Abstract
Submerged macrophytes play an important role in maintaining the structure and function of shallow lakes. Under eutrophication, the community of submerged macrophytes shows a shift of growth forms from rosette-like to canopy-forming macrophytes and a further decline due to the increasing shading from epiphyton and phytoplankton. However, at the early phase of eutrophication, the population of submerged macrophytes may increase due to increased nutrient availability, and the responses of submerged macrophytes to eutrophication are expected to be growth-form dependent. To explore the direct effects of nutrient enrichment on the submerged macrophytes of both growth forms, we constructed a mesocosm study with rosette-like macrophytes (Vallisneria denseserrulata and V. spinulosa) and canopy-forming macrophytes (Potamogeton lucens and P. wrightii) under two nutrient levels but maintained low phytoplankton and epiphyton biomass. Nutrient enrichment had a positive effect on the plant size for both macrophyte growth forms under low algal shading. Based on the 21 plant traits determined, the same growth form responded similarly to the increase in nutrient availability with few exceptions. Interestingly, increased nutrient levels induced different allocation strategies between canopy-forming (especially for ‘magnopotamid’) and rosette-like submerged macrophytes. The increased nutrients promoted leaf growth in rosette-like macrophytes and ramet production in canopy-forming macrophytes. These results provide a case study on the direct effects of increased nutrient levels on submerged macrophytes during the early phase of eutrophication in shallow lakes.
1. Introduction
As primary producers, submerged macrophytes play an important role in maintaining the structure and function of shallow lakes [1,2]. In shallow lakes with moderate nutrient loading, submerged macrophytes can inhibit phytoplankton growth by competing for nutrients [2,3], releasing allelopathic substances [4,5], and providing habitat and predation refuge for zooplankton and invertebrate grazers [1,6], thus maintaining clear water conditions. With nutrient enrichment in the water column, epiphyton and phytoplankton proliferation reduces light availability for macrophyte growth. Specifically, phytoplankton grows in the upper layer of the water body and affects the transmission of light in the water, while epiphyton grows on the leaves of submerged macrophytes and forms a layer of shading film, thus affecting the use of light by submerged macrophytes [4,7]. The decline of submerged plants leads to the enhancement of sediment re-suspension and transforms water from a macrophyte-dominated clear state to a turbid state [4], which are so-called alternative stable states in shallow lakes [8]. However, Sayer et al. [9] argued that the loss of submerged macrophytes in many shallow lakes was coupled with changes in the composition of species in the macrophyte community. Notably, macrophyte communities show succession from rosette-like macrophytes (such as Vallisneria) to canopy-forming macrophytes (such as Potamogeton and Myriophyllum), which differs from the sudden decay of macrophytes proposed by the theory of alternative stable states and reflects the changes in macrophyte growth forms during long-term community succession [10,11,12].
This long-term community succession of submerged macrophytes driven by eutrophication is one of the research hot topics in shallow lake ecosystems [13,14] and is commonly documented by means of a field investigation, remote sensing, or paleolimnology [10]. For example, paleolimnological records can be used to reconstruct the submerged vegetation with the help of biological indicators (e.g., pollen and the phytolites of aquatic plants) to provide continuous information on the succession of macrophyte communities in historical periods [15]. Recent paleolimnological studies have found that abundant pollen and plant debris (macro-fossils) of submerged macrophyte remain in the sediments of the early stage of lake eutrophication [9,16,17], suggesting that submerged plants may grow and reproduce prosperously during this period [18,19]. However, there is a lack of experimental evidence to show how different types of submerged macrophytes expand rapidly during the early eutrophication period.
Submerged macrophytes can directly absorb nutrients from water through stems, leaves, and roots, but there are significant differences in biomass distribution among submerged macrophytes with different growth types. The stems of rosette-like macrophytes are shortened, the aboveground part is mainly composed of leaves, and the biomass is mainly distributed near the sediment. For the canopy-forming type, the erect stem extends to the surface of the water along with the leaves to form a canopy. At present, there are still great differences in the research on the mechanism of the direct effect of nutrients on submerged macrophytes. Chamber and Kalff [20] concluded that the changes in submerged macrophytes in eutrophic water bodies are mainly related to the decrease in water clarity, epiphyton shading, and plant growth forms but not by the changes in nitrogen and phosphorus concentrations directly. However, other studies have shown that higher nitrogen and phosphorus concentrations in water (especially ammonium nitrogen) are an abiotic stress for submerged macrophytes, affecting their normal physiological activities [21,22]. A higher nutrient load is one of the mechanisms affecting the degradation of submerged macrophytes during eutrophication.
In summary, the effects of water nutrients on different types of submerged macrophytes in the early stage of rapid eutrophication need to be investigated. Therefore, we constructed an initially clear-water mesocosm system dominated by submerged macrophytes and set two water-nutrient levels to simulate the direct impact of a water-nutrient increase on the growth and reproduction of canopy-forming and rosette-like submerged macrophytes. We hypothesized that 1) during the early phase of eutrophication in shallow lakes, with limited phytoplankton and epiphyton shading, the increase in nutrient availability would have a positive effect on submerged macrophyte growth (H1); 2) submerged macrophytes of the same growth form would respond similarly to the increase in nutrient availability due to their similarity in plant structure. However, rosette-like and canopy-forming macrophytes would respond differently to the increase in nutrient availability due to their dissimilarity in plant structure (H2).
