Research on the Properties of DOM from the Microalgal Treatment Process for Leachate from Incineration Fly Ash Based on EEM-PARAFAC Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Leachate Sampling
2.3. Selection and Treatment of Microalgae
2.3.1. Cultivation of Microalgae
2.3.2. Pretreatment of Microalgae
2.4. Experimental Setup
2.4.1. Dilution of Fly Ash Leachate
2.4.2. Nutrients
2.5. Determination of DOM in Leachate
2.6. Parallel Factor Analysis
3. Results
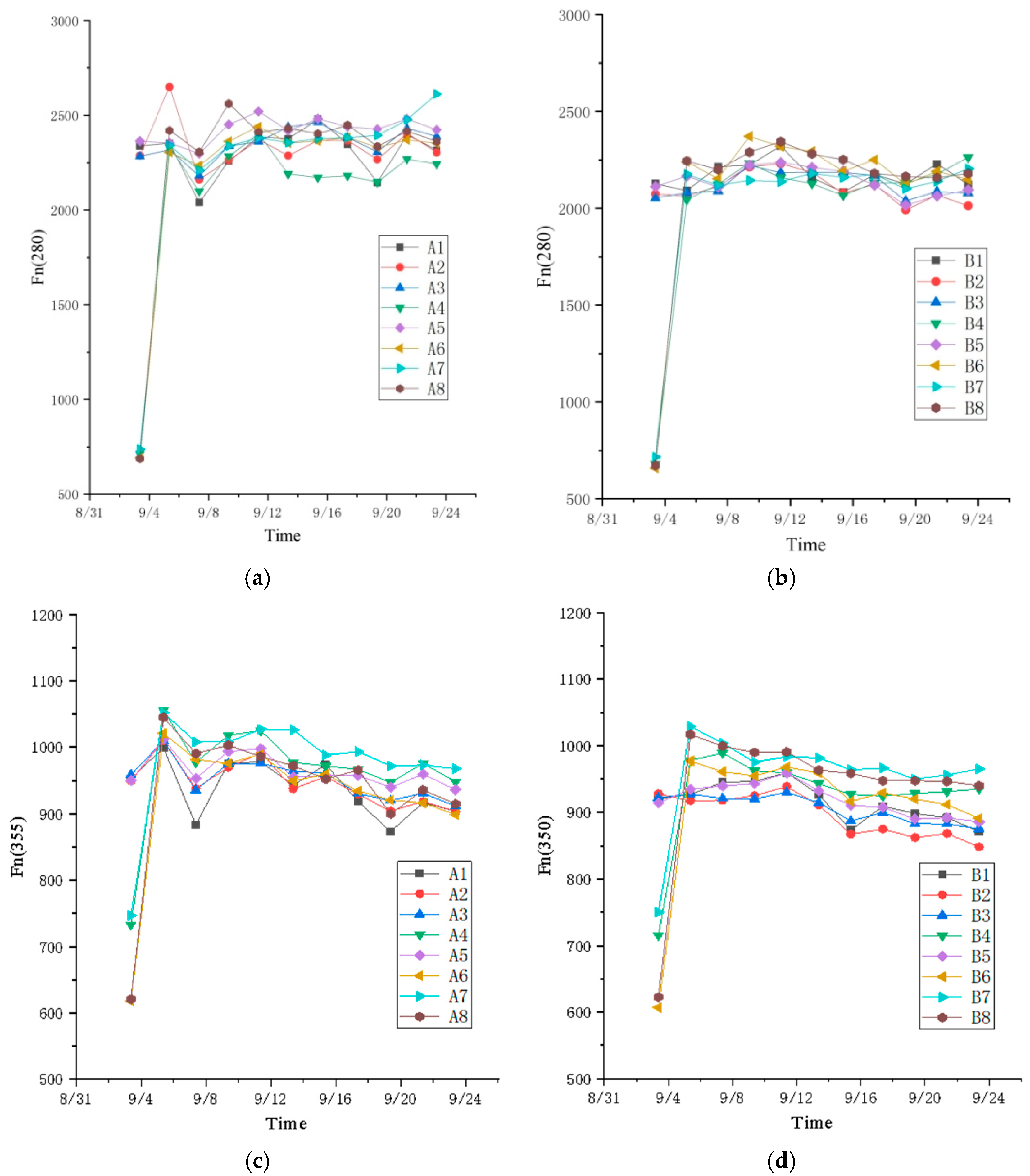
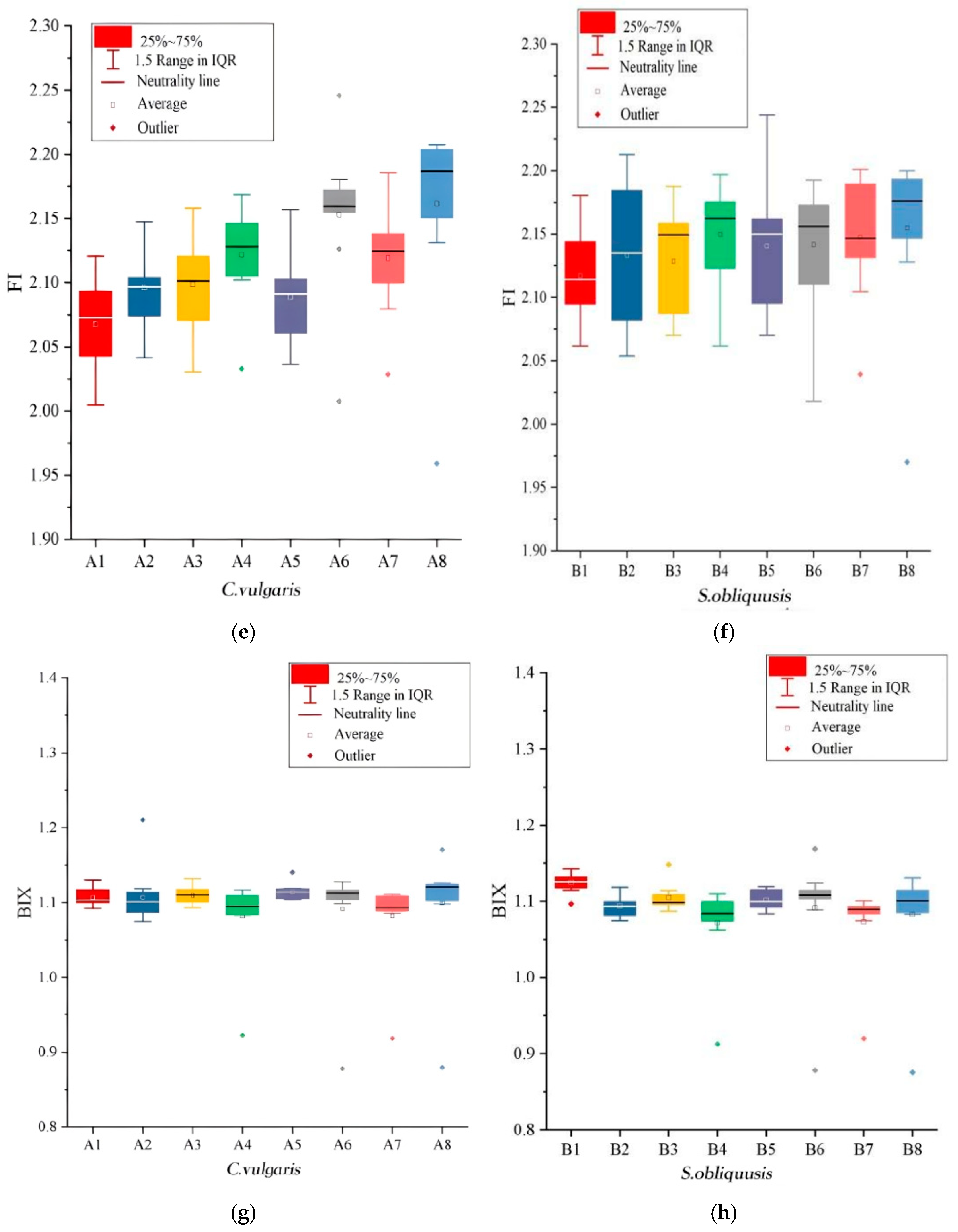
3.1. Fluorescence Characteristic Parameter
3.2. Influence of Leachate Dilution Concentration on Fluorescence Index
3.3. The Impact of Additional Nutrients
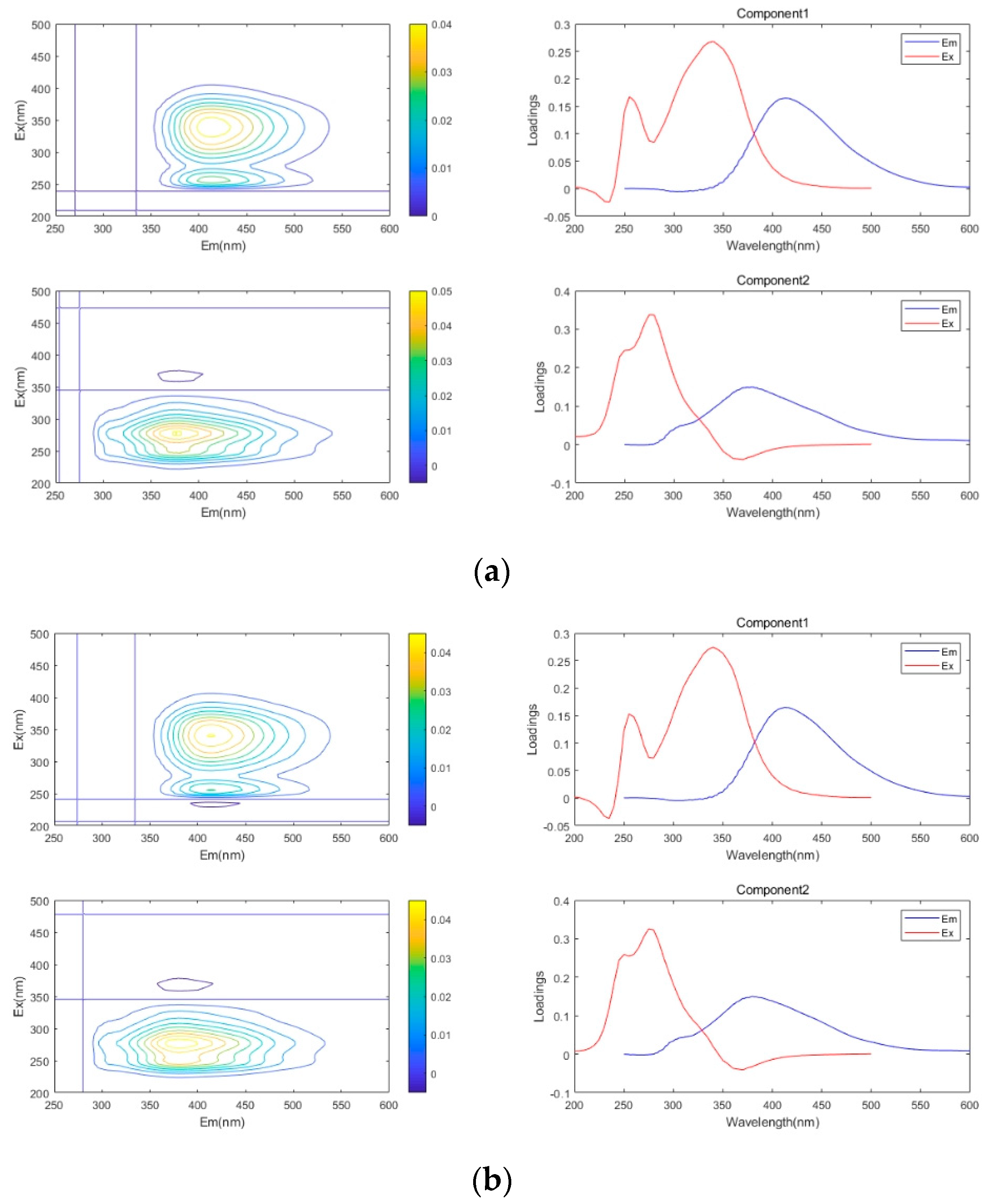
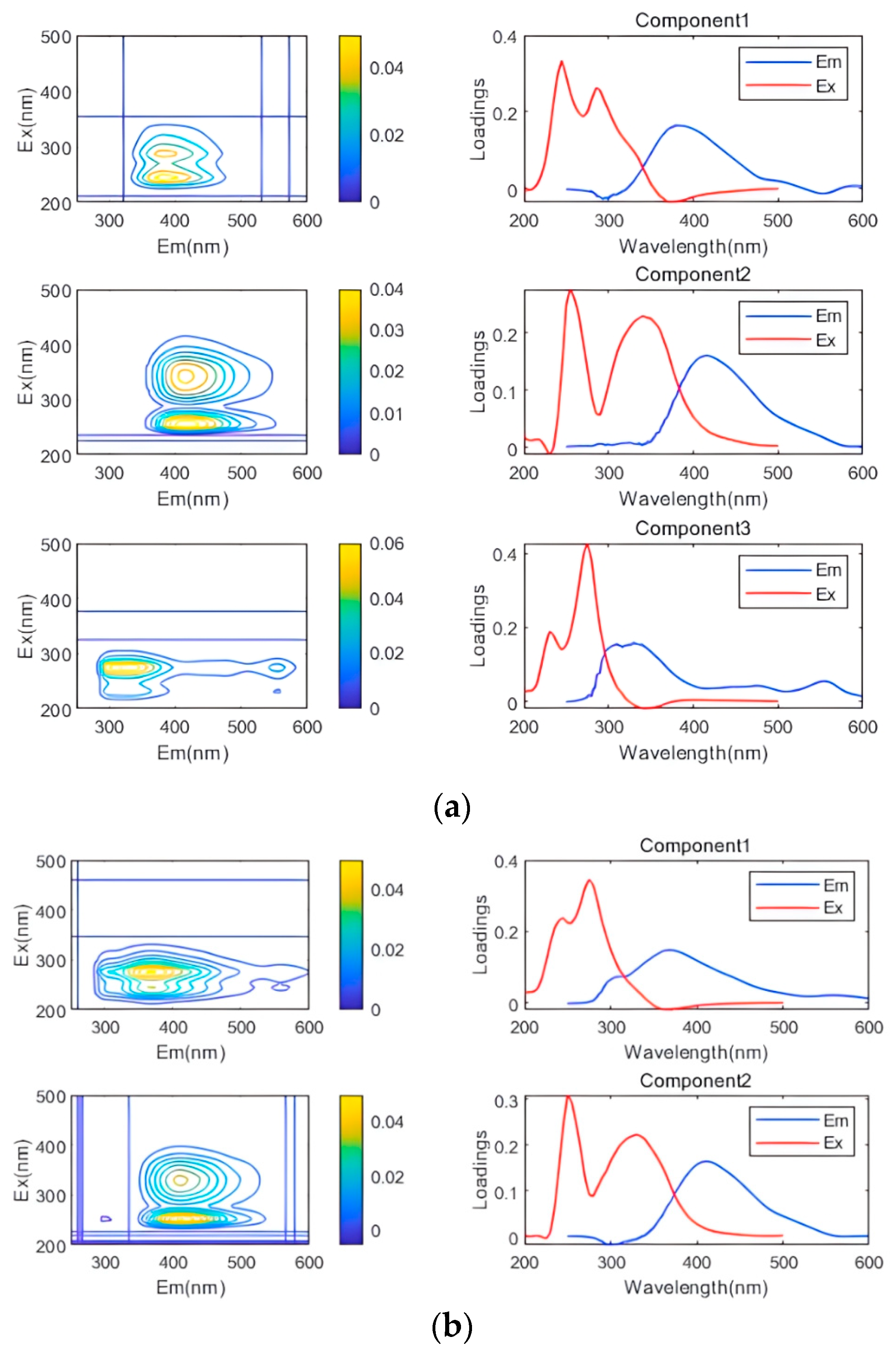
3.4. PARAFAC
3.4.1. Influence of Leachate Concentration
3.4.2. Impact of Additional Nutrients
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Kirkelund, G.M.; Jensen, P.E.; Ottosen, L.M. Comparison of different MSWI fly ash treatment processes on the thermal behavior of As, Cr, Pb and Zn in the ash. Waste Manag. 2017, 68, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Luo, G.; Liu, H.; Qiao, Y.; Xu, M.; Yao, H. Fate of chromium during thermal treatment of municipal solid waste incineration (MSWI) fly ash. Proc. Combust. Inst. 2013, 34, 2795–2801. [Google Scholar] [CrossRef]
