1. Introduction
Due to climate change and increasing periods of drought and extensive rainfall, among others, general interest in the macroscopic behaviour of water is rising. In tandem with this general backdrop, there is increasing interest in understanding water in terms of its meso- and micro-scale phenomena. Nowhere is this more apparent than in the case of bubble dynamics on these scales, e.g., in terms of gas transfer dynamics and Stokes’ law for bubble dissipation [
1]. Indeed, the (re)discovery of the floating water bridge—and the missing context to explain it theoretically—shows us that there is much to learn vis-à-vis “anomalous” or simply surprising water flow and macroscopic structural behaviour [
2]. With emerging puzzles in our understanding of metastable microscopic bubbles that alter the time-resolved behaviour of water, perhaps even the concept of liquid-like nature needs a re-examination in the light of bubble populations [
3]; given that microscopic bubble dissipation will occur over the Stokes’ law timescale of hours, or longer, this may serve to rationalise the observations of changes in macroscopic properties over time.
The Austrian forester, inventor, and engineer Viktor Schauberger might be considered a natural scientist in the literal sense, in that he studied water in unspoiled natural environments that were still abundant in his lifetime about a century ago when he formulated many of his biomimetic hydrodynamic flow pattern ideas. Based on his observations, Schauberger developed many biomimetic water vortex devices and distinguished between various types of water like “old”, “juvenile”, and “fresh” [
4]. Schauberger stated that water should be allowed to flow in vortex-like, “corkscrew”, inward swirling movements, akin to the eddy-current flow around stones in a fast-flowing river (as he observed), to keep it “alive”, although there were few widely available macroscopic observables in his time by which to gauge this emotive-sounding observation.
Of course, in science, the vitality of water is a non-existent concept; effectively, it is “spiritual” and heuristic in nature as an explanation and not useful for mechanistic studies or insights into any physical rigour. Nonetheless, scientific interest in “Schauberger’s technology” of such a vortex-like flow of water/“processing” with inward swirling patterns is growing, despite any such erroneous and risible assertions of vitality, which arguably has since hampered more serious research efforts. The present authors are agnostic and indifferent to the emotive controversy surrounding “life” explanations. In any event, this situation as regards the greater interest in more in-depth mechanistic study of such centuries-old inward swirling flow patterns is now changing; for instance, Agostinho and colleagues measured a marked increase in oxygen mass transfer by an inward vortex flow pattern device that was designed according to Schauberger’s principles [
5]. A plasma reactor with a constrained inward swirling vortex according to the same Schauberger design appears to be a highly efficient tool for the removal of micropollutants, e.g., PFAS [
6,
7]. These vortex flow arrangements clearly require further study for us to understand the physically realistic, mechanistic explanations as to their occasionally observed effectiveness, other than the more “spiritual” ones.
Natural shapes, or vortex flow, was the basis for Schauberger’s work. So, in a quest to study the validity of Schauberger’s claim that natural vortices enhance water to retain its “lively” qualities, one of the authors (C.K.) grew interested in whether the ideas of Schauberger could, in fact, be applied to the various questions and challenges posed by modern-day water treatment, e.g., the distribution of gases in liquids. This author studied the various types of natural flow-enhancing systems [
8,
9,
10], always bearing in mind that Schauberger’s claims would encounter scientific scepticism, so the concepts needed to be simple and straightforward. Various concepts were thus deemed unsuitable. From the ideas, however, the following concept, simple yet elegant in its novelty, arose. This author allowed tap water to flow through a vortex shape that occurs in nature. A Turritella shell was cut perpendicular to its longest axis in order to attach it to a tap and allow tap water to flow through, with apparently positive results in terms of taste enhancement, although this is subjective (vide infra). Initial experiments indicated changes in the DO as well, pointing to a rather wide field of possible applications, as well as an answer to the questions concerning the application of Schauberger’s principles.
More generally, the concept of “age” in vortex-processed water, despite emotive—and somewhat erroneous-seeming—claims of “life” in water per se, points to a heuristic observation of the possibility of time-dependent and time-resolved macroscopic properties. One natural explanation—indeed, a potential hypothesis to explain such an observation—may be that meso- to microbubbles generated via vortex–swirl dynamics in the water simply persist for a time by the virtue of their intrinsic metastability and dissipate over minutes to hours via Stokes’ law for rising. Therefore, in the present work, we investigate this phenomenon.
