-doped diamond electrode modified with titanium dioxide as a photoanode. Environ. Res. 2022, 212, 113362. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Szopińska, M.; Wilk, B.K.; Sobaszek, M.; Łuczkiewicz, A.; Bogdanowicz, R.; Fudala-Książek, S. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J. Hazard. Mater. 2021, 403, 123606. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Dettlaff, A.; Szopińska, M.; Karpienko, K.; Wróbel, M.; Łuczkiewicz, A.; Bogdanowicz, R. Simultaneous opto-electrochemical monitoring of carbamazepine and its electro-oxidation by-products in wastewater. J. Hazard. Mater. 2021, 419, 126509. [Google Scholar] [CrossRef]
- Brocenschi, R.F.; Rocha-Filho, R.C.; Duran, B.; Swain, G.M. The analysis of estrogenic compounds by flow injection analysis with amperometric detection using a boron-doped diamond electrode. Talanta 2014, 126, 12–19. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Rycewicz, M.; Łuczkiewicz, A.; Fudala-Książek, S.; Bogdanowicz, R.; Ruello, M.L. Electrodes criticality: The impact of CRMs in the leachate electrochemical oxidation. Manuf. Rev. 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Swain, G.M. The Use of CVD Diamond Thin Films in Electrochemical Systems. Adv. Mater. 1994, 6, 388–392. [Google Scholar] [CrossRef]
- Swain, G.M.; Ramesham, R. The Electrochemical Activity of Boron-Doped Polycrystalline Diamond Thin Film Electrodes. Anal. Chem. 1993, 65, 345–351. [Google Scholar] [CrossRef]
- Martin, H.B.; Argoitia, A.; Landau, U.; Anderson, A.B.; Angus, J.C. Hydrogen and Oxygen Evolution on Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 1996, 143, L133–L136. [Google Scholar] [CrossRef]
- Wilk, B.K.; Szopińska, M.; Sobaszek, M.; Pierpaoli, M.; Błaszczyk, A.; Luczkiewicz, A.; Fudala-Ksiazek, S. Electrochemical oxidation of landfill leachate using boron-doped diamond anodes: Pollution degradation rate, energy efficiency and toxicity assessment. Environ. Sci. Pollut. Res. 2022, 29, 65625–65641. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Water Works Association (AWWA) & Water Environment Federation (WEF): Washington, DC, USA, 2005. [Google Scholar]
- Panizza, M.; Delucchi, M.; Sirés, I. Electrochemical process for the treatment of landfill leachate. J. Appl. Electrochem. 2010, 40, 1721–1727. [Google Scholar] [CrossRef]
- Panizza, M.; Kapalka, A.; Comninellis, C. Oxidation of organic pollutants on BDD anodes using modulated current electrolysis. Electrochim. Acta 2008, 53, 2289–2295. [Google Scholar] [CrossRef]
- Urtiaga, A.; Ortiz, I.; Anglada, A.; Mantzavinos, D.; Diamadopoulos, E. Kinetic modeling of the electrochemical removal of ammonium and COD from landfill leachates. J. Appl. Electrochem. 2012, 42, 779–786. [Google Scholar] [CrossRef]
- Serrano, K.; Michaud, P.A.; Comninellis, C.; Savall, A. Electrochemical preparation of peroxodisulfuric acid using boron doped diamond thin film electrodes. Electrochim. Acta 2002, 48, 431–436. [Google Scholar] [CrossRef]
- Ding, J.; Bu, L.; Zhao, Q.; Kabutey, F.T.; Wei, L.; Dionysiou, D.D. Electrochemical activation of persulfate on BDD and DSA anodes: Electrolyte influence, kinetics and mechanisms in the degradation of bisphenol A. J. Hazard. Mater. 2020, 388, 121789. [Google Scholar] [CrossRef]
- Lacasa, E.; Llanos, J.; Cañizares, P.; Rodrigo, M.A. Electrochemical denitrification with chlorides using DSA and BDD anodes. Chem. Eng. J. 2012, 184, 66–71. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Xia, G.; Lu, Y.; Xu, D.; Sun, N.; Wang, J.; Chen, J. Process optimization of electrochemical oxidation of ammonia to nitrogen for actual dyeing wastewater treatment. Int. J. Environ. Res. Public Health 2019, 16, 2931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jiang, Y.; Li, Y.; Hu, Z.; Zhou, L.; Zhou, M. Three-dimensional electrochemical process for wastewater treatment: A general review. Chem. Eng. J. 2013, 228, 455–467. [Google Scholar] [CrossRef]
