Insights on the Abiotic/Biotic Interactive Impacts on the Occurrence of PFASs in Municipal Solid Waste Landfill Leachate
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of PFASs in Leachate
2.3. DOM Spectroscopic Analysis
2.4. Physicochemical Factor Analysis
2.5. Determination of Microbial Diversity in Leachate
2.6. Statistical Analysis
3. Results and Discussion
3.1. Leachate Composition Difference in Physical and Chemical Properties
3.2. Occurrence Characteristics of PFASs in Leachate
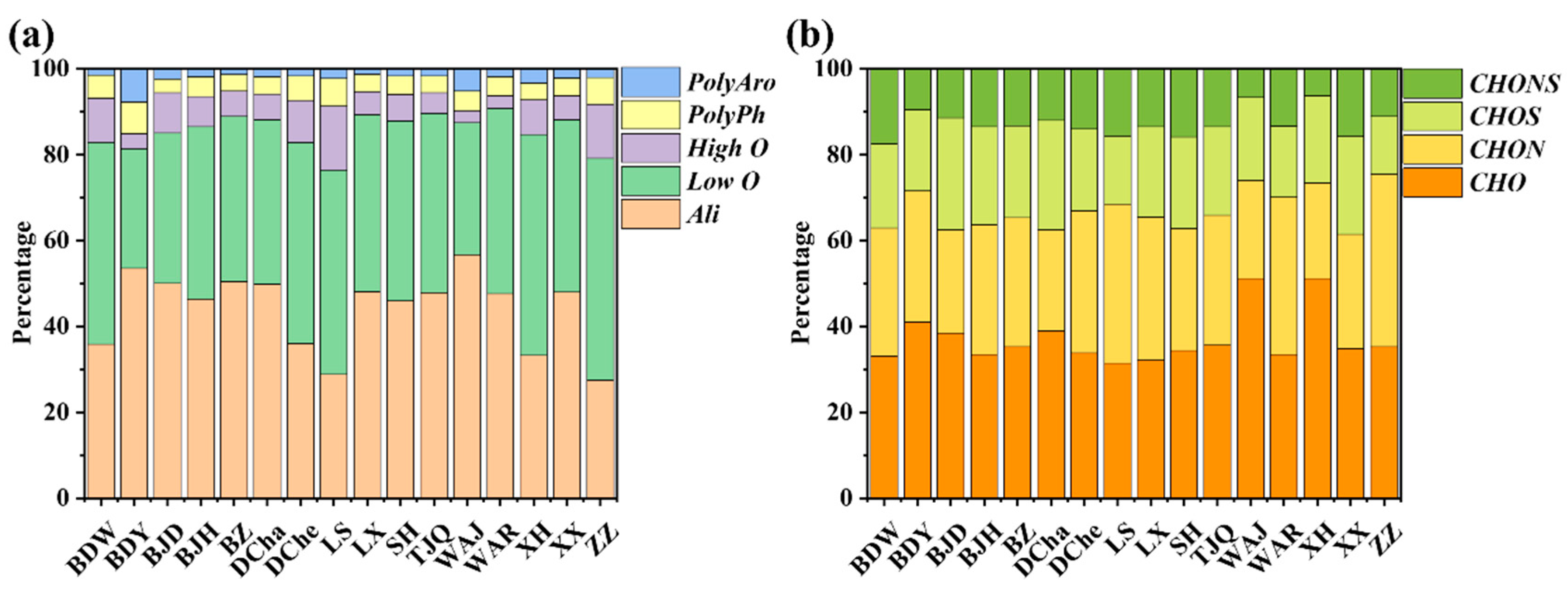
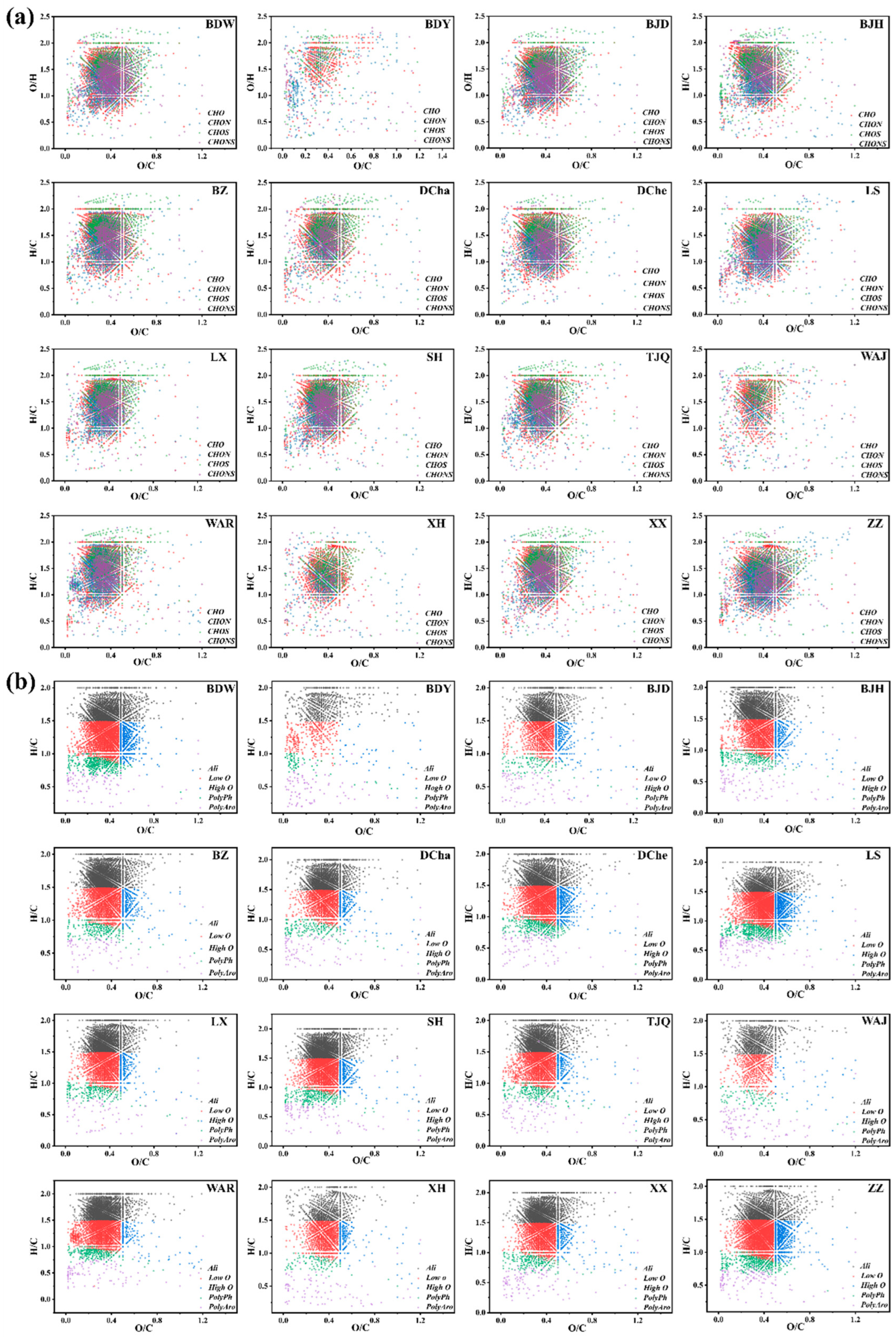
3.3. Composition and Molecular Characteristics of DOM in Leachate
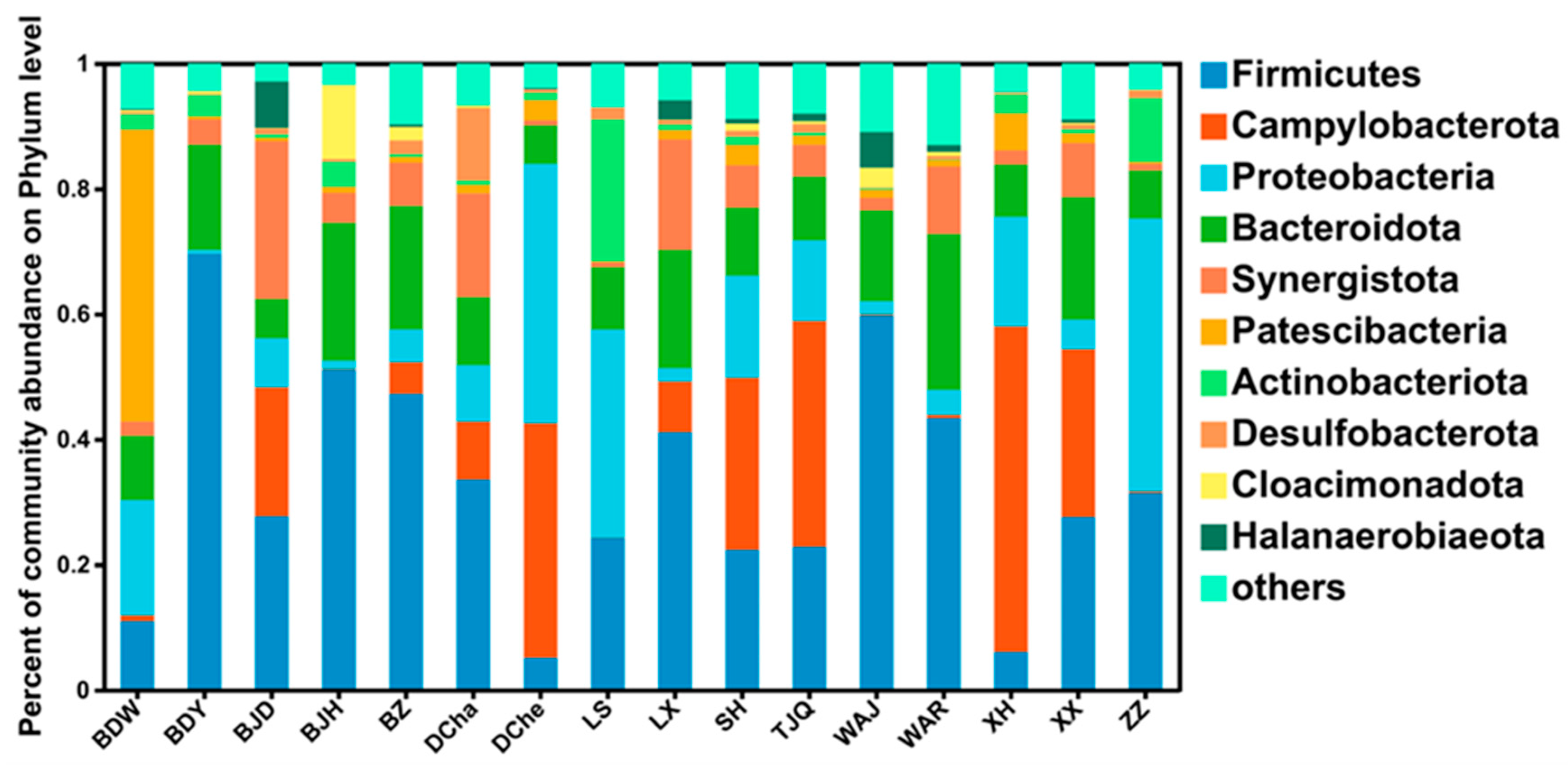
3.4. Species Composition and Function of Microbial Community in Leachate
3.5. Influence of Biotic/Abiotic Factors on the Occurrence of PFASs in Leachate
3.5.1. Influence of Abiotic Factors on the Occurrence of PFASs in Leachate
3.5.2. The Effects of Biotic Factors on the Occurrence of PFASs in Leachate
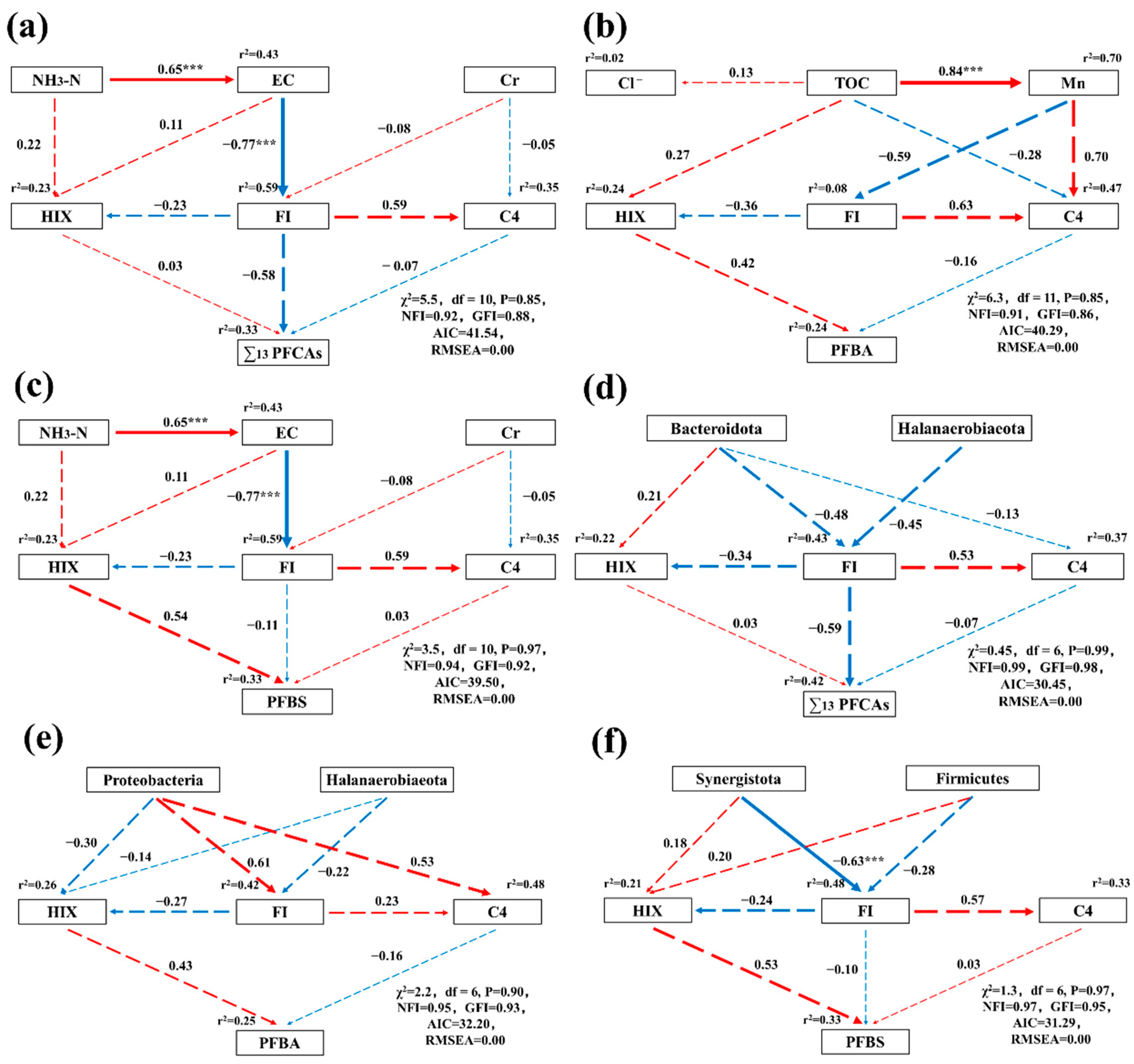
3.5.3. The Path of Influence of Biotic/Abiotic Factors on the Occurrence of PFASs in Leachate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abass, K.; Almusleh, Y.; Shanableh, A.; Semerjian, L. PFAS in the GCC: Towards environmental sustainability and public health protection. Emerg. Contam. 2024, 10, 100360. [Google Scholar] [CrossRef]
- Hansen, K.J.; Johnson, H.O.; Eldridge, J.S.; Butenhoff, J.L.; Dick, L.A. Quantitative characterization of trace levels of pfos and pfoa in the tennessee river. Environ. Sci. Technol. 2002, 36, 1681–1685. [Google Scholar] [CrossRef] [PubMed]