Tap water that had passed through this cut-off shell appeared to change in taste. This was confirmed by a taste session in which a blind panel scored the water as fresher (
p < 0.023) and less thick (
p < 0.037) compared to the water that had not been passed through the device. Three other parameters—freshness, purity, and overall judgment—did not differ significantly [
11].
To overcome and address these challenges and open questions about the “age” of water, we were keen to develop a novel and original approach towards the efficient generation of fine bubbles, ideally in the “small-micro” range in the order of, or less than, about 1 micron, to allow us to test a hypothesis of Stokesian fine-bubble dissipation arising putatively from Schauberger-style vortices. Driven by these observations, a product was developed by bio-mimicking the original shell. However, the actual shape of the inside channel of a Turritella prevents the manufacture of it by injection moulding since the channel cannot be released from a mould. Therefore, 3D printing would be a more logical choice. However, at the time of conceptualization of the design, no food-safe, let alone drinking-water-safe, materials for 3D printing were available. Hence, the adaptation of the design was heavily constrained. It appeared that the geometry of the channel was critical, and many designs did not produce any effect on the taste of the water. After the 23rd prototype, a design was conceived that could be injection moulded and produced the same effects on taste as the original. That design was tried and tested and finally manufactured in ceramics. Hence, the Turritap was born. The Turritap consists of a ceramic core enveloped by a steel hull, with a thread that fits most water taps. Users have reported that the water becomes lighter and easier to drink. The Turritap can also be equipped with a funnel for use with other liquids, e.g., tea, wine, and coffee. Laymen, as well as connoisseurs, have reported differences in taste, with a general trend that the separate components are easier to distinguish; they become more balanced. Not everyone experiences this as positive. In experiments performed to pinpoint any physical differences in the liquids themselves, a slight change in dissolved oxygen was measured. Parameters like pH and ORP did not appear to differ.
