Sustainability Prediction by Evaluating the Emergy of a Co-Treatment System for Municipal Wastewater and Acidic Water Using Intermittent Electrocoagulation
Abstract
1. Introduction
2. Materials and Methods
2.1. A Description of the Case Study
2.2. Characterization of Urban Wastewater and Acid Water
2.3. Study Scenarios
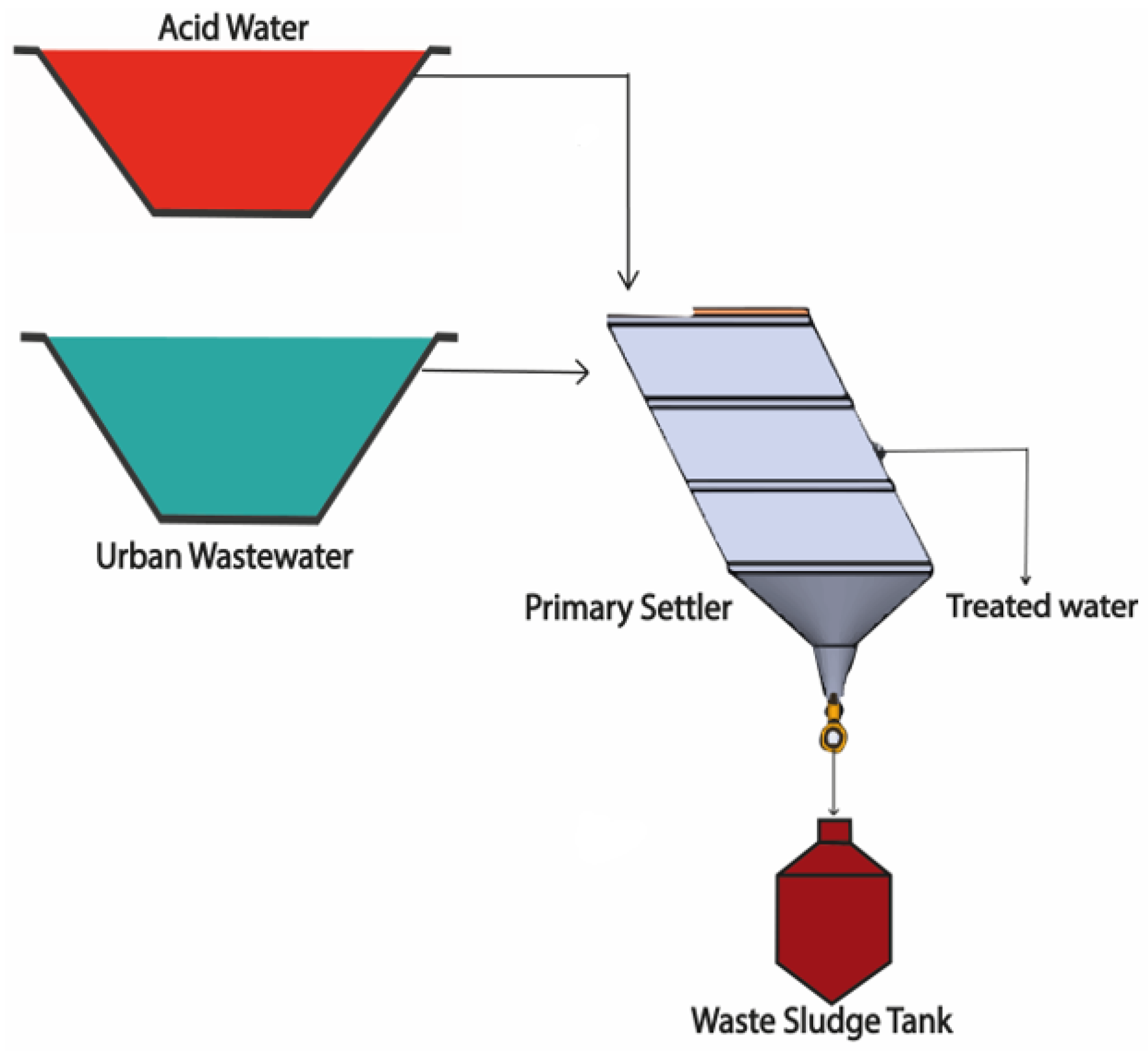
2.3.1. Scheme of Treatment I
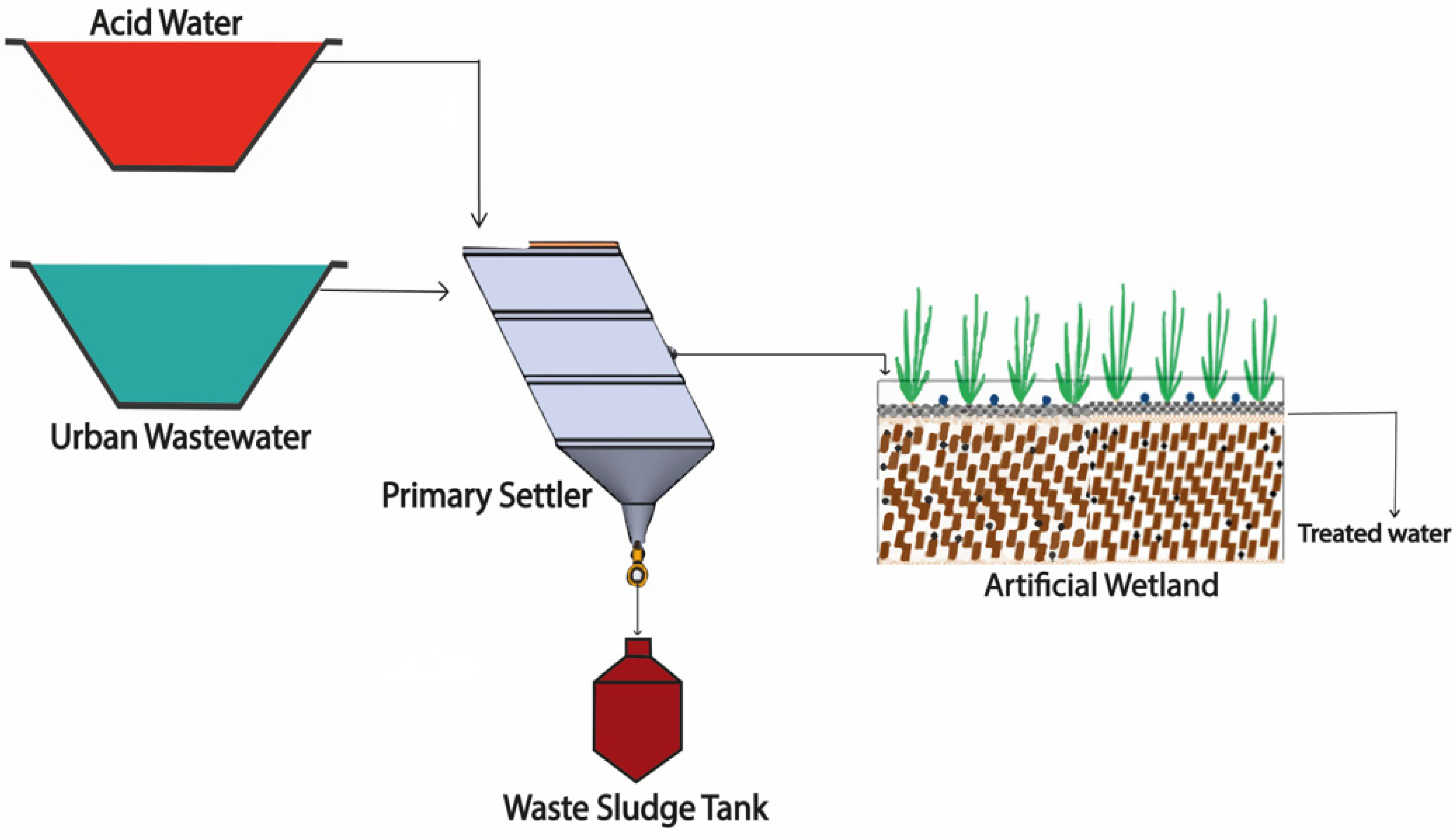
2.3.2. Scheme of Treatment II
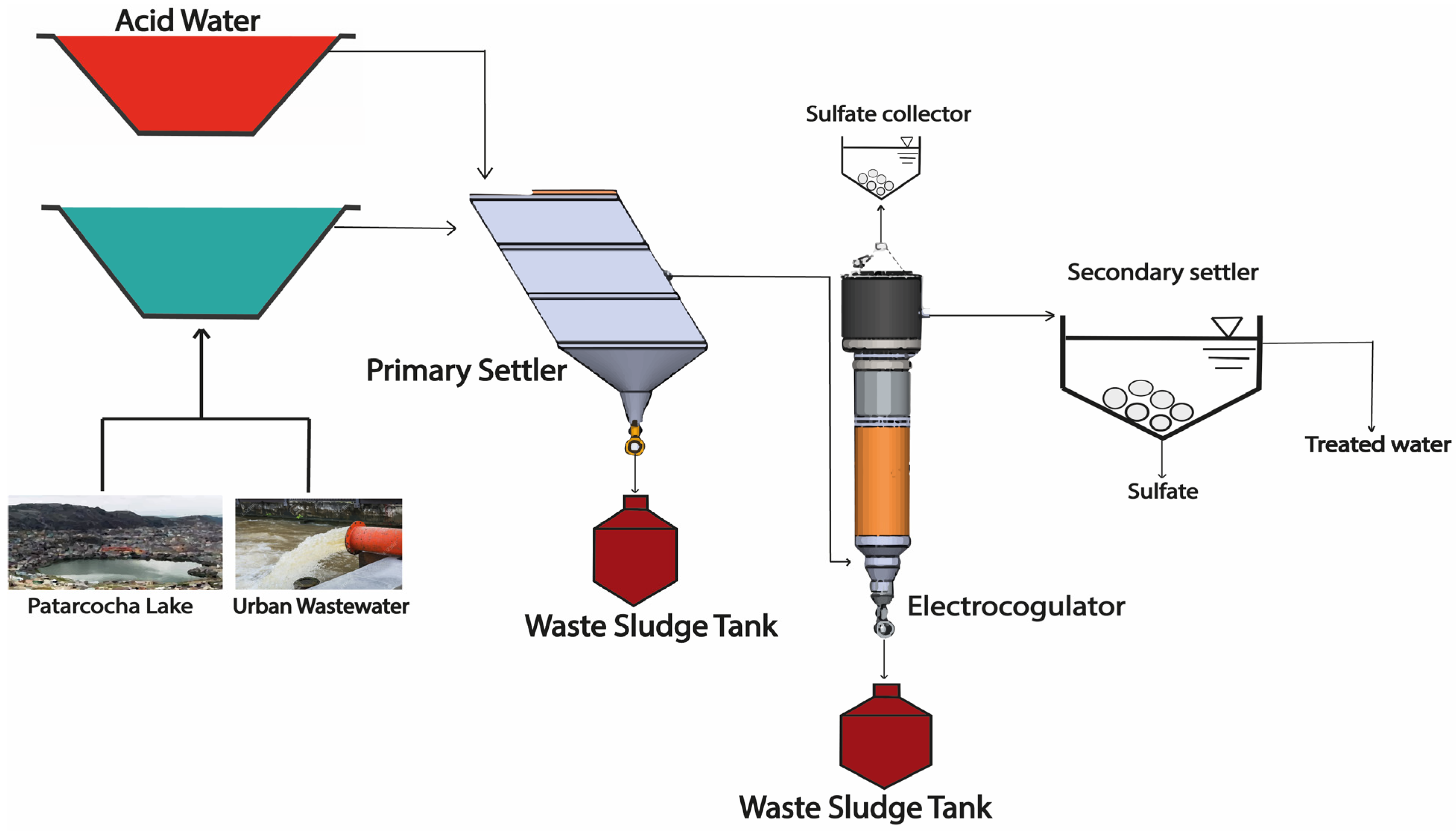
2.3.3. Treatment IIIa and IIIb
2.4. Estimation of Sustainability Indexes of Emergy
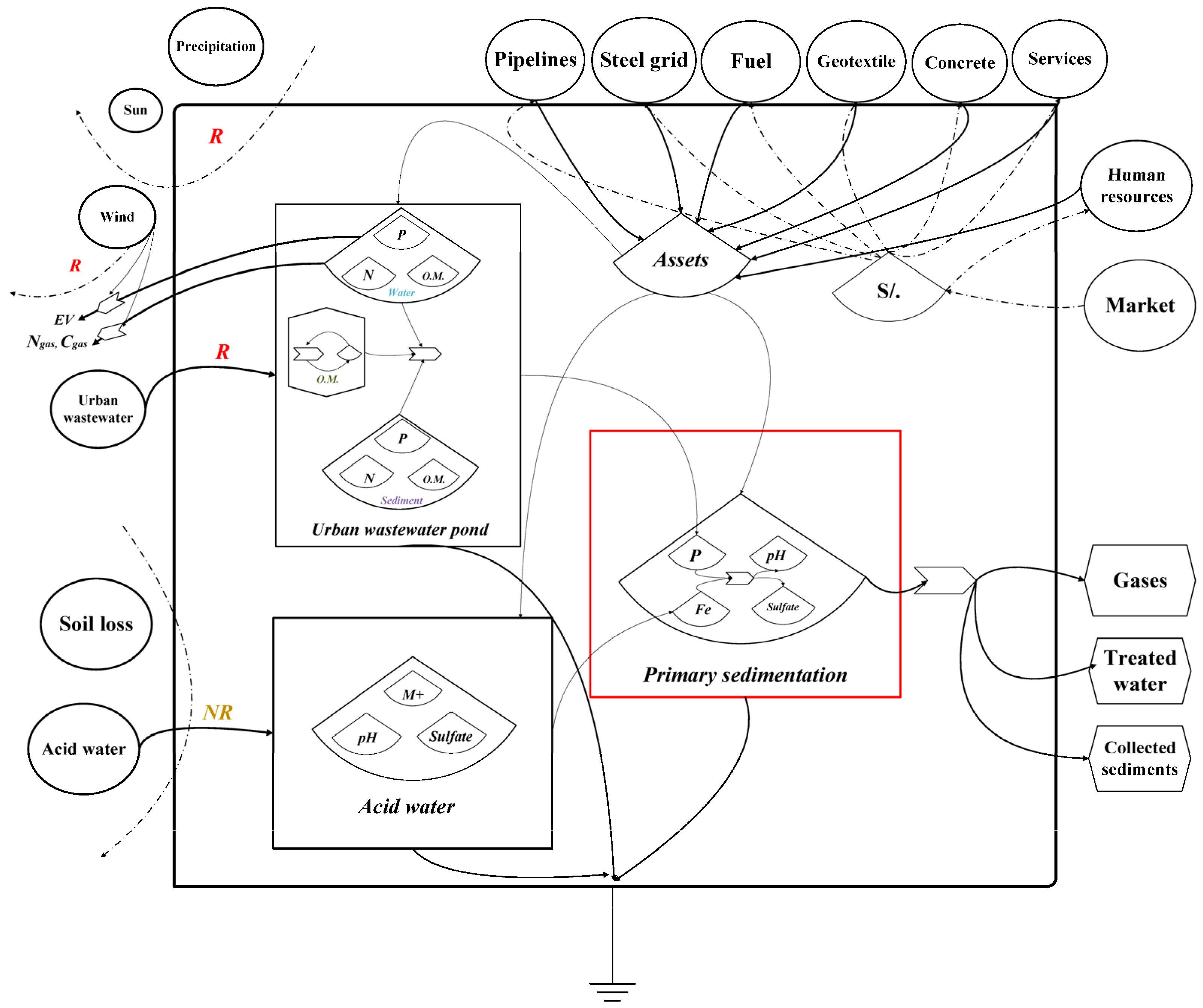
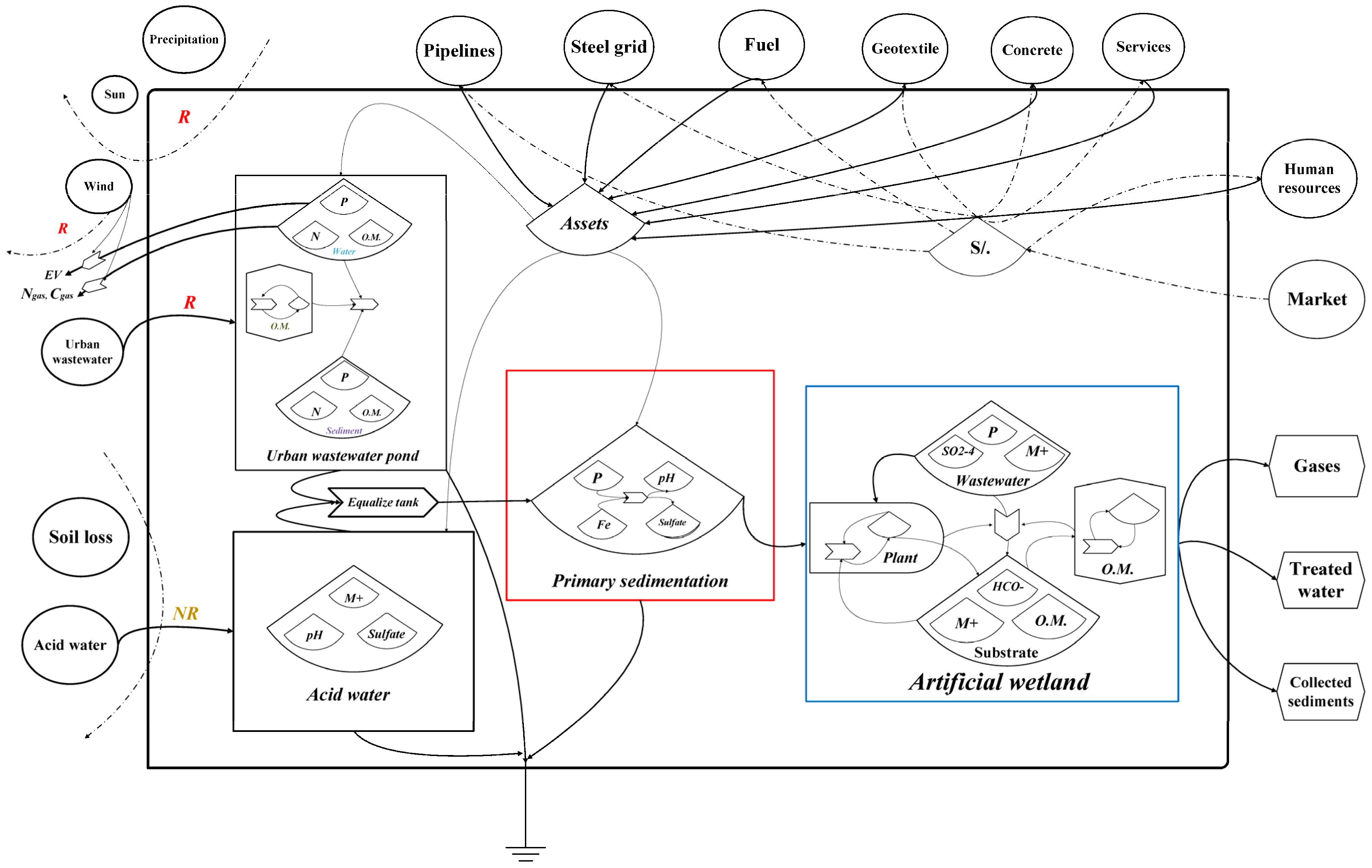
2.5. Process Flow Diagrams
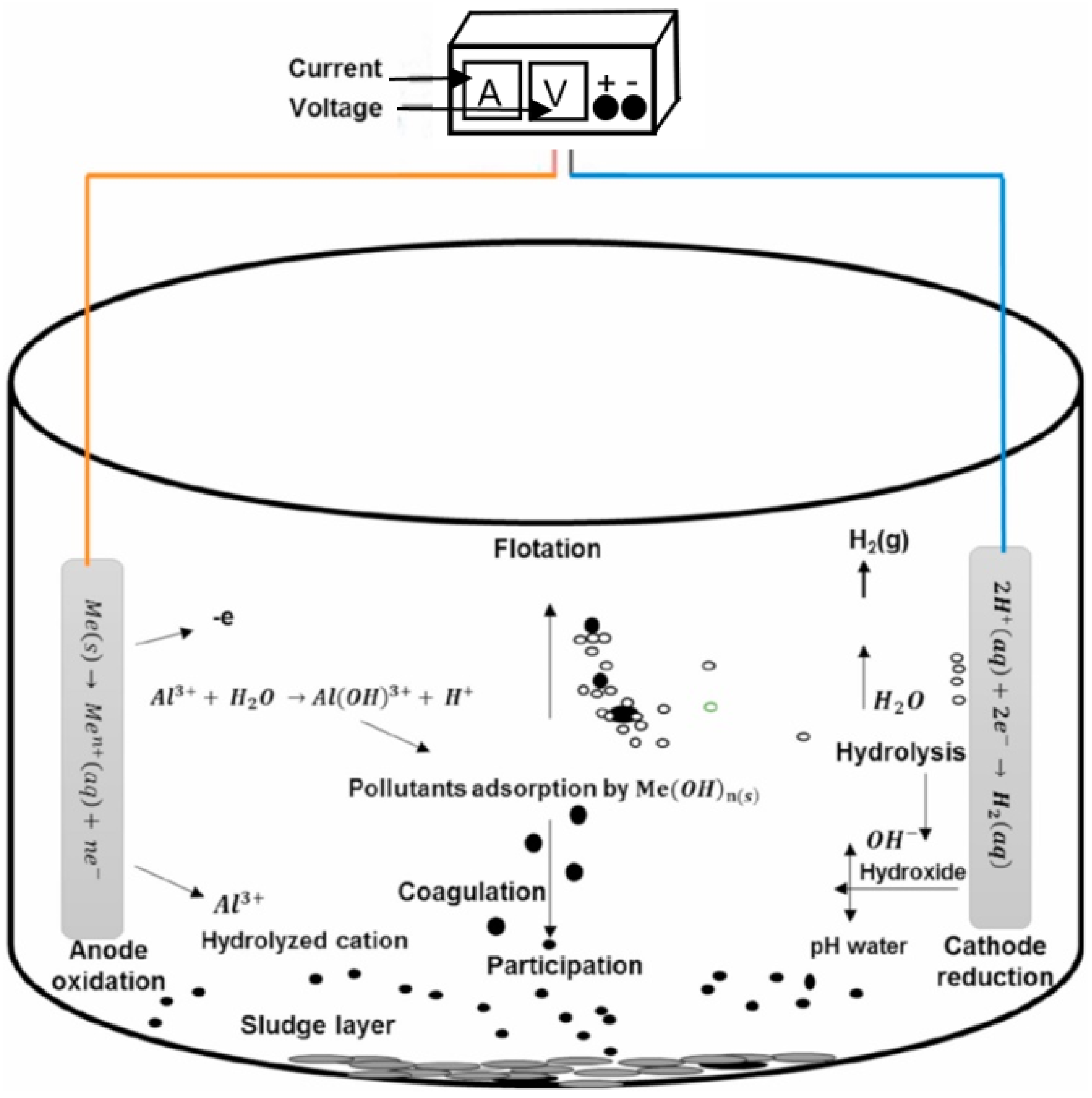
2.6. Intermittent Flow Electrocoagulation
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nepfumbada, C.; Tavengwa, N.T.; Masindi, V.; Foteinis, S.; Chatzisymeon, E. Recovery of phosphate from municipal wastewater as calcium phosphate and its subsequent application for the treatment of acid mine drainage. Resour. Conserv. Recycl. 2023, 190, 106779. [Google Scholar] [CrossRef]
- Strosnider, W.H.J.; Nairn, R.W. Effective passive treatment of high-strength acid mine drainage and raw municipal wastewater in Potosí, Bolivia using simple mutual incubations and limestone. J. Geochem. Explor. 2010, 105, 34–42. [Google Scholar] [CrossRef]
- Suxo, R.L. Evaluación Integral de la Infraestructura Sanitaria (Agua y Alcantarillado) de las Comunidades Amachuma Grande, Palca, Pacuani, Cebolullo, Tahuapalca y Cohoni del Municipio de Palca. 2022. Available online: https://repositorio.umsa.bo/handle/123456789/30800 (accessed on 19 October 2024).
- Chávez, R. Evaluación Geoquímica e Identificación de Drenaje Ácido de Roca de los Desmontes, Mineral y Pared de Tajo de la UEA-Cerro de Pasco Compañía Volcán. Bachelor’s Thesis, Universidad Nacional de Cajamarca, Cajamarca, Peru, 2018. [Google Scholar]
- Custodio, M.; Álvarez, D.; Cuadrado, W.; Montalvo, R.; Ochoa, S. Potentially toxic metals and metalloids in surface water intended for human consumption and other uses in the Mantaro River watershed, Peru. Soil Water Res. 2020, 15, 237–245. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Jones, B.W.; Millar, G.J. Alternative neutralisation materials for acid mine drainage treatment. J. Water Process Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef]
- Tolonen, E.-T.; Sarpola, A.; Hu, T.; Rämö, J.; Lassi, U. Acid mine drainage treatment using by-products from quicklime manufacturing as neutralization chemicals. Chemosphere 2014, 117, 419–424. [Google Scholar] [CrossRef]
- Mokgehle, T.M.; Tavengwa, N.T. Recent developments in materials used for the removal of metal ions from acid mine drainage. Appl. Water Sci. 2021, 11, 42. [Google Scholar] [CrossRef]
