Trihalomethane Formation Potential at the Barekese Water Treatment Plant and the Related Cancer Risk to Consumers in the Kumasi Metropolis of Ghana
Abstract
1. Introduction
2. Materials and Methods
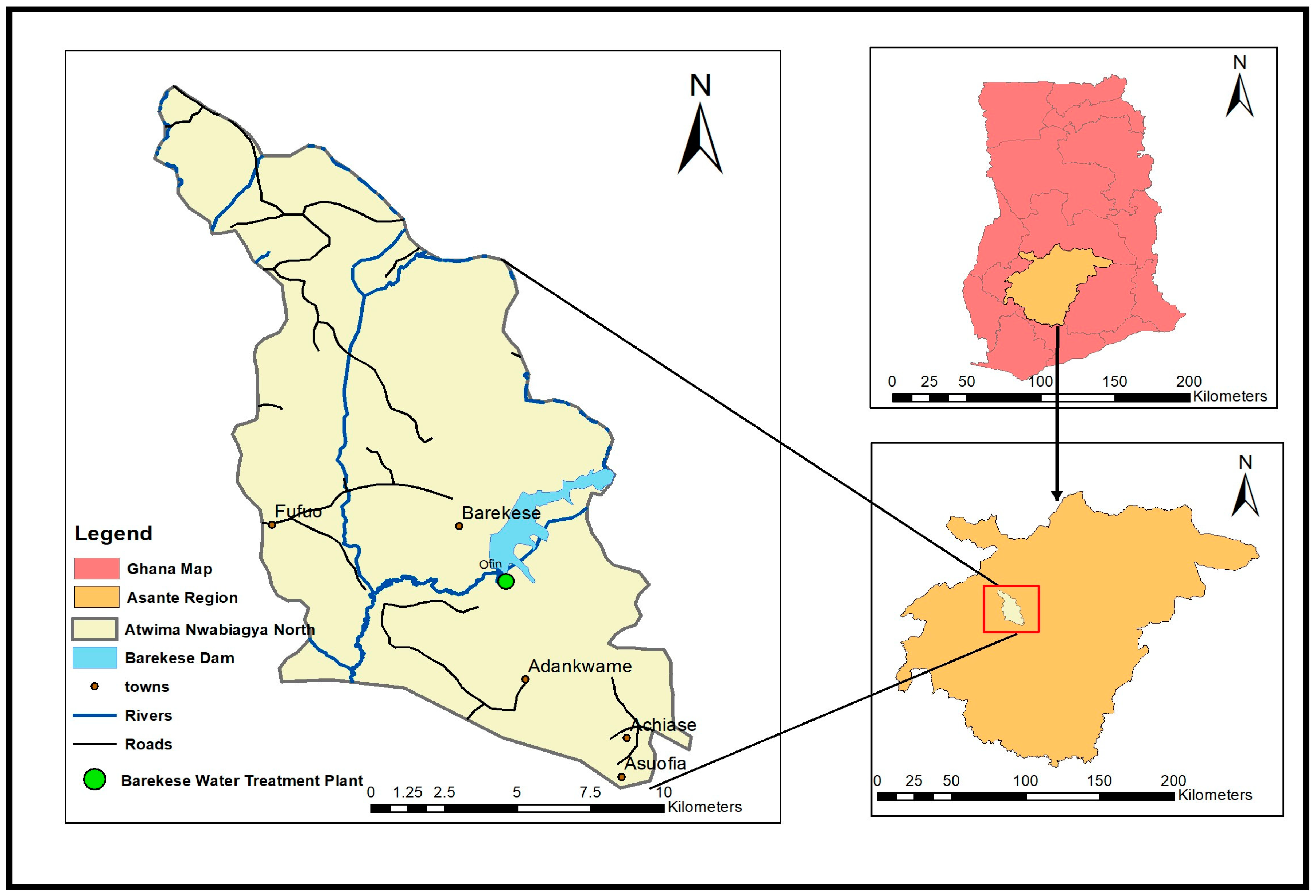
2.1. Study Area
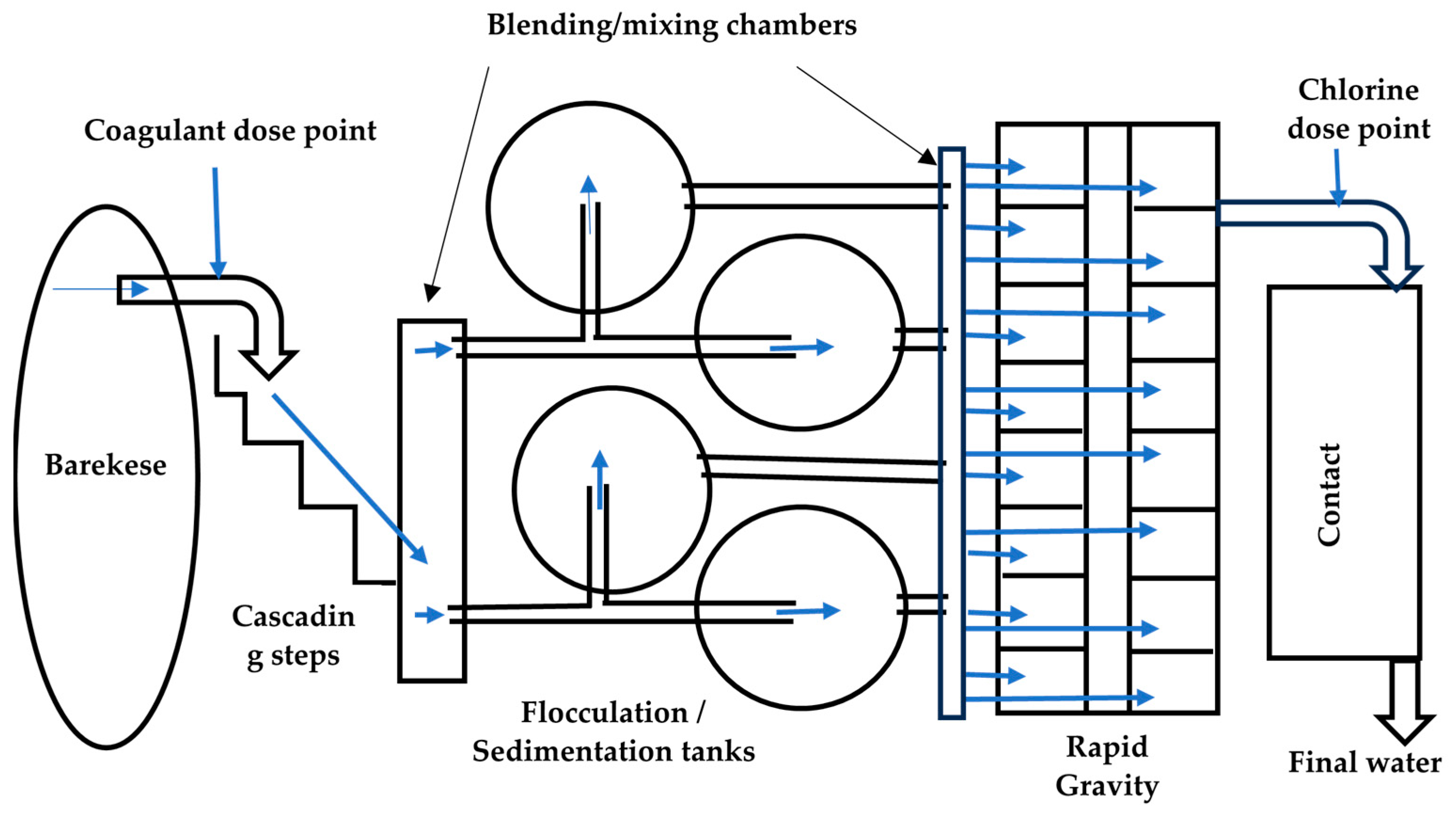
2.2. Barekese Water Treatment Plant Layout and Processes
2.3. Sampling
2.4. Analysis of Water Quality Parameters Relevant for THM Formation Potential
2.4.1. pH
2.4.2. Temperature
2.4.3. Dissolved Organic Carbon (DOC) Concentration and Color
2.4.4. Residual Chlorine Concentration
2.4.5. Contact Time
2.5. Data Analysis
2.5.1. Conversion of Chemical Oxygen Demand to Dissolved Organic Carbon Concentration
2.5.2. Determination of Trihalomethane Formation Potential (THMFP)
2.5.3. Human Health Risk Analysis
2.6. Limitations of the Study
3. Results and Discussion
3.1. Trihalomethane Formation Potential (THMFP)
3.2. Correlations Between THM Formation Potential and Water Quality Parameters
3.3. Principal Component Analysis (PCA) of Parameters Influencing THMFP
3.4. Correlations Between THMFP and Water Quality Parameters
3.5. Predicted Impact of Climate Change on THM Formation Potential at the Barekese WTP
3.6. Estimated Health Risk from the Predicted THM Concentration at the Barekese WTP
4. Conclusions and Recommendations
4.1. Conclusions
4.2. Recommendations
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carlsen, L.; Bruggemann, R. The 17 United Nations’ sustainable development goals: A status by 2020. Int. J. Sustain. Dev. World Ecol. 2021, 29, 219–229. [Google Scholar] [CrossRef]
- Bandoh, D.A.; Kenu, E.; Dwomoh, D.; Afari, E.A.; Dzodzomenyo, M. A study to evaluate WASH interventions and risk factors of diarrhoea among children under five years, Anloga district, Ghana: A research protocol. PLoS ONE 2024, 19, e0302754. [Google Scholar] [CrossRef]
