Assessment of Water Status, Bottom Sediments, Macrophytes in the Light of Index Analysis and Geochemical Parameters of Selected Dam Reservoirs of Kielce Upland (Poland)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Sampling and Chemical Analysis
2.3. Statistical Analysis
2.4. Assessment of the Contamination of Bottom Sediments
2.4.1. Geo-Accumulation Index (Igeo)
2.4.2. CF Contamination Factor
2.4.3. PLI Index
3. Results
3.1. Physical and Chemical Properties of Water
3.2. Physicochemical Properties of Bottom Sediments
3.3. Concentrations of PTEs in Macrophytes
3.4. Correlations Among PTEs Concentrations in Water, Sediments, and Macrophytes
3.5. Assessment of Pollution of Bottom Sediments
4. Discussion
5. Conclusions
- The study showed that the water quality status of selected dam reservoirs allows them to be classified in the first class of water quality in accordance with the Regulation of the Minister of Infrastructure of June 25, 2021, on the classification of ecological status, ecological potential, and chemical status, as well as the method of classifying the surface water bodies status, and environmental quality standards for priority substances (Journal of Laws 2021 item 1475).
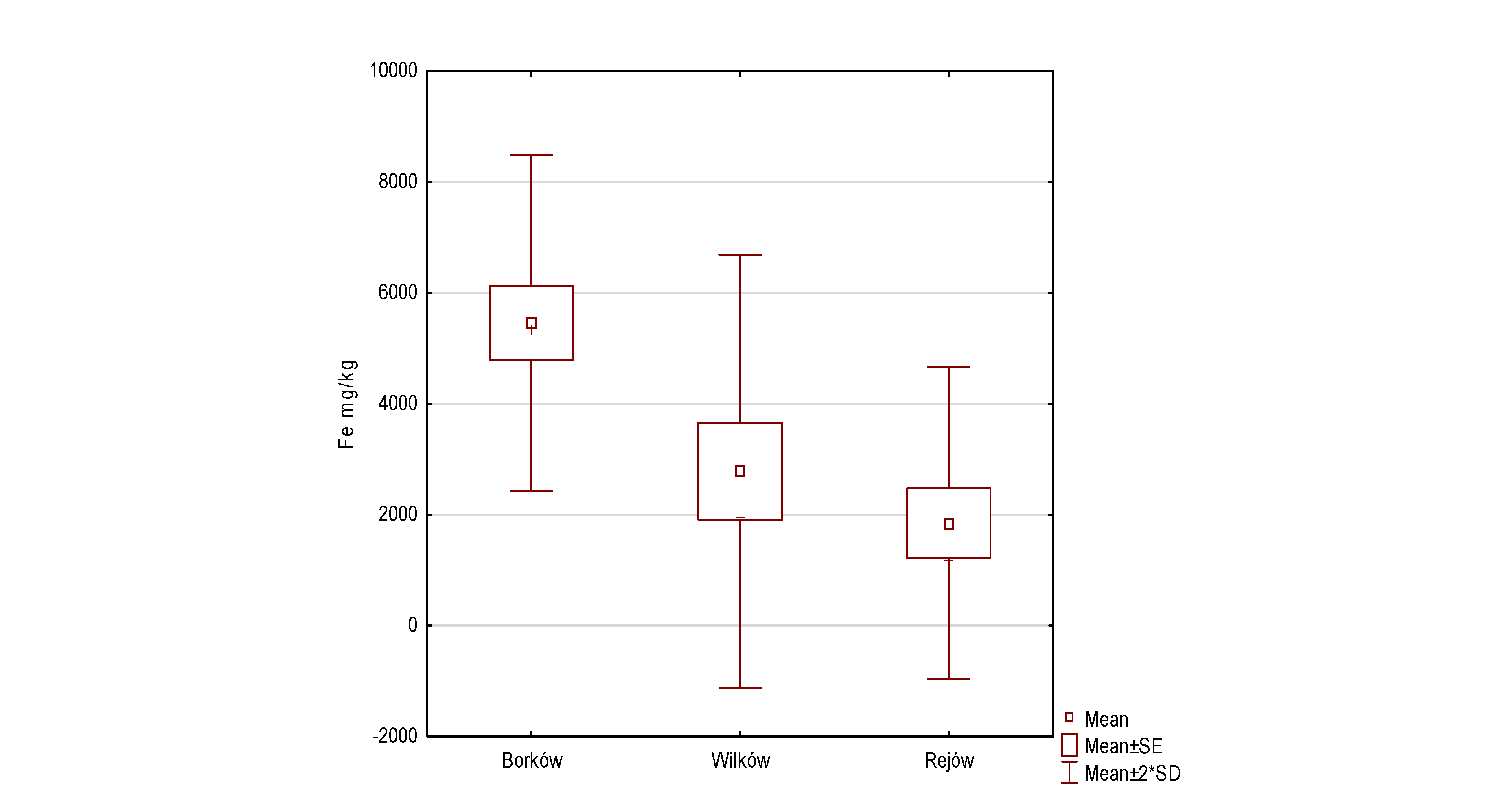