- Houde, M.; Martin, J.W.; Letcher, R.J.; Solomon, K.R.; Muir, D.C. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006, 40, 3463–3473. [Google Scholar] [CrossRef] [PubMed]
- Beisner, K.R.; Travis, R.E.; Alvarez, D.A.; Barber, L.B.; Fleck, J.A.; Jasmann, J.R. Temporal variability and sources of PFAS in the Rio Grande, New Mexico through an arid urban area using multiple tracers and high-frequency sampling. Emerg. Contam. 2024, 10, 100314. [Google Scholar] [CrossRef]
- Cornelsen, M.; Weber, R.; Panglisch, S. Minimizing the environmental impact of PFAS by using specialized coagulants for the treatment of PFAS polluted waters and for the decontamination of firefighting equipment. Emerg. Contam. 2021, 7, 63–76. [Google Scholar] [CrossRef]
- Batayi, B.; Okonkwo, J.O.; Daso, P.A.; Rimayi, C.C. Poly- and perfluoroalkyl substances (PFASs) in sediment samples from Roodeplaat and Hartbeespoort Dams, South Africa. Emerg. Contam. 2020, 6, 367–375. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Liu, L.; Jin, Y.; Dai, J.; Ran, X.; Zhang, Z.; Tsuda, S. Perfluorinated compounds in the environment and the blood of residents living near fluorochemical plants in Fuxin, China. Environ. Sci. Technol. 2011, 45, 8075–8080. [Google Scholar] [CrossRef]
- Calore, F.; Badetti, E.; Bonetto, A.; Pozzobon, A.; Marcomini, A. Non-conventional sorption materials for the removal of legacy and emerging PFAS from water: A review. Emerg. Contam. 2024, 10, 100303. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Meng, J.; Liu, S.; Lu, Y.; Khim, J.S.; Giesy, J.P. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 2015, 129, 87–99. [Google Scholar] [CrossRef]