In an effort to rationalise these observations of flow through the Turritap—a modern embodiment of Schauberger’s hydrodynamic engineering—the current study measures the changes in microbubble distribution over time after their generation by flowing through such a device. Indeed, a boost in the microbubble population served as a potent motivation for the present study, in that gauging how nano-, micro- and fine-bubble enhancement could benefit many other often disparate fields of application is important; advanced oxidation processes, for instance, wherein finer bubbles serve to enhance oxygen transfer and hence benefit reaction kinetics, e.g., [
1,
2,
3], or [
4,
5,
6]. This is a timely study, owing to the surge in interest in both nano- [
12,
13,
14,
15,
16] and micro- [
12,
13,
16,
17] bubbles, particularly for promoting advanced oxidation and ozonation processes.
2. Materials and Methods
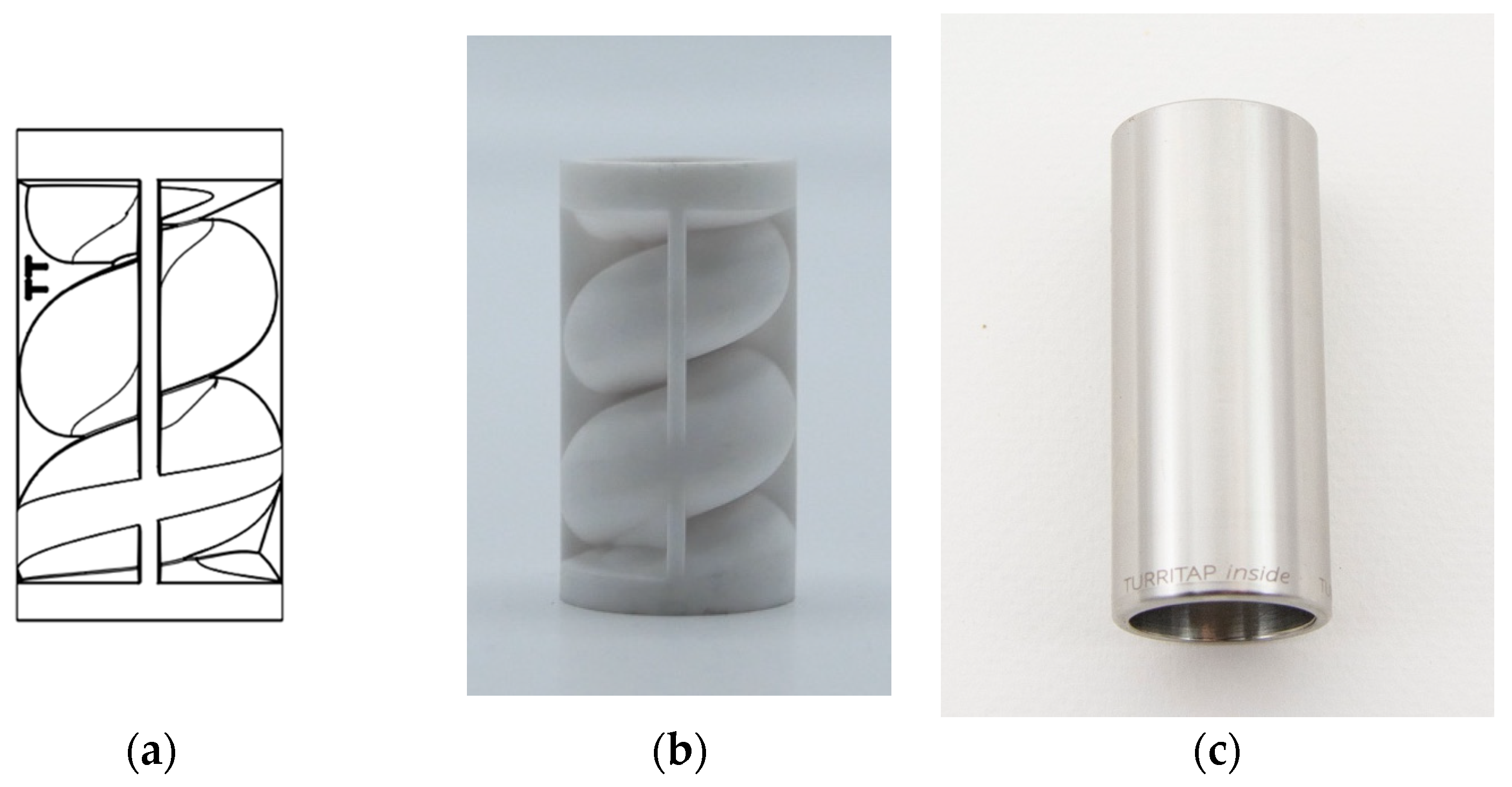
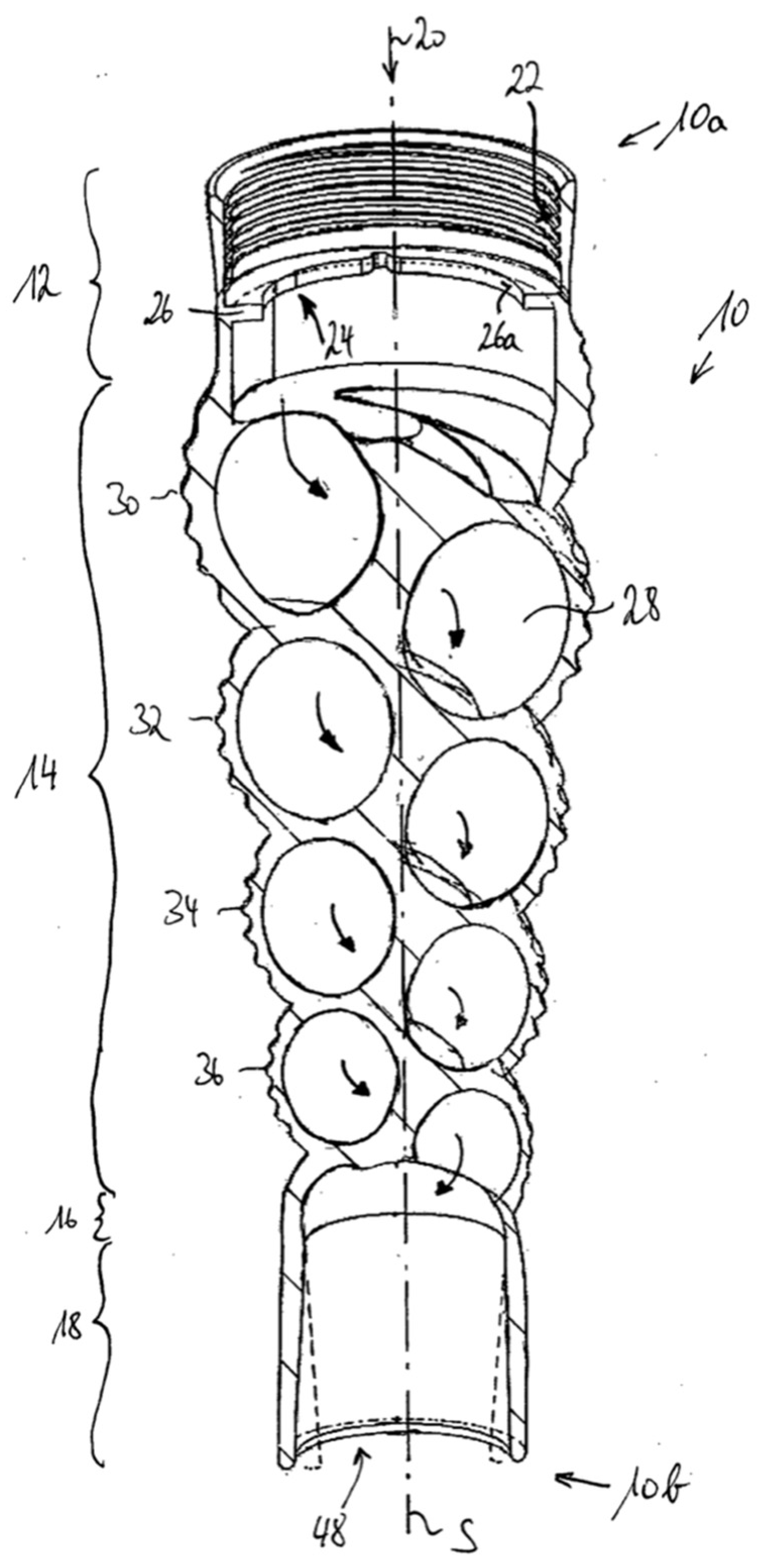
The Turritap is a steel end fitting, suitable for a tap, with a ceramic swirling element and a perlator (Neoperl GmbH, Müllheim Germany) added downstream of the element (cf.
Figure 1 and
Figure 2). The steel fitting is 57.5 mm long and has an ID of 21 mm. The upstream threading is M22x1, so it will fit most common taps. The ceramic element is supported by a seal on either side to prevent leakage. The patented design of the swirling channel inside of the ceramic element mimics a Turritella shell [
18]. The ceramic element is 38.5 mm long with an outer diameter of 20.8 mm to fit the hull accurately. The added perlator reduces the tangential velocity component of the exiting water, ensuring that the outflow is essentially longitudinal along the length axis of the Turritap; this prevent spraying in other directions, which would be impractical in day-to-day use of the Turritap as a tap extension.
In terms of the water sources, both deionised (DI) and filtered tap water were used. DI water was from CarPlan (Rochdale, UK). In the case of the latter, a three-stage water reverse osmosis filter from AquaClear Ltd. (Dublin, Irleand) was used for Dublin tap water, and filtration was carried out until the derived count rate in a Malvern Zetasizer Pro (from Malvern Panalytical, Malvern, UK) was just below the 100 k.c.p.s. threshold to minimise the level of (light-scattering) impurities. The use of DI and filtered tap water is important for reproducibility and to allow for reasonable levels of statistical error to be found for each of these water types. Given that the Turritap is to be deployed almost universally for drinking water, usually municipal water from taps—although often also filtered and/or bottled drinking water—it was felt that municipal tap water which was consistently well-filtered would blend the best of statistical reproducibility with as close to the real-world experience as Turritap would need for eventual deployment.