- Hairuddin, M.N.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Walvekar, R.; Karri, R.R. Magnetic palm kernel biochar potential route for phenol removal from wastewater. Environ. Sci. Pollut. Res. 2019, 26, 35183–35197. [Google Scholar] [CrossRef]
- Buzzi, D.C.; Viegas, L.S.; Rodrigues, M.A.; Bernardes, A.M.; Tenório, J.A.S. Water recovery from acid mine drainage by electrodialysis. Miner. Eng. 2013, 40, 82–89. [Google Scholar] [CrossRef]
- Gervais, M.; Dubuc, J.; Paquin, M.; Gonzalez-Merchan, C.; Genty, T.; Neculita, C.M. Comparative efficiency of three advanced oxidation processes for thiosalts oxidation in mine-impacted water. Miner. Eng. 2020, 152, 106349. [Google Scholar] [CrossRef]
- Skousen, J.; Ziemkiewicz, P. Performance of 116 passive treatment systems for acid mine drainage. Am. Soc. Min. Reclam. 2005, 2005, 1100–1133. [Google Scholar]
- Ferraz, R.M.; Costa, J.M.; Verola, L.M.; Sancinetti, G.P.; Rodriguez, R.P. Effects of the inoculum source, electron donor, and immobilization on the microbial community of sulfidogenic bioreactors. Chem. Eng. J. 2021, 404, 126549. [Google Scholar] [CrossRef]
- Han, L.J.; Li, J.S.; Xue, Q.; Guo, M.Z.; Wang, P.; Poon, C.S. Enzymatically induced phosphate precipitation (EIPP) for stabilization/solidification (S/S) treatment of heavy metal tailings. Constr. Build Mater. 2022, 314, 125577. [Google Scholar] [CrossRef]
- Hameed, B.H.; Rahman, A.A. Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J. Hazard Mater. 2008, 160, 576–581. [Google Scholar] [CrossRef]
- Isik, O.; Erbil, M.C.; Abdelrahman, A.M.; Ersahin, M.E.; Koyuncu, I.; Ozgun, H.; Demir, I. Removal of micropollutants from municipal wastewater by membrane bioreactors: Conventional membrane versus dynamic membrane. J. Environ. Manag. 2022, 303, 114233. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Mao, R.; Shi, Y.; Lin, S.; Qiao, M.; Zhao, X. Removal of phosphate in secondary effluent from municipal wastewater treatment plant by iron and aluminum electrocoagulation: Efficiency and mechanism. Sep. Purif. Technol. 2022, 286, 120439. [Google Scholar] [CrossRef]
- Aslam, A.; Khan, S.J.; Shahzad, H.M.A. Anaerobic membrane bioreactors (AnMBRs) for municipal wastewater treatment-potential benefits, constraints, and future perspectives: An updated review. Sci. Total Environ. 2022, 802, 149612. [Google Scholar] [CrossRef]
- Hossain, M.Z. Water: The Most Precious Resource of Our Life. Glob. J. Adv. Res. 2015, 2, 1436–1445. [Google Scholar]
