Are Water Filters Effective Against Microplastics?
Abstract
1. Introduction
2. Materials and Methods
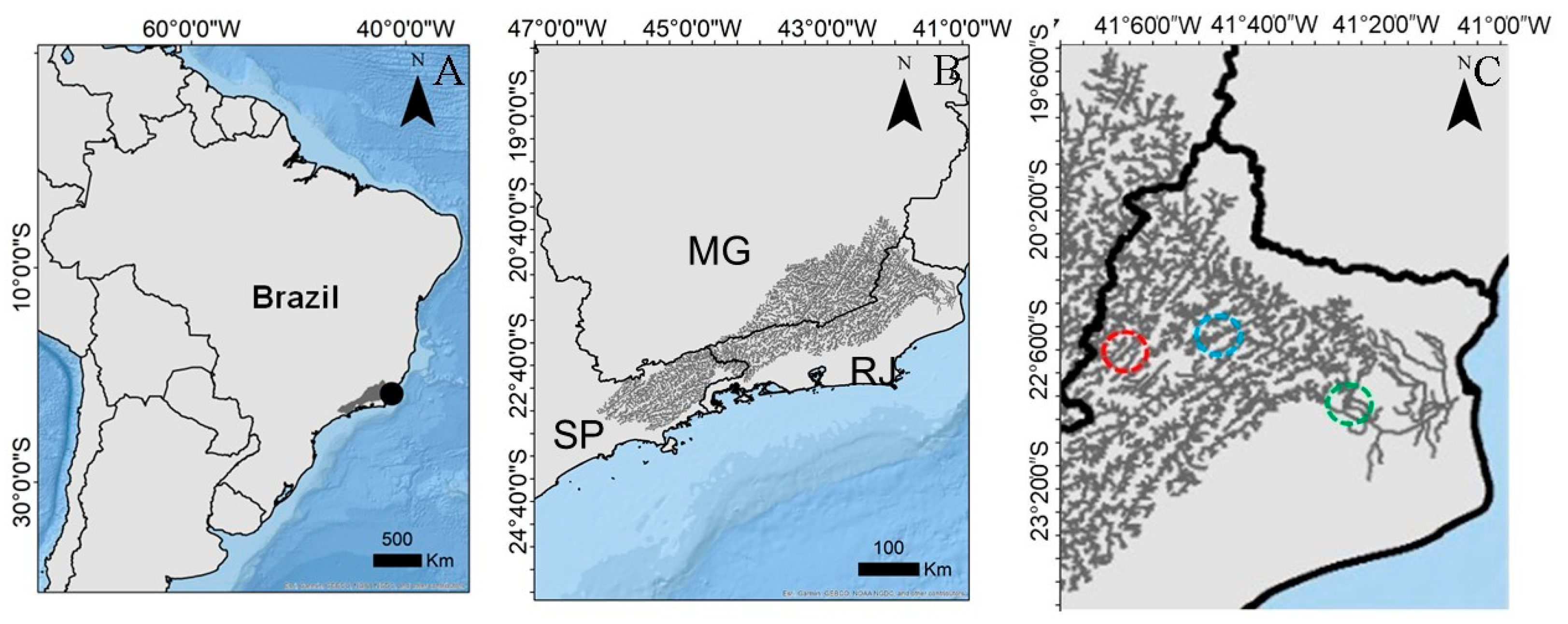
2.1. Study Area
2.2. Sampling
2.3. Sample Processing
2.4. Observation and Identification of MPs
2.5. Procedures for Quality Assurance and Quality Control (QA/QC)
2.6. Data Analysis
3. Results
3.1. MP Abundance and Concentration
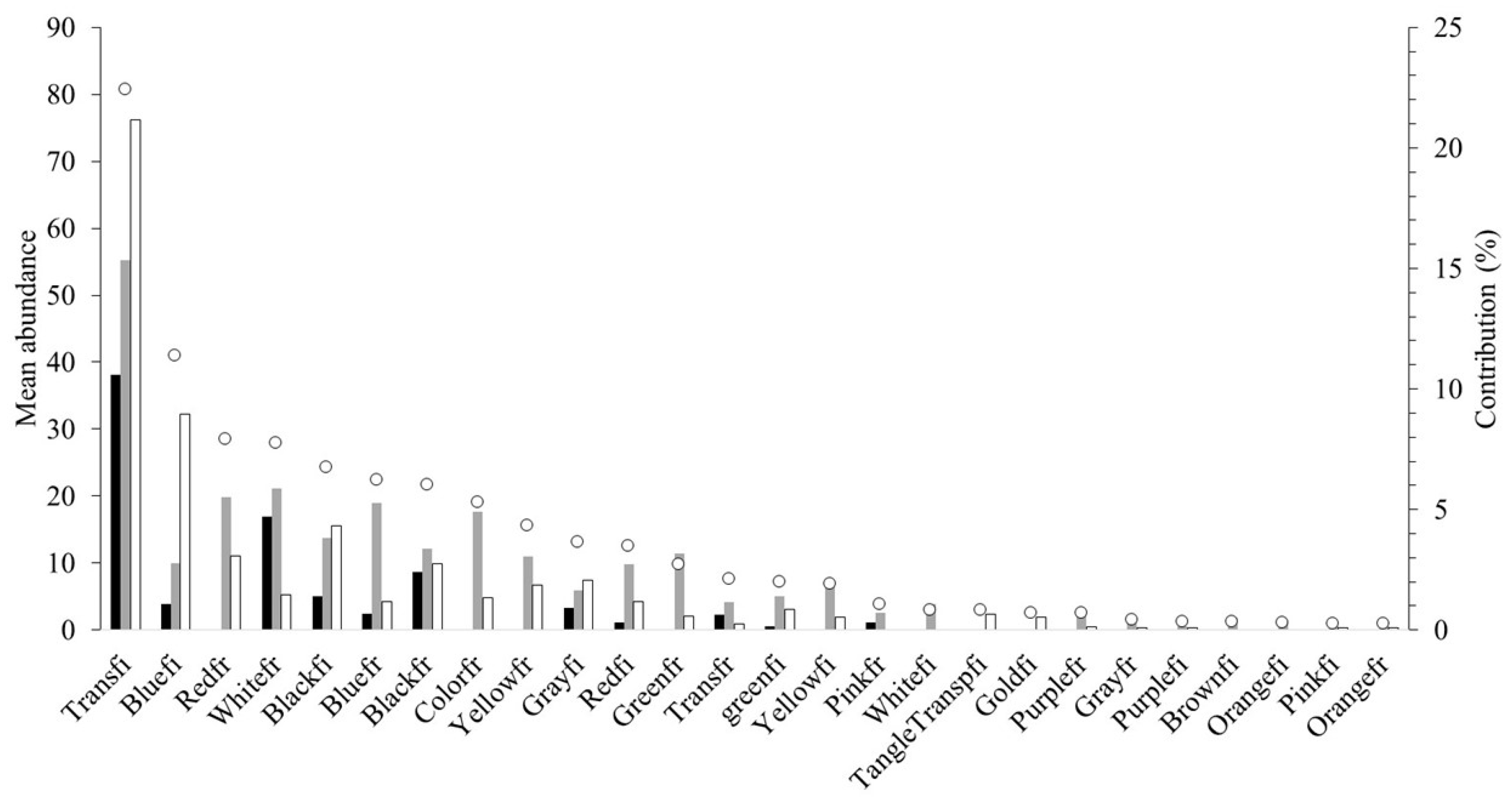
3.2. MP Characteristics
3.3. The Relationship Between MP Morphotype Abundance and Filter Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Global Plastics Outlook: Economic Drivers, Environmental Impacts and Policy Options; OECD: Paris, France, 2022; Available online: http://www.oecd-ilibrary.org/environment/global-plastics-outlook_de747aef-en (accessed on 12 July 2024).