- Amadu, I.; Seidu, A.-A.; Agyemang, K.K.; Arthur-Holmes, F.; Duku, E.; Salifu, I.; Bolarinwa, O.A.; Hagan, J.E.; Ahinkorah, B.O. Joint effect of water and sanitation practices on childhood diarrhoea in sub-Saharan Africa. PLoS ONE 2023, 18, e0283826. [Google Scholar] [CrossRef]
- Alemayehu, K.; Oljira, L.; Demena, M.; Birhanu, A.; Workineh, D. Prevalence and Determinants of Diarrheal Diseases among Under-Five Children in Horo Guduru Wollega Zone, Oromia Region, Western Ethiopia: A Community-Based Cross-Sectional Study. Can. J. Infect. Dis. Med Microbiol. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Li, P.; Wu, J. Drinking Water Quality and Public Health. Expo. Health 2019, 11, 73–79. [Google Scholar] [CrossRef]
- Chaukura, N.; Marais, S.S.; Moyo, W.; Mbali, N.; Thakalekoala, L.C.; Ingwani, T.; Mamba, B.B.; Jarvis, P.; Nkambule, T.T.I. Contemporary issues on the occurrence and removal of disinfection byproducts in drinking water—A review. J. Environ. Chem. Eng. 2020, 8, 103659. [Google Scholar] [CrossRef]
- Srivastav, A.L.; Kaur, T. Chapter 18—Factors affecting the formation of disinfection by-products in drinking water: Human health risk. In Disinfection By-Products in Drinking Water, Detection and Treatment; Butterworth-Heinemann: Oxford, UK, 2020; pp. 433–450. [Google Scholar] [CrossRef]
- Li, X.-F.; Mitch, W.A. Drinking Water Disinfection Byproducts (DBPs) and Human Health Effects: Multidisciplinary Challenges and Opportunities. Environ. Sci. Technol. 2018, 52, 1681–1689. [Google Scholar] [CrossRef]
- Wu, J. Challenges for Safe and Healthy Drinking Water in China. Curr. Environ. Health Rep. 2020, 7, 292–302. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Zhao, X.; Wang, J.; Wang, Y.; Xian, Q. Disinfection byproducts and their cytotoxicity contribution from dissolved black carbon in source water during chlor(am)ination. Sci. Total. Environ. 2024, 930, 172834. [Google Scholar] [CrossRef]
- Freeman, L.E.B.; Kogevinas, M.; Cantor, K.P.; Villanueva, C.M.; Prokunina-Olsson, L.; Florez-Vargas, O.; Figueroa, J.D.; Ward, M.H.; Koutros, S.; Baris, D.; et al. Disinfection By-Products in Drinking Water and Bladder Cancer: Evaluation of Risk Modification by Common Genetic Polymorphisms in Two Case–Control Studies. Environ. Health Perspect. 2022, 130, 57006. [Google Scholar] [CrossRef]