- Wang, K.; Chen, X.; Yan, D.; Xu, Z.; Hu, P.; Li, H. Petrochemical and municipal wastewater treatment plants activated sludge each own distinct core bacteria driven by their specific incoming wastewater. Sci. Total Environ. 2022, 826, 153962. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, L. Understanding the relationships between rheology and chemistry of asphalt binders: A review. Constr. Build. Mater. 2022, 329, 127161. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Z.; Jin, S.; Xu, C.; Deng, H.; Zhang, M. Runoff Changes from Urumqi Glacier No. 1 over the Past 60 Years, Eastern Tianshan, Central Asia. Water 2020, 12, 1286. [Google Scholar] [CrossRef]
- Johnson, K.L.; Younger, P.L. The co-treatment of sewage and mine waters in aerobic wetlands. Eng. Geol. 2006, 85, 53–61. [Google Scholar] [CrossRef]
- Younger, P.L.; Henderson, R. Synergistic wetland treatment of sewage and mine water: Pollutant removal performance of the first full-scale system. Water Res. 2014, 55, 74–82. [Google Scholar] [CrossRef]
- Mamelkina, M.A.; Tuunila, R.; Sillänpää, M.; Häkkinen, A. Systematic study on sulfate removal from mining waters by electrocoagulation. Sep. Purif. Technol. 2019, 216, 43–50. [Google Scholar] [CrossRef]
- Mickova, I. Advanced electrochemical technologies in wastewater treatment part I: Electrocoagulation. Am. Sci. Res. J. Eng. Technol. Sci. 2015, 14, 233–257. [Google Scholar]
- Mousazadeh, M.; Naghdali, Z.; Al-Qodah, Z.; Alizadeh, S.M.; Karamati Niaragh, E.; Malekmohammadi, S.; Nidheesh, P.V.; Roberts, E.P.L.; Sillanpää, M.; Emamjomeh, M.M. A systematic diagnosis of state of the art in the use of electrocoagulation as a sustainable technology for pollutant treatment: An updated review. Sustain. Energy Technol. Assess. 2021, 47, 101353. [Google Scholar] [CrossRef]
- Mayta Lovaton, J.Y. Análisis del Método de Electrocoagulación en la Eliminación de Microplásticos en Aguas Residuales a Nivel de Laboratorio. Master’s Thesis, Universidad Nacional del Centro del Perú, Huancayo, Peru, 2023. [Google Scholar]
- Ensano, B.M.B.; Borea, L.; Naddeo, V.; Belgiorno, V.; De Luna, M.D.G.; Ballesteros, F.C., Jr. Removal of pharmaceuticals from wastewater by intermittent electrocoagulation. Water 2017, 9, 85. [Google Scholar] [CrossRef]
- Hughes, T.A.; Gray, N.F. Co-treatment of acid mine drainage with municipal wastewater: Performance evaluation. Environ. Sci. Pollut. Res. 2013, 20, 7863–7877. [Google Scholar] [CrossRef]
- Arden, S.; Ma, X.; Brown, M. Holistic analysis of urban water systems in the Greater Cincinnati region: (2) resource use profiles by emergy accounting approach. Water Res. X 2019, 2, 100012. [Google Scholar] [CrossRef]
- Pan, H.; Geng, Y.; Tian, X.; Wilson, J.; Chen, W.; Zhong, S.; Song, X. Emergy-based environmental accounting of one mining system. Environ. Sci. Pollut. Res. 2019, 26, 14598–14615. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z.M.; Zhou, Y.; Zhou, J.B.; Chen, G.Q. Emergy as embodied energy based assessment for local sustainability of a constructed wetland in Beijing. Commun. Nonlinear Sci. Numer. Simul. 2009, 14, 622–635. [Google Scholar] [CrossRef]