2. Material and Methods
2.1. Experimental Design
We ran the mesocosm experiment in the experimental greenhouse facilities of Wuhan Botanical Garden (Chinese Academy of Sciences) (114.43° E, 30.54° N) in Wuhan, Hubei province, China. To investigate the responses of submerged macrophytes to increased nutrients, two pairs of submerged macrophytes and two nutrient levels were established. Submerged macrophytes included two canopy-forming species (Potamogeton lucens (Pl) and P. wrightii (Pw)) and two rosette-like species (Vallisneria denseserrulata (Vd) and V. spinulosa (Vs)). The two growth forms were selected based on the leaf type and plant structure, i.e., canopy-forming magnopotamids and rosette-like vallisneriids, respectively (Figure 1). Canopy-forming magnopotamids are monoecious stoloniferous macrophytes with large elliptical leaves and long shoots that usually reach the water surface to form a canopy for light harvesting. In contrast, rosette-like vallisneriids are dioecious stoloniferous macrophytes with long, linear, low-light-adapted leaves and higher biomass allocation near the sediment.
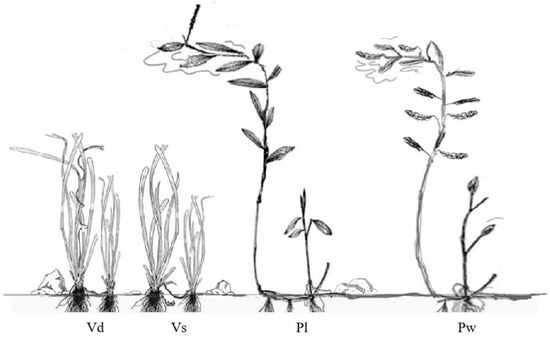
Figure 1.
Differences in structure between rosette-like vallisneriids (VAs) and canopy-forming magnopotamids (POs). Vallisneria denseserrulata (Vd) and V. spinulosa (Vs) belong to VAs; Potamogeton lucens (Pl) and P. wrightii (Pw) belong to POs.
2.1.1. Treatment 1: Growth Forms
The canopy-forming magnopotamids and rosette-like vallisneriid macrophytes were collected from small ponds in the Wuhan Botanical Garden. In July 2019, individuals (shoots for canopy-forming magnopotamids and ramets for rosette-like vallisneriids) of similar size (length ca. 15 cm, fresh weight ca. 1.5 g) of each species were collected, and the epiphyton on leaves was carefully removed by a soft brush [23]. To mimic natural plant communities, a plant coverage of ca. 30% was established in each mesocosm (length: 2m, width: 2 m, depth: 1 m) in eight plastic boxes (length: 0.38 m, width: 0.28 m, height: 0.15 m) (Figure 2A). Macrophyte species of the same growth form were cultivated in the same mesocosm, with four boxes for each species. Five individuals of each species were planted in one box filled with 8 cm of pre-soaked soil and covered with 0.5 cm of pre-washed sand. Thus, the canopy-forming magnopotamids mesocosms contained Pl and Pw, and the rosette-like vallisneriids mesocosms contained Vd and Vs. The macrophyte communities created clear water conditions for the experiments below. Temperatures during the experiment were as follows: September (26.7~28.4 °C), October (21.4~21.8 °C), and November (18.9~20.0 °C).
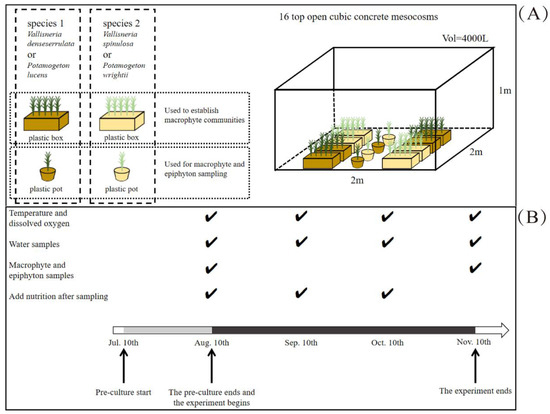
Figure 2.
Schematic diagram of the experiment design (A) and the sampling arrangement (B). Symbols “√” indicate that the sample was collected.
After the macrophyte communities were established, two plastic pots (top diameter: 0.12 m, bottom diameter: 0.08 m, height: 0.07 m) containing Pl and Pw were placed in the canopy-forming magnopotamids mesocosm, with Vd and Vs placed in the rosette-like vallisneriids mesocosm. The plastic pots were filled with soil pre-soaked in lake water for one week [24], and each pot contained one seedling. All seedlings were pre-cultured at a water depth of 1 m (20% lake water + 80% tap water) in a greenhouse for one month (modified from Landkildehus et al. [24]).