In the case of DI water, one may speculate that the relative absence of impurities allows for more reproducible levels of bubble nucleation in convective flow situations. In the case of tap water, the light-scattering micro-particulates present may allow for some scope of heterogeneous bubble nucleation to occur, which might, ceteris paribus, allow for the promotion of the rate of microbubble (MB) nucleation.
The tap perlator and the Turritap were put on 30 L tanks of each of these water sources, and five experiments were carried out and monitored over time, at a flowrate of ~5 L min−1. The pH and DO were monitored over time from outflow samples, and dynamic light scattering (DLS) was also performed with a Malvern Zetasizer Pro to establish the level of bubble population and the associated size distributions by comparison with a number of background water-type samples which were not passed through the Turritap, so as to identify genuine light-scattering features.
It is useful to briefly critique the methodology here with respect to the putative detection of microbubbles, in terms of being “fit-for-purpose”, in addition to the other methods used, e.g., pH and DO measurement, etc. In essence, the light-scattering approach, despite being the tool of choice for MB detection and population/size characterisation, does have some potential drawbacks or, at least, caveats in some form. The most obvious of these is the minimal viable MB population for detection of the order of 106 MBs per mL, such that variations in the population over time suddenly decreases to nought after declining in a measurable way with Stokes’ law for bubble-rising dissipation. In addition, it is not always clear, for the bubble populations, what the detection distinction might be with respect to hydrodynamic radius or, indeed, the more fundamental question of what the bubble dimension represents in terms of the gas–liquid interface or, in other words, where the phases meet in (radial) space. Another point to bear in mind is that the DO probes do not measure MB populations directly, whilst pH shifts often depend on the underlying water quality (although less so in the context of the present study, owing to careful filtering).