- Parvez, S.; Ashby, J.L.; Kimura, S.Y.; Richardson, S.D. Exposure Characterization of Haloacetic Acids in Humans for Exposure and Risk Assessment Applications: An Exploratory Study. Int. J. Environ. Res. Public Health 2019, 16, 471. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lucas, G.; Martínez-Menchón, M.; Vela, N.; Navarro, S. Removal assessment of disinfection by-products (DBPs) from drinking water supplies by solar heterogeneous photocatalysis: A case study of trihalomethanes (THMs). J. Environ. Manag. 2022, 321, 115936. [Google Scholar] [CrossRef] [PubMed]
- Ullberg, M.; Lavonen, E.; Köhler, S.J.; Golovko, O.; Wiberg, K. Pilot-scale removal of organic micropollutants and natural organic matter from drinking water using ozonation followed by granular activated carbon. Environ. Sci. Water Res. Technol. 2021, 7, 535–548. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Kim, J.; Kang, B. DBPs removal in GAC filter-adsorber. Water Res. 2008, 42, 145–152. [Google Scholar] [CrossRef]
- Domfeh Anyemedu, M.K.; FO, K.; Anornu, G.K.; Adjei, K.A.; Odai, S.N. Assessment of the water balance of the Barekese reservoir in Kumasi, Ghana. J. Sci. Technol. 2016, 35, 34–51. [Google Scholar] [CrossRef][Green Version]
- Xing, X.; Li, T.; Bi, Z.; Qi, P.; Li, Z.; Wang, H.; Lyu, L.; Gao, Y.; Hu, C. Efficient removal of disinfection by-products precursors and inhibition of bacterial detachment by strong interaction of EPS with coconut shell activated carbon in ozone/biofiltration. J. Hazard. Mater. 2020, 392, 122077. [Google Scholar] [CrossRef]
- Golea, D.M.; Jarvis, P.; Jefferson, B.; Moore, G.; Sutherland, S.; Parsons, S.A.; Judd, S.J. Influence of granular activated carbon media properties on natural organic matter and disinfection by-product precursor removal from drinking water. Water Res. 2020, 174, 115613. [Google Scholar] [CrossRef]