- Oliaei, F.; Kriens, D.; Weber, R.; Watson, A. PFOS and PFC releases and associated pollution from a PFC production plant in Minnesota (USA). Environ. Sci. Pollut. Res. Int. 2013, 20, 1977–1992. [Google Scholar] [CrossRef]
- Mei, W.; Sun, H.; Song, M.; Jiang, L.; Li, Y.; Lu, W.; Ying, G.G.; Luo, C.; Zhang, G. Per- and polyfluoroalkyl substances (PFASs) in the soil-plant system: Sorption, root uptake, and translocation. Environ. Int. 2021, 156, 106642. [Google Scholar] [CrossRef] [PubMed]
- He, P.J.; Xue, J.F.; Shao, L.M.; Li, G.J.; Lee, D.J. Dissolved organic matter (DOM) in recycled leachate of bioreactor landfill. Water Res. 2006, 40, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, Z.; Parisi, V.A.; Kang, S.; Deng, Y.; Van Nostrand, J.D.; Masoner, J.R.; Cozzarelli, I.M.; Suflita, J.M.; Zhou, J. GeoChip-Based Analysis of Microbial Functional Gene Diversity in a Landfill Leachate-Contaminated Aquifer. Environ. Sci. Technol. 2012, 46, 5824–5833. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; He, W.; Wu, J.Y.; Wu, W.J.; Xu, F.L. Effects of fluorescent dissolved organic matters (FDOMs) on perfluoroalkyl acids (PFAAs) in lake and river water. Sci. Total Environ. 2019, 666, 598–607. [Google Scholar] [CrossRef]
- Li, J.; Sha, H.Q.; Liu, W.J.; Yuan, Y.; Zhu, G.H.; Meng, F.H.; Xi, B.D.; Tan, W.B. Transport of per-/polyfluoroalkyl substances from leachate to groundwater as affected by dissolved organic matter in landfills. Environ. Res. 2024, 247, 118230. [Google Scholar] [CrossRef]