2.1.2. Treatment 2: Nutrient Levels
We used two nutrient-level treatments: i.e., with nutrient addition (high-nutrient level, HN) and control without nutrient addition (low-nutrient level, LN). After pre-culture, the HN concentrations of total nitrogen (TN) and total phosphorus (TP) were maintained at 6 mg L−1 and 600 μg L−1, respectively [25,26], by the addition of KNO3 and KH2PO4 solutions [25], which were replenished once a month.
According to the experimental design, 16 concrete mesocosms (two growth forms and two nutrient levels, each treatment with four replicates) were used for a total of 64 pots, 128 boxes, and 704 individuals.
One month after pre-culture, water samples were collected to determine TN, TP, and phytoplankton chlorophyll a (Chla) concentrations (Figure 2B). The following day, nutrients were added to the HN treatment to achieve target TN and TP concentrations, and the experiment lasted for three months. Water samples were collected monthly with dissolved oxygen (DO) and temperature (Temp) first determined using a ProODO optical dissolved oxygen instrument (YSI, Yellow Springs, OH, USA) at a depth of 30 cm between 14:00 and 16:00. The water samples were collected using a tube sampler and thoroughly mixed before analysis. Concentrations of TN and TP were measured using spectrophotometry after digestion with a K2S2O8 solution, and phytoplankton Chla was determined by filtering 1 L of water on a Whatman GF/C filter following ethanol extraction [27].
Macrophyte and epiphyton samples were taken one month after pre-culture to determine the initial state and at the end of the experiment to determine the final state. For these samples, we first randomly selected one mature leaf to determine chlorophyll fluorescence parameters using a PAM-2500 fluorometer (Walz, Effeltrich, Germany) [23]. To avoid midday depression, two chlorophyll fluorescence parameters (i.e., Fv/Fm and Yield) were determined at 09:00–11:00 and 16:00–18:00. According to the PAM-2500 manual, Fv/Fm is the maximum potential quantum efficiency of photosystem II after 15 min of dark adaptation, and the Yield refers to the effective quantum efficiency of photosystem II under 2 min of active light adaptation (100 μmol m–2 s–1 was used here as it was close to our experimental conditions). After this, two randomly selected mature leaves were stored in a plastic bag at 4 °C for the determination of epiphyton biomass in the lab [23]. The remaining macrophytes were harvested and cleaned in the lab, together with the selected leaves for epiphyton biomass, to determine plant trait indicators. The maximum plant height and ramet number were first recorded. The macrophytes were then divided into leaves, stems, stolons, roots, and flowers. The leaf number, leaf area, stolon length, and flower number were measured, respectively. Finally, the macrophyte parts were oven-dried for 48 h at 80 °C and weighed for the leaf mass, stem mass, stolon mass, root mass, and flower mass, with the total plant mass then calculated.
Additional variables were derived based on the above data, including the leaf mass ratio (leaf mass/total plant mass, g g–1), root mass ratio (root mass/total plant mass, g g–1), stolon mass ratio (stolon mass/total plant mass, g g–1), flower biomass ratio (flower mass/total plant mass, g g–1), root/leaf ratio (root mass/leaf mass, g g−1), specific leaf area (leaf area /leaf mass, cm2 mg−1), leaf area root mass ratio (total leaf area/root mass, dm2 g–1), and mean leaf size (total leaf area/total leaf number, cm2).
The 22 indicators were divided into eight groups [28], including biological interactions (epiphyton biomass), plant sizes (ramet number, total plant mass, leaf mass, root mass, and stolon mass), plant spatial architecture (maximum plant height and stolon length), biomass allocation (leaf mass ratio, root mass ratio, and stolon mass ratio), trade-offs between roots and leaves (root/leaf ratio and leaf area root mass ratio), leaf photosynthesis (Yield and Fv/Fm), leaf morphology (leaf number, leaf area, mean leaf size, and specific leaf area), and sexual reproduction (flower number, flower mass, and flower mass ratio).
2.2. Data Analysis
For the water physical–chemical parameters (TN, TP, Temp, DO, and phytoplankton Chla), a two-way analysis of variance (ANOVA) was used to indicate any differences between the initial conditions in August, with the growth form and nutrient level as the two main factors. For the physicochemical parameters collected thereafter, a repeated measure ANOVA was used to compare differences in different treatments, with the growth form and nutrient level as the two main factors. If the interaction between the growth form and nutrient level was significant, a Student’s t-test was used to compare the two growth form treatments or two nutrient-level treatments.
Epiphyton biomass and plant traits were analyzed by a two-way ANOVA, with macrophyte species (two species of each growth form) and the nutrient level as the two main factors. We first tested whether the indicators showed significant differences between nutrient-level treatments to verify H1. We then determined whether the interactions between species and nutrient-level treatments were significant (H2). If the interaction was not significant, H2 was well supported; if the interaction was significant, a Student’s t-test was used to compare the two species treatments or the two nutrient-level treatments. Finally, we investigated whether the chosen indicator differed between the two nutrient-level treatments in both rosette-like vallisneriids and canopy-forming magnopotamids (H2).