- Ma, W.; Chen, D.; Pan, M.; Gu, T.; Zhong, L.; Chen, G.; Yan, B.; Cheng, Z. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. J. Environ. Manag. 2019, 247, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cheng, Q.; Liu, M.; Li, L.; Ru, Y.; Yan, D. Industrial disposal processes for treatment of polychlorinated dibenzo-p-dioxins and dibenzofurans in municipal solid waste incineration fly ash. Chemosphere 2020, 243, 125351. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, P.; Ni, H. Emission characteristics of parent and halogenated PAHs in simulated municipal solid waste incineration. Sci. Total Environ. 2019, 665, 11–17. [Google Scholar] [CrossRef]
- Huber, F.; Fellner, J. Integration of life cycle assessment with monetary valuation for resource classification: The case of municipal solid waste incineration fly ash. Resour. Conserv. Recycl. 2018, 139, 17–26. [Google Scholar] [CrossRef]
- Nazir, M.A.; Naseer, M.; Ullah, S.; Ahmad, K.; Ismail, M.A.; Iqbal, R.; Najam, T.; Rosaiah, P.; Raza, M.A.; Shah, S.S.A. Designing MOF-COF hybrid materials for energy, biomedical and environment applications. Inorg. Chem. Commun. 2024, 170, 113262. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, Z.; Zhou, J.; Zhao, J.; Ruan, X.; Liu, J.; Qian, G. Chemical characteristics and risk assessment of typical municipal solid waste incineration (MSWI) fly ash in China. J. Hazard. Mater. 2013, 261, 269–276. