The Bioaccumulation of Potentially Toxic Elements in the Organs of Phragmites australis and Their Application as Indicators of Pollution (Bug River, Poland)
Abstract
1. Introduction
2. Materials and Methods
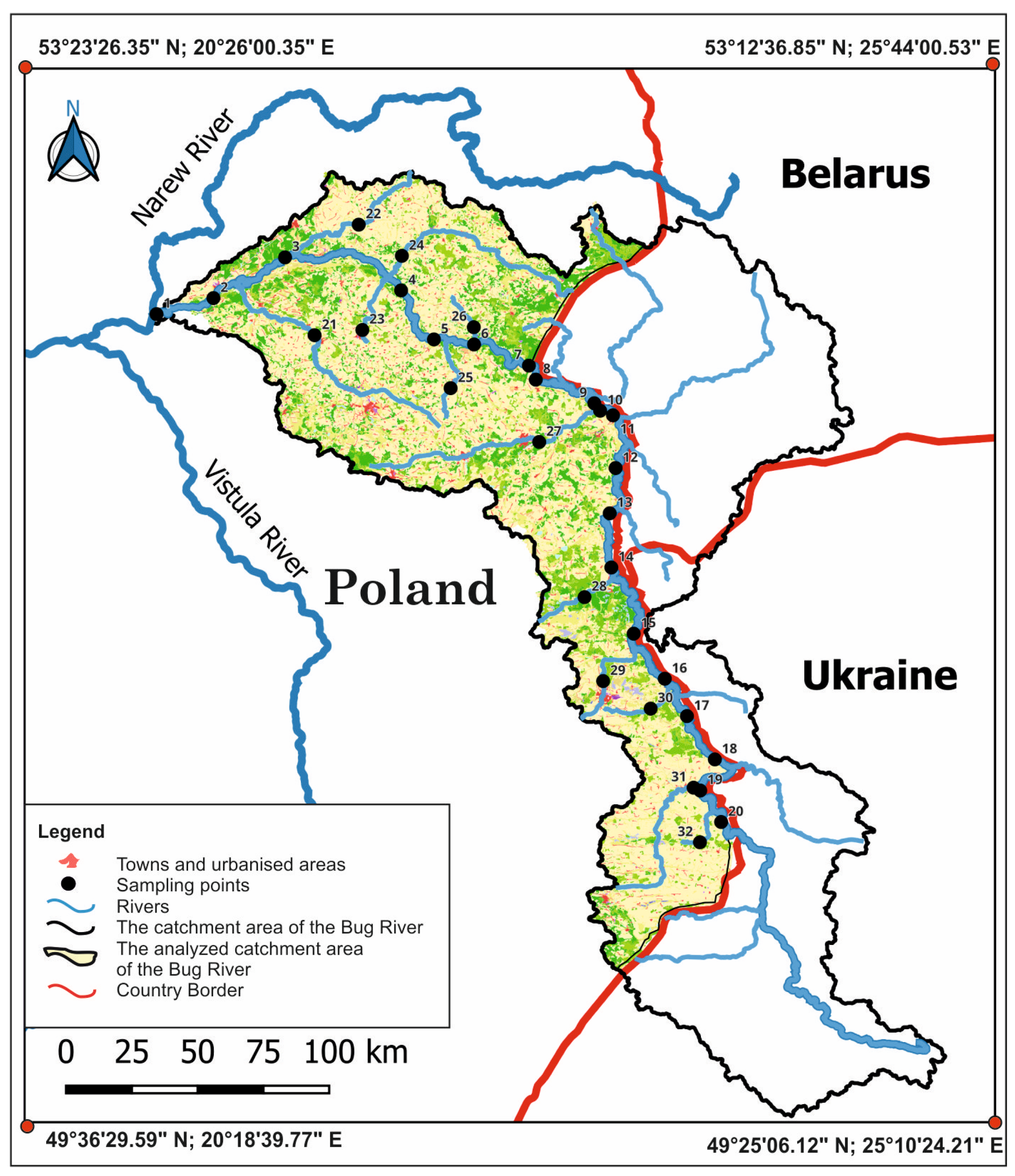
2.1. Study Area
2.2. Plant Sampling and Analytical Procedures
2.3. Statistical Analysis
3. Results and Discussion
3.1. Content of Potentially Toxic Elements (PTEs) in Phragmites australis
3.2. Spatial Distribution of PTEs in Phragmites australis
3.3. Identification of Local PTE Sources in P. australis Based on Land Use in the Catchment
- Urban: Fe > Mn > Zn > Pb > Ni > Cu > Cr > Cd
- Agricultural and forested: Fe > Mn > Zn > Ni > Pb > Cu > Cr > Cd
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tikadar, K.K.; Jahan, F.; Mia, R.; Rahman, M.D.Z.; Sultana, M.A.; Islam, S.; Kunda, M. Assessing the potential ecological and human health risks of trace metal pollution in surface water, sediment, and commercially valuable fish species in the Pashur River, Bangladesh. Environ. Monit. Assess. 2024, 196, 1042. [Google Scholar] [CrossRef] [PubMed]
- Badawy, W.M.; Ghanim, E.H.; Duliu, O.G.; El Samman, H.; Frontasyeva, M.V. Major and trace element distribution in soil and sediments from the Egyptian central Nile Valley. J. Afr. Earth Sci. 2017, 131, 53–61. [Google Scholar] [CrossRef]
- Kicińska, A.; Wikar, J. Health risk associated with soil and plant contamination in industrial areas. Plant Soil 2024, 498, 295–323. [Google Scholar] [CrossRef]
- Ali, M.M.; Rahman, S.; Islam, M.S.; Rakib, M.R.J.; Hossen, S.; Rahman, M.Z.; Kormoker, T.; Idris, A.M.; Phoungthong, K. Distribution of heavy metals in water and sediment of an urban river in a developing country: A probabilistic risk assessment. Int. J. Sediment. Res. 2022, 37, 173–187. [Google Scholar] [CrossRef]
- Barszcz, A.A.; Sidoruk, M. Impacts of water management in aquaculture on heavy metal accumulation in rainbow trout muscles and associated health risks from consumption. Food Control 2024, 164, 110617. [Google Scholar] [CrossRef]
- Jayasree, B.; Naidu, M.V.S.; Bhaskar, B.P.; Madhuri, K.V.N.; Sagar, G.K.; Kumari, P.L. Transect approach for assessing major and trace elemental contamination in agricultural soils of the palamaner division, chittoor district, andhra pradesh, India. Environ. Monit. Assess. 2024, 196, 1040. [Google Scholar] [CrossRef]
- Skorbiłowicz, E.; Ofman, P.; Skorbiłowicz, M.; Sidoruk, M.; Tarasiuk, U. Geochemical Assessment of Heavy Metal Distribution in Bug River Sediments, Poland: The Impacts of Urbanization and Agricultural Practices. Water 2024, 16, 1573. [Google Scholar] [CrossRef]
- Alsubih, M.; El Morabet, R.; Khan, R.A.; Khan, N.A.; Khan, M.H.; Ahmed, S.; Qadir, A.; Changani, F. Occurrence and health risk assessment of arsenic and heavy metals in groundwater of three industrial areas in Delhi, India. Environ. Sci. Pollut. Res. 2021, 28, 63017–63031. [Google Scholar] [CrossRef]
- Guittonny-Philippe, A.; Masotti, V.; Höhener, P.; Boudenne, J.-L.; Viglione, J.; Laffont-Schwob, I. Constructed wetlands to reduce metal pollution from industrial catchments in aquatic Mediterranean ecosystems: A review to overcome obstacles and suggest potential solutions. Environ. Int. 2014, 64, 1–16. [Google Scholar] [CrossRef]