- Polyakova, O.S.; Semenov, S.Y. Emergy analysis of wastewater treatment technology. Eur. Proc. Soc. Behav. Sci. 2017, 26, 784–791. [Google Scholar]
- Londoño, N.A.C.; Suárez, D.G.; Velásquez, H.I.; Ruiz-Mercado, G.J. Emergy analysis for the sustainable utilization of biosolids generated in a municipal wastewater treatment plant. J. Clean. Prod. 2017, 141, 182–193. [Google Scholar] [CrossRef]
- Arguello Delago, J.G.; Gonzales Aguinaca, P.J. Evaluación de Emergía de Medio Ambiente y la Económia de Nicaragua (1995–1999); Universidad Nacional Agraria: Managua, Nicaragua, 1999. [Google Scholar]
- Giannetti, B.F.; Silva, C.C.; Agostinho, F.; Almeida, C. Emergy evaluation of Domestic Wastewater Treatments: The role of Energy and Materials Consumption and Carbon Emissions. J. Environ. Account. Manag. 2016, 4, 317–338. [Google Scholar] [CrossRef]
- Lv, C.; He, Y.; Zhang, W.; Gu, C.; Li, Y.; Yan, D. Quantitative analysis of eco-economic benefits of urban reclaimed water greening based on emergy theory. Water Resour. Manag. 2021, 35, 5029–5047. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Yan, Y. A Dynamic comparison sustainability study of standard wastewater treatment system in the straw pulp papermaking process and printing & dyeing papermaking process based on the hybrid neural network and emergy framework. Water 2020, 12, 1781. [Google Scholar] [CrossRef]
- Winfrey, B.K.; Tilley, D.R. An emergy-based treatment sustainability index for evaluating waste treatment systems. J. Clean. Prod. 2016, 112, 4485–4496. [Google Scholar] [CrossRef]
- Grönlund, E.; Billgren, C.; Tonderski, K.; Raburu, P.O. Emergy assessment of a wastewater treatment pond system in the Lake Victoria Basin. J. Environ. Account. Manag. 2017, 5, 11–26. [Google Scholar] [CrossRef]
- Al-Raad, A.A.; Hanafiah, M.M. Removal of inorganic pollutants using electrocoagulation technology: A review of emerging applications and mechanisms. J. Environ. Manag. 2021, 300, 113696. [Google Scholar] [CrossRef]
- Odum, H.T. Environmental Accounting: Emergy and Environmental Decision Making; Wiley: New York, NY, USA, 1996; Volume 707. [Google Scholar]
- Björklund, J.; Geber, U.; Rydberg, T. Emergy analysis of municipal wastewater treatment and generation of electricity by digestion of sewage sludge. Resour. Conserv. Recycl. 2001, 31, 293–316. [Google Scholar] [CrossRef]
- Agostinho, F.; Diniz, G.; Siche, R.; Ortega, E. The use of emergy assessment and the Geographical Information System in the diagnosis of small family farms in Brazil. Ecol. Model. 2008, 210, 37–57. [Google Scholar] [CrossRef]
