Abstract
Water pollution caused by fluoranthene (FLN), phenanthrene (PHE), and pyrene (PYR) has become an increasingly serious issue in recent years. Consequently, finding effective methods to remove these pollutants from aquatic environments is of paramount importance. This study investigated the removal rate of FLN, PHE, and PYR from simulated wastewater using persulfate (PS) and explored the effects of PS catalyzed by three different forms of schwertmannite (sch): sch-1, sch-2, and sch@BC (schwertmannite-biochar composite), and the BET of sch-1, sch-2, sch@BC has been detected, which were 1.09 cm3/g, 11.30 cm3/g, and 6.10 cm3/g. The results showed varying removal rates after a 1 h reaction time for different treatments: For FLN: sch-1+PS (98.5%), sch-2+PS (54.2%), sch@BC+PS (21.1%), and PS alone (14.8%). For PHE: sch-1+PS (94.3%), sch-2+PS (44.1%), sch@BC+PS (28.4%), and PS alone (7.6%). For PYR: sch-1+PS (97.2%), sch-2+PS (52.5%), sch@BC+PS (14.2%), and PS alone (1.7%). Among the catalysts tested, sch-2 (added 0.36 mL H2O2 five times) demonstrated excellent catalytic ability in enhancing the PS removal of FLN, PHE, and PYR. This research provides theoretical support for treating polycyclic aromatic hydrocarbon (PAH)-containing wastewater via persulfate oxidation catalyzed by schwertmannite.
1. Introduction
Water plays a vital role in human lives; however, water pollution and clean water shortage have become global issues which are the result of industrial and agricultural activities [1]. In recently years, the water pollution caused by polycyclic aromatic hydrocarbons (PAHs) is a typical water pollution issue caused by organic compounds [2,3], and it has attracted widespread attention around the world. And many researchers found the presence of PAHs in various water environments, such as wastewater from chemical treatment plant, surface water, groundwater and even in the ocean [4].
PAHs are aromatic hydrocarbons containing two or more benzene rings [5]. PAHs could be produced by natural combustion such as forest or brush fires and human activities such as the burning of fossil fuels [6], automobile exhaust [7], cigarette smoke [8], and industrial activities [9]. Generally, PAHs produced by combustion and vehicle emissions diffuse into the atmosphere first, and a part of it enters into soil or water as deposits. Different from the PAH pollution in the atmosphere, another important pollution source in water is the discharge of wastewater caused by industrial activities, increasing the concentration of PAHs in a water environment. Fluoranthene (FLN), phenanthrene (PHE), and pyrene (PYR) are frequently in high quantities in water environments around the world according to many references [10,11,12,13]; these studies study the PAH concentration of drinking water, rivers, and lakes, chemical wastewater and even groundwater. The reports show that the concentration of FLN, PHE, and PYR in drinking water in China [11] ranged from 0.004 mg/L to 0.02 mg/L, and in Poland [14] it ranged from 0.01 mg/L to 0.05 mg/L. Liu et al. [15] and Pan et al. [16] evaluated the PAHs in rivers or lakes from China and found that the concentrations of FLN, PHE, and PYR ranged from 0.002 mg/L to 0.73 mg/L. Nasher et al. [17] have found that the concentration of FLN, PHE, and PYR in seawater of the South China Sea is 1.4, 0.6, and 1.8 mg/L, respectively. Therefore, removing FLN, PHE, and PYR from the water environment is an urgent issue.
The treatments currently used to remove FLN, PHE, and PYR from wastewater include biological, physical, and chemical methods [18,19]. The biological methods include phytoremediation and bioremediation. According to the Reynoso-cuevas et al.’s [20] study, 6.99% of PAHs could be accumulated at the stem of F. arundinacea by phytoremediation methods, meanwhile, 20.66% accumulated at the root. The physical and chemical methods include adsorption and advanced oxidation processes (AOPs): common adsorbents include activated carbon, bentonite, biochar, nano-tubes, and so on [21,22,23], and AOPs including Fenton oxidation, UV irradiation, ozone oxidation, photocatalysis, electrochemical oxidation, and persulfate (PS) oxidation [24,25]. Among them, the persulfate oxidation method is popular due to its economy and durability. And, activated methods are used to improve the oxidation efficiency of PS [26], including heat activation [27], microwave activation [28], and transition metal [29,30,31] (Fe, Cu, Zn, Mn, etc.) activation.
The special hydrated iron oxyhydroxide rich in sulfate [32] (schwertmannite, Fe8O8(OH)x(SO4)y) was used with weak crystallinity and large specific surface area, it has been often used as an adsorbent and catalyst. The schwertmannite (sch) is usually used for adsorption due to its large specific surface area. Liao et al. [33] adsorbed As(III) by sch from groundwater and found that the maximum amount of adsorption was 113.9 mg/g, and the adsorption of arsenic was not affected by other competing ions in the system. In recent years, sch has also been used in the field of the catalytic degradation of organic pollutants [34,35,36]. The study found that the introduction of sch could make the removal rate of 4-nitrophenol (4-NP) in the Fenton system reach 88.9% in 1 h [37], and the synergistic effect of sch and oxalic acid could remove 97% methyl orange from wastewater [34]. Biochar has also been extensively employed as a versatile adsorbent, and bamboo biochar showed a high adsorption rate of its microcrystalline structure with high porosity, fast adsorption, and high active surface area [38].
Sch has been popular as a catalyst and adsorbent in recent years, and PS oxidation has been widely used for the removal of organic pollutants from wastewater. However, there are no reports on schwertmannite-catalyzed PS removing FLN, PHE, and PYR from wastewater. Furthermore, the sch synthesis with bamboo biochar used to remove FLN, PHE, and PYR from wastewater has not been studied yet. Therefore, this study focuses on (1) different methods to synthesize schwertmannite; (2) comparing the effects of schwertmannite synthesized with different methods (including treatment added bamboo biochar) in catalyzed PS on the removal of FLN, PHE, and PYR from wastewater. The goals of this study have practical significance for controlling and removing FLN, PHE, and PYR from wastewater.
2. Materials and Methods
2.1. Chemical Reagents
Phenanthrene (>95%), FeSO4·7H2O (>99.0%), Na2S2O8 (>97%), acetone (>99.5%), and methanol (>99.5%) are the Wako 1st Grade reagents, and they were provided by Fujifilm Wako Pure Chemical Corporation, Osaka, Japan; Fluoranthene (>98%) provided by Tokyo Chemical Industry, Japan; dichloromethane (>99.5%) and pyrene (>97%) are Wako Special Grade, and they were obtained from Fujifilm Wako Pure Chemical Corporation, Osaka, Japan; hexane (>99.5%) was Wako Special Grade, and it was purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan; pyrene-d10 (>99.99%) was supplied by AccuStandard, New Haven, CT, USA and it was Wako Special Grade; silica was purchased from Nacalai Tesque, Inc., Kyoto, Japan; Deionized water was prepared by a water purifier (Direct-Q 3 UV, Millipore, Burlington, MA, USA).