As Vd and Pl flowered during the experiment, a Student’s t-test was used to analyze differences in sexual reproduction indicators between the two nutrient levels (Table S4).
Prior to statistical analysis, the data were log (x + 1)-transformed when necessary to satisfy variance homogeneity. SPSS v25.0 was used for statistical analyses. All data are presented as the mean ± standard deviation, with the significance level set to 0.05.
3. Results
3.1. Water Physical–Chemical Parameters
Prior to nutrient additions, no significant differences between the growth form or nutrient-level treatments were found for TN (1.21 ± 0.08 mg L−1), TP (20.3 ± 5.3 μg L−1), and phytoplankton Chla (4.50 ± 1.79 μg L−1) after one month of pre-culturing (two-way ANOVA, F < 0.962, p > 0.05 for all; see Table S1).
The concentrations of TN and TP were significantly higher in the HN treatment than in the LN treatment (Table 1; Figure 3A,B). Although phytoplankton Chla in canopy-forming magnopotamids mesocosms was significantly higher than that in rosette-like vallisneriids mesocosms, phytoplankton Chla was low in all mesocosms, ranging from 0.5 to 5.1 μg L−1 (Figure 3C). Results showed no significant differences in DO between the two nutrient-level treatments, but there was significantly higher DO in canopy-forming magnopotamids mesocosms than in rosette-like vallisneriids mesocosms (Figure 3D). The temperature decreased significantly from September (27.8 ± 0.5 °C) to November (19.5 ± 0.4 °C), but there was no significant difference between the growth form treatments or between the nutrient-level treatments.

Table 1.
Statistical significance level tests on changes in total nitrogen, total phosphorus, phytoplankton Chla, dissolved oxygen and temperature in growth form (GF) and nutrient-level (NL) treatments during the experiment, as determined by repeated measures ANOVA. If the interaction is significant, Student’s t-test was used for comparison between the two GF treatments or between the two NL treatments.
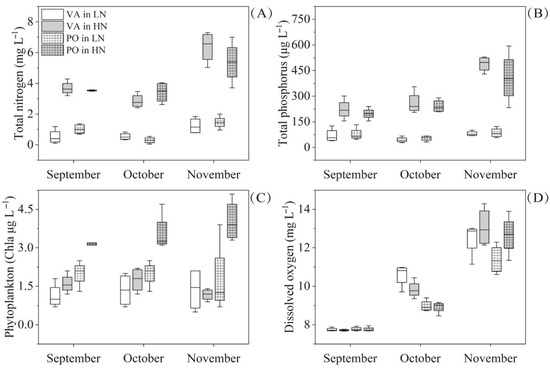
Figure 3.
Changes in TN (A), TP (B), phytoplankton Chla (C), and DO (D) from September 2019 to November 2019 under two nutrient levels (LN and HN) and in two growth forms (VAs and POs). Values are displayed as mean ± standard deviation of four replicates. LN, HN, VAs, POs, TN, TP, and DO refer to low-nutrient level, high-nutrient level, rosette-like vallisneriids, canopy-forming magnopotamids, total nitrogen, total phosphorus, and dissolved oxygen, respectively.
3.2. Response of Two Macrophytes Growth Forms to Increased Nutrient Levels
Under initial conditions, the interactions between species and nutrient-level treatments were not significant for any indicator in the eight pre-defined groups (see Tables S2 and S3). No significant differences were found in any plant trait between the two nutrient-level treatments (two-way ANOVA, F < 2.695, p > 0.05 for all). For macrophyte species of the same growth form, the leaf mass ratio was higher in Pl than in Pw, while the mean leaf size was significantly higher, and the specific leaf area was lower in Vd than in Vs (Figure S1). For the remaining indicators, no significant differences were observed between macrophyte species of the same growth form.
At the end of the experiment, the epiphyton biomass was low (ca. 1.02 ± 0.63 mg Chla m−2 in canopy-forming magnopotamids mesocosms and < 1 mg Chla m−2 in rosette-like vallisneriids) mesocosms and showed no significant difference between macrophytes of the same growth form or between the two nutrient-level treatments (Figure 4A; Table 2 and Table 3).
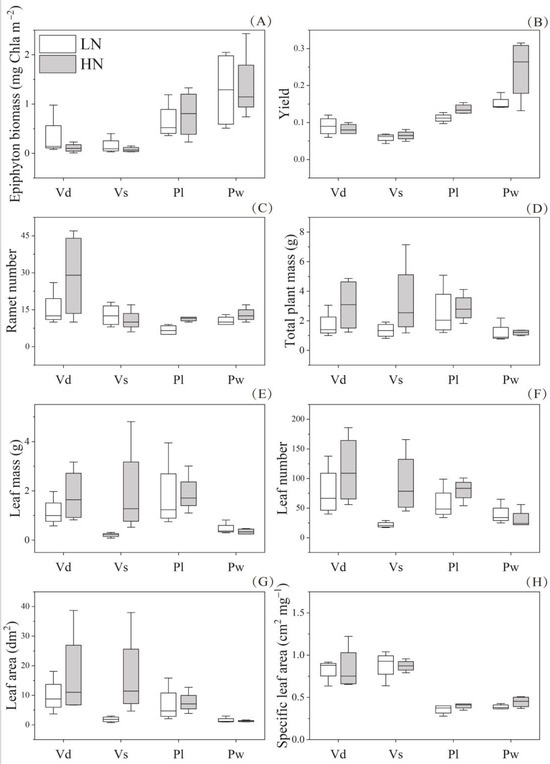
Figure 4.