3. Results and Discussion
For general reference with the two above-described water sources, we carried out the Turritap water passage experiments for regular, unfiltered tap water. If anything, the results for this water source showed a greater level of variability than for the two studied water sources, and the results were relatively close to those for the filtered case, albeit with somewhat higher populations (of the order of 10–15%), owing to a greater opportunity for heterogeneous bubble nucleation on micro- and mesoscopic particulates, with the presence of a lower MB-nucleation free-energy barrier.
However, the level of noise in the DLS spectra was often rather high, which made a definitive conclusion more difficult; this is only to be expected with higher levels of light-scattering micro-particulates present in unfiltered municipal tap water.
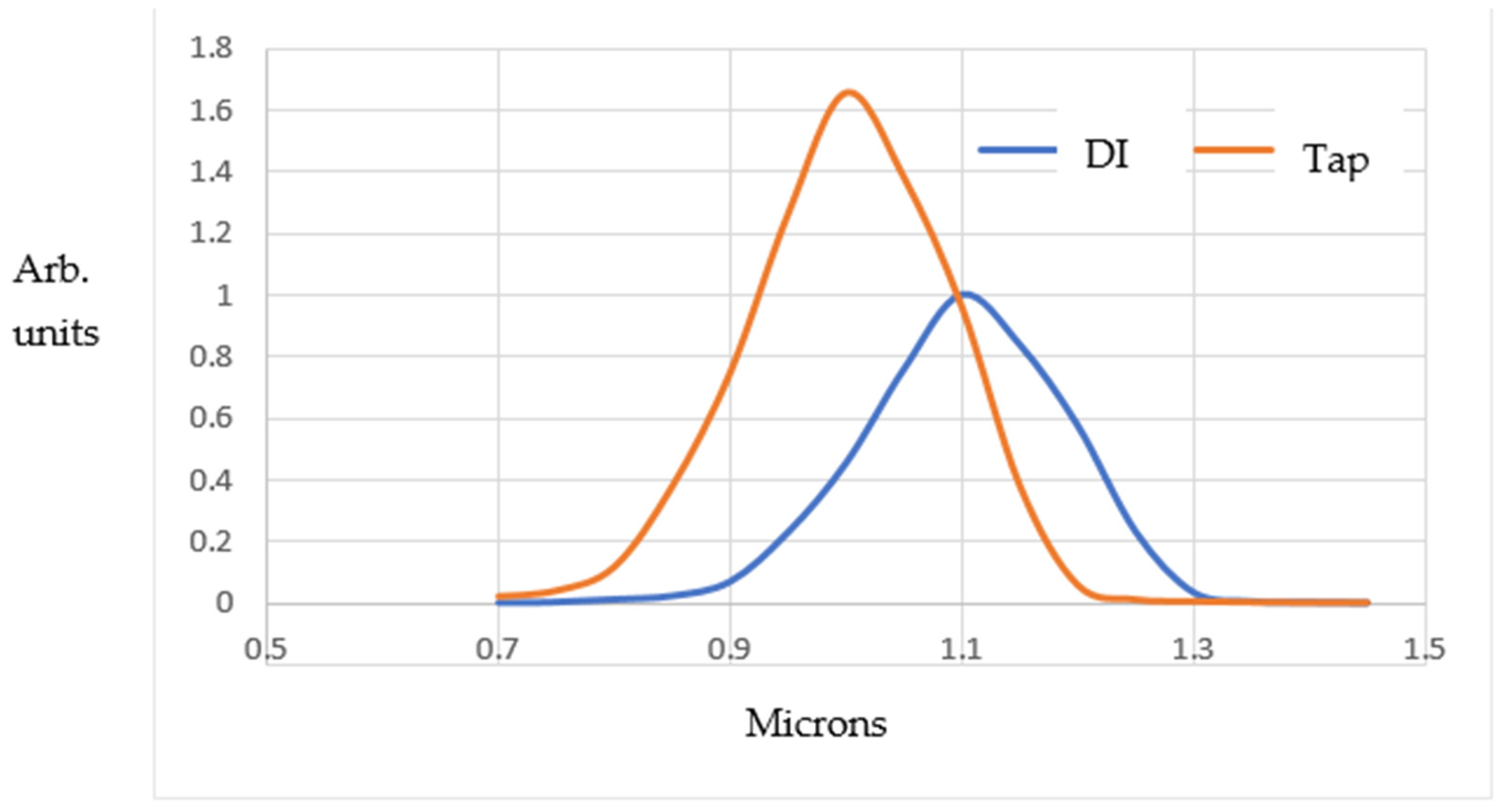
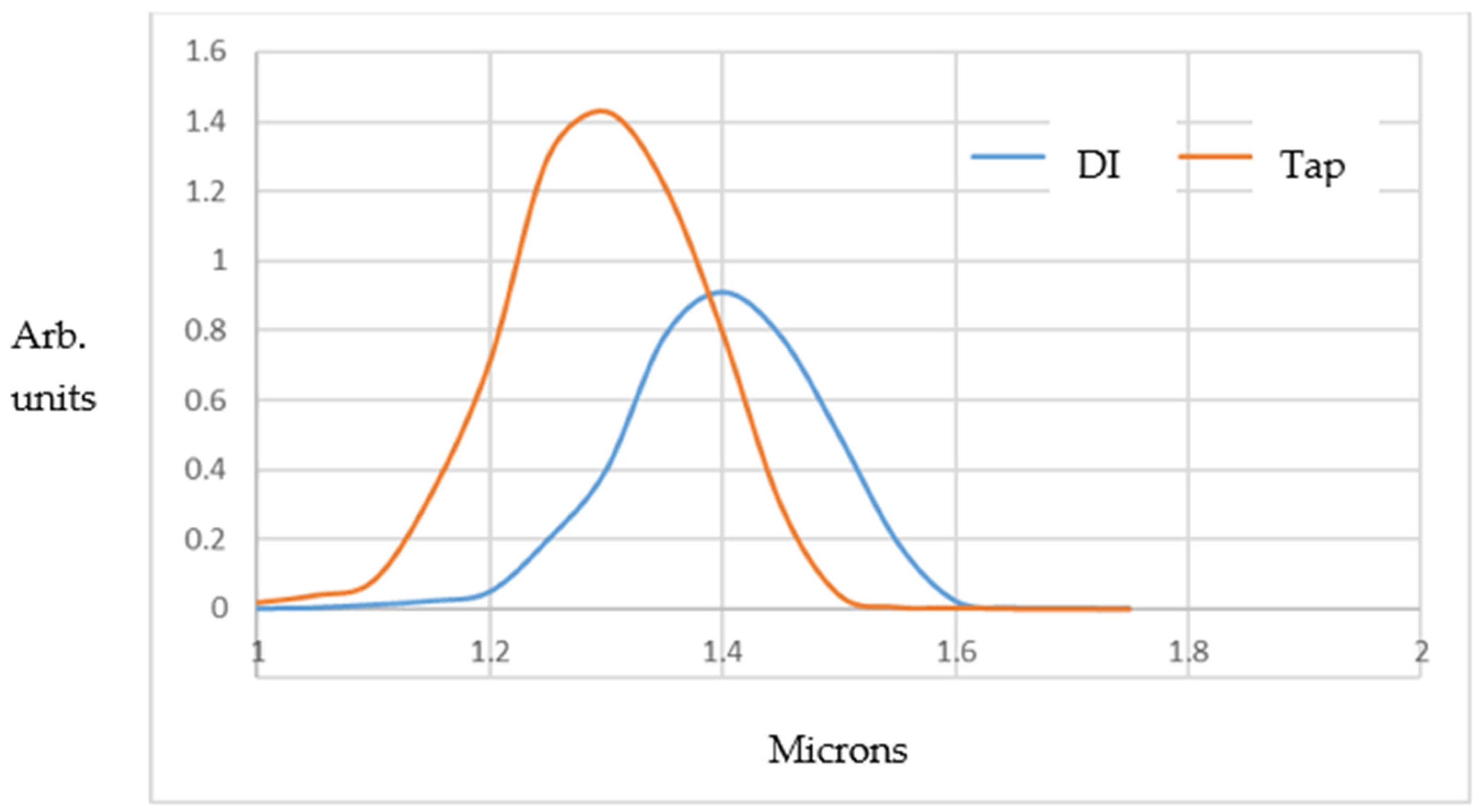
The time-resolved DLS results for the bubble population/size distributions are shown in
Figure 3,
Figure 4 and
Figure 5 for both water types, evolving over up to 3 h after passage through the Turritap at a typical tap flowrate (~5 L min