- Zhou, J.B.; Jiang, M.M.; Chen, B.; Chen, G.Q. Emergy evaluations for constructed wetland and conventional wastewater treatments. Commun. Nonlinear Sci. Numer. Simul. 2009, 14, 1781–1789. [Google Scholar] [CrossRef]
- Wang, L.K.; Hung, Y.-T.; Lo, H.H.; Yapijakis, C. Hazardous Industrial Waste Treatment; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Brown, M.T.; McClanahan, T.R. Emergy analysis perspectives of Thailand and Mekong River dam proposals. Ecol. Model. 1996, 91, 105–130. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, S.; Wu, J.; Jiang, W. A sustainability analysis of a municipal sewage treatment ecosystem based on emergy. Ecol. Eng. 2010, 36, 685–696. [Google Scholar] [CrossRef]
- Chen, B.; Chen, G.Q.; Hao, F.H.; Yang, Z.F. The water resources assessment based on resource exergy for the mainstream Yellow River. Commun. Nonlinear Sci. Numer. Simul. 2009, 14, 331–344. [Google Scholar] [CrossRef]
- Grönlund, S.E. Indicators and methods to assess sustainability of wastewater sludge management in the perspective of two systems ecology models. Ecol. Indic. 2019, 100, 45–54. [Google Scholar] [CrossRef]
- Chen, D.; Luo, Z.; Webber, M.; Chen, J.; Wang, W. Emergy evaluation of the contribution of irrigation water, and its utilization, in three agricultural systems in China. Front. Earth Sci. 2014, 8, 325–337. [Google Scholar] [CrossRef]
- Giannetti, B.F.; Barrella, F.A.; Almeida, C.A. Combined tool for environmental scientists and decision makers: Ternary diagrams and emergy accounting. J. Clean. Prod. 2006, 14, 201–210. [Google Scholar] [CrossRef]
- Cano, N.A.; Céspedes-Zuluaga, S.; Guerrero-Martin, C.; Gallego, D. Exergy and emergy: Complementary tools for assessing the environmental sustainability use of biosolids generated in wastewater-treatment plant for energy-production. Quim. Nova. 2022, 45, 4–15. [Google Scholar] [CrossRef]
- Shao, S.; Mu, H.; Yang, F.; Zhang, Y.; Li, J. Application of emergy analysis to the sustainability evaluation of municipal wastewater treatment plants. Sustainability 2016, 9, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Y.; Li, M.; Deng, S.; Wu, J.; Zhang, Y.; Xiao, H. Emergy evaluation of an integrated livestock wastewater treatment system. Resour. Conserv. Recycl. 2014, 92, 95–107. [Google Scholar] [CrossRef]
- Moss, A.R.; Lansing, S.A.; Tilley, D.R.; Klavon, K.H. Assessing the sustainability of small-scale anaerobic digestion systems with the introduction of the emergy efficiency index (EEI) and adjusted yield ratio (AYR). Ecol. Eng. 2014, 64, 391–407. [Google Scholar] [CrossRef]
- Theregowda, R.B.; González-Mejía, A.M.; Ma, X.; Garland, J. Nutrient recovery from municipal wastewater for sustainable food production systems: An alternative to traditional fertilizers. Environ. Eng. Sci. 2019, 36, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, C.; Si, X.; Zhang, C.; Liu, F.; Yu, L.; Chen, G. Study on the choice of wastewater treatment process based on the emergy theory. Processes 2021, 9, 1648. [Google Scholar] [CrossRef]
