Effects of Low-Molecular-Weight Organic Acids on the Transport of Polystyrene Nanoplastics in Saturated Goethite-Coated Sand Columns
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of PS-NPs Suspensions
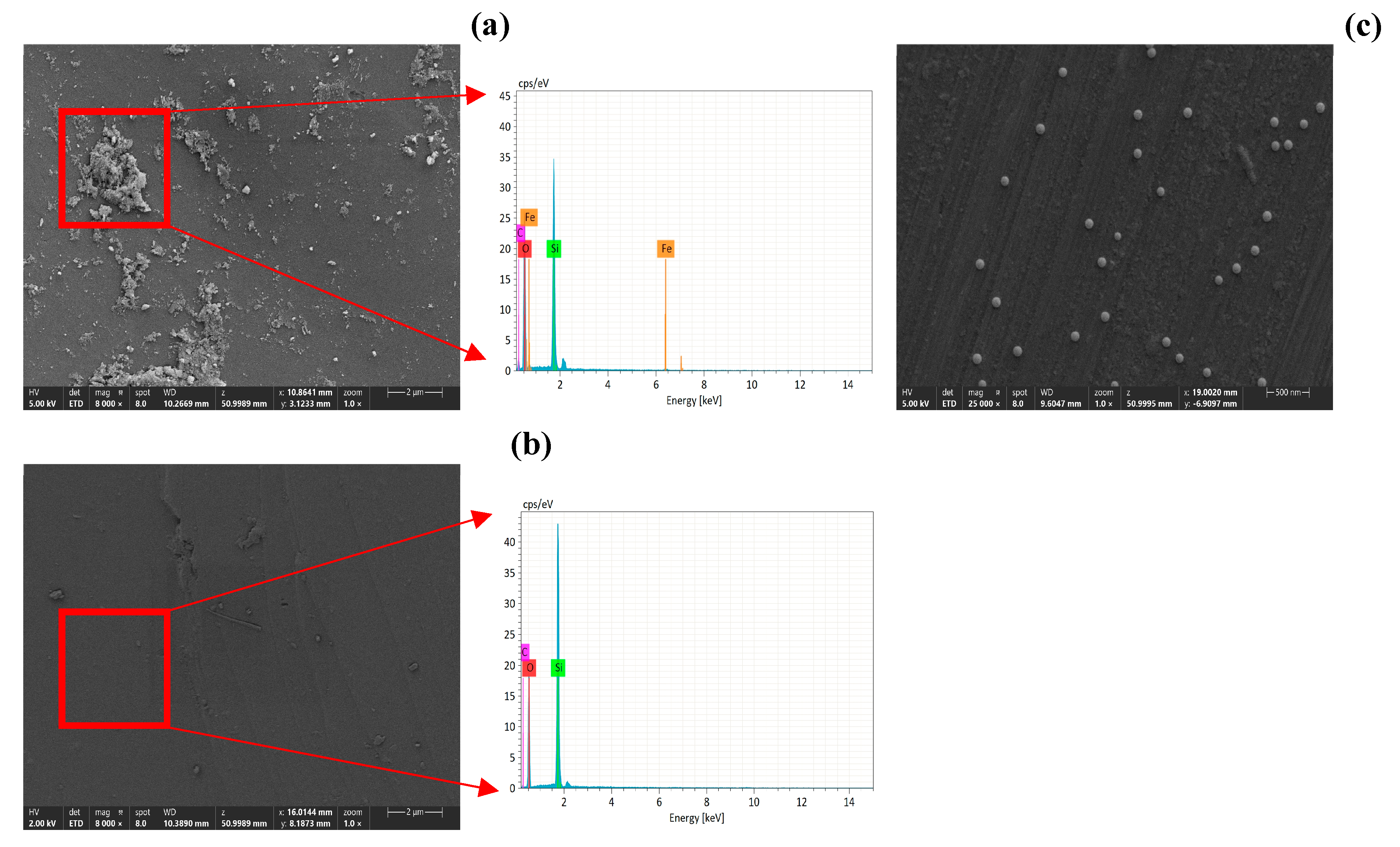
2.3. Preparation of Goethite-Coated Sand
2.4. Column Experiments
2.5. DLVO Calculations
3. Results and Discussion
3.1. Impacts of the Content of Goethite-Coated Sand on PS-NPs Migration
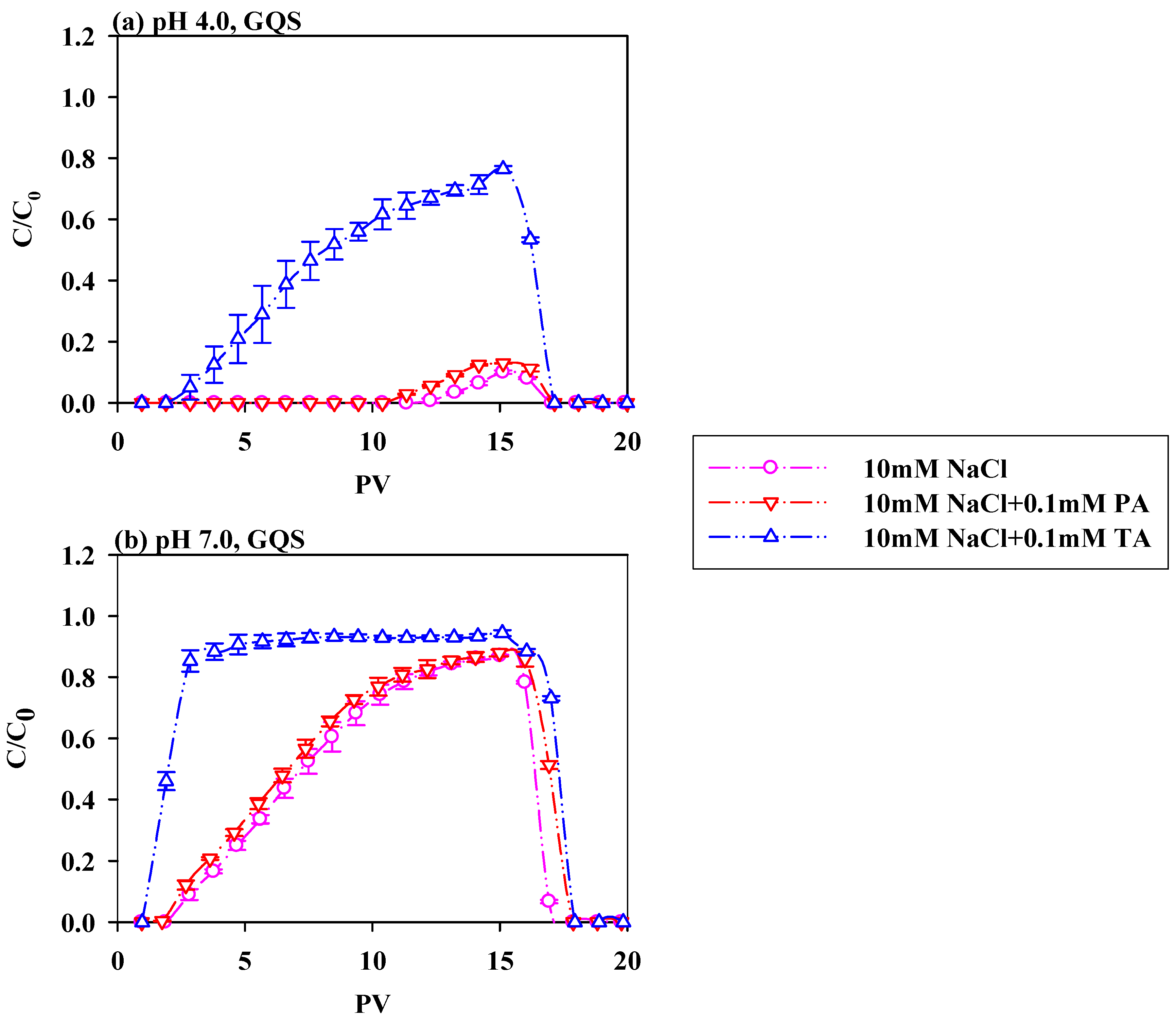
3.2. Impacts of LMWOAs on PS-NPs Migration Under Varying pH Conditions
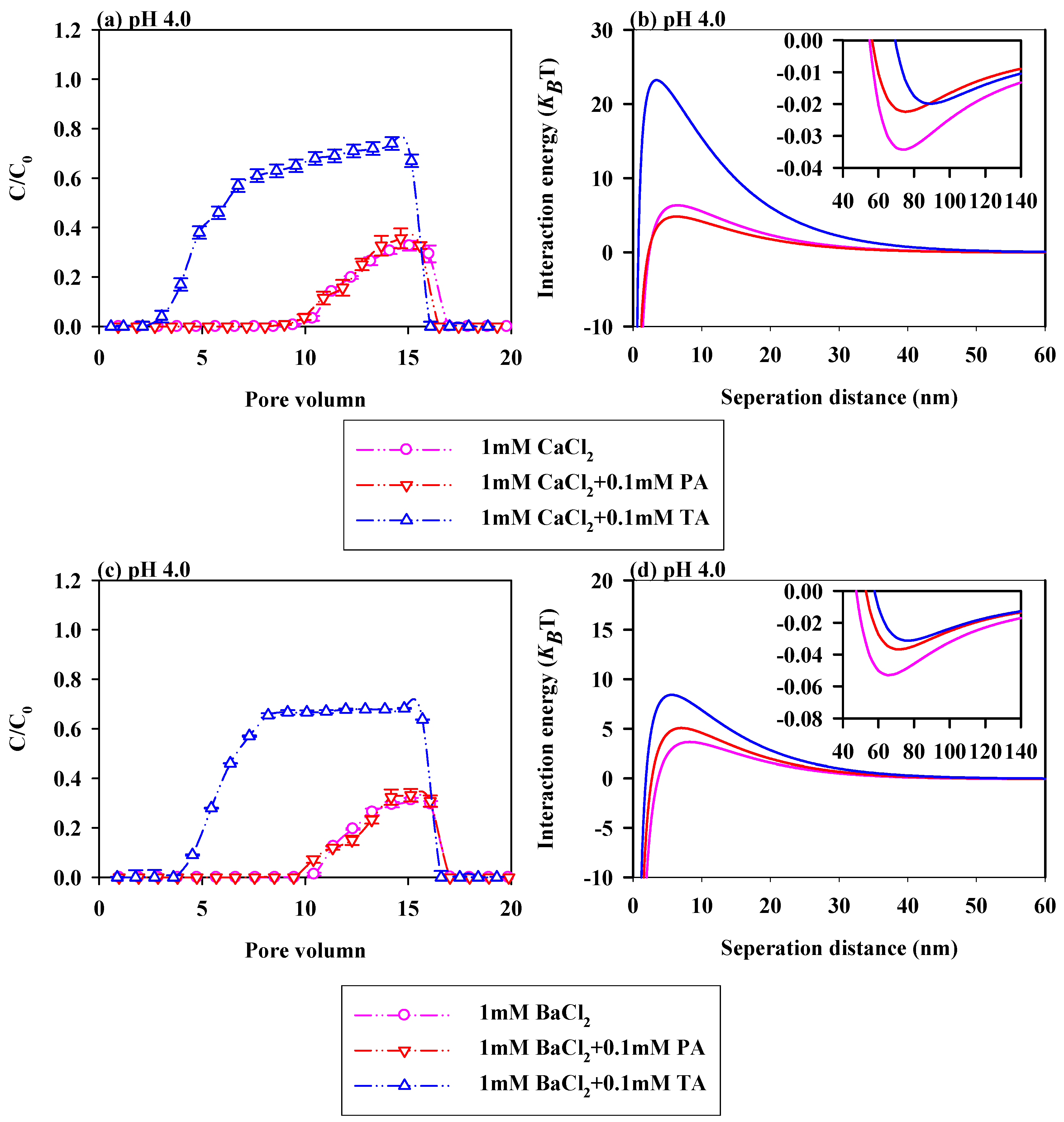
3.3. Effects of LMWOAs on PS-NPs Migration in the Presence of Cation Species
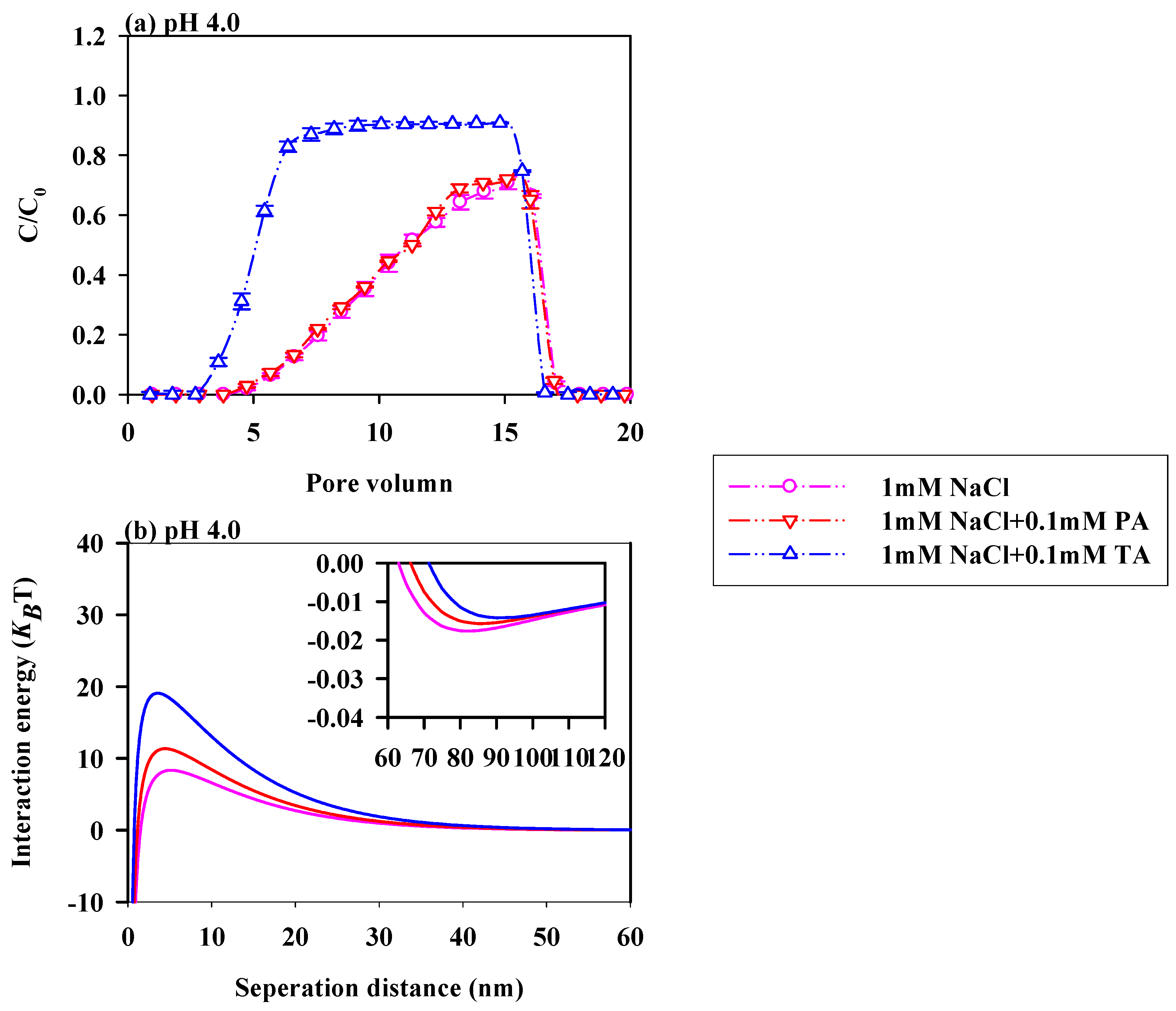
3.4. Impacts of LMWOAs on the Migration of PS-NPs with Various Ionic Strength
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.; Abrantes, N.; Gonçalves, F.; Nogueira, H.; Marques, J.C.; Gonçalves, A.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Ghoshal, S.; Tufenkji, N.; Adamowski, J.F.; Bayen, S.; Chen, Q.; Demokritou, P.; Flury, M.; Hüffer, T.; Ivleva, N.P. Plastics can be used more sustainably in agriculture. Commun. Earth Environ. 2023, 4, 332. [Google Scholar] [CrossRef]
- Ng, E.L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Gigault, J.; Ter Halle, A.; Baudrimont, M.; Pascal, P.Y.; Gauffre, F.; Phi, T.L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Wright, S.L.; Kelly, F. Plastic and human health: A micro issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef]
- Sangkham, S.; Faikhaw, O.; Munkong, N.; Sakunkoo, P.; Arunlertaree, C.; Chavali, M.; Mousazadeh, M.; Tiwari, A. A review on microplastics and nanoplastics in the environment: Their occurrence, exposure routes, toxic studies, and potential effects on human health. Mar. Pollut. Bull. 2022, 181, 113832. [Google Scholar] [CrossRef]
- Surendran, U.; Jayakumar, M.; Raja, P.; Gopinath, G.; Chellam, P.V. Microplastics in terrestrial ecosystem: Sources and migration in soil environment. Chemosphere 2023, 318, 137946. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Junhao, C.; Xining, Z.; Xiaodong, G.; Li, Z.; Qi, H.; Siddique, K.H. Extraction and identification methods of microplastics and nanoplastics in agricultural soil: A review. J. Environ. Manag. 2021, 294, 112997. [Google Scholar] [CrossRef] [PubMed]