- Ghazouani, M.; Akrout, H.; Bousselmi, L. Nitrate and carbon matter removals from real effluents using Si/BDD electrode. Environ. Sci. Pollut. Res. 2017, 24, 9895–9906. [Google Scholar] [CrossRef]
- Zhou, B.; Yu, Z.; Wei, Q.; Long, H.Y.; Xie, Y.; Wang, Y. Electrochemical oxidation of biological pretreated and membrane separated landfill leachate concentrates on boron-doped diamond anode. Appl. Surf. Sci. 2016, 377, 406–415. [Google Scholar] [CrossRef]
- Fudala-Ksiazek, S.; Sobaszek, M.; Łuczkiewicz, A.; Pieczynska, A.; Ofiarska, A.; Fiszka-Borzyszkowska, A.; Sawczak, M.; Ficek, M.; Bogdanowicz, R.; Siedlecka, E.M. Influence of the boron doping level on the electrochemical oxidation of raw landfill leachates: Advanced pre-treatment prior to the biological nitrogen removal. Chem. Eng. J. 2018, 334, 1074–1084. [Google Scholar] [CrossRef]
- del Rosario Salas-Sandoval, E.; Pérez-Segura, T.; Garcia-Segura, S.; Dos Santos, A.J. Innovative approaches to electrochemical oxidation of bisphenol B in synthetic and complex water environments. Sci. Total Environ. 2024, 955, 176762. [Google Scholar] [CrossRef]
- Marselli, B.; Garcia-Gomez, J.; Michaud, P.-A.; Rodrigo, M.A.; Comninellis, C. Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J. Electrochem. Soc. 2003, 150, D79. [Google Scholar] [CrossRef]
- Herraiz-Carboné, M.; Santos, A.; Hayat, A.; Domínguez, C.M.; Cotillas, S. Remediation of groundwater polluted with lindane production wastes by conductive-diamond electrochemical oxidation. Sci. Total Environ. 2024, 926, 171848. [Google Scholar] [CrossRef]
- Zhi, D.; Lin, Y.; Jiang, L.; Zhou, Y.; Huang, A.; Yang, J.; Luo, L. Remediation of persistent organic pollutants in aqueous systems by electrochemical activation of persulfates: A review. J. Environ. Manag. 2020, 260, 110125. [Google Scholar] [CrossRef]
- Sharma, J.; Mishra, I.M.; Kumar, V. Degradation and mineralization of bisphenol A (BPA) in aqueous solution using advanced oxidation processes: UV/H2O2 and UV/S2O82− oxidation systems. J. Environ. Manag. 2015, 156, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Moussavi, G.; Pourakbar, M.; Shekoohiyan, S.; Satari, M. The photochemical decomposition and detoxification of bisphenol A in the VUV/H2O2 process: Degradation, mineralization, and cytotoxicity assessment. Chem. Eng. J. 2018, 331, 755–764. [Google Scholar] [CrossRef]
- Särkkä, H.; Bhatnagar, A.; Sillanpää, M. Recent developments of electro-oxidation in water treatment—A review. J. Electroanal. Chem. 2015, 754, 46–56. [Google Scholar] [CrossRef]
- Liu, Y.J.; Hu, C.Y.; Lo, S.L. Direct and indirect electrochemical oxidation of amine-containing pharmaceuticals using graphite electrodes. J. Hazard. Mater. 2019, 366, 592–605. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Fenti, A.; Blotevogel, J.; Musmarra, D. An innovative approach for atrazine electrochemical oxidation modelling: Process parameter effect, intermediate formation and kinetic constant assessment. Chem. Eng. J. 2023, 474, 146022. [Google Scholar] [CrossRef]
- Iovino, P.; Fenti, A.; Galoppo, S.; Najafinejad, M.S.; Chianese, S.; Musmarra, D. Electrochemical removal of nitrogen compounds from a simulated saline wastewater. Molecules 2023, 28, 1306. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Arts, A.; de Groot, M.T.; van der Schaaf, J. Current efficiency and mass transfer effects in electrochemical oxidation of C1 and C2 carboxylic acids on boron-doped diamond electrodes. Chem. Eng. J. Adv. 2021, 6, 100093. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and mediated anodic oxidation of organic pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Blais, J.F.; Mercier, G.; Lafrance, P. Experimental design methodology applied to electrochemical oxidation of the herbicide atrazine using Ti/IrO2 and Ti/SnO2 circular anode electrodes. J. Hazard. Mater. 2011, 185, 1499–1507. [Google Scholar] [CrossRef]