2.2. Preparation of Sch with Three Synthesis Methods
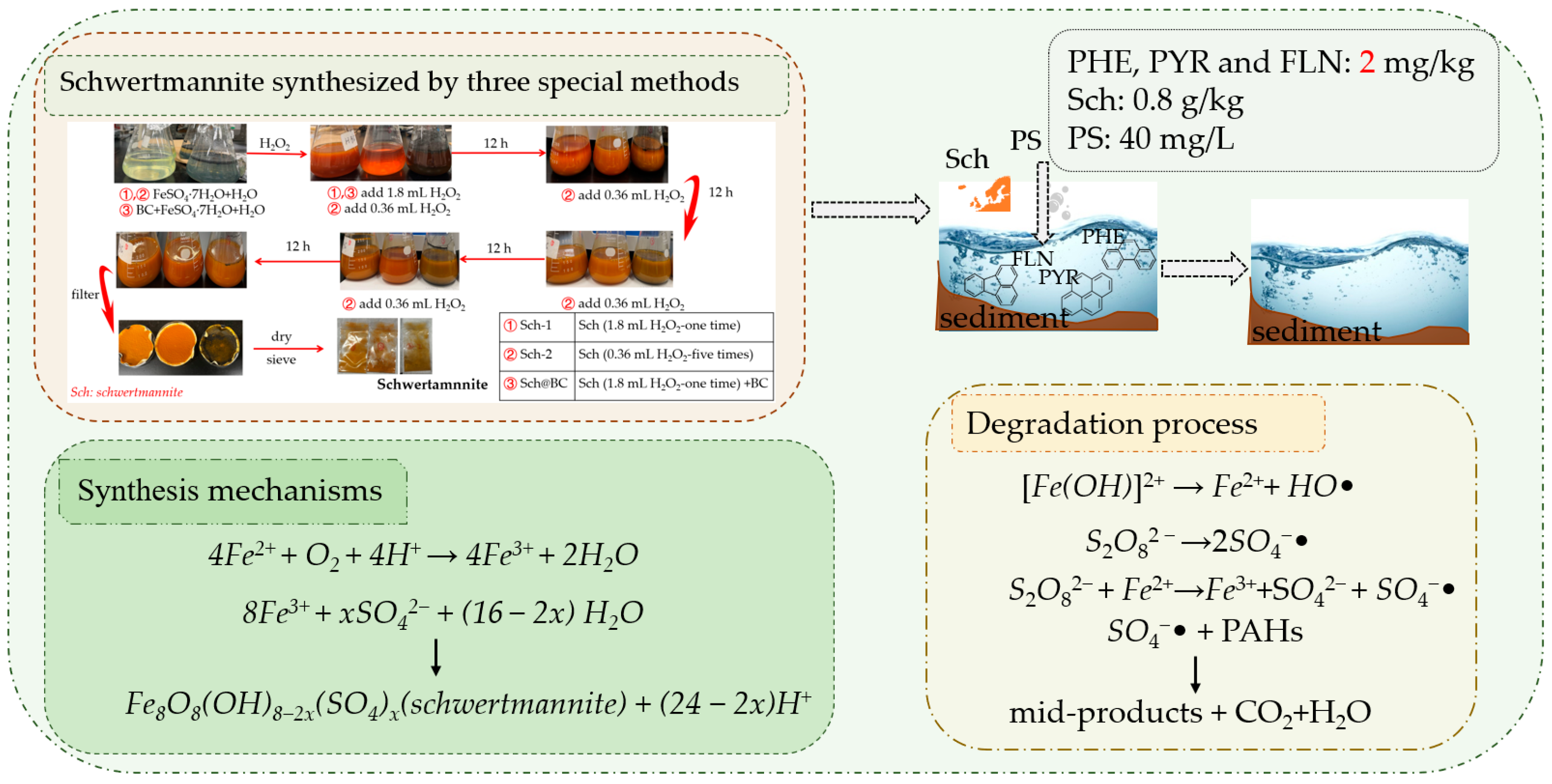
The different types of sch were prepared by the oxidation of ferrous sulfate solution with H2O2 following the methods of Liu et al. [39]. In short, 1.80 mL of H2O2 was added into a flask with 150 mL of 160 mmol/L FeSO4·7H2O solution. In this research, we used three methods to synthesize the special sch; first of all, we prepared FeSO4∙7H2O solution (160 mmol/L) following three methods:
- (1)
- sch-1: add 1.8 mL H2O2 at 2 h;
- (2)
- sch-2: add 0.36 mL H2O2 at 2, 14, 26, 38, and 50 h;
- (3)
- sch@BC: add 1.8 mL H2O2 at 2 h, and add biochar (mFe2+:mBC = 1:20) at 3 h.
The precipitates formed by different treatments were washed three times by H2SO4 solution (pH = 2.0) and then washed three times by deionized water. Then, they were collected and oven-dried at 50 °C to constant weight (around 60 h). The mineral was identified by scanning electron microscopy (SEM), and X-ray diffraction (XRD). Moreover, the BET of Sch was also evaluated. The research process and degradation mechanisms of this study are shown in Figure 1.
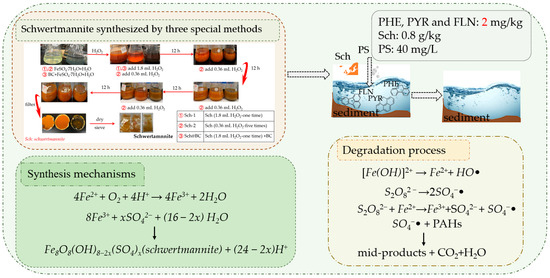
Figure 1.
Research process and degradation mechanisms. Notes: FLN: fluoranthene; PHE: phenanthrene; PYR: pyrene; PS: persulfate; sch: schwertmannite.
The preparation method of biochar (BC) is as follows: bamboo was ground through a 250 μm sieve and was collected from Anhui, China. Using single-step activation to synthesize biochar, a ceramic boat was used to put 5 g bamboo powder in a stainless horizontal tubular furnace the N2 atmosphere condition was 200 mL/min flow rate for 10 min to exhaust air and then the temperature was set as 800 °C for 1 h with a heating rate of 10 °C/min to activated [22].