- Moeck, C.; Davies, G.; Krause, S.; Schneidewind, U. Microplastics and nanoplastics in agriculture—A potential source of soil and groundwater contamination? Grundwasser 2023, 28, 23–35. [Google Scholar] [CrossRef]
- Guo, H.; Barnard, A.S. Naturally occurring iron oxide nanoparticles: Morphology, surface chemistry and environmental stability. J. Mater. Chem. A 2013, 1, 27–42. [Google Scholar] [CrossRef]
- Tao, Z.; Zhou, Q.; Zheng, T.; Mo, F.; Ouyang, S. Iron oxide nanoparticles in the soil environment: Adsorption, transformation, and environmental risk. J. Hazard. Mater. 2023, 459, 132107. [Google Scholar] [CrossRef]
- Li, Q.; Hu, W.; Li, L.; Li, Y. Interactions between organic matter and Fe oxides at soil micro-interfaces: Quantification, associations, and influencing factors. Sci. Total Environ. 2023, 855, 158710. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qiu, Y.; Zhao, J.; Ouyang, X.; Zhao, Y.; Weng, L.; MD Yasir, A.; Chen, Y.; Li, Y. Effect of agricultural organic inputs on nanoplastics transport in saturated goethite-coated porous media: Particle size selectivity and role of dissolved organic matter. Environ. Sci. Technol. 2022, 56, 3524–3534. [Google Scholar] [CrossRef]
- Oriekhova, O.; Stoll, S. Heteroaggregation of nanoplastic particles in the presence of inorganic colloids and natural organic matter. Environ. Sci.-Nano 2018, 5, 792–799. [Google Scholar] [CrossRef]
- Peña, A. A comprehensive review of recent research concerning the role of low molecular weight organic acids on the fate of organic pollutants in soil. J. Hazard. Mater. 2022, 434, 128875. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, T.; Shang, Z.; Li, Y.; He, J.; Liu, S.; Li, D.; Zhou, Y.; Qi, Z. Transport of Cd2+ through saturated porous media: Insight into the effects of low-molecular-weight organic acids. Water Res. 2020, 168, 115182. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Fu, Y. Effects of organic acids on Cd adsorption and desorption by two anthropic soils. Front. Environ. Sci. Eng. 2013, 7, 19–30. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, M.K.; Chiu, C.Y.; Huang, P.M.; King, H.B. Determination of low molecular weight dicarboxylic acids and organic functional groups in rhizosphere and bulk soils of Tsuga and Yushania in a temperate rain forest. Plant Soil 2001, 231, 37–44. [Google Scholar] [CrossRef]