- Geber, U.; Björklund, J. The relationship between ecosystem services and purchased input in Swedish wastewater treatment systems—A case study. Ecol. Eng. 2002, 19, 97–117. [Google Scholar] [CrossRef]
- Tilley, D.R.; Brown, M.T. Dynamic emergy accounting for assessing the environmental benefits of subtropical wetland stormwater management systems. Ecol. Model. 2006, 192, 327–361. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, X.; Yang, X.; Lv, Y.; Wu, J.; Lin, L.; Xiao, Y.; Qi, H.; Yu, X.; Zhang, Y. The environmental sustainability evaluation of an urban tap water treatment plant based on emergy. Ecol. Indic. 2018, 94, 28–38. [Google Scholar] [CrossRef]
- Sulca, L.; Paulino, C. Estimación de la Radiación Solar Global Mediante Temperaturas Extremas, Aplicando el Modelo Bristow–Campbell en la Región Pasco; Universidad Nacional Daniel Alcides Carrión: Cerro De Pasco, Peru, 2019. [Google Scholar]
- SENHAMI. Pronóstico de Cobertura Nacional del Indice de Radiación Solar Ultravioleta. 2016. Available online: https://www.senamhi.gob.pe/pdf/estudios/meteo_2016_pro_cob_nac_ind_rad_sol_ult.pdf (accessed on 19 October 2024).
- Mendoza Artica, E.Y. Potencial de la Energía Eólica para la Generación de Electricidad y su Relación con el Cambio Climático, en la Comunidad Campesina de Huaraucaca, Distrito de Tinyahuarco, Provincia de Pasco-2017; Daniel Alcides Carrión National University: Cerro de Pasco, Peru, 2018. [Google Scholar]
- MEM. Estudio de Impacto Ambiental del Proyecto “Línea de Transmisión 220 kV C.H. Chancay 2—C.H. Rucuy—S.E. Francoise. 2014. Available online: http://www.minem.gob.pe/minem/archivos/4_4_1ClimayMetRev0.pdf (accessed on 19 October 2024).
- Alania, V.; Orializ, Y. Caracterización Ambiental de Los Ecosistemas, Zonas de Vida y Vegetación Natural de la Provincia de Pasco; Universidad Nacional Daniel Alcides Carrión: Cerro De Pasco, Peru, 2019. [Google Scholar]
- Huiza, W.; Quispe, J. Evaluación de las Propiedades Físicas y Químicas del Suelo de los Tipos de Pastizal del Centro de Investigación de Camélidos Sudamericanos-LACHOCC. Tesis de Grado; Universidad Nacional de Huancavelica: Huancavelica, Peru, 2017. [Google Scholar]
- Bezuijen, A.; Pilarczyk, K.W. Geosintéticos en ingeniería hidráulica y costera: Filtros, revestimientos y estructuras llenas de arena. Ing. Civ. 2013, 171, 39–56. [Google Scholar]
- INGEMECANICA. Tablas de Pesos Específicos y Densidades de Materiales. 2020. Available online: https://ingemecanica.com/tutoriales/pesos.html#concreto (accessed on 19 October 2024).