- Kushwaha, A.; Rani, R.; Kumar, S.; Gautam, A. Heavy metal detoxification and tolerance mechanisms in plants: Implications for phytoremediation. Environ. Rev. 2016, 24, 39–51. [Google Scholar] [CrossRef]
- Telahigue, K.; Antit, M.; Rabeh, I.; Chouba, L.; Kheriji, S.; Cafsi, M.E.; Hajji, T.; Mhadhbi, L. Heavy Metal Bioaccumulation and Oxidative Stress Profile in Brachidontes pharaonis (Bivalvia: Mytilidae) from the Tunisian Coast: Insight into Its Relevance as Bioindicator of Marine Pollution. Bull. Environ. Contam. Toxicol. 2022, 109, 831–838. [Google Scholar] [CrossRef] [PubMed]
- El-Metwally, M.E.A. Heavy metal accumulation and interaction dynamics in Brachidontes pharaonis: A bioindicator study in the Red Sea. Environ. Monit. Assess. 2024, 196, 1011. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Sharma, A. Phragmites australis as a model organism for phytoremediation: Opportunities and constraints. Environ. Sci. Pollut. Res. 2018, 25, 21734–21748. [Google Scholar]
- No, D. Directive 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 establishing the list of priority substances in the field of water policy and amending Directive 2000/60/EC (Text with EEA relevance). Off. J. Eur. Communities 2001, 15, 1–5. [Google Scholar]
- Srivastava, J.; Gupta, A.; Chandra, H. Phytoremediation of urban wastewater: Potentials and prospects. Int. J. Phytoremediation 2021, 23, 34–52. [Google Scholar]
- Marmiroli, M.; Pigoni, V.; Savo-Sardaro, M.L. The role of macrophytes in assessing water quality in Italian river basins: Towards a new biomonitoring index? Ecol. Indic. 2019, 106, 105457. [Google Scholar]
- Franzaring, J.; Klumpp, A.; Fangmeier, A. Air quality bio-monitoring: Successes, challenges and perspectives of biomonitoring programs in Europe. Environ. Pollut. 2017, 230, 994–1008. [Google Scholar]
- Ruiz, M.; Velasco, J. Nutrient Bioaccumulation in Phragmites australis: Management Tool for Reduction of Pollution in the Mar Menor. Water Air Soil. Pollut. 2010, 205, 173–185. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Al-Sodany, Y.M.; Haroun, S.A.; Galal, T.M.; Ayed, H.; Khedher, K.M.; Jensen, K. Seasonal potential of Phragmites australis in nutrient removal to eliminate the eutrophication in Lake Burullus. Egypt. J. Freshw. Ecol. 2020, 35, 135–155. [Google Scholar] [CrossRef]
- Du Laing, G.; Tack, F.M.G.; Verloo, M.G. Performance of selected destruction methods for the determination of heavy metals in reed plants (Phragmites australis). Anal. Chim. Acta 2003, 497, 191–198. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, X.; Li, L.; Chen, H. Multivariate statistical analysis of heavy metals in street dust of Baoji, NW China. J. Hazard. Mater. 2010, 173, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, X.; Zhu, M.; Zhao, K.; Wu, J.; Xu, J.; Huang, P. Identification of trace element sources and associated risk assessment in vegetable soils of the urban-rural transitional area of Hangzhou, China. Environ. Pollut. 2011, 159, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Markert, B. Plants as Biomonitors: Indicators for Heavy Metals in the Terrestrial Environment; VCH Publishers: Weinheim, Germany, 1992. [Google Scholar]