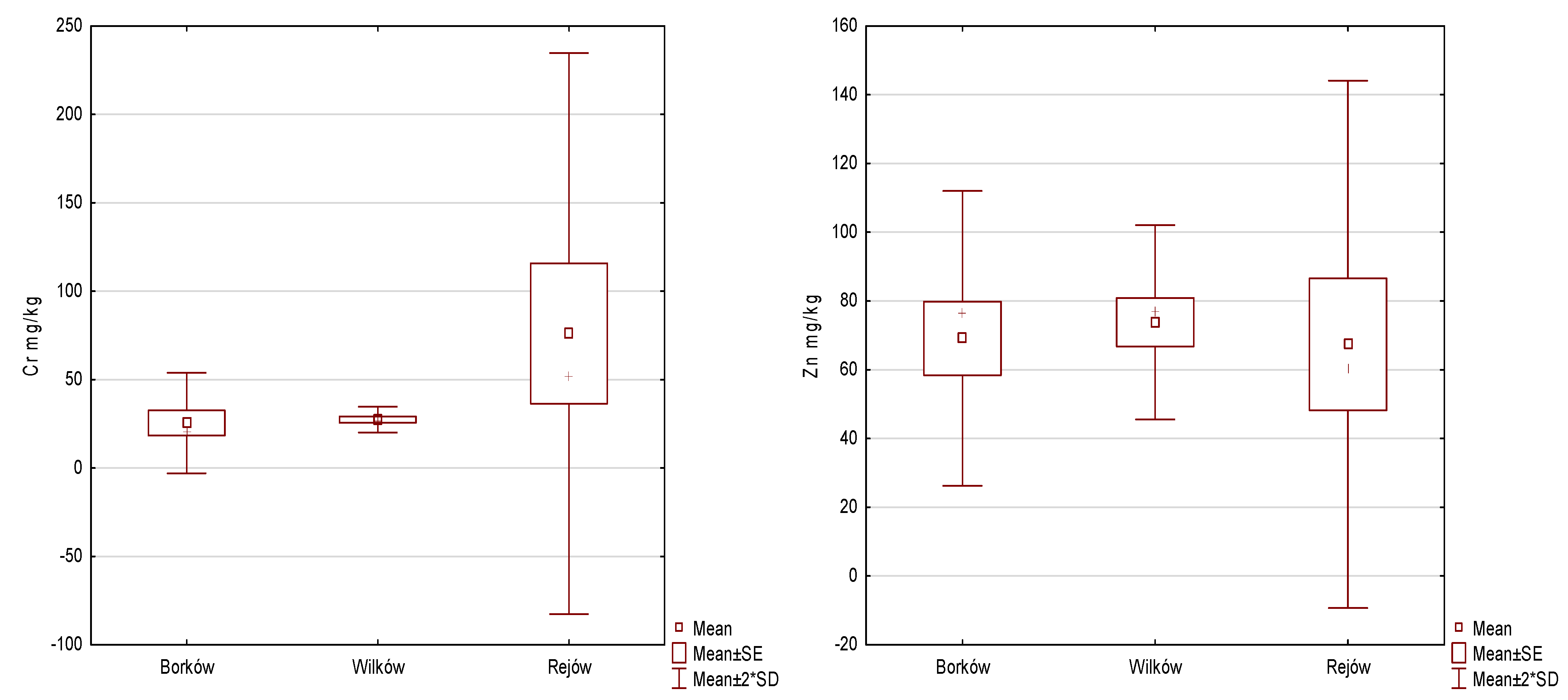
- The analyses showed that the accumulation of trace elements in the surface layer of reservoir sediments increased in the following order in Borków: Cd < Co < Ni < Cu < Pb < Cr < Zn < Mn < Fe; Wilków: Cd < Co < Cu < Ni < Pb < Cr < Zn < Mn < Fe; and Rejów: Cd < Co = Cu = Ni = Pb < Zn < Cr < Mn < Fe, respectively.
- It was indicated that the average distribution of metals in the bottom sediments of the studied reservoirs was as follows Borków > Wilków > Rejów.
- The concentration of Mn, Fe, Pb, Zn (Wilków and Rejów), Cu (Wilków and Rejów), Ni (Rejów) in the bottom sediments of reservoirs was at levels below the geochemical background. This indicates their origin from geogenic sources. Increased values of Cr, Cd, Co, Ni (Borków and Wilków), Cu (Borków), and Zn (Borków) indicate the supply of these elements from anthropogenic sources.
- The values of Igeo, CF, and PLI indices indicate low contamination of the bottom sediments of reservoirs with heavy metals.
- The content of PTEs in the macrophytes of reservoirs can be arranged in ascending order in Borków: Cd < Co < Pb < Cu < Ni < Cr < Zn < Mn < Fe; Wilków: Cd < Co < Pb < Cu < Ni < Cr < Zn < Mn < Fe; and Rejów: Cd < Co = Pb < Cu < Ni < Zn < Cr < Fe < Mn.