Difference in epiphyton biomass (A), Yield (B), ramet number (C), total plant mass (D), leaf mass (E), leaf number (F), leaf area (G), and specific leaf area (H) of four macrophyte species (Vd, Vs, Pl, and Pw) in response to two nutrient levels at the end of experiment. Values are displayed as mean ± standard deviation of four replicates. LN, HN, Vd, Vs, Pl, and Pw refer to low-nutrient level, high-nutrient level, Vallisneria denseserrulata, V. spinulosa, Potamogeton lucens, and P. wrightii, respectively.

Table 2.
Statistical significance tests on changes in epiphyton biomass and plant traits of canopy-forming magnopotamids (POs) in species (SP) and nutrient-level (NL) treatments at the end of experiment, as determined by two-way ANOVA.

Table 3.
Student’s t-test on changes in stolon length of canopy-forming magnopotamids (POs) in species (SP) and nutrient-level (NL) treatments at the end of experiment, as determined by two-way ANOVA.
For plant size indicators, the ramet number of the two canopy-forming macrophytes differed between the two nutrient-level treatments, while the leaf mass of the two rosette-like macrophytes differed between the two nutrient-level treatments (Table 2, Table 3, Table 4 and Table 5). The ramet number of the two canopy-forming magnopotamids species and leaf mass of the two rosette-like vallisneriids species were significantly higher under high-nutrient levels (Figure 4B,C). The average total plant mass of the two rosette-like vallisneriids species under high-nutrient treatment was more than twice that under low-nutrient treatment (Figure 4D). For leaf photosynthesis indicators, Yield differed significantly between the two nutrient levels in the canopy-forming macrophytes (Table 2 and Table 3; Figure 4E). For leaf morphology indicators, the leaf number and leaf area of Vd and Vs were significantly higher under high-nutrient treatment than under low-nutrient treatment (Table 4 and Table 5; Figure 4F,G). The other four indicator groups did not show significant differences between the nutrient-level treatments.

Table 4.
Statistical significance tests on changes in epiphyton biomass and plant traits of rosette-like vallisneriids (VAs) in species (SP) and nutrient-level (NL) treatments at the end of experiment, as determined by two-way ANOVA. If the interaction is significant, Student’s t-test was used for comparison between the two SP treatments or between the two NL treatments.

Table 5.
Student’s t-test on changes in plant traits of rosette-like vallisneriids (VAs) in species (SP) and nutrient-level (NL) treatments at the end of experiment.
For the two canopy-forming magnopotamids species, interactions between species and nutrient-level treatments were not significant for any of the plant traits, except for the stolon length, which showed a significant interaction between macrophyte species and nutrient levels (Table 2 and Table 3). However, for the two rosette-like vallisneriids plants, interactions between species and nutrient-level treatments were significant for indicators related to biomass allocation and trade-offs between roots and leaves (Table 4 and Table 5). Further t-test analysis showed that the leaf mass ratio and leaf area root mass ratio of Vs were significantly increased, while the root mass ratio, stolon mass ratio, and root/leaf ratio were significantly decreased under high-nutrient treatment compared with low-nutrient treatment (Figure S2). However, these indicators did not differ in Vd under the two nutrient levels.
4. Discussion
In the mesocosms, increased nutrient levels resulted in an increase in the plant size of both growth forms, thus supporting H1. Furthermore, most traits of macrophytes with the same growth form showed similar responses to the increase in nutrients. In addition, nutrient enrichment promoted more leaves in the rosette-like macrophytes but more ramets in the canopy-forming macrophytes, thus supporting H2.
Based on a 40-day mesocosm study of V. spinulosa, Zhao et al. [29] found that high N concentrations (5 mg L−1) did not cause physiological stress on macrophyte growth, whereas phytoplankton and epiphyton shading resulted in a significant decrease in plant growth. In our experiments, phytoplankton and epiphyton biomass was relatively low, with bottom-view transparency in the mesocosm with a water depth of 1 m. Therefore, the shading effects on macrophyte growth were negligible, and the difference in submerged plant growth between the two nutrient treatments was mainly ascribed to the direct effects of increased nutrients in the water column. Previous studies on V. natans have shown that increased nutrients in the water column promote leaf growth, as leaves are the main organ for nutrient uptake [18,30,31]. In our study, increased nutrient levels significantly promoted leaf mass in the rosette-like macrophytes, potentially reflecting the short-term prosperity of submerged plants in the early stage of eutrophication in natural lakes. Wolfer and Straile [32] also found that the number of ramets in P. perfoliatus increased markedly after sediment fertilization (250 g of slow-release N-P-K-fertilizer). Consistently, in our experiment, nutrient enrichment in the water column also promoted ramet production in the two canopy-forming macrophytes.