- Cymes, I.; Glińska-Lewczuk, K.; Szymczyk, S.; Sidoruk, M.; Potasznik, A. Distribution and potential risk assessment of heavy metals and arsenic in sediments of a dam reservoir: A case study of the Łoje retention reservoir, NE Poland. J. Elementol. 2017, 22, 843–856. [Google Scholar] [CrossRef]
- Lu, Q.; He, Z.-L.; Graetz, D.A.; Stoffella, P.J.; Yang, X. Uptake, and distribution of metals by water lettuce (Pistia stratiotes L.). Environ. Sci. Pollut. Res. 2011, 18, 978–986. [Google Scholar] [CrossRef]
- Sawidis, T.; Chettri, M.K.; Zachariadis, G.A.; Stratis, J.A. Heavy metals in aquatic plants and sediments from water systems in Macedonia, Greece. Ecotoxicol. Environ. Saf. 1995, 32, 73–80. [Google Scholar] [CrossRef]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Cicero-Fernández, D.; Peña-Fernández, M.; Expósito-Camargo, J.A.; Antizar-Ladislao, B. Role of Phragmites australis (common reed) for heavy metals phytoremediation of estuarine sediments. Int. J. Phytoremediation 2016, 18, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Batty, L.C.; Baker, A.J.M.; Wheeler, B.D. Aluminium and phosphate uptake by Phragmites australis: The role of Fe, Mn and Al root plaques. Ann. Bot. 2002, 89, 443–449. [Google Scholar] [CrossRef]
- Mazmudar, K.; Das, S. Phytoremediation of Pb, Zn, Fe, and Mg with 25 wetland species from a papermill contaminated site in North East India. Environ. Sci. Pollut. Res. 2015, 22, 701–710. [Google Scholar] [CrossRef]
- Zhou, C.; Ge, N.; Guo, J.; Zhu, L.; Ma, Z.; Cheng, S.; Wang, J. Enterobacter asburiae Reduces Cadmium Toxicity in Maize Plants by Repressing Iron Uptake-Associated Pathways. J. Agric. Food Chem. 2019, 67, 10126–10136. [Google Scholar] [CrossRef]
- Zhou, C.; Ge, N.; Guo, J.; Zhu, L.; Ma, Z.; Cheng, S.; Wang, J. Silicon supplementation ameliorated the inhibition of photosynthesis and nitrate metabolism by cadmium (Cd) toxicity in Cucumis sativus L. Sci. Hortic. 2010, 123, 521–530. [Google Scholar]
- Ederli, L.; Reale, L.; Ferranti, F.; Pasqualini, S. Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol. Plantarum 2004, 121, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Feng, Q. Comparison of the Potential Ecological and Human Health Risks of Heavy Metals from Sewage Sludge and Livestock Manure for Agricultural Use. Toxics 2021, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Verkleij, J.A. The Effects of Heavy Metals Stress on Higher Plants and Their Use as Biomonitors. In Plant as Bioindicators: Indicators of Heavy Metals in the Terrestrial Environment; Markert, B., Ed.; VCH Publishers: Weinheim, Germany, 1993; pp. 415–442. [Google Scholar]
- Gope, M.; Masto, R.E.; George, J.; Hoque, R.R.; Balachandran, S. Bioavailability and health risk of some potentially toxic elements (Cd, Cu, Pb and Zn) in street dust of Asansol, India. Ecotoxicol. Environ. Saf. 2017, 138, 231–241. [Google Scholar] [CrossRef]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. New Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef]