- Van Schöll, L.; Hoffland, E.; Van Breemen, N. Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol. 2006, 170, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, T. Low-molecular-weight organic acids in soils: Sources, composition, concentrations, and functions: A review. Eurasian Soil Sci. 2020, 53, 580–594. [Google Scholar] [CrossRef]
- Xiao, M.; Wu, F. A review of environmental characteristics and effects of low-molecular weight organic acids in the surface ecosystem. J. Environ. Sci. 2014, 26, 935–954. [Google Scholar] [CrossRef]
- Xu, B.; Lu, L.; Liu, M.; Zhang, Q.; Farooq, U.; Lu, T.; Qi, Z.; Ge, C. Low-molecular-weight organic acids-mediated transport of neonicotinoid pesticides through saturated soil porous media: Combined effects of the molecular structures of organic acids and the chemical properties of contaminants. Chemosphere 2024, 349, 140870. [Google Scholar] [CrossRef]
- Qin, F.; Shan, X.Q.; Wei, B. Effects of low-molecular-weight organic acids and residence time on desorption of Cu, Cd, and Pb from soils. Chemosphere 2004, 57, 253–263. [Google Scholar] [CrossRef]
- Chen, J.; Lu, T.; Wang, Y.; Li, J.; Fu, X.; Qi, Z.; Zhang, Q. Transport of graphene oxide nanoparticles in saturated kaolinite-and goethite-coated sand columns: Effects of low-molecular-weight organic acids. Environ. Sci. Pollut. Res. 2019, 26, 24922–24932. [Google Scholar] [CrossRef]
- Chen, F.; Wei, X.; Gong, Y.; Chen, D.; Lu, T. Effects of low-molecular-weight organic acids on the transport of polystyrene nanoplastics: An insight at the structure of organic acids. Sci. Total Environ. 2024, 949, 175204. [Google Scholar] [CrossRef]
- Mattison, N.T.; O’Carroll, D.M.; Rowe, R.K.; Petersen, E.J. Impact of Porous Media Grain Size on the Transport of Multi-walled Carbon Nanotubes. Environ. Sci. Technol. 2011, 45, 9765–9775. [Google Scholar] [CrossRef]
- Stahl, R.S.; James, B.R. Zinc sorption by iron-oxide-coated sand as a function of pH. Soil Sci. Soc. Am. J. 1991, 55, 1287–1290. [Google Scholar] [CrossRef]
- Lanphere, J.D.; Luth, C.J.; Walker, S.L. Effects of Solution Chemistry on the Transport of Graphene Oxide in Saturated Porous Media. Environ. Sci. Technol. 2013, 47, 4255–4261. [Google Scholar] [CrossRef] [PubMed]
- Leij, F.J.; Bradford, S.A. Combined physical and chemical nonequilibrium transport model: Analytical solution, moments, and application to colloids. J. Contam. Hydrol. 2009, 110, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Dultz, S.; Steinke, H.; Mikutta, R.; Woche, S.K.; Guggenberger, G. Impact of organic matter types on surface charge and aggregation of goethite. Colloids Surf. A Physicochem. Eng. Aspects 2018, 554, 156–168. [Google Scholar] [CrossRef]