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Z.; Li, C.; Yue, J.; Su, Y.; Cheng, W.; Sun, S.; Chen, X.; Shi, D.; Liu, B. Emerging Contaminants in Landfill Leachate and Groundwater: A Case Study of Hazardous Waste Landfill and Municipal Solid Waste Landfill in Northeastern China. Water 2024, 16, 2575. [Google Scholar] [CrossRef]
- Budi, S.; Suliasih, B.A.; Othman, M.S.; Heng, L.Y.; Surif, S. Toxicity identification evaluation of landfill leachate using fish, prawn and seed plant. Waste Manag. 2016, 55, 231–237. [Google Scholar] [CrossRef]
- Toufexi, E.; Tsarpali, V.; Efthimiou, I.; Vidali, M.-S.; Vlastos, D.; Dailianis, S. Environmental and human risk assessment of landfill leachate: An integrated approach with the use of cytotoxic and genotoxic stress indices in mussel and human cells. J. Hazard. Mater. 2013, 260, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xue, X.; Dong, L.; Nai, C.; Liu, Y.; Huang, Q. Long-term dynamics of leachate production, leakage from hazardous waste landfill sites and the impact on groundwater quality and human health. Waste Manag. 2018, 82, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Duan, L.; Gao, Q.; Zhang, H.; Yu, H.; Zhang, J. Three-dimensional fluorescence characteristics analysis of DOMs in water treated by the ultrafiltration-reverse osmosis process. J. Mol. Liq. 2024, 398, 124297. [Google Scholar] [CrossRef]