- Jeon, J.; Kannan, K.; Lim, B.J.; An, K.G.; Kim, S.D. Effects of salinity and organic matter on the partitioning of perfluoroalkyl acid (PFAs) to clay particles. J. Environ. Monit. 2011, 13, 1803–1810. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, H.; Pan, W.; Wang, C.; Liang, Y. The role of dissolved organic matter during Per- and Polyfluorinated Substance (PFAS) adsorption, degradation, and plant uptake: A review. J. Hazard. Mater. 2022, 436, 129139. [Google Scholar] [CrossRef]
- Groele, J.R.; Sculley, N.; Olson, T.M.; Foster, J.E. An investigation of plasma-driven decomposition of per- and polyfluoroalkyl substances (PFAS) in raw contaminated ground water. J. Appl. Phys. 2021, 130, 053304. [Google Scholar] [CrossRef]
- Kidd, J.; Fabricatore, E.; Jackson, D. Current and future federal and state sampling guidance for per- and polyfluoroalkyl substances in environmental matrices. Sci. Total Environ. 2022, 836, 155523. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, W.; Zhou, K.; Teng, C. Utilization of UV–Vis and synchronous fluorescence heterospectral two-dimensional correlation analysis on the structural variation of landfill leachate during membrane bioreactor-reverse osmosis process. J. Water Process Eng. 2024, 59, 105047. [Google Scholar] [CrossRef]
- Fichot, C.G.; Benner, R. The spectral slope coefficient of chromophoric dissolved organic matter (S275-295) as a tracer of terrigenous dissolved organic carbon in river-influenced ocean margins. Limnol. Oceanogr. 2012, 57, 1453–1466. [Google Scholar] [CrossRef]