- Zhang, J.; Stanforth, R. Slow adsorption reaction between arsenic species and goethite (α-FeOOH): Diffusion or heterogeneous surface reaction control. Langmuir 2005, 21, 2895–2901. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, Q.; Cai, P.; Rong, X.; Chen, W. Adsorption of Pseudomonas putida on clay minerals and iron oxide. Colloids Surf. B 2007, 54, 217–221. [Google Scholar] [CrossRef]
- Rundberg, R.; Albinsson, Y.; Vannerberg, K. Sodium adsorption onto goethite as a function of pH and ionic strength. Radiochim. Acta 1994, 66, 333–340. [Google Scholar] [CrossRef]
- Lövgren, L.; Sjöberg, S.; Schindler, P.W. Acid/base reactions and Al (III) complexation at the surface of goethite. Geochim. Cosmochim. Acta 1990, 54, 1301–1306. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, Z.; Chen, W. Effects of several low-molecular weight organic acids and phosphate on the adsorption of acid phosphatase by soil colloids and minerals. Chemosphere 2003, 52, 571–579. [Google Scholar] [CrossRef]
- Carstens, J.; Bachmann, J.; Guggenberger, G. Aggregation and transport behavior of goethite colloids as affected by dissolved organic matter and pH: Electrostatic vs. hydrophilic interactions. Colloids Surf. A Physicochem. Eng. Aspects 2021, 609, 125639. [Google Scholar] [CrossRef]
- Dong, P.; Liang, Y.; Shen, C.; Jiang, E.; Bradford, S.A. Dual roles of goethite coating on the transport of plastic nanoparticles in heterogeneous porous media: The significance of collector surface roughness. J. Hazard. Mater. 2024, 470, 134153. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Bradford, S.A.; Harvey, R.W.; Gao, B.; Cang, L.; Zhou, D. Humic acid facilitates the transport of ARS-labeled hydroxyapatite nanoparticles in iron oxyhydroxide-coated sand. Environ. Sci. Technol. 2012, 46, 2738–2745. [Google Scholar] [CrossRef] [PubMed]
- Zinko, U.; Dynesius, M.; Nilsson, C.; Seibert, J. The role of soil pH in linking groundwater flow and plant species density in boreal forest landscapes. Ecography 2006, 29, 515–524. [Google Scholar] [CrossRef]
- Wamelink, G.W.; Walvoort, D.J.; Sanders, M.E.; Meeuwsen, H.A.; Wegman, R.M.; Pouwels, R.; Knotters, M. Prediction of soil pH patterns in nature areas on a national scale. Appl. Veg. Sci. 2019, 22, 189–199. [Google Scholar] [CrossRef]