- Gichner, T.; Žnidar, I.; Száková, J. Evaluation of DNA damage and mutagenicity induced by lead in tobacco plants. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2008, 652, 186–190. [Google Scholar] [CrossRef]
- Reddy, A.M.; Kumar, S.G.; Jyothsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead induced changes in antioxidant metabolism of horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and bengalgram (Cicer arietinum L.). Chemosphere 2005, 60, 97–104. [Google Scholar] [CrossRef]
- Siedlecka, A.; Tukendorf, A.; Skórzyńska-Polit, E.; Maksymiec, W.; Wójcik, M.; Baszyński, T.; Krupa, Z. Angiosperms (Asteraceae, Convolvulaceae, Fabaceae and Poaceae; other than Brassicaceae). In Metals in the Environment; Prasad, M.N.V., Ed.; Analysis by Biodiversity; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 171–217. [Google Scholar]
- Bonanno, G. Comparative performance if trace element bioaccumulation and biomonitoring in the plant species Typha domingensis, Phragmites australis and Arundo donax. Ecotoxicol. Environ. Saf. 2013, 97, 124–130. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, H.; Zhou, T.; Lin, C.; Guo, S. The estimated atmospheric lead emissions in China, 1990–2009. Atmos. Environ. 2012, 60, 1–8. [Google Scholar] [CrossRef]
- Ali, S.; Mir, R.A.; Tyagi, A.; Manzar, N.; Kashyap, A.S.; Mushtaq, M.; Raina, A.; Park, S.; Sharma, S.; Mir, Z.A.; et al. Chromium Toxicity in Plants: Signaling, Mitigation, and Future Perspectives. Plants 2023, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants: Tansley review. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmitt, R.K.; Tiecher, T.L.; Facco, D.B.; Silva, L.O.S.; Schwalbert, R.; Drescher, G.L.; Trentin, E.; Somavilla, L.M.; Kulmann, M.S.S.; Silva, I.C.B.; et al. Copper and zinc distribution and toxicity in ‘Jade’/‘Genovesa’ young peach tree. Sci. Hortic. 2020, 259, 108763. [Google Scholar] [CrossRef]
- Kumari, M.; Tripathi, B.D. Efficiency of Phragmites australis and Typha latifolia for heavy metal removal from wastewater. Ecotoxicol. Environ. Saf. 2015, 112, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Kelepertzis, E. Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin, Peloponnese, Greece. Geoderma 2014, 221-222, 82–90. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Chakraborty, S.; Tuteja, D.; Patel, M. Zinc and Chromium Load in Road Dust, Suspended Particulate Matter and Foliar Dust Deposits of Anand City, Gujarat. Open J. Met. 2013, 3, 42–50. [Google Scholar] [CrossRef]
- Ozaki, H.; Watanabe, I.; Kuno, K. Investigation of the heavy metal sources in relation to automobiles. Water Air Soil Pollut. 2004, 157, 209–223. [Google Scholar] [CrossRef]
- Vardanyan, L.G.; Ingole, B.S. Studies on heavy metal accumulation in aquatic macrophytes from Sevan (Armenia) and Carambolim (India) lake systems. Environ. Int. 2006, 32, 208–218. [Google Scholar] [CrossRef]
- Milke, J.; Gałczyńska, M.; Wróbel, J. The Importance of Biological and Ecological Properties of Phragmites Australis (Cav.) Trin. Ex Steud., in Phytoremendiation of Aquatic Ecosystems—The Review. Water 2020, 12, 1770. [Google Scholar] [CrossRef]