- Kulkarni, H.V.; Mladenov, N.; Johannesson, K.H.; Datta, S. Contrasting dissolved organic matter quality in groundwater in Holocene and Pleistocene aquifers and implications for influencing arsenic mobility. Appl. Geochem. 2017, 77, 194–205. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Z.; Ye, Q.; Liang, Y.; Liu, M.; Dang, Z.; Wang, Y.; Liu, C. Chemodiversity of Soil Dissolved Organic Matter. Environ. Sci. Technol. 2020, 54, 6174–6184. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Podgorski, D.C.; Rodgers, R.P.; Blakney, G.T.; Hendrickson, C.L. 21 Tesla FT-ICR Mass Spectrometer for Ultrahigh-Resolution Analysis of Complex Organic Mixtures. Anal. Chem. 2018, 90, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dang, Q.; Wang, Y.; Zhang, C.; Chen, Y.; He, L.; Xi, B. Linking Redox Characteristics to Dissolved Organic Matter Derived from Different Biowaste Composts: A Theoretical Modeling Approach Based on FT-ICR MS Analysis. Environ. Sci. Technol. 2023, 57, 15076–15086. [Google Scholar] [CrossRef]
- Seidel, M.; Beck, M.; Riedel, T.; Waska, H.; Suryaputra, I.G.N.A.; Schnetger, B.; Niggemann, J.; Simon, M.; Dittmar, T. Biogeochemistry of dissolved organic matter in an anoxic intertidal creek bank. Geochim. Cosmochim. Acta 2014, 140, 418–434. [Google Scholar] [CrossRef]
- GB 11894-89; Water Quaility-Determination of Total Nitrigon-Alkaline Potassium Persulfate Digestion UV Spectrophotometric Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1989.