- Wang, L.; Ni, W.; Li, Z. Emergy evaluation of combined heat and power plant eco-industrial park (CHP plant EIP). Resour. Conserv. Recycl. 2006, 48, 56–70. [Google Scholar] [CrossRef]
- Aceros Valval. Caracteristicas de Planchas de Aluminio. 2018. Available online: https://acerosvalval.cl/plancha-de-aluminio/ (accessed on 8 November 2020).



| N° | Parameter | Concentration (mg/L) | |
|---|---|---|---|
| Urban Wastewater | Eutrophic Lake Patarcocha | ||
| N: 8820489.96 E: 361900.75 | N: 8818441.49 E: 363081.15 | ||
| 1 | BOD5 | 151.20 | 66.50 |
| 2 | COD | 255.50 | 111.30 |
| 4 | Total phosphorus | 8.50 | 5.46 |
| 5 | Sulfates | 1542.8 | - |
| 6 | pH | 5.7 | 8.81 |
| 7 | Nitrate | 20.95 | 1.66 |
| 8 | Turbidez | 3.6 | - |
| 9 | Ammonia nitrogen | - | 1.30 |
| 10 | |||
| 11 | |||
| N: 8820489.96 E: 361900.75 | AW from the Contaminated Lake Quiulacocha | |||||||
|---|---|---|---|---|---|---|---|---|
| N° | Parameter | Concentration (mg/L) | N° | Parameter | Concentration (mg/L) | N° | Parameter | Concentration (mg/L) |
| 1 | Sulfate | 6000.00 | 13 | Total cadmium | 0.0001 | 24 | Total nickel | 0.001 |
| 2 | pH | 1.80 | 14 | Total calcium | 284.81 | 25 | Total silver | 0.005 |
| 4 | Total iron | 1316.65 | 15 | Total cesium | 0.02 | 26 | Total potassium | 8.19 |
| 5 | Total zinc | 1.02 | 16 | Total cobalt | 0.002 | 27 | Total selenium | 0.001 |
| 6 | Total copper | 2.69 | 17 | Total chromium | 0.0003 | 28 | Total silica | 22 |
| 7 | Total lead | 335.87 | 18 | Total phosphorus | 4.6 | 29 | Total sodium | 20.39 |
| 8 | Total arsenic | 0.002 | 19 | Total lithium | 0.12 | 30 | Total titanium | 0.0007 |
| 9 | Total aluminum | 19.59 | 20 | Total magnesium | 1023.72 | 31 | Total uranium | 0.005 |
| 10 | Total barium | 0.0002 | 21 | Total manganese | 498.94 | 32 | Total vanadium | 0.0002 |
| 11 | Total bismuth | 0.009 | 22 | Total mercury | 0.001 | |||
| 12 | Total boron | 0.002 | 23 | Total molybdenum | 0.001 | |||
| Index | Description | Formula | Acceptability Range | References |
|---|---|---|---|---|
| Net Emergy Yield Ratio (EYR) | Measures the efficiency with which the system transforms inputs into outputs. A higher value indicates that the system produces significantly more emergy than it invests, which is desirable in sustainable processes. | EYR = (Total Emergy Used)/(Imported Emergy) | >1 is acceptable. Higher values indicate a more efficient system. | [35,37] |
| Emergy Investment Ratio (EIR) | This index measures the system’s dependence on imported emergy compared to local emergy. A high value indicates greater dependence on external inputs, while a low value suggests a self-sufficient or efficient system in using local resources. | EIR = (Imported Emergy)/(Local Emergy) | A low value is favorable, preferably close to 1. A high EIR reflects a high dependence on external inputs. | [34] |
| Environmental Loading Ratio (ELR) | Compares the use of renewable resources with non-renewable and imported ones. A low value indicates that the system is more environmentally friendly, relying primarily on renewable resources, while a high value suggests greater environmental pressure due to reliance on non-renewable or imported inputs. | ELR = (Non-renewable Emergy + Imported Emergy)/(Renewable Emergy) | A low value (<2) is ideal, reflecting lower environmental pressure. High values (>5) indicate high dependence on non-renewable resources. | [40] |
| Sustainability Index (SI) | The sustainability index is a ratio between the system’s efficiency (EYR) and its environmental impact (ELR). A high value indicates that the system is both efficient and sustainable, while a low value reflects unsustainability due to high environmental impact compared to emergy production efficiency. | SI = EYR/ELR | Values above 1 indicate a sustainable system. Low values (<1) indicate unsustainable processes. | [41] |
| Item | Description | Quantities | Units | Solar Transfomicity (sej/year) | References | Emergy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario I | Scenario II | Scenario IIIa | Scenario IIIb | Scenario I | Scenario II | Scenario IIIa | Scenario IIIb | |||||
| Renewable resources | ||||||||||||
| Construction and operation stage | ||||||||||||
| 1 | Sunlight | 5.64 × 1012 | 7.52 × 1012 | 8.27 × 1012 | 8.27 × 1012 | J | 1 | [43] | 5.64 × 1012 | 7.52 × 1012 | 8.27 × 1012 | 8.27 × 1012 |
| 2 | Wind, kinetic | 5.45 × 107 | 7.27 × 107 | 8.00 × 107 | 8.00 × 107 | J | 6.23 × 102 | [43] | 3.40 × 1010 | 4.53 × 1010 | 4.98 × 1010 | 4.98 × 1010 |
| 3 | Rainfall, geopotential | 1.50 × 1010 | 2.00 × 1010 | 2.20 × 1010 | 2.20 × 1010 | J | 8.89 × 103 | [43] | 1.33 × 1014 | 1.78 × 1014 | 1.96 × 1014 | 1.96 × 1014 |
| 4 | Chemical rain | 1.62 × 1010 | 2.16 × 1010 | 2.38 × 1010 | 2.38 × 1010 | J | 1.54 × 104 | [43] | 2.51 × 1014 | 3.34 × 1014 | 3.67 × 1014 | 3.67 × 1014 |
| 5 | Earth cycle | 8.25 × 108 | 1.10 × 109 | 1.21 × 109 | 1.21 × 109 | J | 5.80 × 104 | [43] | 4.79 × 1013 | 6.38 × 1013 | 7.02 × 1013 | 7.02 × 1013 |