Based on most plant indicators, such as the ramet number, plant biomass, and leaf number, submerged macrophytes of the same growth form responded similarly to increased nutrient levels (Table 2, Table 3, Table 4 and Table 5). Growth forms, i.e., morphologically comparable plant types [33], represent a combination of multiple functional traits. Kraft et al. [34] suggested that the growth form, as a group of functional traits, is a more suitable indicator of plant function in an ecosystem than a single functional trait. Our results indicated that the growth form played an important role in the responses of submerged macrophytes to eutrophication [35]. Submerged macrophytes with the same growth form may have similar growth strategies in eutrophic water, but there are still a few exceptions. For example, significant differences were found in the biomass allocation and root/leaf ratio between V. denseserrulata and V. spinulosa in response to the same increased nutrient. This may be related to their different reproductive strategies, with V. spinulosa reproducing by tubers and seeds and V. denseserrulata reproducing by aboveground evergreen parts [36]. In contrast, the two Potamogeton species use the same reproductive strategy, which showed very similar responses to eutrophication.
The four chosen submerged macrophytes are also clonal plants, which tend to show clonal integration under heterogenous conditions [37,38]. Increased nutrient availability in the water column increases the homogeneity of the nutrients, and thus, macrophytes may allocate more resources for growth rather than clonal architecture (e.g., ramet and stolon length). In the current study, the rosette-like macrophytes produced more leaves in response to nutrient additions. In contrast, a higher ramet number (i.e., clonal investment) was observed for magnopotamids at higher nutrient levels. Magnopotamids formed a canopy near the water surface in our mesocosm. Though nutrient additions in the water column promoted homogeneity, the structure of magnopotamids offset this effect and exacerbated spatial heterogeneity, thereby inducing higher clonal investment in these magnopotamids macrophytes. In addition, the higher specific leaf area in these macrophytes (Figure 4H) suggests higher leaf construction costs and a longer leaf lifespan [39], which may explain why these magnopotamids macrophytes did not show a significant leaf biomass response to increased nutrient levels compared to the rosette-like macrophytes. Moreover, other canopy-forming macrophytes (e.g., Myriophyllum spicatum) increase by branching and, therefore, may respond to nutrient additions in a different way [40]. The response of phytoplankton to increased water nutrients was also affected by the growth form of submerged macrophytes. We discovered that there was no significant difference in the TN and TP concentrations, but a significant difference in phytoplankton Chla, between the two growth forms. This indicated that the variation in phytoplankton Chla was directly related with the growth form of submerged macrophytes rather than water-nutrient levels.
5. Conclusions
Under low algal shading conditions, nutrient enrichment could increase macrophyte sizes and directly promote leaf growth in rosette-like macrophytes and ramet production in canopy-forming macrophytes. These results provide a case study of the direct effects of increased nutrients on submerged macrophytes during early eutrophication in shallow lakes. However, as our studies were conducted in mesocosms, interactions between macrophyte growth and nutrient increases in natural lakes warrant further investigation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16223196/s1, Table S1: Concentration of total nitrogen, total phosphorus, and phytoplankton Chla in nutrient level (NL) and growth form (GF) treatments before nutrient addition (August). Table S2: Initial index of plant traits in different nutrient-level treatments before nutrient addition (August). Table S3: Statistical significance tests on changes in epiphyton biomass and plant traits in species (SP) and nutrient-level (NL) treatments before nutrient addition (August), as determined by two-way ANOVA. Table S4: Statistical significance tests on changes in flower number, flower mass, and flower mass ratio of Vallisneria denseserrulata and Potamogeton lucens between the two nutrient levels at the end of experiment, as determined by Student’s t-test. Figure S1: Variation in four plant traits in response to nutrient levels (LN and HN) in two macrophytes with same growth form. (A) Leaf mass ratio in POs; (B) Mean leaf size in VAs; and (C) Leaf mass per area in VAs. Values are displayed as mean ± standard deviation of at least three replicates. POs, VAs, Pl, Pw, Vd, Vs, LN, and HN refer to canopy-forming magnopotamids, rosette-like vallisneriids, Potamogeton lucens, P. wrightii, Vallisneria denseserrulata, V. spinulosa, low-nutrient level, and high-nutrient level, respectively. Figure S2: The differential response of six plant traits of Vd and Vs to nutrient levels. (A) Leaf mass ratio; (B) Root mass ratio; (C) Stolon mass ratio; (D) Root/leaf ratio; and (E) Leaf area rot mass ratio. Values are displayed as mean ± standard deviation of at least three replicates. LN, HN, Vd, and Vs refer to low-nutrient level, high-nutrient level, Vallisneria denseserrulata, and V. spinulosa, respectively.