- Ryberg, M.W.; Hauschild, M.Z.; Wang, F.; Averous-Monnery, S.; Laurent, A. Global environmental losses of plastics across their value chains. Resour. Conserv. Recycl. 2019, 151, 104459. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Redondo-Hasselerharm, P.E.; Nor, N.H.M.; de Ruijter, V.N.; Mintenig, S.M.; Kooi, M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022, 7, 138–152. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Hofman-Caris, R.C.; Pieke, E.N.; Ter Laak, T.L. Fate of microplastics in the drinking water production. Water Res. 2022, 221, 118790. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, X. Impact of microplastics from polyethylene and biodegradable mulch films on rice (Oryza sativa L.). Sci. Total Environ. 2024, 828, 154579. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean. 2020, 125, 123–135. [Google Scholar] [CrossRef]
- Nirmala, K.; Rangasamy, G.; Ramya, M.; Shankar, V.U.; Rajesh, G. A critical review on recent research progress on microplastic pollutants in drinking water. Environ. Res. 2023, 222, 115312. [Google Scholar] [CrossRef]
- Gao, S.; Yan, K.; Liang, B.; Shu, R.; Wang, N.; Zhang, S. The different ways microplastics from the water column and sediment accumulate in fish in Haizhou Bay. Sci. Total Environ. 2023, 854, 158575. [Google Scholar] [CrossRef]
- Zuri, G.; Karanasiou, A.; Lacorte, S. Microplastics: Human exposure assessment through air, water, and food. Environ. Int. 2023, 179, 108150. [Google Scholar] [CrossRef]
- World Health Organization. Microplastics in Drinking-Water; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Kaushik, A.; Singh, A.; Gupta, V.K.; Mishra, Y.K. Nano/Micro-Plastic, an invisible threat getting into the Brain. Chemosphere 2024, 361, 142380. [Google Scholar] [CrossRef]
- Alijagic, A.; Suljević, D.; Fočak, M.; Sulejmanović, J.; Šehović, E.; Särndahl, E.; Engwall, M. The triple exposure nexus of microplastic particles, plastic-associated chemicals, and environmental pollutants from a human health perspective. Environ. Int. 2024, 108736. [Google Scholar] [CrossRef]
- Choudhary, S.; Neelavanan, K.; Saalim, S.M. Microplastics in the surface sediments of Krossfjord-Kongsfjord system, Svalbard, Arctic. Mar. Pollut. Bull. 2022, 176, 113452. [Google Scholar] [CrossRef] [PubMed]
- Oßmann, B.E.; Sarau, G.; Holtmannspötter, H.; Pischetsrieder, M.; Christiansen, S.H.; Dicke, W. Small-sized microplastics and pigmented particles in bottled mineral water. Water Res. 2018, 141, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guevara, F.; Roy, P.D.; Elizalde-Martínez, I.; Kutralam-Muniasamy, G.; Shruti, V.C. Human exposure to microplastics from urban decentralized pay-to-fetch drinking-water refill kiosks. Sci. Total Environ. 2022, 848, 157722. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, H.; Ghayebzadeh, M.; Ganji, F.; Mousavi, S.; Azizi, N. Tracking microplastics contamination in drinking water in Zahedan, Iran: From source to consumption taps. Sci. Total Environ. 2023, 872, 162121. [Google Scholar] [CrossRef]
- Pratesi, C.B.; Almeida, M.A.S.; Paz, G.S.C.; Teotonio, M.H.R.; Gandolfi, L.; Pratesi, R.; Zandonadi, R.P. Presence and quantification of microplastic in urban tap water: A pre-screening in Brasilia, Brazil. Sustainability 2021, 13, 6404. [Google Scholar] [CrossRef]
- Ferraz, M.; Bauer, A.L.; Valiati, V.H.; Schulz, U.H. Microplastic concentrations in raw and drinking water in the Sinos River, Southern Brazil. Water 2020, 12, 3115. [Google Scholar] [CrossRef]
- Fernandes, C.V.; Misael, C.G.A.; Chaves, F.J.F.; Santos, J.S.B.; Cavalcante, J.N.A. Estudo da qualidade das águas processadas em filtros de barro tradicionais contrapondo os filtros modernos. Blucher Mater. Sci. Proc. 2015, 3, 1–7. [Google Scholar]