| 6 | Urban wastewater (eutrophic) | 6.88 × 1012 | 6.88 × 1012 | 6.88 × 1012 | 2.99 × 1012 | J | 3.76 × 106 | [44] | 2.59 × 1019 | 2.59 × 1019 | 2.59 × 1019 | 1.12 × 1019 |
| Non-renewable system resources | ||||||||||||
| Construction and operation stage | ||||||||||||
| 7 | Loss of soil | 1.12 × 109 | 1.29 × 109 | 1.55 × 109 | 1.55 × 109 | g | 1.24 × 105 | [45] | 1.39 × 1014 | 1.59 × 1014 | 1.92 × 1014 | 1.92 × 1014 |
| 8 | AW | 2.60 × 1012 | 2.60 × 1012 | 2.60 × 1012 | 1.30 × 1012 | J | 3.76 × 106 | [44] | 9.79 × 1018 | 9.79 × 1018 | 9.79 × 1018 | 4.90 × 1018 |
| Imports | ||||||||||||
| Construction stage operation and maintenance | ||||||||||||
| 9 | PVC pipes for wastewater | 1.37 × 107 | 1.72 × 107 | 3.00 × 107 | 3.35 × 107 | g | 5.87 × 109 | [44] | 8.01 × 1016 | 1.01 × 1017 | 1.76 × 1017 | 1.97 × 1017 |
| 10 | Geotextile | 1.93 × 101 | 2.78 × 101 | 3.42 × 101 | 3.42 × 101 | J | 1.11 × 105 | [46] | 2.14 × 106 | 3.09 × 106 | 3.80 × 106 | 3.80 × 106 |
| 11 | Concrete | 5.14 × 107 | 5.57 × 107 | 6.22 × 107 | 6.22 × 107 | g | 7.05 × 109 | [47] | 3.62 × 1017 | 3.93 × 1017 | 4.39 × 1017 | 4.39 × 1017 |
| 12 | Fuel | 2.85 × 1010 | 5.87 × 1010 | 7.65 × 1010 | 7.65 × 1010 | J | 1.11 × 105 | [46] | 3.16 × 1015 | 6.52 × 1015 | 8.49 × 1015 | 8.49 × 1015 |
| 13 | Steel grids | 3.30 × 103 | 4.40 × 103 | 4.84 × 103 | 4.84 × 103 | g | 4.13 × 109 | [48] | 1.36 × 1013 | 1.82 × 1013 | 2.00 × 1013 | 2.00 × 1013 |
| 14 | Metals (Aluminum) | 1.32 × 105 | 1.32 × 105 | g | 1.00 × 109 | [43] | 1.32 × 1014 | 1.32 × 1014 | ||||
| 15 | Gravel (Zeolite) | 9.88 × 105 | g | 1.00 × 109 | [46] | 9.88 × 1014 | ||||||
| 16 | Phytoremediation | 8.65 × 105 | $ | 8.70 × 101 | [36] | 7.53 × 107 | ||||||
| 17 | Machinery rental service | 9.56 × 104 | 2.58 × 105 | 4.65 × 105 | 4.65 × 105 | $ | 3.38 × 1012 | [49] | 3.23 × 1017 | 8.72 × 1017 | 1.57 × 1018 | 1.57 × 1018 |
| 18 | Material rental service | 1.45 × 105 | 3.93 × 105 | 7.07 × 105 | 7.07 × 105 | $ | 3.30 × 1012 | [50] | 4.80 × 1017 | 1.30 × 1018 | 2.33 × 1018 | 2.33 × 1018 |
| 19 | Construction labor service | 3.45 × 104 | 6.21 × 104 | 1.12 × 105 | 1.12 × 105 | $ | 3.38 × 1012 | [49] | 1.17 × 1017 | 2.10 × 1017 | 3.78 × 1017 | 3.78 × 1017 |
| 20 | Labor service for operation | 1.12 × 105 | 2.02 × 105 | 3.64 × 105 | 3.64 × 105 | $ | 3.38 × 1012 | [51] | 3.79 × 1017 | 6.83 × 1017 | 1.23 × 1018 | 1.23 × 1018 |
| 21 | Maintenance and operation service | 2.03 × 104 | 3.65 × 105 | 5.47 × 104 | 5.47 × 104 | $ | 3.46 × 1012 | [47] | 7.01 × 1016 | 1.26 × 1017 | 1.89 × 1017 | 1.89 × 1017 |
| 22 | Energy flow in the electrodes | 1.13 × 1013 | 1.13 × 1013 | J | 1.11 × 105 | [46] | 1.25 × 1018 | 1.25 × 1018 | ||||
| Exports+ | ||||||||||||
| 23 | Treated wastewater | 1.55 × 1012 | 1.55 × 1012 | 1.55 × 1012 | 1.55 × 1012 | J | 1.92 × 107 | [50] | 2.97 × 1019 | 2.97 × 1019 | 2.97 × 1019 | 2.97 × 1019 |
| Study Scenarios | Net Yield Ratio (EYR) | Emergy Inversion Ratio (EIR) | Environmental Loading Ratio (ELR) | Sustainability Index (SI) |
|---|---|---|---|---|
| Treatment I | 15.26 | 0.05 | 0.45 | 33.99 |
| Treatment II | 10.67 | 0.10 | 0.52 | 20.48 |
| Treatment IIIa | 5.09 | 0.18 | 0.62 | 8.17 |
| Treatment IIIb | 2.82 | 0.55 | 1.22 | 2.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo-Toledo, L.; Virú-Vásquez, P.; Rodriguez-Flores, R.; Sierra-Flores, L.; Flores-Salinas, J.; Tineo-Cordova, F.; Palomino-Vildoso, R.; Madueño-Sulca, C.; Rios-Varillas de Oscanoa, C.; Pilco-Nuñez, A. Sustainability Prediction by Evaluating the Emergy of a Co-Treatment System for Municipal Wastewater and Acidic Water Using Intermittent Electrocoagulation. Water 2024, 16, 3081. https://doi.org/10.3390/w16213081
Bravo-Toledo L, Virú-Vásquez P, Rodriguez-Flores R, Sierra-Flores L, Flores-Salinas J, Tineo-Cordova F, Palomino-Vildoso R, Madueño-Sulca C, Rios-Varillas de Oscanoa C, Pilco-Nuñez A. Sustainability Prediction by Evaluating the Emergy of a Co-Treatment System for Municipal Wastewater and Acidic Water Using Intermittent Electrocoagulation. Water. 2024; 16(21):3081. https://doi.org/10.3390/w16213081
Chicago/Turabian StyleBravo-Toledo, Luigi, Paul Virú-Vásquez, Ruben Rodriguez-Flores, Luis Sierra-Flores, José Flores-Salinas, Freddy Tineo-Cordova, Rolando Palomino-Vildoso, César Madueño-Sulca, Cecilia Rios-Varillas de Oscanoa, and Alex Pilco-Nuñez. 2024. "Sustainability Prediction by Evaluating the Emergy of a Co-Treatment System for Municipal Wastewater and Acidic Water Using Intermittent Electrocoagulation" Water 16, no. 21: 3081. https://doi.org/10.3390/w16213081
APA StyleBravo-Toledo, L., Virú-Vásquez, P., Rodriguez-Flores, R., Sierra-Flores, L., Flores-Salinas, J., Tineo-Cordova, F., Palomino-Vildoso, R., Madueño-Sulca, C., Rios-Varillas de Oscanoa, C., & Pilco-Nuñez, A. (2024). Sustainability Prediction by Evaluating the Emergy of a Co-Treatment System for Municipal Wastewater and Acidic Water Using Intermittent Electrocoagulation. Water, 16(21), 3081. https://doi.org/10.3390/w16213081