- Li, J.; Leng, Z.; Jia, H.; Wei, L.; Yuguda, T.K.; Du, D. Effect of Seawall Embankment Reclamation on the Distribution of Cr, Cu, Pb and Zn Pollution in Invasive Spartina alterniflora and Native Phragmites australis Coastal Saltmarshes of East China. Biology 2023, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Llagostera, I.; Pérez, M.; Romero, J. Trace metal content in the seagrass Cymodocea nodosa: Differential accumulation in plant organs. Aquat. Bot. 2011, 95, 124–128. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Rizwan, M.; Zaheer, I.E.; Yavaş, İ.; Ünay, A.; Abdel-DAIM, M.M.; Bin-Jumah, M.; Hasanuzzaman, M.; Kalderis, D. Application of Floating Aquatic Plants in Phytoremediation of Heavy Metals Polluted Water: A Review. Sustainability 2020, 12, 1927. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, Q.; Lin, Q.; Zeng, C.; Zhong, C. Cadmium Source Identification in Soils and High-Risk Regions Predicted by Geographical Detector Method. Environ. Pollut. 2020, 263, 114338. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing Anthropogenic Cadmium Emissions: From Sources to Pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Sun, J.; She, J.; Yin, M.; Fang, F.; Xiao, T.; Song, G.; Liu, J. Geochemical Transfer of Cadmium in River Sediments near a Lead-Zinc Smelter. Ecotoxicol. Environ. Saf. 2020, 196, 110529. [Google Scholar] [CrossRef] [PubMed]
- Skorbiłowicz, E.; Skorbiłowicz, M.; Rogowska, W. Temporal and spatial changes in metal contents of arable soils in the Bug river catchment in 1995-2015 (Poland). Int. J. Conserv. Sci. 2022, 13, 895–914. [Google Scholar]
- European Commission (EC). EU Biodiversity Strategy for 2030: Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Brabec, E.; Schulte, S.; Richards, P.L. Impervious surfaces and water quality: A review of current literature and its implications for watershed planning. J. Plan. Lit. 2002, 16, 499–514. [Google Scholar] [CrossRef]
- USEPA. National Water Quality Inventory: Report to Congress; USEPA, Office of Water: Washington, DC, USA, 2017.
- Lin, B.Q.; Chen, X.; Yao, H.; Chen, Y.; Liu, M.; Gao, L.; James, A. Analyses of landuse change impacts on catchment runoff using different time indicators based on SWAT model. Ecol. Indic. 2015, 58, 55–63. [Google Scholar] [CrossRef]
- Steinnes, E.; Friedland, A.J. Metal contamination of natural surface soils from long-range atmospheric transport: Existing and missing knowledge. Environ. Rev. 2006, 14, 169–186. [Google Scholar] [CrossRef]

| Basic Statistics | Pb | Cr | Cu | Zn | Ni | Cd | Fe | Mn |
|---|---|---|---|---|---|---|---|---|
| Roots n = 96 | ||||||||
| Min | 2.59 | 0.70 | 0.58 | 5.15 | 2.98 | 0.29 | 360.55 | 104.58 |
| Max | 6.47 | 2.45 | 2.45 | 19.51 | 6.00 | 0.48 | 632.24 | 358.75 |
| Average | 7.1 ab | 2.9 | 3.3 ef | 21.7 h | 8.2 ij | 0.40 lł | 1361.9 mno | 430.1 rs |
| Stalks n = 96 | ||||||||
| Min | 3.32 | 0.97 | 0.55 | 7.31 | 2.36 | 0.06 | 360.55 | 111.52 |
| Max | 7.36 | 3.05 | 3.22 | 26.27 | 6.48 | 0.24 | 791.68 | 403.88 |