- GB 11893-89; Water Quality-Determination of Total Phosphorus-Ammonium Molybdate Spectrophotometric Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 1989.
- HJ 828—2017; Water Quality-Determination of the Chemical Oxygen Demand-Dichromate Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2017.
- HJ 505—2009; Water Quality—Determination of Biochemical Oxygen Demand After 5 Days (BOD5) for Dilution and Seeding Method. Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2009.
- Ma, Y.; Zhao, H.; Shan, Q.; Xu, Y.; Yu, M.; Cui, J.; Liu, T.; Qiao, L.; He, X. K-strategy species plays a pivotal role in the natural attenuation of petroleum hydrocarbon pollution in aquifers. J. Hazard. Mater. 2021, 420, 126559. [Google Scholar] [CrossRef]
- Sinan Bilgili, M.; Demir, A.; İnce, M.; Özkaya, B. Metal concentrations of simulated aerobic and anaerobic pilot scale landfill reactors. J. Hazard. Mater. 2007, 145, 186–194. [Google Scholar] [CrossRef]
- Prechthai, T.; Parkpian, P.; Visvanathan, C. Assessment of heavy metal contamination and its mobilization from municipal solid waste open dumping site. J. Hazard. Mater. 2008, 156, 86–94. [Google Scholar] [CrossRef]
- Kabiri, S.; Tucker, W.; Navarro, D.A.; Bräunig, J.; Thompson, K.; Knight, E.R.; Nguyen, T.M.H.; Grimison, C.; Barnes, C.M.; Higgins, C.P.; et al. Comparing the Leaching Behavior of Per- and Polyfluoroalkyl Substances from Contaminated Soils Using Static and Column Leaching Tests. Environ. Sci. Technol. 2021, 56, 368–378. [Google Scholar] [CrossRef]
- Chen, W.; Yang, F.; Hu, E.; Yang, C.; Sun, C.; Li, M. Occurrence, fate and risk assessment of per- and polyfluoroalkyl substances in wastewater treatment plants in Shaanxi, China. Environ. Pollut. 2022, 314, 120226. [Google Scholar] [CrossRef]
- Tian, S.; Xu, T.; Fang, L.; Zhu, Y.; Li, F.; Leary, R.N.; Zhang, M.; Zhao, D.; Soong, T.-Y.; Shi, H. A ‘Concentrate-&-Destroy’ technology for enhanced removal and destruction of per- and polyfluoroalkyl substances in municipal landfill leachate. Sci. Total Environ. 2021, 791, 148124. [Google Scholar] [CrossRef] [PubMed]
- Dahlbom, S.; Bjarnemark, F.; Nguyen, B.; Petronis, S.; Mallin, T. Analysis of per- and polyfluoroalkyl substances (PFAS) extraction from contaminated firefighting materials: Effects of cleaning agent, temperature, and chain-length dependencies. Emerg. Contam. 2024, 10, 100335. [Google Scholar] [CrossRef]
- Capozzi, S.L.; Leang, A.L.; Rodenburg, L.A.; Chandramouli, B.; Delistraty, D.A.; Carter, C.H. PFAS in municipal landfill leachate: Occurrence, transformation, and sources. Chemosphere 2023, 334, 138924. [Google Scholar] [CrossRef] [PubMed]
- Robey, N.M.; Liu, Y.; Crespo-Medina, M.; Bowden, J.A.; Solo-Gabriele, H.M.; Townsend, T.G.; Tolaymat, T.M. Characterization of per- and polyfluoroalkyl substances (PFAS) and other constituents in MSW landfill leachate from Puerto Rico. Chemosphere 2024, 358, 142141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Z.H.; Xi, B.D.; Li, W.T.; Xu, Y.Q.; Zhao, H.Z.; Chen, Z.Q.; He, X.S.; Xing, B. Molecular structure and evolution characteristics of dissolved organic matter in groundwater near landfill: Implications of the identification of leachate leakage. Sci. Total Environ. 2021, 787, 147649. [Google Scholar] [CrossRef]
- Jiang, B.-C.; Tian, Y.-C.; Ji, W.-X.; Cai, M.-H.; Han, Y.-Z.; Zuo, Y.-T.; Shuang, C.-D.; He, X.-S.; Liu, F.-Q.; Li, W.-T.; et al. Identifying and Monitoring the Landfill Leachate Contamination in Groundwater with SEC-DAD-FLD-OCD and a Portable Fluorescence Spectrometer. ACS EST Water 2021, 2, 165–173. [Google Scholar] [CrossRef]