- Heim, C.; Rajab, M.; Greco, G.; Grosse, S.; Drewes, J.E.; Letzel, T.; Helmreich, B. Fate of diclofenac and its transformation and inorganic by-products in different water matrices during electrochemical advanced oxidation process using a boron-doped diamond electrode. Water 2020, 12, 1686. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S.; Skoumal, M.; Arias, C. Electrochemical incineration of diclofenac in neutral aqueous medium by anodic oxidation using Pt and boron-doped diamond anodes. Chemosphere 2010, 79, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, B.; Guan, X. Oxidative removal of bisphenol A by permanganate: Kinetics, pathways and influences of co-existing chemicals. Sep. Purif. Technol. 2013, 107, 48–53. [Google Scholar] [CrossRef]
- Watanabe, I.; Harada, K.; Matsui, T.; Miyasaka, H.; Okuhata, H.; Tanaka, S.; Nakayama, H.; Kato, K.; Bamba, T.; Hirata, K. Characterization of bisphenol A metabolites produced by Portulaca oleracea cv. by liquid chromatography coupled with tandem mass spectrometry. Biosci. Biotechnol. Biochem. 2012, 76, 1015–1017. [Google Scholar] [CrossRef]
- Ye, X.; Zhou, X.; Needham, L.L.; Calafat, A.M. In-vitro oxidation of bisphenol A: Is bisphenol A catechol a suitable biomarker for human exposure to bisphenol A? Anal. Bioanal. Chem. 2011, 399, 1071–1079. [Google Scholar] [CrossRef]
- Bakr, A.R.; Rahaman, M.S. Removal of bisphenol A by electrochemical carbon-nanotube filter: Influential factors and degradation pathway. Chemosphere 2017, 185, 879–887. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Ignatev, A.N.; Frimmel, F.H.; Bräse, S.; Horn, H.; Revelsky, A.I. Formation of genotoxic quinones during bisphenol A degradation by TiO2 photocatalysis and UV photolysis: A comparative study. Appl. Catal. B Environ. 2014, 160, 106–114. [Google Scholar] [CrossRef]
- Jewell, K.S.; Falås, P.; Wick, A.; Joss, A.; Ternes, T.A. Transformation of diclofenac in hybrid biofilm–activated sludge processes. Water Res. 2016, 105, 559–567. [Google Scholar] [CrossRef]
- Calza, P.; Sakkas, V.A.; Medana, C.; Baiocchi, C.; Dimou, A.; Pelizzetti, E.; Albanis, T. Photocatalytic degradation study of diclofenac over aqueous TiO2 suspensions. Appl. Catal. B Environ. 2006, 67, 197–205. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; d’Ischia, M. Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res. 2004, 38, 414–422. [Google Scholar] [CrossRef]
- Coelho, A.D.; Sans, C.; Agüera, A.; Gómez, M.J.; Esplugas, S.; Dezotti, M. Effects of ozone pre-treatment on diclofenac: Intermediates, biodegradability and toxicity assessment. Sci. Total Environ. 2009, 407, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fernández-Alba, A.R. Photo-Fenton degradation of diclofenac: Identification of main intermediates and degradation pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shao, Y.; Gao, N.; Chen, J.; Zhang, Y.; Xiang, H.; Guo, Y. Degradation of diclofenac by UV-activated persulfate process: Kinetic studies, degradation pathways and toxicity assessments. Ecotoxicol. Environ. Safety 2017, 141, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Faber, H.; Melles, D.; Brauckmann, C.; Wehe, C.A.; Wentker, K.; Karst, U. Simulation of the oxidative metabolism of diclofenac by electrochemistry/(liquid chromatography/) mass spectrometry. Anal. Bioanal. Chem. 2012, 403, 345–354. [Google Scholar] [CrossRef]
- Agüera, A.P.E.L.A.; Pérez Estrada, L.A.; Ferrer, I.; Thurman, E.M.; Malato, S.; Fernández-Alba, A.R. Application of time-of-flight mass spectrometry to the analysis of phototransformation products of diclofenac in water under natural sunlight. J. Mass Spectrom. 2005, 40, 908–915. [Google Scholar] [CrossRef]
- Cheng, H.; Song, D.; Liu, H.; Qu, J. Permanganate oxidation of diclofenac: The pH-dependent reaction kinetics and a ring-opening mechanism. Chemosphere 2015, 136, 297–304. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).