The wastewater in this study is simulated (synthetic) wastewater. The preparation process is as follows: in a series of 250 mL Erlenmeyer flasks, 50 mL total solution volume and a total of 12 treatments were set up as Table 1. All the treatments were repeated 3 times and reacted for 3 h on a magnetic stirrer (RS-6DN, AS ONE company, Osaka, Japan) with 1000 r·min−1. Sampling was carried out at 1 h, 2 h, and 3 h, and passed through a 0.20 μm membrane filter. We added 150 μL methanol to quench free radicals, and then extracted FLN, PHE, and PYR from wastewater, and GC-MS was used to determine the contents of FLN, PHE, and PYR according to the method [40].

Table 1.
Different treatments of the special sch-catalyzed persulfate remove FLN, PHE, and PYR from simulated wastewater. Notes: ——: not added; FLN: fluoranthene; PHE: phenanthrene; PYR: pyrene; PS: persulfate; sch: schwertmannite; sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
2.3. Analytical Methods
We used Rotavapor (R-100, Buchi, Essen, Germany) to concentrate the extraction of FLN, PHE, and PYR; we used GC-MS instrument (6890A (G1530A), Agilent, Redwood City, CA, USA) to determine the concentration of FLN, PHE, and PYR in the extract. Gas Chromatography–Mass Spectrometry (GC-MS) investigations was conducted by a 0.25 mm internal diameter (i.d.) × 30 m length column with 0.25 μm film thickness, specifically an Intertcap 17 column. An HP6890 GC interface was used in the GC system with a 5973 MSD detector. A temperature of 40 °C was set as the initial temperature of the column and it was kept for 2 min; after that it was raised to 200 °C with a ramp rate of 15 °C/min, and then we changed the ramp rate to 5 °C /min till it increased to 320 °C. The carrier gas utilized was helium with high purity and the flow was set at 1 mL/min. The electron ionization mode with an electron multiplier voltage of 70 eV was acquired by MS spectra. The m/z range between 45 and 350 has been scanned by mass scanning. Finally, the samples’ chromatographic peaks were identified by the NIST 98 mass spectra library.
Furthermore, a scanning electron microscope (SEM, S-4800, Hitachi Co., Ltd., Tokyo, Japan) was used to observe morphology. Power X-ray diffraction (XRD, MiniFlex II, Tokyo, Japan) was applied for mineral phase analysis. Fourier transform infrared spectroscopy (FTIR) (IR-6100, JASCO Co., Ltd., Tokyo, Japan) was used to determine the chemical bonds of sch. The automatic specific surface area and pore analyzer (BELSORP-miniX-TKS0, MicrotracBEL Corp., Osaka, Japan) was used to obtain the mineral-specific surface area. An integrated sulfur analyzer (HYDL-9, Hebi Huayu Instrument Co., Ltd., Cangzhou, China) was applied to determine the sulfur. Descriptive statistics were performed using Microsoft Excel 2019. Data analysis was conducted with the Origin 2016 software package.
3. Results
3.1. Characterization of Sch
The scanning electron microscope (SEM) image (×5000) of sch-1 are shown in Figure 2a; sch-1 has spherical aggregates with smaller particle diameters, and it is smooth, which is the typical morphology of chemically synthesized schwertmannite. Figure 2b shows the SEM of sch-2; it can be found that the sch-2 surface is rough and has burrs, which is the same as the morphology of Sch observed by Liu et al. [39]. The SEM of sch@BC is displayed in Figure 2c; the composites have a prominent aggregate structure, and the sch has covered the surface of BC, which is similar to the morphology of sch synthesized by others [41]. The cell diameters of sch-1, sch-2, and sch@BC were measured under an SEM image (×30,000): 1.11 μm, 1.36 μm, and 1.32 μm, respectively.

Figure 2.
Scanning electron microscopy (SEM) of (a) sch-1; (b) sch-2; (c) sch@BC. Notes: sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
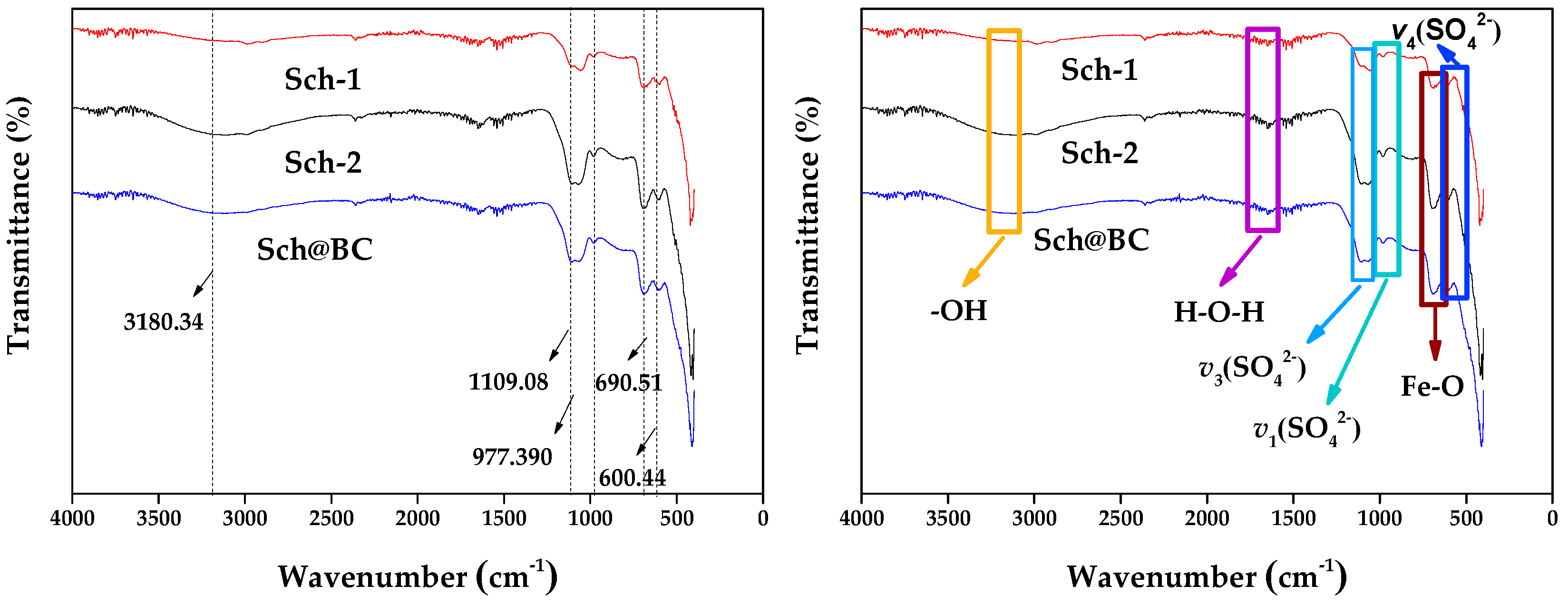
The Fourier transform infrared spectroscopy (FTIR) is shown in Figure 3; the contraction vibration peak at 3180.34 cm−1 is the hydroxyl group (-OH) on the sch surface. The triple degenerate asymmetric absorption peak ν3(SO42−) of the sulfate ion of schwertmannite is shown as a broad peak of contraction vibration at 1109.08 cm−1. The inner symmetric contraction peak at 977.39 cm−1 is ν1(SO42−). Both ν1(SO42−) and ν3(SO42−) are considered to be the sulfate groups externally complexed by schwertmannite. The absorption peak of ν4(SO42−) at 600.44 cm−1 is the sulfate inside the tunnel of schwertmannite. The results show that the contraction vibration peak at 690.51 cm−1 is the Fe-O absorption peak. These peaks are similar to the morphology of sch synthesized by others [42].