- Khan, M.H.R.; Adachi, T.J.S.S.; Nutrition, P. Effects of selected natural factors on soil pH and element dynamics studied in columns of pyritic sediments. Soil Sci. Plant Nutr. 1999, 45, 783–793. [Google Scholar] [CrossRef]
- Roelofsen, H.D.; van Bodegom, P.M.; Kooistra, L.; van Amerongen, J.J.; Witte, J.P.M. An evaluation of remote sensing derived soil pH and average spring groundwater table for ecological assessments. Int. J. Appl. Earth Obs. 2015, 43, 149–159. [Google Scholar] [CrossRef]
- Li, Z.; Xie, D.; Xu, R. Influence of goethite colloid retention on the zeta potential of saturated porous media. J. Soils Sediments 2018, 18, 1844–1852. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, J.; Wu, X.; Weng, L.; Li, Y. Sedimentation and transport of different soil colloids: Effects of goethite and humic acid. Water 2020, 12, 980. [Google Scholar] [CrossRef]
- Wang, M.; Lu, T.; Chen, W.; Zhang, H.; Qi, W.; Song, Y.; Qi, Z. Enhanced role of humic acid on the transport of iron oxide colloids in saturated porous media under various solution chemistry conditions. Colloids Surf. A 2020, 607, 125486. [Google Scholar] [CrossRef]
- Wei, Q.; Zhou, K.; Chen, J.; Zhang, Q.; Lu, T.; Farooq, U.; Chen, W.; Li, D.; Qi, Z. Insights into the molecular mechanism of tetracycline transport in saturated porous media affected by low-molecular-weight organic acids: Role of the functional groups and molecular size. Sci. Total Environ. 2021, 799, 149361. [Google Scholar] [CrossRef]
- Filius, J.D.; Hiemstra, T.; Van Riemsdijk, W.H. Adsorption of small weak organic acids on goethite: Modeling of mechanisms. J. Colloid Interface Sci. 1997, 195, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Deng, Y.; Gong, D.; Luo, H.; Zhou, X.; Jiang, F. Effects of low molecular weight organic acids on adsorption of quinclorac by sepiolite. Environ. Sci. Pollut. Res. 2021, 28, 9582–9597. [Google Scholar] [CrossRef] [PubMed]
- Visser, J. The adhesion of colloidal polystyrene particles to cellophane as a function of pH and ionic strength. J. Colloid Interface Sci. 1976, 55, 664–677. [Google Scholar] [CrossRef]
- Ye, X.; Cheng, Z.; Wu, M.; Hao, Y.; Hu, B.X.; Mo, C.; Li, Q.; Xiang, L.; Zhao, H.; Wu, J. Investigating transport kinetics of polystyrene nanoplastics in saturated porous media. Ecotox. Environ. Safe 2022, 241, 113820. [Google Scholar] [CrossRef] [PubMed]