- Gan, S.; Heuer, V.B.; Schmidt, F.; Wörmer, L.; Hinrichs, K.-U. A simple guideline to apply excitation-emission matrix spectroscopy (EEMs) for the characterization of dissolved organic matter (DOM) in anoxic marine sediments. Acta Oceanol. Sin. 2023, 42, 109–119. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Gao, L.; Wu, Y.; Lu, H.; Yuan, M.; Cui, J.; Wei, F. Fluorescence Analysis of River DOM Spectra Using PARAFAC in Combination with a Self-Organizing Map to Distinguish Organic Matter Sources. Int. J. Environ. Res. 2024, 18, 20. [Google Scholar] [CrossRef]
- Rehman, M.U.; Taj, M.B.; Carabineiro, S.A.C. Biogenic adsorbents for removal of drugs and dyes: A comprehensive review on properties, modification and applications. Chemosphere 2023, 338, 139477. [Google Scholar] [CrossRef]
- Han, G.; Ma, L.; Zhang, C.; Wang, B.; Sheng, X.; Wang, Z.; Wang, X.; Wang, L. Research on the Tolerance and Degradation of o-Cresol by Microalgae. Water 2023, 15, 1522. [Google Scholar] [CrossRef]
- Mohsenpour, S.F.; Willoughby, N. Effect of CO2 aeration on cultivation of microalgae in luminescent photobioreactors. Biomass Bioenergy 2016, 85, 168–177. [Google Scholar] [CrossRef]
- Fragoso-Fuentes, S.; Rico-Martínez, R.; Arzate-Cárdenas, M.A. Effect of the Non-Steroidal Anti-Inflammatory Drug Ibuprofen on the Chydorid Alona guttata (Chydoridae: Aloninae) and the Rotifer Lecane papuana (Monogononta: Lecanidae) Fed on Different Algal Densities. Pol. J. Environ. Stud. 2024, 33, 4083–4094. [Google Scholar] [CrossRef]