- Bellingieri, J.C. Água de beber: A filtração doméstica e a difusão do filtro de água em São Paulo. An. Mus. Paul. História Cult. Mater. 2004, 12, 161–191. [Google Scholar] [CrossRef]
- Bellingieri, J.C. Uma análise da indústria de filtros de água no Brasil. Cerâmica Ind. 2006, 11, 31–35. [Google Scholar]
- Ali, M.U.; Lin, S.; Yousaf, B.; Abbas, Q.; Munir, M.A.M.; Ali, M.U.; Wong, M.H. Environmental emission, fate and transformation of microplastics in biotic and abiotic compartments: Global status, recent advances and future perspectives. Sci. Total Environ. 2021, 791, 148422. [Google Scholar] [CrossRef] [PubMed]
- Emenike, P.C.; Araoye, O.V.; Academe, S.O.; Unokiwedi, P.; Omole, D.O. The Effects of Microplastics in Oceans and Marine Environment on Public Health—A Mini-Review; IOP Publishing: Philadelphia, PA, USA, 2022. [Google Scholar]
- Liang, R.; Sun, F.; Zhang, C.; Zhang, R.; Wang, H.; Wang, X. Interaction between microplastics and microorganisms in soil environment: A review. Chin. J. Biotechnol. 2023, 39, 500–515. [Google Scholar] [CrossRef]
- Suzuki, G.; Uchida, N.; Tanaka, K.; Higashi, O.; Takahashi, Y.; Kuramochi, H.; Osako, M. Global discharge of microplastics from mechanical recycling of plastic waste. Environ. Pollut. 2024, 348, 123855. [Google Scholar] [CrossRef] [PubMed]
- da Costa, I.D.; Nunes, N.N.; Costa, L.L.; Zalmon, I.R. Is the Paraíba do Sul River colourful? Prevalence of microplastics in freshwater, south-eastern Brazil. Mar. Freshw. Res. 2022, 73, 1439–1449. [Google Scholar] [CrossRef]
- da Costa, I.D.; Costa, L.L.; Zalmon, I.R. Microplastics in water from the confluence of tropical rivers: Overall review and a case study in Paraíba do Sul River basin. Chemosphere 2023, 338, 139493. [Google Scholar] [CrossRef]
- Cotovicz Jr, L.C.; Vidal, L.O.; de Rezende, C.E.; Bernardes, M.C.; Knoppers, B.A.; Sobrinho, R.L.; Abril, G. Carbon dioxide sources and sinks in the delta of the Paraíba do Sul River (Southeastern Brazil) modulated by carbonate thermodynamics, gas exchange and ecosystem metabolism during estuarine mixing. Mar. Chem. 2020, 226, 103869. [Google Scholar] [CrossRef]
- Marengo, J.A.; Alves, L.M. Tendências hidrológicas da bacia do rio Paraíba do Sul. Rev. Bras. Meteorol. 2005, 20, 215–226. [Google Scholar]
- Comitê de Integração da Bacia Hidrográfica do Rio Paraíba do Sul. Rio que te quero vivo—Conheça mais sobre a bacia do rio Paraíba do Sul; CEIVAP: Rio de Janeiro, Brazil, 2024; Available online: https://www.ceivap.org.br/arquivos/CartilhaVirtual.pdf (accessed on 20 July 2024).
- Lima, F.P.; Azevedo-Santos, V.M.; Santos, V.M.; Vidotto-Magnoni, P.; Soares, C.L.; Manzano, F.V.; Nobile, A.B. Plastic ingestion by commercial and non-commercial fishes from a Neotropical river basin. Water Air Soil Pollut. 2021, 232, 29. [Google Scholar] [CrossRef]
- Meneguelli-Souza, A.C.; Pestana, I.A.; Azevedo, L.S.; Almeida, M.G.; Souza, C.M.M. Arsenic in the lower drainage basin of the Paraíba do Sul River (Southeast Brazil): Dynamics between the water column and sediment. Environ. Monit. Assess. 2021, 193, 1–15. [Google Scholar] [CrossRef]
- Oliveira, E.C. Mudanças na sedimentação do delta do Rio Paraíba do Sul nos últimos 40 anos: Resultados do manejo excessivo. In O Homem e as Zonas Costeiras; Fundação de Amparo a Pesquisa do Estado do rio de Janeiro: Rio de Janeiro, Brazil, 2015; pp. 174–178. [Google Scholar]
- FEAM. Programa Minas Trata Esgoto, Relatório de progresso do Programa Minas Trata Esgoto; Fundação Estadual do Meio Ambiente: Belo Horizonte, Brazil, 2013; Available online: http://www.feam.br/minas-trata-esgoto (accessed on 12 July 2024).