- Quevedo, I.R.; Tufenkji, N. Mobility of functionalized quantum dots and a model polystyrene nanoparticle in saturated quartz sand and loamy sand. Environ. Sci. Technol. 2012, 46, 4449–4457. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, J.; Wu, J.; Miao, L.; Zeng, Y. Effects of input concentration, media particle size, and flow rate on fate of polystyrene nanoplastics in saturated porous media. Sci. Total Environ. 2023, 881, 163237. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Fu, W.; Xia, X.; Liu, C.; Min, J.; Zhang, W.; Crittenden, J.C. Interactions between nano/micro plastics and suspended sediment in water: Implications on aggregation and settling. Water Res. 2019, 161, 486–495. [Google Scholar] [CrossRef]
- Lu, T.; Gilfedder, B.S.; Peng, H.; Peiffer, S.; Papastavrou, G.; Ottermann, K.; Frei, S.J.W. Relevance of iron oxyhydroxide and pore water chemistry on the mobility of nanoplastic particles in water-saturated porous media environments. Water Air Soil Pollut. 2021, 232, 168. [Google Scholar] [CrossRef]
- Swedlund, P.J.; Webster, J.G.; Miskelly, G.M. Goethite adsorption of Cu (II), Pb (II), Cd (II), and Zn (II) in the presence of sulfate: Properties of the ternary complex. Geochim. Cosmochim. Acta 2009, 73, 1548–1562. [Google Scholar] [CrossRef]
- Nilsson, N.; Persson, P.; Lövgren, L.; Sjöberg, S. Competitive surface complexation of o-phthalate and phosphate on goethite (α-FeOOH) particles. Geochim. Cosmochim. Acta 1996, 60, 4385–4395. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Wang, S.; Fang, H.; Wang, D. Aquatic behavior and toxicity of polystyrene nanoplastic particles with different functional groups: Complex roles of pH, dissolved organic carbon and divalent cations. Chemosphere 2019, 228, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Shen, M.; Li, S.; Fu, Y.; Zhang, D.; Liu, H.; Liu, J. Aggregation kinetics of different surface-modified polystyrene nanoparticles in monovalent and divalent electrolytes. Environ. Pollut. 2019, 255, 113302. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Fortner, J.D.; Zhu, D.; Qi, Z.; Chen, W. Transport of sulfide-reduced graphene oxide in saturated quartz sand: Cation-dependent retention mechanisms. Environ. Sci. Technol. 2015, 49, 11468–11475. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.; Duch, M.C.; Mansukhani, N.D.; Hersam, M.C.; Bouchard, D. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment. Environ. Sci. Technol. 2013, 47, 6288–6296. [Google Scholar] [CrossRef] [PubMed]