Author Contributions
Y.C., M.X. and Y.Z. discussed and designed the experiment. Y.Z. completed the collection and assembly of data, and drafted the article. W.L., M.X., W.W. and Y.C. provided guidance for the establishment of the mesocosm system. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by National Natural Science Foundation of China (31870345) and the Joint Fund of Water Science of the Yangtze River set up by National Natural Science Fund of China, Ministry of Water Resources of the People’s Republic of China and the China Three Gorges Corporation (no. U2240213). The authors declare that this study received funding from the China Three Gorges Corporation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank Tingting Li for her assistance during plant cultivation and harvesting. We are grateful to Hang Wu and Wei Liu for providing Figure 1 and Graphical abstract. Yu Cao is supported by the Yangtze River Joint Research Project (Phase II).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carpenter, S.R.; Lodge, D.M. Effects of submersed macrophytes on ecosystem processes. Aquat. Bot. 1986, 26, 341–370. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Søndergaard, M.; Christoffersen, K. The Structuring Role of Submerged Macrophytes in Lakes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Mjelde, M.; Faafeng., B. Ceratophyllum demersum hampers phytoplankton development in some small Norwegian lakes over a wide range of phosphorus concentrations and geographical latitude. Freshw. Biol. 1997, 37, 355–365. [Google Scholar] [CrossRef]
- Phillips, G.L.; Eminson, D.; Moss, B. A mechanism to account for macrophyte decline in progressively eutrophicated freshwaters. Aquat. Bot. 1978, 4, 103–126. [Google Scholar] [CrossRef]
- Gross, E.M.; Hilt, S.; Lombardo, P.; Mulderij, G. Searching for allelopathic effects of submerged macrophytes on phytoplankton—State of the art and open questions. Hydrobiologia 2007, 584, 77–88. [Google Scholar] [CrossRef]
- Lauridsen, T.; Pedersen, L.J.; Jeppesen, E.; Sønergaard, M. The importance of macrophyte bed size for cladoceran composition and horizontal migration in a shallow lake. J. Plankton Res. 1996, 18, 2283–2294. [Google Scholar] [CrossRef]
- Sand-Jensen, K.; Borum, J. Interactions among phytoplankton, periphyton, and macrophytes in temperate freshwaters and estuaries. Aquat. Bot. 1991, 41, 137–175. [Google Scholar] [CrossRef]
- Scheffer, M.; Hosper, S.H.; Meijer, M.L.; Moss, B.; Jeppesen, E. Alternative equilibria in shallow lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Sayer, C.D.; Burgess, A.; Kari, K.; Davidson, T.A.; Peglar, S.; Yang, H.; Rose, N. Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: Implications for the stability of macrophyte-dominance. Freshw. Biol. 2010, 55, 565–583. [Google Scholar] [CrossRef]
- Sayer, C.D.; Davidson, T.A.; Jones, J.I. Seasonal dynamics of macrophytes and phytoplankton in shallow lakes: A eutrophication-driven pathway from plants to plankton? Freshw. Biol. 2010, 55, 500–513. [Google Scholar] [CrossRef]
- Madgwick, G.; Emson, D.; Sayer, C.D.; Willby, N.J.; Rose, N.L.; Jackson, M.J.; Kelly, A. Centennial-scale changes to the aquatic vegetation structure of a shallow eutrophic lake and implications for restoration. Freshw. Biol. 2011, 56, 2620–2636. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, X.; Yang, X.; Odgaard, B.V.; Jeppesen, E. Hydrologic and anthropogenic influences on aquatic macrophyte development in a large, shallow lake in China. Freshw. Biol. 2019, 64, 799–812. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.; Jeppesen, E. The response of Vallisneria spinulosa (Hydrocharitaceae) to different loadings of ammonia and nitrate at moderate phosphorus concentration: A mesocosm approach. Freshw. Biol. 2008, 53, 2321–2330. [Google Scholar] [CrossRef]
- Olsen, S.; Chan, F.; Li, W.; Zhao, S.; Søndergaard, M.; Jeppesen, E. Strong impact of nitrogen loading on submerged macrophytes and algae: A long-term mesocosm experiment in a shallow Chinese lake. Freshw. Biol. 2015, 60, 1525–1536. [Google Scholar] [CrossRef]
- Davidson, T.A.; Sayer, C.D.; Bennion, H.; David, C.; Rose, N.; Wade, M.P. A 250 year comparison of historical, macrofossil and pollen records of aquatic plants in a shallow lake. Freshw. Biol. 2005, 50, 1671–1686. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, K.; Yang, X. Long-term succession of aquatic plants reconstructed from palynological records in a shallow freshwater lake. Sci. Total Environ. 2018, 643, 312–323. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, K.; Huang, S.; Lin, Q. Patterns and trajectories of macrophyte change in East China’s shallow lakes over the past one century. Sci. China Earth Sci. 2021, 64, 1735–1745. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, B.; Zhang, X.; Liu, Z. Nutrient addition delivers growth advantage to Hydrilla verticillata over Vallisneria natans: A mesocosm study. Knowl. Manag. Aquat. Ecosyst. 2019, 420, 12. [Google Scholar] [CrossRef]