- Guedes, H.A.; Silva, D.D.; Elesbon, C.; Ribeiro, A.T.D.; Soares, J.H. Aplicação da análise estatística multivariada no estudo da qualidade da água do Rio Pomba, MG. Rev. Bras. Eng. Agric. Ambient. 2012, 16, 558–563. [Google Scholar] [CrossRef]
- Pittroff, M.; Müller, Y.K.; Witzig, C.S.; Scheurer, M.; Storck, F.R.; Zumbülte, N. Microplastic analysis in drinking water based on fractionated filtration sampling and Raman microspectroscopy. Environ. Sci. Pollut. Res. 2021, 28, 59439–59451. [Google Scholar] [CrossRef] [PubMed]
- Feld, L.; Silva, V.H.D.; Murphy, F.; Hartmann, N.B.; Strand, J. A study of microplastic particles in Danish tap water. Water 2021, 13, 2097. [Google Scholar] [CrossRef]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is it or isn’t it: The importance of visual classification in microplastic characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Hollman, P.; Mendoza-Hill, J. Microplastics in Fisheries and Aquaculture: Status of Knowledge on Their Occurrence and Implications for Aquatic Organisms and Food Safety; Food and Agriculture Organization of United Nations: Rome, Italy, 2017. [Google Scholar]
- Galgani, F.; Hanke, G.; Werner, H.; De Vrees, L. Marine litter within the European Marine Strategy Framework Directive. ICES Mar. Sci. 2013, 70, 1055–1064. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 18, 143–159. [Google Scholar] [CrossRef]
- Schymanski, D.; Oßmann, B.E.; Benismail, N.; Boukerma, K.; Dallmann, G.; Von der Esch, E.; Ivleva, N.P. Analysis of microplastics in drinking water and other clean water samples with micro-Raman and micro-infrared spectroscopy: Minimum requirements and best practice guidelines. Anal. Bioanal. Chem. 2021, 413, 5969–5994. [Google Scholar] [CrossRef]
- Bessa, F.; Barría, P.; Neto, J.M.; Frias, J.P.; Otero, V.; Sobral, P.; Marques, J.C. Occurrence of microplastics in commercial fish from a natural estuarine environment. Mar. Pollut. Bull. 2018, 128, 575–584. [Google Scholar] [CrossRef]
- Zar, J.H. Bioestatistical Analysis; Prentice Hall: Saddle River, New Jersey, USA, 1999. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9–25. [Google Scholar]
- Wheeler, B.; Torchiano, M.; Torchiano, M.M. Package ‘lmPerm’. R Package Version 2.1.0. Available online: https://cran.r-project.org/web/packages/lmPerm/lmPerm.pdf (accessed on 2 July 2024).