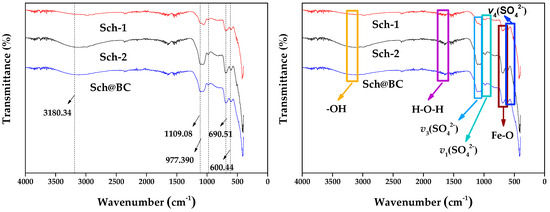
Figure 3.
Fourier transform infrared spectroscopy (FTIR) of sch-1; sch-2; sch@BC. Notes: sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
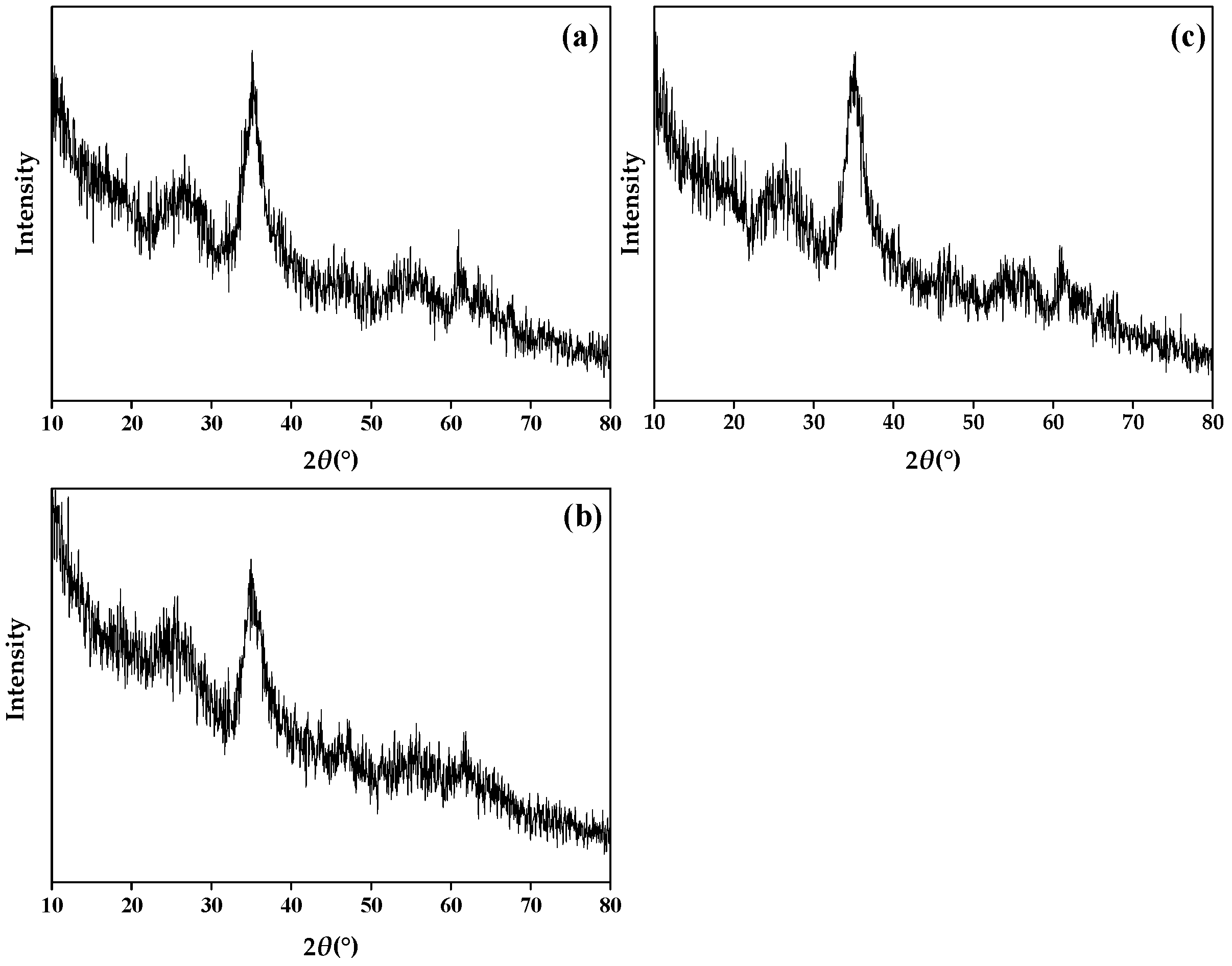
Figure 4 shows the X-ray diffraction (XRD) of sch, and the peaks of 2θ = 18.24°, 26.26°, 35.16°, 39.50°, 46.54°, 55.30°, and 61.34° are the characteristic diffraction peaks of sch. It can see from Figure 4 that the three types of minerals have no sharp and strong peaks; they have wide peaks and more burrs. Compare this with the standard XRD peaks of sch (JCPDS: PDF47-1775). It is certain the three minerals synthesized in this study are sch.
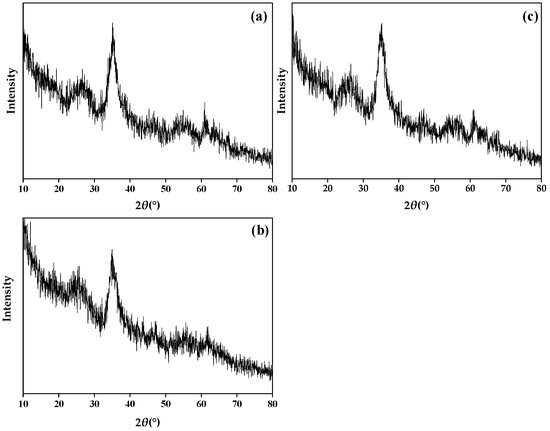
Figure 4.