- Chrysikopoulos, C.V.; Katzourakis, V.E. Colloid particle size-dependent dispersivity. Water Resour. Res. 2015, 51, 4668–4683. [Google Scholar] [CrossRef]
- Rahnemaie, R.; Hiemstra, T.; van Riemsdijk, W.H. Inner-and outer-sphere complexation of ions at the goethite–solution interface. J. Colloid Interface Sci. 2006, 297, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Geng, J.; Wang, X.; Gu, X.; Gao, S. Adsorption of tetracycline onto goethite in the presence of metal cations and humic substances. J. Colloid Interface Sci. 2011, 361, 247–251. [Google Scholar] [CrossRef]
- Katzourakis, V.E.; Chrysikopoulos, C.V. Advanced mathematical model for the transport of aggregating nanoparticles in water saturated porous media: Nonlinear attachment and particle size-dependent dispersion. Water Resour. Res. 2024, 60, e2024WR037056. [Google Scholar] [CrossRef]
- Rasmusson, M.; Wall, S. Surface electrical properties of polystyrene latex: 1. electrophoresis and static conductivity. J. Colloid Interface Sci. 1999, 209, 312–326. [Google Scholar] [CrossRef]
- Elimelech, M.; O’Melia, C.R. Effect of particle size on collision efficiency in the deposition of Brownian particles with electrostatic energy barriers. Langmuir 1990, 6, 1153–1163. [Google Scholar] [CrossRef]
- Gregory, J. Approximate expressions for retarded van der waals interaction. J. Colloid Interface Sci. 1981, 83, 138–145. [Google Scholar] [CrossRef]
- Hogg, R.; Healy, T.W.; Fuerstenau, D.W. Mutual coagulation of colloidal dispersions. Trans. Faraday Soc. 1966, 62, 1638–1651. [Google Scholar] [CrossRef]
- Russel, W.B.; Saville, D.A.; Schowalter, W.R. Colloidal Dispersions; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Tufenkji, N.; Elimelech, M. Deviation from the classical colloid filtration theory in the presence of repulsive DLVO interactions. Langmuir 2004, 20, 10818–10828. [Google Scholar] [CrossRef] [PubMed]

| Organic Acid | Structure | Molecular Weight | pka1 | pka2 |
|---|---|---|---|---|
| Propanoic acid (PA) | 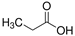 | 74.08 | 4.87 | |
| Tartaric acid (TA) | 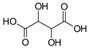 | 150.09 | 3.04 | 4.37 |
| Column No. | θ | Porous Media | Background Solution | pH | ζ Potential of PS-NPs a (mV) | ζ Potential of Sand b (mV) | Zave-PS-NPs c (nm) |
|---|---|---|---|---|---|---|---|