- Zhao, R.; Pang, W.; Wang, C.; Chen, Q.; Ke, Q.; Wang, Q. Optimization of Culture Conditions for Microalgae Treatment Fly Ash Leachate System. Water 2024, 16, 2223. [Google Scholar] [CrossRef]
- Ji, M.-K.; Abou-Shanab, R.A.; Kim, S.-H.; Salama, E.-S.; Lee, S.-H.; Kabra, A.-N.; Lee, Y.-S.; Hong, S.; Jeon, B.-H. Cultivation of microalgae species in tertiary municipal wastewater supplemented with CO2 for nutrient removal and biomass production. Ecol. Eng. 2013, 58, 142–148. [Google Scholar] [CrossRef]
- Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Xu, X.-Q.; Wang, X.-X.; Hu, H.-Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yang, J.; Marrakchi, F.; Wei, M.; Liu, Y.; Xiao, Y.; Li, C.; Wang, S. Macro- and micro-algae-based carbon composite for pharmaceutical wastewater treatment: Batch adsorption and mechanism study. Process Saf. Environ. Prot. 2023, 176, 641–652. [Google Scholar] [CrossRef]
- Strezov, A.; Nonova, T. Influence of macroalgal diversity on accumulation of radionuclides and heavy metals in Bulgarian Black Sea ecosystems. J. Environ. Radioact. 2009, 100, 144–150. [Google Scholar] [CrossRef]