X-ray diffraction (XRD) of (a) sch-1; (b) sch-2; (c) sch@BC. Notes: sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
Table 2 shows the BET and formulae of different sch. The BET of sch-1, sch-2, and sch@BC is 1.09 m2/g, 11.30 m2/g, and 6.10 m2/g, respectively. And, the sch-2 has highest pore volume which is 7.35 × 10−2 cm3/g, followed by sch@BC which is 1.50 × 10−2 cm3/g, and the pore volume of sch-1 is 1.09 × 10−2 cm3/g. The mean pore diameter of sch-1, sch-2, and sch-BC is 22.85 nm, 25.99 nm, and 9.86 nm, respectively. It also could be found that the molar ratios of Fe/S in sch-1, sch-2, and sch@BC were 4.66, 4.72, and 6.59, respectively. And, the formulae of sch-1, sch-2, and sch@BC were determined as Fe8O8(OH)4.56(SO4)1.72, Fe8O8(OH)4.61(SO4)1.70, and Fe8O8(OH)5.48(SO4)1.21, respectively.

Table 2.
BET and formulae of different sch. Notes: sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
3.2. Sch with Different Methods Catalyze Persulfate to Control FLN, PHE, and PYR from Simulated Wastewater
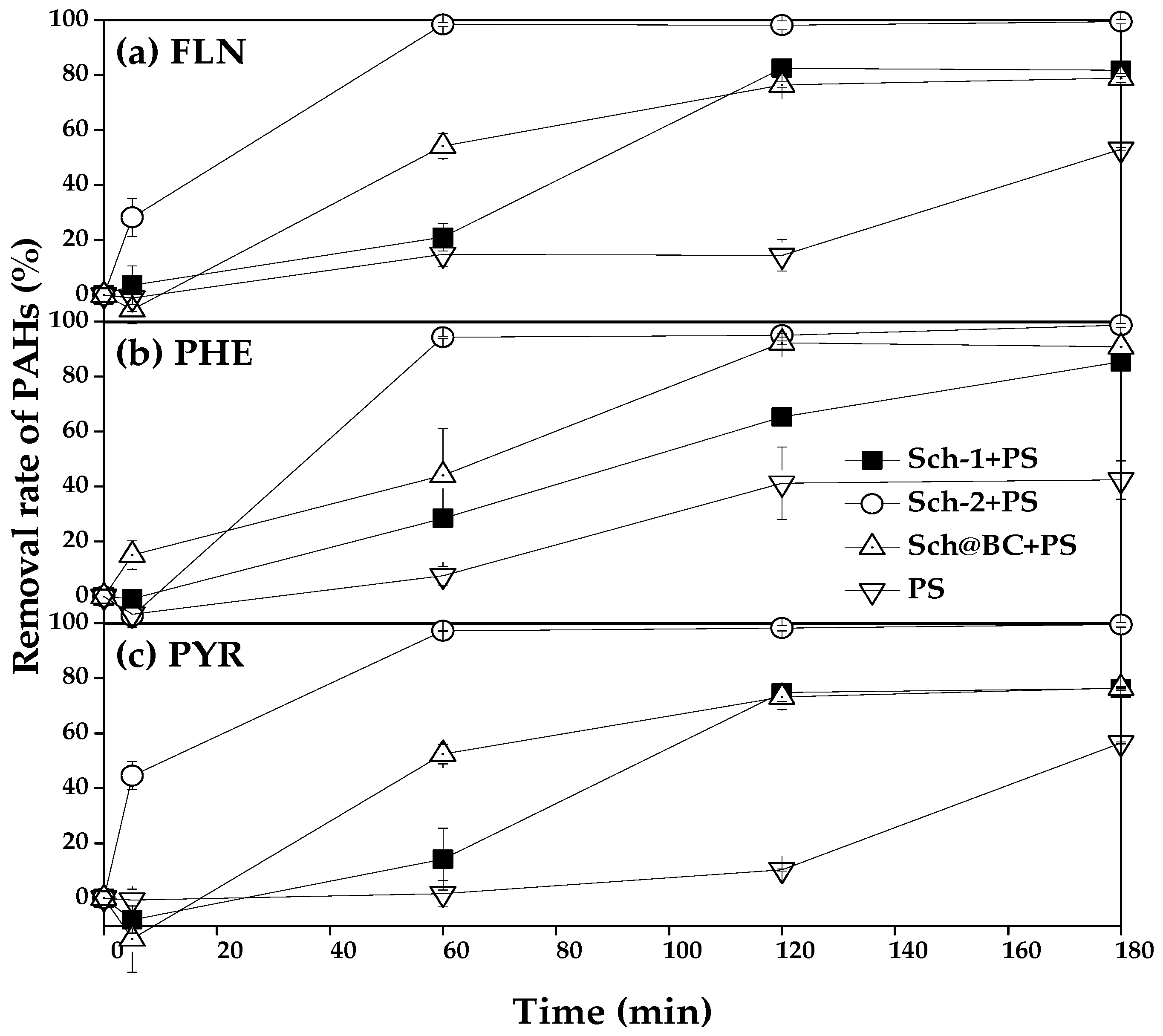
PS remove FLN, PHE, and PYR catalyzed by different sch as shown in Figure 5; it can be seen that the removal rate of FLN in treatment which added sch-1+PS, sch-2+PS, and sch@BC+PS after reacting for 1 h was 21.1%, 98.5%, and 54.2%, respectively, from Figure 5a; meanwhile, the removal rate of FLN was 14.8% in the treatment which only added PS. After reacting for 2 h, the removal rate of FLN in treatment which added sch-1+PS, sch-2+PS, sch@BC+PS, and PS was 82.6%, 98.1%, 76.5%, and 14.5%, respectively. After reacting for 3 h, the removal rate of FLN in the treatment which only added PS increased to 53.0%, and in other treatments which added sch-1+PS, sch-2+PS, and sch@BC, it was 81.8%, 99.5%, 78.9%, respectively.
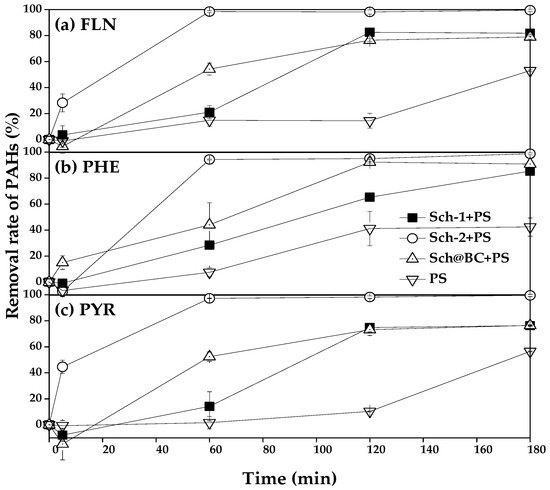
Figure 5.