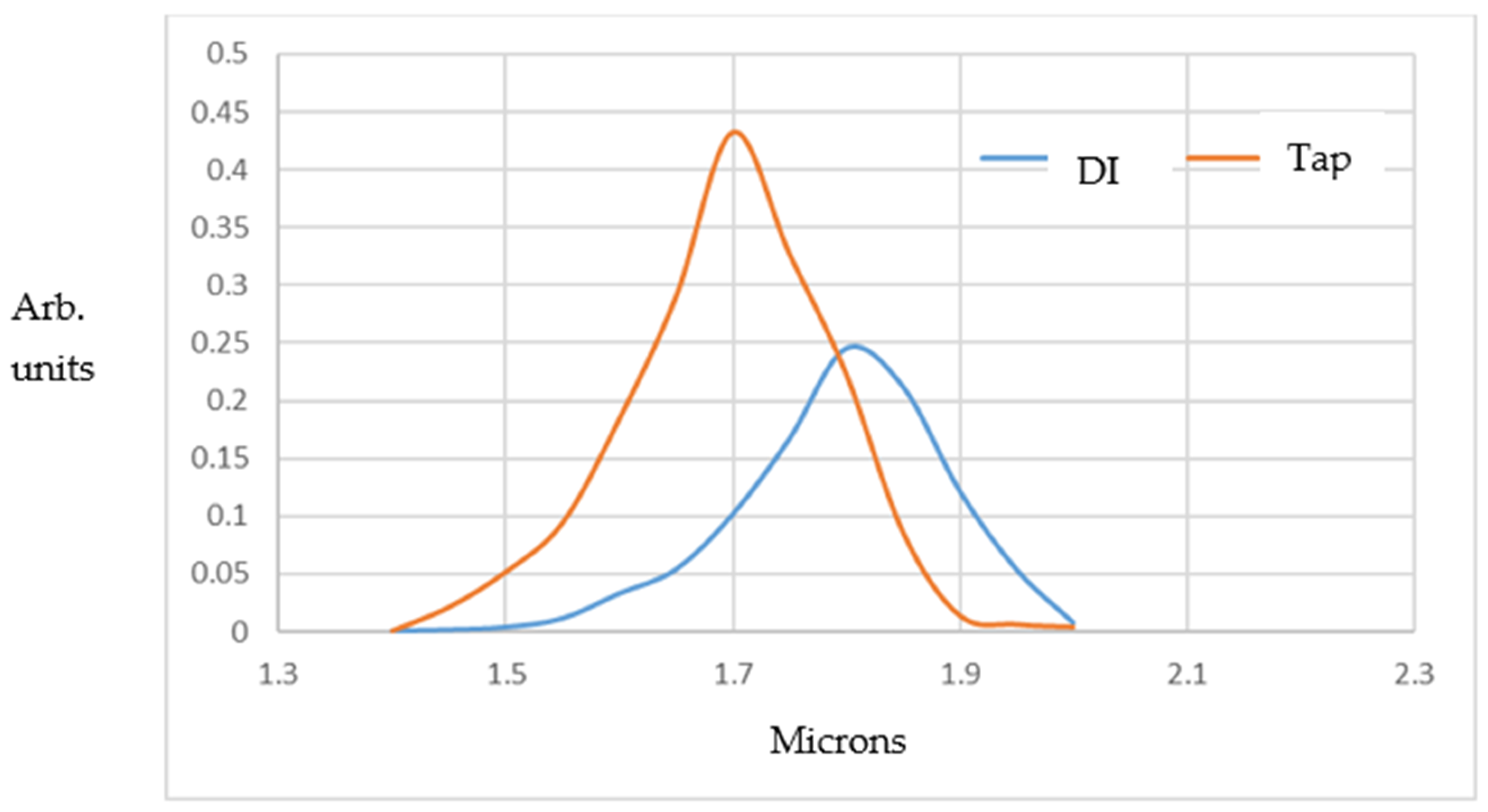
−1). It can be seen that the MB population is about 1.5 times larger at peak height for filtered tap water compared to DI water (i.e., about 9.7 × 10
6 versus 6.8 × 10
6 MBs/mL “fresh” out of the Turritap, within the ~2–3 min DLS measurement period). In addition, the filtered tap water shows smaller bubble sizes, owing, no doubt, to a more rapid nucleation rate on the residual micro- and mesoscopic particulates present when the derived count rate drops somewhat below 100 k.c.p.s.