- Tedetti, M.; Cuet, P.; Guigue, C.; Goutx, M. Characterization of dissolved organic matter in a coral reef ecosystem subjected to anthropogenic pressures (La Reunion Island, Indian Ocean) using multi-dimensional fluorescence spectroscopy. Sci. Total Environ. 2011, 409, 2198–2210. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Peiris, K.H.S.; Pumphrey, M.O.; Dong, Y.; Maghirang, E.B.; Berzonsky, W.; Dowell, F.E. Near-Infrared Spectroscopic Method for Identification of Fusarium Head Blight Damage and Prediction of Deoxynivalenol in Single Wheat Kernels. Cereal Chem. 2010, 87, 511–517. [Google Scholar] [CrossRef]
- Wilson, H.F.; Xenopoulos, M.A. Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2008, 2, 37–41. [Google Scholar] [CrossRef]
- Yan, P.; Wei, S.; Chen, Y.; Ning, Q.; Hu, Z.; Guo, Z.; Xie, H.; Wu, H.; Zhang, J. Fluorescence spectroscopic characterization of dissolved organic matter in the wastewater treatment plant and hybrid constructed wetlands coupling system in winter: A case study in eastern China. Environ. Technol. Innov. 2023, 32, 103399. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, H.; Chang, C.H.; Lee, D.J.; He, P.J.; Shao, L.M.; Su, A. Dissolved organic matter and estrogenic potential of landfill leachate. Chemosphere 2008, 72, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Musadji, N.Y.; Lemee, L.; Caner, L.; Porel, G.; Poinot, P.; Geffroy-Rodier, C. Spectral characteristics of soil dissolved organic matter: Long-term effects of exogenous organic matter on soil organic matter and spatial-temporal changes. Chemosphere 2020, 240, 124808. [Google Scholar] [CrossRef] [PubMed]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Goranov, A.I.; Swinton, M.W.; Winkler, D.A.; Farrell, J.L.; Nierzwicki-Bauer, S.A.; Wagner, S. Assessing the spatiotemporal variability of dissolved organic matter fluorescence composition in the Lake George, NY watershed. Biogeochemistry 2024, 167, 849–870. [Google Scholar] [CrossRef]
- Li, W.; Liu, N.; Li, J.; Wang, B.; Shi, X.; Liang, X.; Yang, M.; Xu, S.; Liu, C.Q. Chemodiversity of Dissolved Organic Matter Is Governed by Microbial Biogeography in Inland Waters. Environ. Sci. Technol. 2023, 57, 7753–7763. [Google Scholar] [CrossRef]
- She, Z.; Wang, J.; He, C.; Pan, X.; Li, Y.; Zhang, S.; Shi, Q.; Yue, Z. The Stratified Distribution of Dissolved Organic Matter in an AMD Lake Revealed by Multi-sample Evaluation Procedure. Environ. Sci. Technol. 2021, 55, 8401–8409. [Google Scholar] [CrossRef]
- Yuan, Z.; He, C.; Shi, Q.; Xu, C.; Li, Z.; Wang, C.; Zhao, H.; Ni, J. Molecular Insights into the Transformation of Dissolved Organic Matter in Landfill Leachate Concentrate during Biodegradation and Coagulation Processes Using ESI FT-ICR MS. Environ. Sci. Technol. 2017, 51, 8110–8118. [Google Scholar] [CrossRef]
- Davis, S.N.; Klumker, S.M.; Mitchell, A.A.; Coppage, M.A.; Labonte, J.M.; Quigg, A. Life in the PFAS lane: The impact of perfluoroalkyl substances on photosynthesis, cellular exudates, nutrient cycling, and composition of a marine microbial community. Sci. Total Environ. 2024, 927, 171977. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, J.; Feng, J.; Li, X.; Li, B. Microbial community composition and metabolic functions in landfill leachate from different landfills of China. Sci. Total. Environ. 2021, 767, 144861. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, W.; Liu, Y.; Ming, Z.; Liu, Y.; Meng, R.; Wang, H. Structure and diversity of bacterial communities in two large sanitary landfills in China as revealed by high-throughput sequencing (MiSeq). Waste Manag. 2017, 63, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Identifying the Dominant Soil Bacterial Taxa in Libraries of 16S rRNA and 16S rRNA Genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, M.; Zhong, Z.; Chen, H.; Wang, H.; Zhou, L.; Cao, L.; Fu, L.; Zhang, H.; Lian, C.; et al. Adaption to hydrogen sulfide-rich environments: Strategies for active detoxification in deep-sea symbiotic mussels, Gigantidas platifrons. Sci. Total Environ. 2022, 804, 150054. [Google Scholar] [CrossRef] [PubMed]