Schwertmannite catalyze persulfate to remove (a) FLN; (b) PHE; (c) PYR. Notes: FLN: fluoranthene; PHE: phenanthrene; PYR: pyrene; PS: persulfate; sch: schwertmannite; sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
Figure 5b shows the PS catalyzed by different sch to remove PHE, and it can be found that 28.4%, 94.3%, 44.1%, and 7.6% of the PHE has been removed after reacting for 1 h in the treatment of sch-1+PS, sch-2+PS, sch@BC, and PS, respectively. The removal rate of PHE in the treatment of sch-1+PS, sch-2+PS, sch@BC, and PS was 65.3%, 95.0%, 92.2%, and 41.2%, respectively, after reacting for 2 h. And after reacting for 3 h, the removal rate of PHE in sch-1+PS, sch-2+PS, and sch@BC was 85.4%, 98.7%, and 90.8%, respectively, but in treatment that added PS only, the removal rate of PHE was only 42.4% after reacting for 3 h. The removal rate of PYR is shown in Figure 5c; it can be found that after reacting for 1 h, sch-1+PS, sch-2+PS, sch@BC, and PS could remove 14.2%, 97.2%, 52.5%, and 1.7% PYR, respectively. And after reacting for 2 h, the removal rate of PYR in the treatment of sch-1+PS, sch-2+PS, sch@BC, and PS was 74.8%, 98.2%, 73.2%, and 10.3%, respectively. After reacting for 3 h, the removal rate of PYR in treatment which added sch-1+PS, sch-2+PS, and sch@BC was 76.3%, 99.5%, and 76.5%, respectively, and the removal rate of PYR was 56.5% in the treatment of PS after reacting for 3 h.
Figure 6 shows the SEM image (×5000) of sch-1, sch-2, and sch@BC after the reaction; the surface of schwertmannite has no obvious change and is still slightly rough, appearing as spherical aggregates with burrs, compared with the SEM of schwertmannite before the reaction. The cell diameters of sch-1, sch-2, and sch@BC after reaction were measured under an SEM image (×30,000): 1.30 μm, 1.44 μm, and 1.40 μm, respectively.

Figure 6.
Scanning electron microscopy (SEM) of (a) sch-1; (b) sch-2; (c) sch@BC after reaction. Notes: sch-1: add 1.8 mL one time; sch-2: add 0.36 mL H2O2 five times; sch@BC: add 1.8 mL H2O2 one time with biochar (mFe2+:mBC = 1:20).
4. Discussion
As is shown in Table 2, sch-2 has a larger BET (11.30 m2/g) compared with sch-1 (1.09 m2/g) and sch@BC (6.10 m2/g); combined with the FTIR shown in Figure 3, the vibration peak of sch-2 is stronger, and the -OH vibration peak of sch-2 fluctuates greatly, which indicates that there are more externally bound hydroxyl functional groups, and the ν3(SO42−) and ν4(SO42−) stretching vibration peaks of sch-2 fluctuate greatly, indicating that it have more internally and externally complexed SO42−. Wang et al. [43] have found that the sch with stronger vibration peaks have more binding sites. Therefore, it could be indicated that sch-2 has more binding sites, which makes it have better adsorption and catalysis performance. According to Figure 5, it can be found that the treatment that only added PS could only remove 53.0% FLN, 42.4% PHE, and 56.5% PYR after reacting for 1 h. It is due to the fact that S2O82− in PS can produce SO4−· (Equation (1)). When adding sch into the wastewater, whatever kind of sch, the removal rate of FLN, PHE, and PYR was all better than the treatment that only added PS; this is because sch can produce Fe (II) (Equation (2)) [44], which can react with S2O82− to produce more SO4−· (Equation (3)) [32,45]. Many researchers [46,47,48] found that Fe can activate S2O82− to produce more SO4−·, which is similar to the results of this study. And, the sch used in this study has a large amount of Fe, which can continuously supply Fe for the activation of S2O82−.
S2O82− → 2SO4−·
[FeIII(OH)]2+ → Fe(II) + HO·
S2O82− + Fe(II) → Fe(III) + SO42− +SO4−·
And, it was found that sch-2 has the best capacity on catalyzed persulfate to remove FLN, PHE, and PYR. Adding sch-2 after 1 h could remove 98.5% FLN, 94.3% PHE, and 97.2% PYR by catalyzing PS, respectively. And after reacting for 3 h, the removal rates of FLN, PHE, and PYR were 99.5%, 98.7%, and 99.5%, respectively. It is due to the fact that sch-2 has more binding sites, which can promote the S2O82− to produce SO4−· more rapidly than others, and then SO4−· can react with FLN, PHE, and PYR to destroy their structures. And, the -OH on the surface of sch also can react with SO4−· to produce HO· (Equation (4)); HO· can also destroy the structure of FLN, PHE, and PYR and control them from wastewater. Compared with the system that added PS only, we found that the system with PS catalyzed by sch greatly promotes the reaction process and affects the reaction mechanism, which is similar to the results of Szabados [49]. Wang et al. [32] discovered the same mechanism when studying the activation of persulfate by sch to remove oxytetracycline.
SO4−· + HO− → SO42− + HO·
In summary, the removal rate of FLN, PHE, and PYR by PS is slow. The addition of sch greatly improves the removal rate of FLN, PHE, and PYR by PS, and the sch with more binding sites has a better catalytic effect.
5. Conclusions
In this study, schwertmannite was synthesized by three special methods, and sch-catalyzed PS was used to remove FLN, PHE, and PYR from wastewater, and we found that (1) Sch synthesized by adding 0.36 mL H2O2 five times (sch-2) has the biggest BET (11.3 m2/g); (2) the system with PS catalyzed by sch greatly promotes the reaction process of removing FLN, PHE, and PYR compared with the system that added PS only; (3) Sch-2 shows the best capacity on catalyzed persulfate to remove FLN, PHE, and PYR. Therefore, sch (synthesized by adding 0.36 mL H2O2 five times) is an excellent catalytic material for PS to remove FLN, PHE, and PYR from chemical wastewater.
Author Contributions
Y.W.: validation and writing—original draft preparation; W.W.: data curation and visualization; F.L.: methodology and writing—reviewing and editing; Q.W.: project administration, writing—review and editing, funding acquisition, and supervision; S.W.: validation. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the Special Funds for Innovative Area Research (Number. 20120015, FY2008-FY2012); Basic Research (B) (Number. 24310005, FY2012-FY2014; Number. 18H03384, FY2017-FY2020; Number. 22H03747, FY2022-FY2024) of Grant-in-Aid for Scientific Research of Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan; and the Shanxi Province 1331 Project funded project (20211331-15).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Y.W., upon reasonable request.
Acknowledgments
We thank the Comprehensive Analysis Center for Science, Saitama University for allowing us to conduct some analyses and providing insight and expertise that greatly assisted the research.