| Average | 6.3 a | 2.2 cd | 2.7 eg | 19.9 | 5.8 ik | 0.29 l | 885.5 mop | 227.9 rtu |
| Leaves n = 96 | ||||||||
| Min | 7.16 | 2.14 | 3.32 | 30.63 | 7.15 | 0.29 | 1381.74 | 491.66 |
| Max | 12.42 | 7.11 | 6.97 | 50.41 | 11.09 | 0.45 | 2919.86 | 790.76 |
| Average | 5.2 ab | 1.6 cd | 1.9 fg | 17.5 h | 3.3 j | 0.18 ł | 489.1 np | 159.4 stu |
| Natural Content | 0.1–5 ** | 0.02–0.5 * | 5–30 * | 10–70 * | 0.1–5 * | 0.05–0.2 * | 50–200 ** | 20–500 * |
| Basic Statistics | Pb | Cr | Cu | Zn | Ni | Cd | Fe | Mn |
|---|---|---|---|---|---|---|---|---|
| Agricultural area n = 39 | ||||||||
| Min | 2.59 | 0.70 | 0.58 | 5.15 | 2.98 | 0.29 | 360.55 | 104.58 |
| Max | 6.47 | 2.45 | 2.45 | 19.51 | 6.00 | 0.48 | 632.24 | 358.75 |
| Average | 4.68 a | 1.33 c | 1.78 e | 13.26 g | 4.30 i | 0.39 k | 477.25 ł | 166.51 n |
| Forest area n = 33 | ||||||||
| Min | 3.32 | 0.97 | 0.55 | 7.31 | 2.36 | 0.06 | 360.55 | 111.52 |
| Max | 7.36 | 3.05 | 3.22 | 26.27 | 6.48 | 0.24 | 791.68 | 403.88 |
| Average | 5.32 b | 1.62 d | 1.95 f | 15.15 h | 4.76 j | 0.13 kl | 520.28 m | 191.07 o |
| Urbanized area n = 24 | ||||||||
| Min | 7.16 | 2.14 | 3.32 | 30.63 | 7.15 | 0.29 | 1381.74 | 491.66 |
| Max | 12.42 | 7.11 | 6.97 | 50.41 | 11.09 | 0.45 | 2919.86 | 790.76 |
| Average | 9.96 ab | 4.58 cd | 4.99 ef | 36.38 gh | 9.45 ij | 0.36 l | 2156.27 łm | 623.20 no |
| Variable | Urbanized Area | Agricultural Area | Forest Area | |||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 1 | Factor 2 | Factor 1 | Factor 2 | |
| Pb | 0.66 | 0.55 | 0.78 | −0.11 | 0.83 | −0.50 |
| Cr | 0.85 | −0.25 | 0.84 | −0.01 | 0.64 | 0.10 |
| Cu | 0.85 | 0.22 | 0.37 | 0.60 | 0.84 | −0.20 |
| Zn | 0.70 | −0.05 | 0.03 | 0.63 | 0.63 | −0.50 |
| Ni | 0.22 | 0.71 | 0.13 | 0.53 | 0.64 | −0.04 |
| Cd | 0.19 | −0.49 | −0.38 | 0.16 | −0.25 | 0.76 |
| Fe | −0.13 | 0.82 | 0.34 | 0.76 | 0.72 | 0.07 |
| Mn | 0.40 | 0.42 | 0.85 | 0.10 | 0.74 | −0.01 |
| Variance explained [%] | 41 | 27 | 30 | 22 | 31 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorbiłowicz, E.; Skorbiłowicz, M.; Sidoruk, M. The Bioaccumulation of Potentially Toxic Elements in the Organs of Phragmites australis and Their Application as Indicators of Pollution (Bug River, Poland). Water 2024, 16, 3294. https://doi.org/10.3390/w16223294
Skorbiłowicz E, Skorbiłowicz M, Sidoruk M. The Bioaccumulation of Potentially Toxic Elements in the Organs of Phragmites australis and Their Application as Indicators of Pollution (Bug River, Poland). Water. 2024; 16(22):3294. https://doi.org/10.3390/w16223294
Chicago/Turabian StyleSkorbiłowicz, Elżbieta, Mirosław Skorbiłowicz, and Marcin Sidoruk. 2024. "The Bioaccumulation of Potentially Toxic Elements in the Organs of Phragmites australis and Their Application as Indicators of Pollution (Bug River, Poland)" Water 16, no. 22: 3294. https://doi.org/10.3390/w16223294
APA StyleSkorbiłowicz, E., Skorbiłowicz, M., & Sidoruk, M. (2024). The Bioaccumulation of Potentially Toxic Elements in the Organs of Phragmites australis and Their Application as Indicators of Pollution (Bug River, Poland). Water, 16(22), 3294. https://doi.org/10.3390/w16223294