- Zainun, M.Y.; Simarani, K. Metagenomics profiling for assessing microbial diversity in both active and closed landfills. Sci. Total Environ. 2018, 616–617, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Köchling, T.; Sanz, J.L.; Gavazza, S.; Florencio, L. Analysis of microbial community structure and composition in leachates from a young landfill by 454 pyrosequencing. Appl. Microbiol. Biotechnol. 2015, 99, 5657–5668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Zhang, H.K.; Liu, Y.L.; Bowden, J.A.; Tolaymat, T.M.; Townsend, T.G.; Solo-Gabriele, H.M. Evaluation of per- and polyfluoroalkyl substances (PFAS) in leachate, gas condensate, stormwater and groundwater at landfills. Chemosphere 2023, 318, 137903. [Google Scholar] [CrossRef]
- Zhang, H.K.; Chen, Y.T.; Liu, Y.L.; Bowden, J.A.; Townsend, T.G.; Solo-Gabriele, H.M. Do PFAS changes in landfill leachate treatment systems correlate with changes in physical chemical parameters. Waste Manag. 2022, 151, 49–59. [Google Scholar] [CrossRef]
- Wu, E.H.; Wang, K.; Liu, Z.Z.; Wang, J.; Yan, H.C.; Zhu, X.Y.; Zhu, X.M.; Chen, B.L. Metabolic and Microbial Profiling of Soil Microbial Community under Per- and Polyfluoroalkyl Substance (PFAS) Stress. Environ. Sci. Technol. 2023, 57, 21855–21865. [Google Scholar] [CrossRef]
- Kang, Y.; Li, M.; Guo, Z.; Zhang, Y.; Li, W.; Wu, H.; Zhang, J. Effect of electron shuttles on typical perfluoroalkyl substance removal via iron oxide reduction in wetland sediment. J. Clean. Prod. 2022, 365, 132821. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, C.; Wang, X.; Wang, N.; Chen, Q.; Cui, G.; Xu, Q. Co-digestion of food waste and hydrothermal liquid digestate: Promotion effect of self-generated hydrochars. Environ. Sci. Ecotechnol. 2023, 15, 100239. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Arcadi, E.; Calogero, R.; Ciro Rappazzo, A.; Caruso, G.; Maimone, G.; Lo Giudice, A.; Romeo, T.; Andaloro, F. Deciphering the evolvement of microbial communities from hydrothermal vent sediments in a global change perspective. Environ. Res. 2024, 240, 117514. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Stackebrandt, E.; Goebel, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Bacteriol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ye, R.; Zhu, G.; Chen, S.; Tan, W.; Liu, W. Insights on the Abiotic/Biotic Interactive Impacts on the Occurrence of PFASs in Municipal Solid Waste Landfill Leachate. Water 2024, 16, 3436. https://doi.org/10.3390/w16233436
Li J, Ye R, Zhu G, Chen S, Tan W, Liu W. Insights on the Abiotic/Biotic Interactive Impacts on the Occurrence of PFASs in Municipal Solid Waste Landfill Leachate. Water. 2024; 16(23):3436. https://doi.org/10.3390/w16233436
Chicago/Turabian StyleLi, Jia, Rongchuan Ye, Ganghui Zhu, Shuhe Chen, Wenbing Tan, and Weijiang Liu. 2024. "Insights on the Abiotic/Biotic Interactive Impacts on the Occurrence of PFASs in Municipal Solid Waste Landfill Leachate" Water 16, no. 23: 3436. https://doi.org/10.3390/w16233436
APA StyleLi, J., Ye, R., Zhu, G., Chen, S., Tan, W., & Liu, W. (2024). Insights on the Abiotic/Biotic Interactive Impacts on the Occurrence of PFASs in Municipal Solid Waste Landfill Leachate. Water, 16(23), 3436. https://doi.org/10.3390/w16233436