- Wang, L.; Renwick, D.V.; Regli, S. Re-assessing ICR GAC Treatment Study Database: Effect of Bromide on DBP Formation. AWWA Water Sci. 2019, 1, 4. [Google Scholar] [CrossRef]
- Liao, X.B.; Cheng, Y.S.; Liu, Z.H.; Shen, L.L.; Zhao, L.; Chen, C.; Li, F.; Zhang, X.J. Performance of BAC for DBPs precursors’ removal for one year with micro-polluted lake water in East-China. Environ. Technol. 2019, 41, 3554–3561. [Google Scholar] [CrossRef]
- van der Aa, L.T.J.; Rietveld, L.C.; van Dijk, J.C. Effects of ozonation and temperature on the biodegradation of natural organic matter in biological granular activated carbon filters. Drink. Water Eng. Sci. 2011, 4, 25–35. [Google Scholar] [CrossRef]
- Verdugo, E.M.; Gifford, M.; Glover, C.; Cuthbertson, A.A.; Trenholm, R.A.; Kimura, S.Y.; Liberatore, H.K.; Richardson, S.D.; Stanford, B.D.; Summers, R.S.; et al. Controlling disinfection byproducts from treated wastewater using adsorption with granular activated carbon: Impact of pre-ozonation and pre-chlorination. Water Res. X 2020, 9, 100068. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xia, W.; Liu, H.; Liu, J.; Cao, S.; Fang, X.; Li, S.; Li, Y.; Chen, C.; Xu, S. Trihalomethanes in global drinking water: Distributions, risk assessments, and attributable disease burden of bladder cancer. J. Hazard. Mater. 2024, 469, 133760. [Google Scholar] [CrossRef]
- Serajuddin, M.; Chowdhury, M.A.I.; Ferdous, T. Correlation among some global parameters describing organic pollutants in River water: A case study. Int. J. Res. Granthaalayah. 2018, 6, 278–289. [Google Scholar] [CrossRef]
- Rodriguez, M.J.; Sérodes, J.; Morin, M. Estimation of water utility compliance with trihalomethane regulations using a modelling approach. J. Water Supply Res. Technol. 2000, 49, 57–73. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, Fourth Edition Incorporation First and Second Addenda. 2022. Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 2 July 2024).