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. Vegan: Community Ecology Package; R Core Team; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Comitê de Integração da Bacia Hidrográfica do Rio Paraíba do Sul. Deliberação CEIVAP nº 259, de 5 de abril de 2018. Dispõe sobre a atualização do Preço Público Unitário—PPU da metodologia da cobrança pelo uso dos recursos hídricos de domínio da União na Bacia Hidrográfica do rio Paraíba do Sul; CEIVAP: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- Santana, G.R.; Santos, E.B.; da Silva, M.G.A.J. Caracterização espaço-temporal das secas na bacia do Rio Paraíba do Sul. Anu. Inst. Geociênc. 2020, 43, 364–375. [Google Scholar] [CrossRef]
- Obraczka, M.; Pinto, S.D.O.M.; Marques, C.F.; Ohnuma, A.A. Jr. Emprego de indicadores na avaliação do saneamento-região hidrográfica médio Paraíba do Sul. Rev. Int. Ciências 2019, 9, 3–21. [Google Scholar] [CrossRef]
- Barchiesi, M.; Chiavola, A.; Di Marcantonio, C.; Boni, M.R. Presence and fate of microplastics in the water sources: Focus on the role of wastewater and drinking water treatment plants. J. Water Process Eng. 2021, 40, 101787. [Google Scholar] [CrossRef]
- Sturm, M.T.; Myers, E.; Schober, D.; Korzin, A.; Schuhen, K. Beyond microplastics: Implementation of a two-stage removal process for microplastics and chemical oxygen demand in industrial wastewater streams. Water 2024, 16, 268. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Hadibarata, T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ. Chall. 2021, 5, 100264. [Google Scholar] [CrossRef]
- Díaz, L.; Huesca, R.H.; Armenta, A.G. Characterization of the Microporous Structure of Activated Carbons through Different Approaches. Ind. Eng. Chem. 1999, 38, 1396. [Google Scholar] [CrossRef]
- Caramello, N.; Furtado, M.S.; Rodrigues, A.B. Monitoramento da qualidade de água residencial e in natura para análise de tratamento alternativo, Amapá, Brasil. Nat. Resour. 2022, 12, 107–122. [Google Scholar]
- Ingram, C. The Drinking Water Book: How to Eliminate Harmful Toxins from Your Water; Celestial Arts: Toronto, Ontario, Canada, 2006. [Google Scholar]
- da Costa, I.D.; Costa, L.L.; Zalmon, I.R. Are fishes selecting the trash they eat? Influence of feeding mode and habitat on microplastic uptake in an artificial reef complex (ARC). Sci. Total Environ. 2023, 904, 166788. [Google Scholar] [CrossRef]
- Frank, Y.A.; Vorobiev, E.D.; Vorobiev, D.S.; Trifonov, A.A.; Antsiferov, D.V.; Soliman Hunter, T.; Strezov, V. Preliminary screening for microplastic concentrations in the surface water of the Ob and Tom Rivers in Siberia, Russia. Sustainability 2020, 13, 80. [Google Scholar] [CrossRef]
- Chinfak, N.; Sompongchaiyakul, P.; Charoenpong, C.; Shi, H.; Yeemin, T.; Zhang, J. Abundance, composition, and fate of microplastics in water, sediment, and shellfish in the Tapi-Phumduang River system and Bandon Bay, Thailand. Sci. Total Environ. 2021, 781, 146700. [Google Scholar] [CrossRef]
- Wan, L.; Cheng, H.; Liu, Y.; Shen, Y.; Liu, G.; Su, X. Global meta-analysis reveals differential effects of microplastics on soil ecosystem. Sci. Total Environ. 2023, 867, 161403. [Google Scholar] [CrossRef]
- Pastorelli, G.; Cucci, C.; Garcia, O.; Piantanida, G.; Elnaggar, A.; Cassar, M.; Strlič, M. Environmentally induced colour change during natural degradation of selected polymers. Polym. Degrad. Stab. 2014, 107, 198–209. [Google Scholar] [CrossRef]
- Yang, J.; Li, L.; Li, R.; Xu, L.; Shen, Y.; Li, S.; Luo, Y. Microplastics in an agricultural soil following repeated application of three types of sewage sludge: A field study. Environ. Pollut. 2021, 289, 117943. [Google Scholar] [CrossRef] [PubMed]