- Adam, C.; Garnier-Laplace, J. Bioaccumulation of silver-110m, cobalt-60, cesium-137, and manganese-54 by the freshwater algae Scenedesmus obliquus and Cyclotella meneghiana and by suspended matter collected during a summer bloom event. Limnol. Oceanogr. 2003, 48, 2303–2313. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption capacity and kinetics of cadmium(II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 211–221. [Google Scholar] [CrossRef]
- Zerbini, M.; Solari, P.L.; Orange, F.; Jeanson, A.; Leblanc, C.; Gomari, M.; Auwer, C.D.; Beccia, M.R. Exploring uranium bioaccumulation in the brown alga Ascophyllum nodosum: Insights from multi-scale spectroscopy and imaging. Sci. Rep. 2024, 14, 1021. [Google Scholar] [CrossRef]
- Hejna, M.; Kapuscinska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- Riaz, M.; Ijaz, B.; Riaz, A.; Amjad, M. Improvement of waste water quality by application of mixed algal inocula. Bangladesh J. Sci. Ind. Res. 2018, 53, 77–82. [Google Scholar] [CrossRef]
- Garbowski, T.; Pietryka, M.; Pulikowski, K.; Richter, D. The use of a natural substrate for immobilization of microalgae cultivated in wastewater. Sci. Rep. 2020, 10, 7915. [Google Scholar] [CrossRef] [PubMed]
- Hernández-García, A.; Velásquez-Orta, S.B.; Novelo, E.; Yáñez-Noguez, I.; Monje-Ramírez, I.; Ledesma, M.T.O. Wastewater-leachate treatment by microalgae: Biomass, carbohydrate and lipid production. Ecotoxicol. Environ. Saf. 2019, 174, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-X.; Huang, Y.; Fu, Q.; Liao, Q.; Zhu, X. Kinetic characteristics and modeling of microalgae Chlorella vulgaris growth and CO2 biofixation considering the coupled effects of light intensity and dissolved inorganic carbon. Bioresour. Technol. 2016, 206, 231–238. [Google Scholar] [CrossRef] [PubMed]