- USEPA. Six-Year Review of Drinking Water Standards. 2022. Available online: https://www.epa.gov/dwsixyearreview/six-year-review-4-drinking-water-standards-information-collection-request (accessed on 13 May 2024).
- APHA. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Saidan, M.; Rawajfeh, K.; Fayyad, M. Investigation of Factors Affecting THMs Formation in Drinking Water. Am. J. Environ. Eng. 2013, 3, 207–212. [Google Scholar]
- Bougeard, C.M.; Goslan, E.H.; Jefferson, B.; Parsons, S.A. Comparison of the disinfection by-product formation potential of treated waters exposed to chlorine and monochloramine. Water Res. 2010, 44, 729–740. [Google Scholar] [CrossRef]
- Diehl, A.C.; Speitel, G.E., Jr.; Symons, J.M.; Krasner, S.W.; Hwang, C.J.; Barrett, S.E. DBP formation during chloramination. J. AWWA 2000, 92, 76–90. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chen, C.-Y.; Wang, G.-S. Temperature dependence of characteristics of organic precursors, bromide, and disinfection byproduct formation. Sci. Total. Environ. 2019, 662, 746–754. [Google Scholar] [CrossRef]
- Valdivia-Garcia, M.; Weir, P.; Graham, D.W.; Werner, D. Predicted Impact of Climate Change on Trihalomethanes Formation in Drinking Water Treatment. Sci. Rep. 2019, 9, 9967. [Google Scholar] [CrossRef] [PubMed]
- Ramavandi, B.; Farjadfard, S.; Ardjmand, M.; Dobaradaran, S. Effect of water quality and operational parameters on trihalomethanes formation potential in Dez River water, Iran. Water Resour. Ind. 2015, 11, 1–12. [Google Scholar] [CrossRef]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Cihlář, Z.; Vojtová, L.; Conte, P.; Nasir, S.; Kučerík, J. Hydration and water holding properties of cross-linked lignite humic acids. Geoderma 2014, 230–231, 151–160. [Google Scholar] [CrossRef]
- Lag, J.; Hadas, A.; Fairbridge, R.W.; Muñoz JC, N.; Pombal, X.P.; Cortizas, A.M.; Almendros, G. Humic Substances. In Encyclopedia of Soil Science; Chesworth, W., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 315–323. [Google Scholar] [CrossRef]
- Ghernaout, D. The hydrophilic/hydrophobic ratio vs. dissolved organics removal by coagulation—A review. J. King Saud Univ. Sci. 2014, 26, 169–180. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A. Sillanpää; M An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef]
- Bond, T.; Huang, J.; Graham, N.J.D.; Templeton, M.R. Examining the interrelationship between DOC, bromide and chlorine dose on DBP formation in drinking water—A case study. Sci. Total Environ. 2014, 470, 469–479. [Google Scholar] [CrossRef]
- Goss, C.D.; Gorczyca, B. Trihalomethane formation potential of DOC fractions isolated from two Canadian Prairie surface water sources. Water Supply 2013, 13, 114–122. [Google Scholar] [CrossRef]
- Sharma, N.; Mohapatra, S.; Padhye, L.P.; Mukherji, S. Role of precursors in the formation of trihalomethanes during chlorination of drinking water and wastewater effluents from a metropolitan region in western India. J. Water Process. Eng. 2021, 40, 101928. [Google Scholar] [CrossRef]