- Zicarelli, G.; Romano, C.; Gallo, S.; Valentino, C.; Pepe Belomo, V.; Leonetti, F.L.; Giglio, G.; Neri, A.; Marsili, L.; Milazzo, C.; et al. Diet and plastic ingestion in the blackmouth catsharkGaleus melastomus, Rafinesque 1810, in Italian waters. Animals 2023, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Ta, A.T.; Babel, S. Occurrence and spatial distribution of microplastic contaminated with heavy metals in a tropical river: Effect of land use and population density. Mar. Pollut. Bull. 2023, 191, 114919. [Google Scholar] [CrossRef] [PubMed]
- Gambino, I.; Bagordo, F.; Grassi, T.; Panico, A.; De Donno, A. Occurrence of microplastics in tap and bottled water: Current Knowledge. Int. J. Environ. Res. Public Health 2022, 19, 5283. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, J.; Wright, S.L.; Dris, R.; Collard, F.; Mandin, C.; Guerrouache, M.; Tassin, B. Microplastics in air: Are we breathing it in? Curr. Opin. Environ. Sci. Health 2018, 1, 1–5. [Google Scholar] [CrossRef]
- Sodré, F.F.; Arowojolu, I.M.; Canela, M.C.; Ferreira, R.S.; Fernandes, A.N.; Montagner, C.C.; Waldman, W.R. How natural and anthropogenic factors should drive microplastic behavior and fate: The scenario of Brazilian urban freshwater. Chemosphere 2023, 340, 139813. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Yang, H.; He, W.; Li, B.; Zhu, X.; Tang, K.H.D. Leaching of chemicals from microplastics: A review of chemical types, leaching mechanisms and influencing factors. Sci. Total Environ. 2024, 906, 167666. [Google Scholar] [CrossRef]
- FAO-ICAC. Food and Agriculture Organization of the United Nations and International Cotton Advisory Committee. Washington, 2013. Available online: https://sewitagain.com/wp-content/uploads/2013/12/FAO-ICAC-Survey-2013-Update-and-2011-Text.pdf (accessed on 1 July 2024).
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Velázquez, D.; Casero-Chamorro, M.C.; Leganés, F.; Quesada, A.; Rosal, R. First detection of microplastics in the freshwater of an Antarctic Specially Protected Area. Mar. Pollut. Bull. 2020, 161, 111811. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, B.; Gu, C.; Shen, C.; Yin, S.; Aamir, M.; Li, F. Are we underestimating the sources of microplastic pollution in terrestrial environment? J. Hazard. Mater. 2020, 400, 123228. [Google Scholar] [CrossRef]
- Lin, L.; Zuo, L.Z.; Peng, J.P.; Cai, L.Q.; Yan, Y.; Xu, X.R. Occurrence and distribution of microplastics in an urban river: A case study in the Pearl River along Guangzhou City, China. Sci. Total Environ. 2018, 644, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Haberstroh, C.J.; Arias, M.E.; Yin, Z.; Sok, T.; Wang, M.C. Plastic transport in a complex confluence of the Mekong River in Cambodia. Environ. Res. Lett. 2021, 16, 231–252. [Google Scholar] [CrossRef]
- Laermanns, H.; Lehmann, M.; Klee, M.; Löder, M.G.; Gekle, S.; Bogner, C. Tracing the horizontal transport of microplastics on rough surfaces. Microplastics Nanoplastics 2021, 1, 11. [Google Scholar] [CrossRef]
- Decreto 10.936/2022. Regulamentação da Política Nacional de Resíduos S´olidos. Presidência da República. Brazil, 2022. Available online: https://www.in.gov.br/web/dou/-/decreto-n-10 (accessed on 20 July 2024).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, I.D.; Nunes, N.N.d.S.; Costa, L.L.; Zalmon, I.R. Are Water Filters Effective Against Microplastics? Water 2024, 16, 3189. https://doi.org/10.3390/w16223189
da Costa ID, Nunes NNdS, Costa LL, Zalmon IR. Are Water Filters Effective Against Microplastics? Water. 2024; 16(22):3189. https://doi.org/10.3390/w16223189
Chicago/Turabian Styleda Costa, Igor David, Natalia Neto dos Santos Nunes, Leonardo Lopes Costa, and Ilana Rosental Zalmon. 2024. "Are Water Filters Effective Against Microplastics?" Water 16, no. 22: 3189. https://doi.org/10.3390/w16223189
APA Styleda Costa, I. D., Nunes, N. N. d. S., Costa, L. L., & Zalmon, I. R. (2024). Are Water Filters Effective Against Microplastics? Water, 16(22), 3189. https://doi.org/10.3390/w16223189