- El Ouaer, M.; Kallel, A.; Kasmi, M.; Hassen, A.; Trabelsi, I. Tunisian landfill leachate treatment using Chlorella sp.: Effective factors and microalgae strain performance. Arab. J. Geosci. 2017, 10, 457. [Google Scholar] [CrossRef]
- Viegas, C.; Nobre, C.; Mota, A.; Vilarinho, C.; Gouveia, L.; Gonçalves, M. A circular approach for landfill leachate treatment: Chemical precipitation with biomass ash followed by bioremediation through microalgae. J. Environ. Chem. Eng. 2021, 9, 105187. [Google Scholar] [CrossRef]
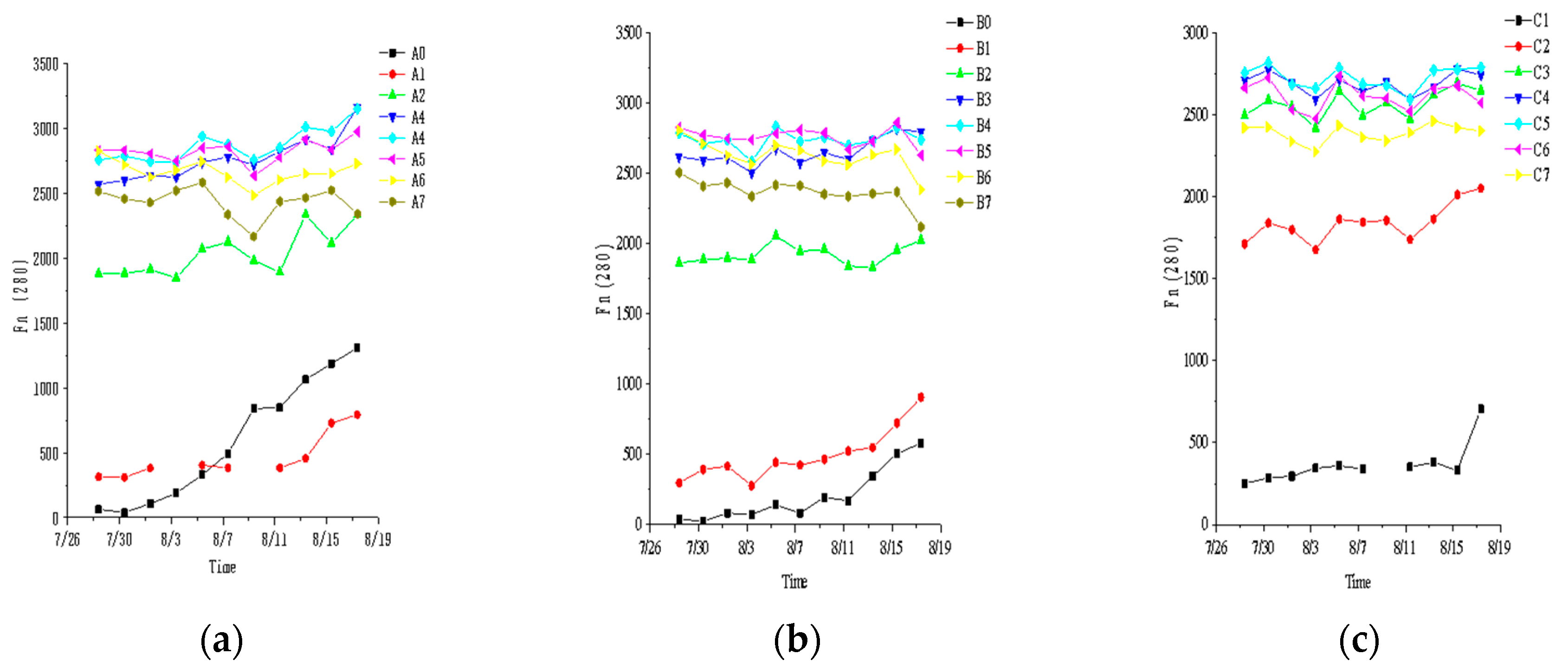
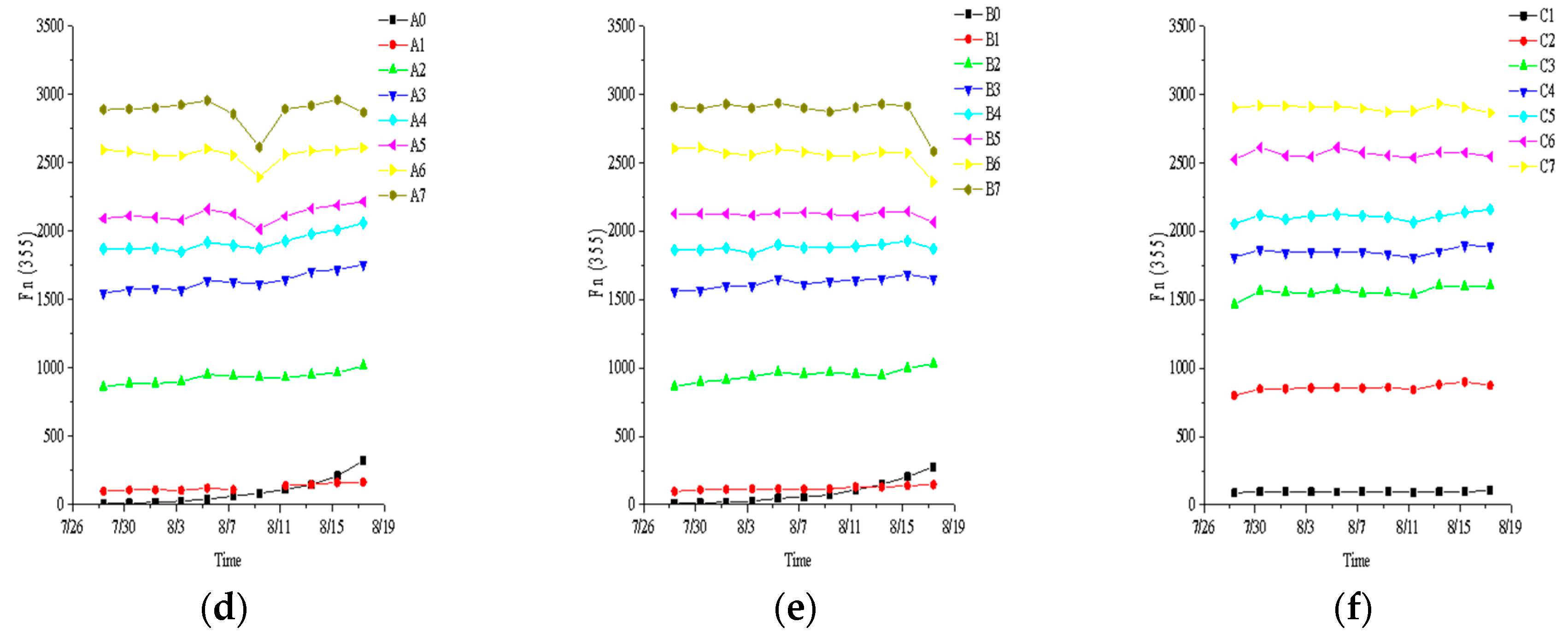
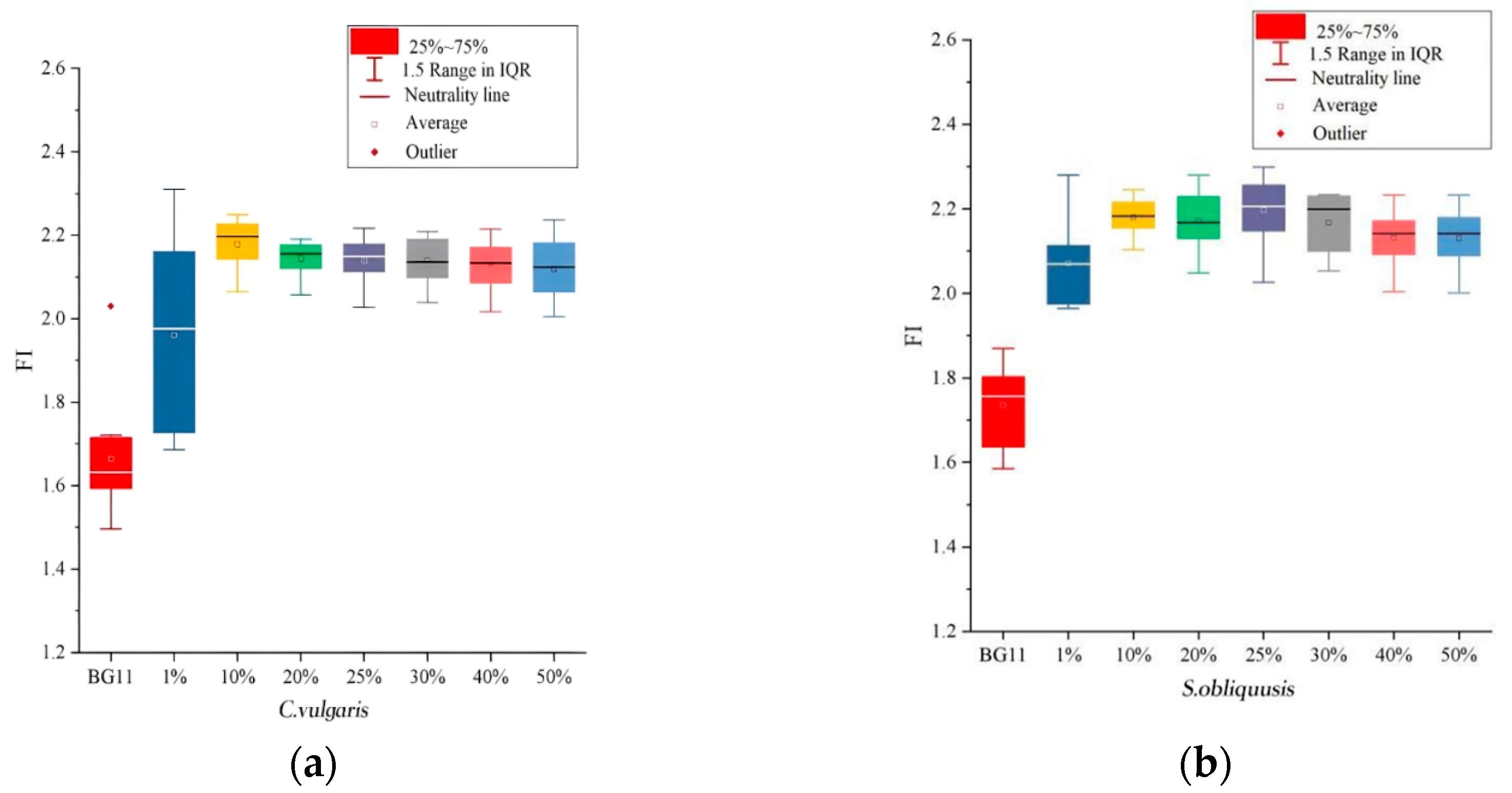
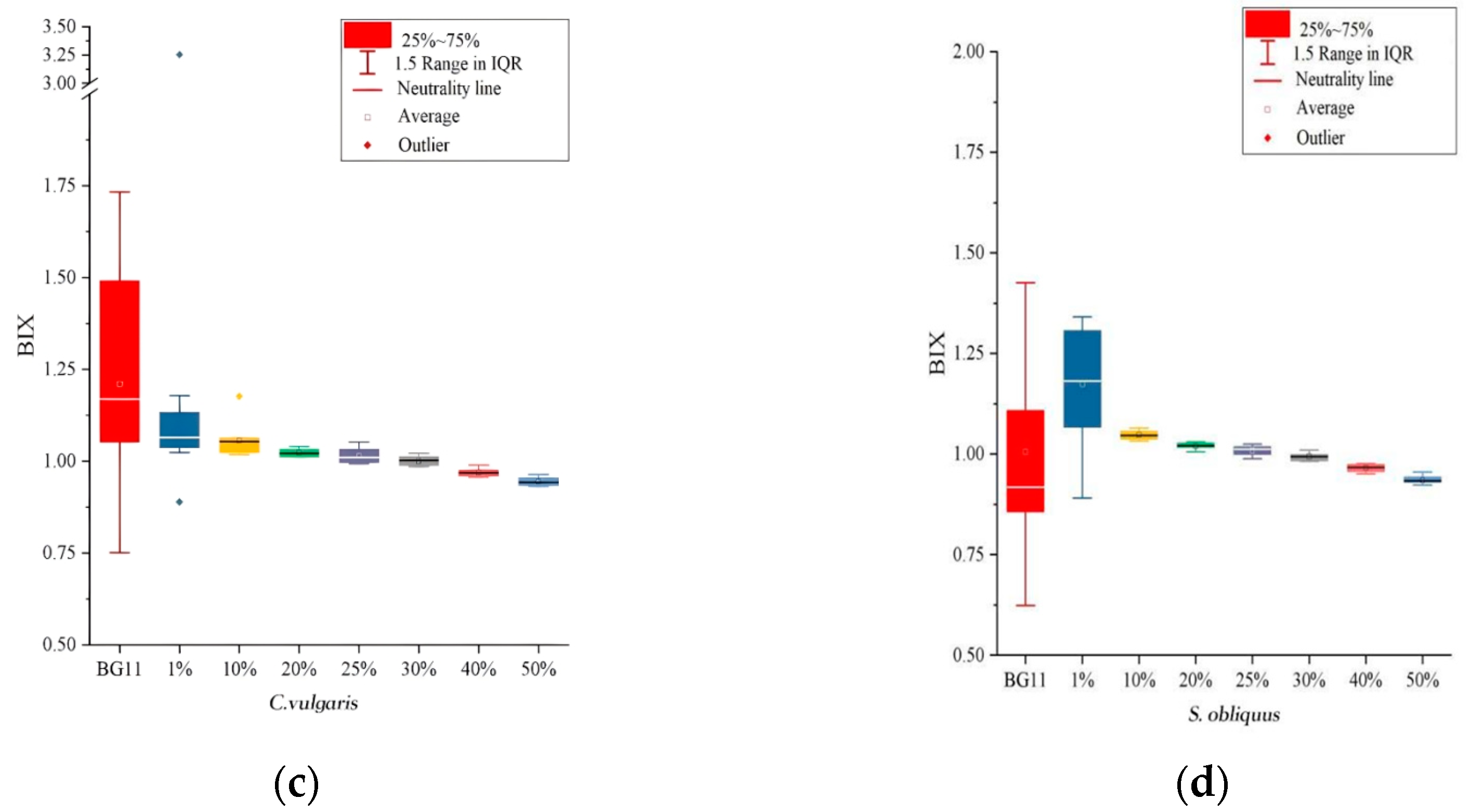
| Base Fluid | BG11 | 1% Leachate | 10% Leachate | 20% Leachate | 25% Leachate | 30% Leachate | 40% Leachate | 50% Leachate |
|---|---|---|---|---|---|---|---|---|
| C. vulgaris | A0 | A1 | A2 | A3 | A4 | A5 | A6 | A7 |
| S. obliquus | B0 | B1 | B2 | B3 | B4 | B5 | B6 | B7 |
| Without algae | / | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
| C. vulgaris | S. obliquus | K2HPO4·3H2O (g/L) | MgSO4·7H2O (g/L) | C6H11FeNO7 (g/L) | Trace Elements (mL/L) |
|---|---|---|---|---|---|
| A1 | B1 | 0.200 | 0.075 | 0 | 0 |