- Sriboonnak, S.; Induvesa, P.; Wattanachira, S.; Rakruam, P.; Siyasukh, A.; Pumas, C.; Wongrueng, A.; Khan, E. Trihalomethanes in Water Supply System and Water Distribution Networks. Int. J. Environ. Res. Public Health Artic. Res. Public Health 2021, 18, 9066. [Google Scholar] [CrossRef]
- Valdivia-Garcia, M.; Weir, P.; Frogbrook, Z.; Graham, D.W.; Werner, D. Climatic, Geographic and Operational Determinants of Trihalomethanes (THMs) in Drinking Water Systems. Sci. Rep. 2016, 6, 35027. [Google Scholar] [CrossRef]
- Rajamohan, R.; Ebenezer, V.; Rajesh, P.; Venugopalan, V.; Natesan, U.; Murugesan, V.; Narasimhan, S.V. Trihalomethane formation potential of drinking water sources in a rural location. Adv. Environ. Res. 2012, 1, 181–189. [Google Scholar] [CrossRef]
- Jutaporn, P.; Armstrong, M.D.; Coronell, O. Assessment of C-DBP and N-DBP formation potential and its reduction by MIEX® DOC and MIEX® GOLD resins using fluorescence spectroscopy and parallel factor analysis. Water Res. 2020, 172, 115460. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Singh, V.P. Chapter 13—Water Quality Modeling. In Developments in Water Science; Elsevier: Amsterdam, The Netherlands, 2003; Volume 51, pp. 743–786. [Google Scholar] [CrossRef]
- Dyck, R.; Cool, G.; Rodriguez, M.; Sadiq, R. Treatment, residual chlorine and season as factors affecting variability of trihalomethanes in small drinking water systems. Front. Environ. Sci. Eng. 2015, 9, 171–179. [Google Scholar] [CrossRef]
- Krishnaiah, D. Free chlorine residual content within the drinking water distribution system. Int. J. Phys. Sci. 2007, 2, 196–201. [Google Scholar]
- Lu, C.; Chung, Y.-L.; Chang, K.-F. Adsorption of trihalomethanes from water with carbon nanotubes. Water Res. 2005, 39, 1183–1189. [Google Scholar] [CrossRef]
- Chowdhury, S. Exposure assessment for trihalomethanes in municipal drinking water and risk reduction strategy. Sci. Total Environ. 2013, 463, 922–930. [Google Scholar] [CrossRef]
- Vuvor, F.; Harrison, O. Correlation between body weight and total body fat composition in adults in a community in Ghana. Mathews J. Nutr. Diet. 2017, 2, 1–5. [Google Scholar]
- Gbadago, B.K.; Antiaye, J.; Boachie, J.; Adu, P. Drinking recommended daily water significantly alters haemato-biochemical parameters in prospective blood donors; a one-center quasi-experimental study in a tropical setting. Blood Cells Mol. Dis. 2023, 102, 102757. [Google Scholar] [CrossRef]
- Mishaqa, E.-S.I.; Radwan, E.K.; Ibrahim, M.; Hegazy, T.A.; Ibrahim, M.S. Multi-exposure human health risks assessment of trihalomethanes in drinking water of Egypt. Environ. Res. 2022, 207, 112643. [Google Scholar] [CrossRef]
- DataEarth. Life Expectancy in Ghana. 2022. Available online: https://database.earth/population/ghana/life-expectancy#:~:text=The%20current%20average%20life%20expectancy%20for%20Ghana%20in,76.0378%20years%20of%20age%2C%20by%20the%20year%202100 (accessed on 12 April 2024).
- Minnesota Department of Health. Health-Based Water Guidance. 2020. Available online: https://www.health.state.mn.us/communities/environment/risk/index.html (accessed on 15 May 2024).