Conflicts of Interest
The authors (Yanyan Wang, Weiqian Wang, Fenwu Liu, Qingyue Wang, and Shangrong Wu) declared that they have no conflicts of interest in this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
- Wang, J.; Liu, X.; Liu, G.; Zhang, Z.; Cui, B.; Bai, J.; Zhang, W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol. Environ. Saf. 2019, 173, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Edokpayi, J.N.; Odiyo, J.O.; Durowoju, O.S. Impact of wastewater on surface water quality in developing countries: A case study of South Africa. In Water Quality; Tutu, H., Ed.; Intech Open: London, UK, 2017. [Google Scholar] [CrossRef]
- Lin, C.; Nguyen, K.A.; Vu, C.T.; Senoro, D.; Villanueva, M.C. Contamination levels and potential sources of organic pollution in an Asian river. Water Sci. Technol. 2017, 76, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Grandclement, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef] [PubMed]
- Rayaroth, M.P.; Marchel, M.; Boczkaj, G. Advanced oxidation processes for the removal of mono and polycyclic aromatic hydrocarbons—A review. Sci. Total Environ. 2023, 857, 159043. [Google Scholar] [CrossRef]
- Uddin, M.M.; Xu, F. Sources, Occurrences, and Risks of Polycyclic Aromatic Hydro-Carbons (PAHs) in Bangladesh: A Review of Current Status. Atmosphere 2024, 15, 233. [Google Scholar] [CrossRef]
- Papadopoulou, P.; Misseyanni, A.; Marouli, C. Current environmental health challenges: Part I—Exposures and research trends. In Handbook of Research on Emerging Developments and Environmental Impacts of Ecological Chemistry; IGI Global: Hershey, PA, USA, 2020; pp. 1–37. [Google Scholar]
- Stejskalova, L.; Dvorak, Z.; Pavek, P. Endogenous and exogenous ligands of aryl hydrocarbon receptor: Current state of art. Curr. Drug. Metab. 2011, 12, 198–212. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Olayinka, O.O.; Adewusi, A.A.; Olarenwaju, O.O.; Aladesida, A.A. Concentration of Polycyclic Aromatic Hydrocarbons and Estimated Human Health Risk of Water Samples around Atlas Cove, Lagos, Nigeria. J. Health Pollut. 2018, 8, 181210. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, B.; He, Z.; Li, L.; Shi, H.; Wang, M. Enantioselective metabolism of four chiral triazole fungicides in rat liver microsomes. Chemosphere 2019, 224, 77–84. [Google Scholar] [CrossRef]
- Adekunle, A.S.; Oyekunle, J.A.O.; Ojo, O.S.; Maxakato, N.W.; Olutona, G.O.; Obisesan, O.R. Determination of polycyclic aromatic hydrocarbon levels of groundwater in Ife north local government area of Osun state, Nigeria. Toxicol. Rep. 2017, 4, 39–48. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Sun, J.; Zhang, Y.; Liu, C. The distribution and sources of polycyclic aromatic hydrocarbons in shallow groundwater from an alluvial-diluvial fan of the Hutuo River in North China. Front. Earth Sci. 2019, 13, 33–42. [Google Scholar] [CrossRef]
- Kabzinski, A.K.M.; Cyran, J.; Juszczak, R. Determination of Polycyclic Aromatic Hydrocarbons in Water (Including Drinking Water) of Lodz. Pol. J. Environ. Stud. 2002, 11, 695–706. [Google Scholar]
- Liu, Y.; Shen, J.; Chen, Z.; Ren, N.; Li, Y. Distribution of polycyclic aromatic hydrocarbons in surface water and sediment near a drinking water reservoir in Northeastern China. Environ. Sci. Pollut. Res. 2013, 20, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Pan, E.C.; Sun, H.; Xu, Q.J.; Zhang, Q.; Liu, L.F.; Chen, X.D.; Xu, Y. Polycyclic Aromatic Hydrocarbons Concentrations in Drinking Water in Villages along the Huai River in China and Their Association with High Cancer Incidence in Local Population. BioMed Res. Int. 2015, 2015, 762832. [Google Scholar] [CrossRef]
- Nasher, E.; Heng, L.Y.; Zakaria, Z.; Surif, S. Concentrations and Sources of Polycyclic Aromatic Hydrocarbons in the Seawater around Langkawi Island, Malaysia. J. Chem. 2013, 2013, 975781. [Google Scholar] [CrossRef]
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Azelee, N.L.W.; Jeon, B.H.; et al. Polyaromatic hydrocarbons (PAHs) in the water environment: A review on toxicity, microbial biodegradation, systematic biological advancements, and environmental fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef]
- Dai, C.; Han, Y.; Duan, Y.; Lai, X.Y.; Fu, R.B.; Liu, S.G.; Leong, K.H.; Tu, Y.J.; Zhou, L. Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environ. Res. 2022, 205, 112423. [Google Scholar] [CrossRef]
- Reynoso-Cuevas, L.; Cruz-Sosa, F.; Gutiérrez-Rojas, M. In vitro phytoremediation mechanisms of PAH removal by two plant species. In Polycyclic Aromatic Hydrocarbons: Pollution, Health; Haines, P.A., Hendrickson, M.D., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Karaca, G.; Baskaya, H.S.; Tasdemir, Y. Removal of polycyclic aromatic hydrocarbons (PAHs) from inorganic clay mineral: Bentonite. Environ. Sci. Pollut. Res. 2016, 23, 242–252. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Q.Y. Investigation of Pyrolysis/Gasification Process Conditions and Syngas Production with Metal Catalysts Through Waste Bam-boo Biomass: Effects and Insights. Sustainability 2023, 15, 14588. [Google Scholar] [CrossRef]
- Paszkiewicz, M.; Sikorska, C.; Leszczyńska, D.; Stepnowski, P. Helical multi-walled carbon nanotubes as an efficient material for the dispersive solid-phase extraction of low and high molecular weight polycyclic aromatic hydrocarbons from water samples: Theoretical study. Water Air Soil Pollut. 2018, 229, 253. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Gaurav, G.K.; Mehmood, T.; Kumar, M.; Cheng, L.; Sathishkumar, K.; Kumar, A.; Yadav, D. Review on polycyclic aromatic hydrocarbons (PAHs) migration from wastewater. J. Contam. Hydrol. 2021, 236, 103715. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Li, C.; Wang, Z.; Zhao, S.; Xu, Y.X.; Wang, S.G. Polycyclic aromatic hydrocarbons degradation mechanisms in methods using activated persulfate: Radical and non-radical pathways. Chem. Eng. J. 2023, 473, 145319. [Google Scholar] [CrossRef]