| A2 | B2 | 0 | 0 | 0 | 1 |
| A3 | B3 | 0.200 | 0.075 | 0 | 1 |
| A4 | B4 | 0.200 | 0 | 0.060 | 1 |
| A5 | B5 | 0 | 0 | 0 | 0 |
| A6 | B6 | 0 | 0.075 | 0.060 | 1 |
| A7 | B7 | 0.200 | 0 | 0.060 | 0 |
| A8 | B8 | 0 | 0.075 | 0.060 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Pang, W.; Zheng, Y.; Wang, C.; Chen, Q.; Ke, Q.; Wang, Q. Research on the Properties of DOM from the Microalgal Treatment Process for Leachate from Incineration Fly Ash Based on EEM-PARAFAC Analysis. Water 2024, 16, 3413. https://doi.org/10.3390/w16233413
Yang Y, Pang W, Zheng Y, Wang C, Chen Q, Ke Q, Wang Q. Research on the Properties of DOM from the Microalgal Treatment Process for Leachate from Incineration Fly Ash Based on EEM-PARAFAC Analysis. Water. 2024; 16(23):3413. https://doi.org/10.3390/w16233413
Chicago/Turabian StyleYang, Yahan, Wenjing Pang, Yuting Zheng, Chuanhua Wang, Qiongzhen Chen, Qiang Ke, and Qi Wang. 2024. "Research on the Properties of DOM from the Microalgal Treatment Process for Leachate from Incineration Fly Ash Based on EEM-PARAFAC Analysis" Water 16, no. 23: 3413. https://doi.org/10.3390/w16233413
APA StyleYang, Y., Pang, W., Zheng, Y., Wang, C., Chen, Q., Ke, Q., & Wang, Q. (2024). Research on the Properties of DOM from the Microalgal Treatment Process for Leachate from Incineration Fly Ash Based on EEM-PARAFAC Analysis. Water, 16(23), 3413. https://doi.org/10.3390/w16233413