Carrying out exponential decay analysis on the DLS-measured MB populations gave relaxation times τ of ~2.4 h and 3.6 h in exp(−t/τ) fits for the DI and filtered tap water, respectively. This is roughly consistent with Stokes’ law for MB-rising dissipation timescales and escape into the atmosphere in the open-to-atmosphere (albeit indoor) samples. Therefore, the heuristic and anecdotal observations of the changes in the taste of Turritap-processed water, as well as the “texture” over minutes to hours (which were summarised briefly in the Introduction section), appear to stem primarily from simple Stokes’ law for MB dissipation.
In stark contrast, in the case of equivalent water not passed through the Turritap, there was no detection of any bubbles, so this would correspond to a “zero” line in
Figure 3,
Figure 4 and
Figure 5. The dissolved oxygen level in the unprocessed background water sample for DI water was about 84 ± 1.1%. It was 86 ± 1.2% for unfiltered tap water.
The downward shift in pH was about 0.08 ± 0.016 and 0.11 ± 0.018 for DI and filtered tap water, respectively. After three hours, this was 0 and 0.025 ± 0.004 lower, respectively, compared to the corresponding unprocessed background samples. This pH “normalisation” over time appears consistent with the DLS decay and Stokes’ law MB-dissipation timescales.
For DI water, the level of dissolved oxygen (DO) at room temperature of 19 °C was ~102% at “t = 0”, and it declined to ~87% within 3 h (with the unprocessed background sample being about 84 ± 1.1%). The respective DO decay results in the case of the filtered tap water were as follows (at 19 °C): ~105% at “t = 0”, declining to 91% within 3 h (background = 86 ± 1.2%). Again, given that the oxygen mass transfer is occurring by Fick’s law from Stokes’ law for dissipating MBs to the regularly dissolved state according to Henry’s law, it is to be expected that the driving force for the supersaturation of oxygen decays over time with MB dissipation.
Measurements on the perlator only, without the ceramic Turritap core, indicated a slight increase in bubble population, often dissipating within the timeframe of the DLS measurement.
In the Methodology section, we mentioned briefly the point about uncertainty over the physical interpretation of the bubble size per se. In this sense, the current results show unambiguously that microbubbles are present, although the size may be “fuzzy” in terms of where the radial gas–water interface lies. However, it must also be kept in mind that there are capillary wave fluctuations present at the MB interfaces occurring over timescales that are markedly shorter than those of the light-scattering impurities (with the light at a similar order of magnitude in terms of wavelength vis-a-vis the MBs), and this serves to contribute to the overall level of uncertainty in the boundary between the hydration layer and the gas density. In the context of the lifetime and survival kinetics of MBs, quite separate from Stokes’ law for dissipation, is the lower population limit for statistically reliable MB detection by light scattering. This can accelerate, to some extent, the observed population dissipation timescales (but not always in a consistent manner, in that the lower detection limit itself can vary in the general 106 range), which emphasizes the importance of repeated measurements to establish uncertainty envelopes for the detection of MB dissipation timescales and may lead to a potential systematic underestimation of MB dissipation timescales (although not by too much, as the presently discussed/determined dissipation kinetics are broadly in line with those expected from Stokes’ law).
4. Conclusions
The inward vortex–swirl-type motion of water flow has been studied vis-à-vis its propensity for bubble formation, with a particular focus on the microbubble region. It has been found that a large population of smaller microbubbles, in the order of 1 μm in diameter, is created in the process of these types of motions, and the time-dependent behaviour of this “micro-bubbly” water is analysed as Stokes’ law for microbubble dissipation occurs.
Schauberger’s flow “legacy” appears to be a population of MBs dissipating according to Stokes’ law; we therefore put forth, in the present work, a simple explanation vis-à-vis controversial views about the “vitality” of water being primarily the phenomenon of MB creation and inevitable dissipation. Naturally, this may well be of interest in gas transfer optimisation in the growing field of “fine-bubble engineering”. In addition, a broad panoply of processes which depend on both diffusion and the promotion of general oxidative processes, e.g., advanced oxidation, gas-limited chemical reaction promotion (e.g., hydrogenation), and pipe-cleaning ozonation, may well expect to benefit from the application of geometrically constricted flow, i.e., vortices or swirls, and their microbubble legacy, as employed in the Turritap.