| 1 | 0.05 | GQS | 10 mM NaCl | 4.0 | - | - | - |
| 2 | 0.25 | GQS | 10 mM NaCl | 4.0 | - | - | - |
| 3 | 0.10 | GQS | 10 mM NaCl | 4.0 | −1.15 | −3.20 | 190.8 |
| 4 | 0.10 | GQS | 10 mM NaCl + 0.1 mM propanoic acid | 4.0 | −2.62 | −3.96 | 150.2 |
| 5 | 0.10 | GQS | 10 mM NaCl + 0.1 mM tartaric acid | 4.0 | −4.65 | −5.71 | 120.0 |
| 6 | 0.10 | GQS | 10 mM NaCl | 7.0 | −10.61 | −21.93 | 182.4 |
| 7 | 0.10 | GQS | 10 mM NaCl + 0.1 mM propanoic acid | 7.0 | −13.68 | −26.00 | 133.9 |
| 8 | 0.10 | GQS | 10 mM NaCl + 0.1 mM tartaric acid | 7.0 | −21.53 | −44.03 | 104.2 |
| 9 | 0.10 | GQS | 1 mM NaCl | 4.0 | −10.33 | −19.20 | 124.6 |
| 10 | 0.10 | GQS | 1 mM NaCl + 0.1 mM propanoic acid | 4.0 | −12.17 | −20.90 | 118.9 |
| 11 | 0.10 | GQS | 1 mM NaCl + 0.1 mM tartaric acid | 4.0 | −15.50 | −24.10 | 119.3 |
| 12 | 0.10 | GQS | 1 mM CaCl2 | 4.0 | −7.98 | −14.17 | 203.7 |
| 13 | 0.10 | GQS | 1 mM CaCl2 + 0.1 mM propanoic acid | 4.0 | −8.15 | −15.27 | 137.2 |
| 14 | 0.10 | GQS | 1 mM CaCl2 + 0.1 mM tartaric acid | 4.0 | −19.20 | −16.87 | 160.9 |
| 15 | 0.10 | GQS | 1 mM BaCl2 | 4.0 | −6.20 | −11.07 | 262.6 |
| 16 | 0.10 | GQS | 1 mM BaCl2 + 0.1 mM propanoic acid | 4.0 | −7.26 | −13.40 | 207.4 |
| 17 | 0.10 | GQS | 1 mM BaCl2 + 0.1 mM tartaric acid | 4.0 | −9.48 | −14.33 | 196.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Peng, X.; Liu, X.; Chen, B.; Chen, L.; Lu, T.; Gong, Y. Effects of Low-Molecular-Weight Organic Acids on the Transport of Polystyrene Nanoplastics in Saturated Goethite-Coated Sand Columns. Water 2024, 16, 3500. https://doi.org/10.3390/w16233500
Chen F, Peng X, Liu X, Chen B, Chen L, Lu T, Gong Y. Effects of Low-Molecular-Weight Organic Acids on the Transport of Polystyrene Nanoplastics in Saturated Goethite-Coated Sand Columns. Water. 2024; 16(23):3500. https://doi.org/10.3390/w16233500
Chicago/Turabian StyleChen, Feiyu, Xiaocheng Peng, Xiaocheng Liu, Biaodian Chen, Lidong Chen, Taotao Lu, and Yi Gong. 2024. "Effects of Low-Molecular-Weight Organic Acids on the Transport of Polystyrene Nanoplastics in Saturated Goethite-Coated Sand Columns" Water 16, no. 23: 3500. https://doi.org/10.3390/w16233500
APA StyleChen, F., Peng, X., Liu, X., Chen, B., Chen, L., Lu, T., & Gong, Y. (2024). Effects of Low-Molecular-Weight Organic Acids on the Transport of Polystyrene Nanoplastics in Saturated Goethite-Coated Sand Columns. Water, 16(23), 3500. https://doi.org/10.3390/w16233500