- Pardakhti, A.R.; Bidhendi, G.R.N.; Torabian, A.; Karbassi, A.; Yunesian, M. Comparative cancer risk assessment of THMs in drinking water from well water sources and surface water sources. Environ. Monit. Assess. 2011, 179, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ghana Standards Authority (GSA). Water Quality—Specification for Drinking Water; Ministry of Trade and Industry: Accra, Ghana, 2021. [Google Scholar]
- Tafesse, N.; Porcelli, M.; Hirpessa, B.B.; Gasana, J.; Padhi, R.K.; Garie, S.R.; Ambelu, A. Exposure and carcinogenic risk assessment of trihalomethanes (THMs) for water supply consumers in Addis Ababa, Ethiopia. Toxicol. Rep. 2023, 10, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Doederer, K.; Gernjak, W.; Weinberg, H.S.; Farré, M.J. Factors affecting the formation of disinfection by-products during chlorination and chloramination of secondary effluent for the production of high quality recycled water. Water Res. 2014, 48, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Andrés, J.; Peperzak, L. Operational and environmental factors affecting disinfection byproducts formation in ballast water treatment systems. Chemosphere 2019, 232, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Reckhow, D.A. DBP formation during chlorination and chloramination: Effect of reaction time, pH, dosage, and temperature. J. AWWA 2008, 100, 82–95. Available online: http://www.jstor.org/stable/41313049 (accessed on 10 May 2024). [CrossRef]
- Zhang, Y.; Zhou, L.; Zeng, G.; Song, Z.; Li, G. Factors affecting the formation of trihalomethanes in the presence of bromide during chloramination. J. Zhejiang Univ. A 2010, 11, 606–612. [Google Scholar] [CrossRef]
- Akcay, M.; Yigit Avdan, Z.; Inan, H. Effect of biofiltration process on the control of THMs and HAAs in drinking water. Desalination Water Treat. 2015, 57, 2546–2554. [Google Scholar] [CrossRef]
- Ayesu, S.; Barnes, V.R.; Agbenyega, O. Threats of Changes in Land-Use and Drivers on Owabi and Barekese Watershed Forests in Ghana. Int. J. Appl. Geospat. Res. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Kyei, M.; Appiah-Effah, E.; Akodwaa-Boadi, K. Mechanistic Interaction between Climate Variables Rainfall and Temperature on Surface Water Quality and Water Treatment Costs at the Barekese Headworks, Ghana: A Time Series and Water Quality Index Modelling approach. Sci. Afr. 2023, 22, e01953. [Google Scholar] [CrossRef]
- Parveen, N.; Chowdhury, S.; Goel, S. Environmental impacts of the widespread use of chlorine-based disinfectants during the COVID-19 pandemic. Environ. Sci. Pollut. Res. 2022, 29, 85742–85760. [Google Scholar] [CrossRef]
- Sinha, R.; Gupta, A.K.; Ghosal, P.S. A review on Trihalomethanes and Haloacetic acids in drinking water: Global status, health impact, insights of control and removal technologies. J. Environ. Chem. Eng. 2021, 9, 106511. [Google Scholar] [CrossRef]

| Variables | Notation | Value | Reference |
|---|---|---|---|
| Average body mass of an adult in Ghana (Kg) | ABM | Male: 72.43 | [54] |
| Female: 68.12 | |||
| Ingestion Rate (L/day) | IR | 2 | [55] |
| Exposure frequency (days/year) | EF | 365 | |
| Exposure duration (days/year) | ED | 365 | [56] |
| Average lifetime in Ghana (days) | AT | Male: 23,170.2 (63.48 years) | [57] |
| Female: 24,761.6 (67.84 years) | |||
| Age-dependent adjustment factor | ADAF | <2 year = 10 | [58] |
| 2 to 16 years = 3 | |||
| >16 years = 1 | |||