- Bakker, E.S.; Nolet, B.A. Experimental evidence for enhanced top-down control of freshwater macrophytes with nutrient enrichment. Oecologia 2014, 176, 825–836. [Google Scholar] [CrossRef]
- Chambers, P.A.; Kalff, J. Light and nutrients in the control of aquatic plant community structure. I. In situ experiments. J. Ecol. 1987, 75, 611–619. [Google Scholar] [CrossRef]
- Rao, Q.; Deng, X.; Su, H.; Xia, W.; Wu, Y.; Zhang, X.; Xie, P. Effects of high ammonium enrichment in water column on the clonal growth of submerged macrophyte Vallisneria natans. Environ. Sci. Pollut. Res. 2018, 25, 32735–32746. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, S.; Chen, D.; Liu, K.; Lu, J. Response of biofilms-leaves of two submerged macrophytes to high ammonium. Chemosphere 2018, 192, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, W.; Jeppesen, E. The response of two submerged macrophytes and periphyton to elevated temperatures in the presence and absence of snails: A microcosm approach. Hydrobiologia 2014, 738, 49–59. [Google Scholar] [CrossRef]
- Landkildehus, F.; Søndergaard, M.; Beklioglu, M.; Adrian, R.; Angeler, D.G.; Hejzlar, J.; Papastergiadou, E.; Zingel, P.; Çakiroglu, A.I.; Scharfenberger, U.; et al. Climate change effects on shallow lakes: Design and preliminary results of a cross-European climate gradient mesocosm experiment. Est. J. Ecol. 2014, 63, 71–89. [Google Scholar] [CrossRef]
- Pacheco, J.P.; Aznarez, C.; Meerhoff, M.; Liu, Y.; Li, W.; Baattrup-Pedersen, A.; Cao, Y.; Jeppesen, E. Small-sized omnivorous fish induce stronger effects on food webs than warming and eutrophication in experimental shallow lakes. Sci. Total Environ. 2021, 797, 148998. [Google Scholar] [CrossRef]
- Liu, Y.; Ndirangu, L.; Li, W.; Pan, J.; Cao, Y.; Jeppesen, E. Response of functional traits of aquatic plants to water depth changes under short-term eutrophic clear-water conditions: A mesocosm study. Plants 2024, 13, 1310. [Google Scholar] [CrossRef]
- Huang, X.F.; Chen, W.M.; Cai, Q.M. Survey, observation and analysis of lake ecology. In Standard Methods for Observation and Analysis in Chinese Ecosystem Research Network, Series V; Standards Press of China: Beijing, China, 1999. [Google Scholar]
- Poorter, L. Growth responses of 15 rain-forest tree species to a light gradient: The relative importance of morphological and physiological traits. Funct. Ecol. 1999, 13, 396–410. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, L.; Chang, F.; Olsen, S.; Søndergaard, M.; Jeppesen, E.; Li, W. Response of Vallisneria spinulosa (Hydrocharitaceae) to contrasting nitrogen loadings in controlled lake mesocosms. Hydrobiologia 2016, 766, 215–223. [Google Scholar] [CrossRef]
- Arisz, W.H. Translocation of salts in Vallisneria leaves. Bull. Res. Coune. Isr. D 1960, 8, 247–257. [Google Scholar]
- Denny, P. Sites of nutrient absorption in aquatic macrophytes. J. Ecol. 1972, 60, 819–829. [Google Scholar] [CrossRef]
- Wolfer, S.R.; Straile, D. Spatio-temporal dynamics and plasticity of clonal architecture in Potamogeton perfoliatus. Aquat. Bot. 2004, 78, 307–318. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Zhong, Y. Theory and Method for Researching Aquatic Macrophytes; Central China Normal University Press: Wuhan, China, 1992. (In Chinese) [Google Scholar]
- Kraft, N.J.B.; Godoy, O.; Levine, J.M. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl. Acad. Sci. USA 2015, 112, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Baattrup-Pedersen, A.; Göthe, E.; Larsen, S.E.; O’Hare, M.; Birk, S.; Riis, T.; Friberg, N. Plant trait characteristics vary with size and eutrophication in European lowland streams. J. Appl. Ecol. 2015, 52, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.; Zhao, Y.; Zhou, W.; Li, L.; Wang, B.; Cui, X.; Chen, J.; Song, Z. Divergences in reproductive strategy explain the distribution ranges of Vallisneria species in China. Aquat. Bot. 2016, 132, 41–48. [Google Scholar] [CrossRef]
- Caraco, T.; Kelly, C.K. On the adaptive value of physiological integration in clonal plants. Ecology 1991, 72, 81–93. [Google Scholar] [CrossRef]
- Alpert, P. Clonal integration in Fragaria chiloensis differs between populations: Ramets from grassland are selfish. Oecologia 1999, 120, 69–76. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Smith, D.H.; Madsen, J.D.; Dickson, K.L.; Beitinger, T.L. Nutrient effects on autofragmentation of Myriophyllum spicatum. Aquat. Bot. 2002, 74, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).