- Li, N.; Wu, S.; Dai, H.X.; Cheng, Z.J.; Peng, W.C.; Yan, B.B.; Chen, G.Y.; Wang, S.B.; Duan, X.G. Thermal activation of persulfates for organic wastewater purification: Heating modes, mechanism and influencing factors. Chem. Eng. J. 2022, 450, 137976. [Google Scholar] [CrossRef]
- Feng, Y.; Tao, Y.; Meng, Q.; Qu, J.; Ma, S.; Han, S.; Zhang, Y. Microwave-combined advanced oxidation for organic pollutants in the environmental remediation: An overview of influence, mechanism, and prospective. Chem. Eng. J. 2022, 441, 135924. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.Q.; Lv, M.Y.; Ye, X.Y.; Ma, J.L.; Lin, Z.; Fu, M.L. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Shi, Q.; Deng, S.; Zheng, Y.; Du, Y.L.; Li, L.; Yang, S.Z.; Zhang, G.X.; Du, L.; Wang, G.F.; Cheng, M.; et al. The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes. Environ. Res. 2022, 212, 113340. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.Q.; Jin, Z.L.; Xiao, J.Y.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhou, J.; Bi, W.L.; Qin, J.M.; Wang, G.H.; Wang, Z.L.; Fu, P.; Liu, F.W. Schwertmannite catalyze persulfate to remove oxytetracycline from wastewater under solar light or UV-254. J. Clean. Prod. 2022, 364, 132572. [Google Scholar] [CrossRef]
- Liao, Y.H.; Liang, J.R.; Zhou, L.X. Adsorptive removal of As(III) by biogenic schwertmannite from simulated As-contaminated groundwater. Chemosphere 2011, 83, 295–301. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Jiang, D.J.; Zhou, P.; Lan, Y.Q.; Zhou, L.X. Heterogeneous photocatalytic degradation of methyl orange in schwertmannite/oxalate suspension under UV irradiation. Environ. Sci. Pollut. Res. 2012, 19, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.Y.; Yu, B. Rapid ferric transformation by reductive sissolution of schwertmannite for highly efficient catalytic degradation of Rhodamine B. Materials 2018, 11, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zheng, G.Y.; Meng, X.Q.; Zhou, L.X. Assessment of catalytic activities of selected iron hydroxysulphates biosynthesized using Acidithiobacillus ferrooxidans for the degradation of phenol in heterogeneous Fenton-like reactions. Sep. Purif. Technol. 2017, 185, 83–93. [Google Scholar] [CrossRef]
- Qiao, X.X.; Yu, K.; Xu, J.Y.; Cai, Y.L.; Li, Y.F.; Cao, H.L.; Lü, J. Engineered nanoscale schwertmannites as Fenton-like catalysts for highly efficient degradation of nitrophenols. Appl. Surf. Sci. 2021, 548, 149248. [Google Scholar] [CrossRef]
- Odega, C.A.; Ayodele, O.O.; Ogutuga, S.O.; Anguruwa, G.T.; Adekunle, A.E.; Fakorede, C.O. Potential application and regeneration of bamboo biochar for wastewater treatment: A review. Adv. Bamboo Sci. 2023, 2, 100012. [Google Scholar] [CrossRef]
- Liu, F.W.; Zhou, J.; Zhang, S.S.; Liu, L.L.; Zhou, L.X.; Fan, W.H. Schwertmannite Synthesis through Ferrous Ion Chemical Oxidation under Different H2O2 Supply Rates and Its Removal Efficiency for Arsenic from Contaminated Groundwater. PLoS ONE 2015, 10, e0138891. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, Q.Y.; Wang, W.Q.; Liu, F.W.; Wu, S.R. Migration of fluoranthene, phenanthrene, and pyrene in soil environment during the growth of Brassica rapa subsp. chinensis. Environ. Toxicol. Phar. 2024, 110, 104535. [Google Scholar] [CrossRef]
- Fu, P.; Wang, X.; Shi, J.; Zhou, L.X.; Hou, Q.J.; Wang, W.Q.; Tian, Y.; Qin, J.M.; Bi, W.L.; Liu, F.W. Enhanced removal of As (III) and Cd (II) from wastewater by alkali-modified Schwertmannite@ Biochar. Environ. Technol. Innov. 2023, 31, 103197. [Google Scholar] [CrossRef]
- Boily, J.F.; Gassman, P.L.; Peretyazhko, T.; Szanyi, J.; Zachara, J.M. FTIR spectral components of schwertmannite. Environ. Sci. Technol. 2010, 44, 1185–1190. [Google Scholar] [CrossRef]
- Wang, C.P.; Chen, K.; Yin, M.L.; Zhou, Y.T.; Zhuang, Q.L.; Cao, Q.Q.; Dang, Z.; Guo, C.L. Surface properties of schwertmannite with different sulfate contents and its effect on Cr (VI) adsorption. Geochim. Cosmochim. Acta 2024, 373, 245–258. [Google Scholar] [CrossRef]
- Bi, W.L.; Wu, Y.L.; Dong, W.B. The degradation of oxytetracycline with low concentration of persulfate sodium motivated by copper sulphate under solar light. Chem. Eng. J. 2019, 393, 122782. [Google Scholar] [CrossRef]
- Ji, Y.F.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.M. Degradation of ciprofloxacin and sulfamethoxazole by ferrous-activated persulfate: Implications for remediation of groundwater contaminated by antibiotics. Sci. Total Environ. 2014, 472, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, S.; Li, N.; Chen, G.Y.; Hou, L.A. Applications of vacancy defect engineering in persulfate activation: Performance and internal mechanism. J. Hazard. Mater. 2023, 449, 130971. [Google Scholar] [CrossRef]
- Yan, H.C.; Lai, C.; Liu, S.Y.; Wang, D.B.; Zhou, X.R.; Zhang, M.M.; Li, L.; Li, X.P.; Xu, F.H.; Nie, J.X. Metal-carbon hybrid materials induced persulfate activation: Application, mechanism, and tunable reaction pathways. Water Res. 2023, 234, 119808. [Google Scholar] [CrossRef]
- Shao, B.; Xu, Y.; Liu, Z.; Wu, T.; Pan, Y.; Zhang, X.S.; He, M.; Ge, L.; Lu, Y.; Liu, Y.; et al. Application of carbon aerogel-based materials in persulfate activation for water treatment: A review. J. Clean. Prod. 2023, 384, 135518. [Google Scholar] [CrossRef]
- Szabados, M.; Szabados, T.; Mucsi, R.; Baán, K.; Kiss, J.; Szamosvölgyi, Á.; Sápi, A.; Kónya, Z.; Kukovecz, Á.; Sipos, P. Directed thermocatalytic CO2 reduction over NiAl4 layered double hydroxide precursors—Activity and selectivity control using different interlayer anions. J. CO2 Util. 2023, 75, 102567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).