| Average carcinogenic slope factor for THM4 (TCM: 0.031; BDCM: 0.062; TBM: 0.0079; CDBM: 0.084) via oral/dermal ingestion | SF | 0.046 | [59] |
| Raw Water | Filtered (Pre-Disinfection) Water | Final (Post-Disinfection) Treated Water | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | Color (PCU) | pH | Temp. (°C) | COD (mg/L) | DOC (mg/L) | R. Cl2 (mg/L) | Contact Time (t) (minutes) | Flow Rate (L/min) | THMs (µg/L) |
| 1 | 240 | 7.03 | 25.80 | 5.00 | 1.58 | 2.50 | 95.34 | 79,403 | 22.42 |
| 2 | 300 | 7.13 | 26.10 | 10 | 2.52 | 2.60 | 100.73 | 75,150 | 38.23 |
| 3 | 260 | 7.08 | 25.70 | 8.00 | 2.14 | 1.90 | 106.66 | 70,974 | 29.38 |
| 4 | 250 | 7.00 | 25.20 | 6.0 | 1.76 | 2.00 | 100.68 | 75,189 | 23.24 |
| 5 | 300 | 7.10 | 26.10 | 11.00 | 2.71 | 2.10 | 103.61 | 73,062 | 38.94 |
| 6 | 300 | 7.20 | 25.00 | 8.00 | 2.14 | 2.05 | 101.26 | 74,756 | 29.38 |
| Mean | 275 | 7.09 | 25.65 | 8.00 | 2.142 | 2.192 | 101.38 | 74,755.7 | 30.265 |
| Min. | 240 | 7.00 | 25.00 | 5 | 1.58 | 1.9 | 95.34 | 70,974 | 22.42 |
| Max. | 300 | 7.2 | 26.1 | 11 | 2.71 | 2.6 | 106.66 | 79,403 | 38.94 |
| STDev | 25.66 | 0.072 | 0.459 | 2.28 | 0.431 | 0.287 | 3.749 | 2553.1 | 7.087 |
| PC1 | PC2 | PC3 | PC4 | PC5 | |
|---|---|---|---|---|---|
| Eigenvalue | 3.681 | 2.092 | 1.014 | 0.154 | 0.058 |
| Variability (%) | 52.589 | 29.881 | 14.491 | 2.204 | 0.836 |
| Cumulative % | 52.589 | 82.470 | 96.961 | 99.164 | 100.000 |
| Variables | pH | Temp | Contact Time | R. Cl2 | COD | DOC |
|---|---|---|---|---|---|---|
| pH | ||||||
| Temperature | −0.110 | |||||
| Contact time | 0.265 | 0.054 | ||||
| R. Cl2 | 0.015 | 0.535 | −0.692 | |||
| COD | 0.576 | 0.516 | 0.583 | 0.000 | ||
| DOC | 0.576 | 0.520 | 0.579 | 0.005 | 1.000 | |
| THMs | 0.560 | 0.615 | 0.466 | 0.174 | 0.984 | 0.985 |
| Estimates | Notation | Value | Reference |
|---|---|---|---|
| Mean THM concentration (μg/L) | THMs | 30.265 | This study |
| 76.31 | [61] | ||
| Lifetime Average Daily Dose (ingestion) (mg/Kg/d) | LADDingestion | Male: 3.949 × 10−4 | This study |
| Female: 3.929 × 10−4 | |||
| Lifestage Integrative Cancer Risk (for over 16-year-old) | LICR | Male: 1.816 × 10−5 | This study |
| Female: 1.808 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellalaga, N.K.W.; Mensah, L.; Agbekey, B.K.; Bentil, E.; Waaley, L.; Anornu, G.K. Trihalomethane Formation Potential at the Barekese Water Treatment Plant and the Related Cancer Risk to Consumers in the Kumasi Metropolis of Ghana. Water 2024, 16, 3089. https://doi.org/10.3390/w16213089
Fellalaga NKW, Mensah L, Agbekey BK, Bentil E, Waaley L, Anornu GK. Trihalomethane Formation Potential at the Barekese Water Treatment Plant and the Related Cancer Risk to Consumers in the Kumasi Metropolis of Ghana. Water. 2024; 16(21):3089. https://doi.org/10.3390/w16213089
Chicago/Turabian StyleFellalaga, Nathaniel Kabral Wezenamo, Lawson Mensah, Bright Kwaku Agbekey, Ethel Bentil, Lilian Waaley, and Geophery Kwame Anornu. 2024. "Trihalomethane Formation Potential at the Barekese Water Treatment Plant and the Related Cancer Risk to Consumers in the Kumasi Metropolis of Ghana" Water 16, no. 21: 3089. https://doi.org/10.3390/w16213089
APA StyleFellalaga, N. K. W., Mensah, L., Agbekey, B. K., Bentil, E., Waaley, L., & Anornu, G. K. (2024). Trihalomethane Formation Potential at the Barekese Water Treatment Plant and the Related Cancer Risk to Consumers in the Kumasi Metropolis of Ghana. Water, 16(21), 3089. https://doi.org/10.3390/w16213089