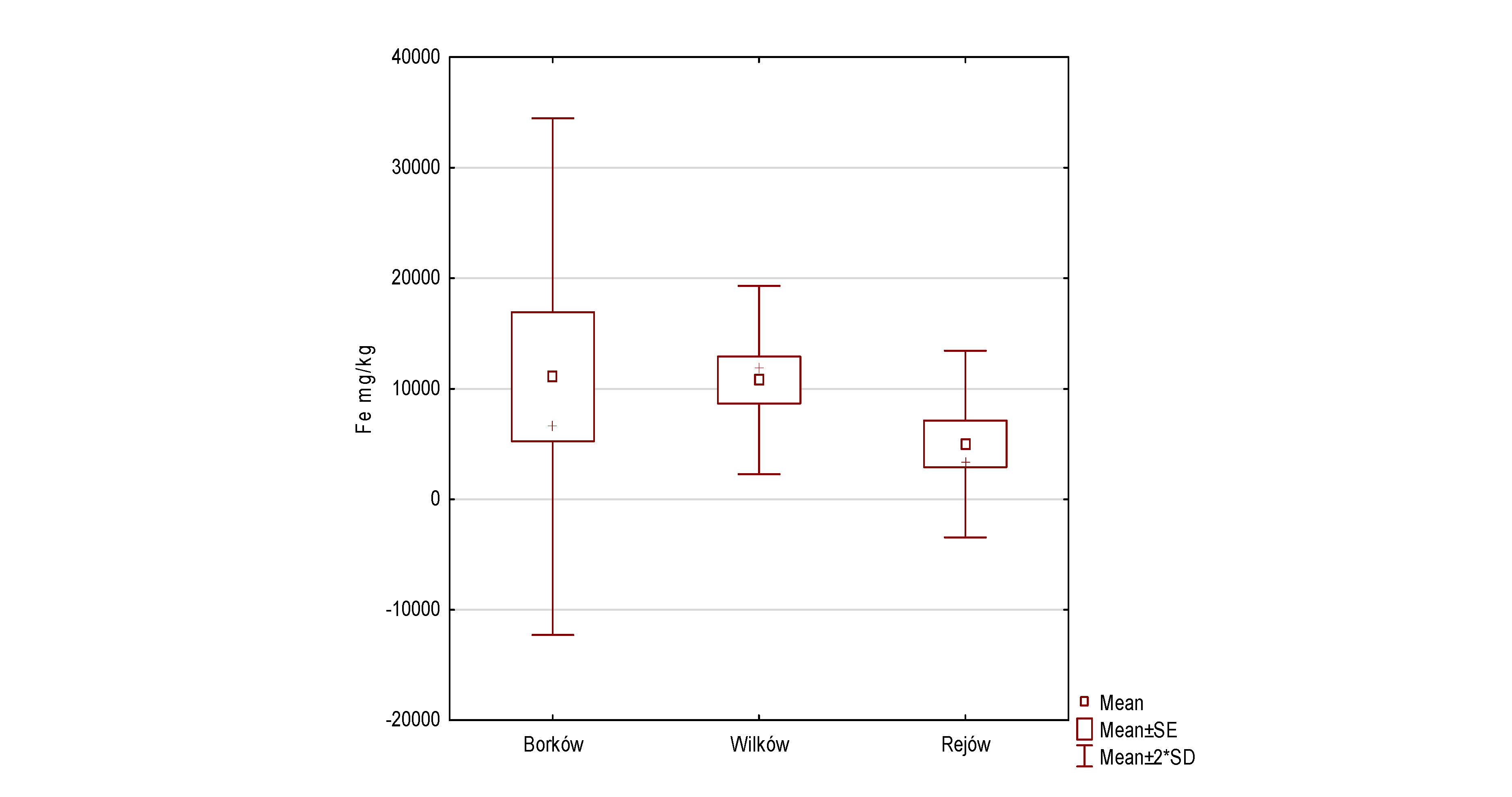
- The metal content of all reservoirs shows significant differences between water, sediment, and macrophytes. The study shows that trace metal accumulation rates in each of the reservoirs selected for the study increase as follows: water < sediment < macrophytes (except for Pb in the Borków reservoir).
- Aquatic plants are an effective barrier to surface water, accumulating heavy metals in their biomass. Macrophytes such as Phragmites australis and Typha latifolia L., among others, are proposed for monitoring reservoirs pollution.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Durán Valsero, J.J.; Morales García, R.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Ardila, P.A.R.; Alonso, R.Á.; Valsero, J.J.D.; García, R.M.; Cabrera, F.Á.; Cosío, E.L.; Laforet, S.D. Assessment of heavy metal pollution in marine sediments from southwest of Mallorca island, Spain. Env. Sci. Pollut. Res. 2023, 30, 16852–16866. [Google Scholar] [CrossRef] [PubMed]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Reston, VA, USA, 1992; Volume 254, p. 263. [Google Scholar]
- Hood, A.P. Trace Elements in Soils; John Wiley and Sons Ltd.: New York, NY, USA, 2010. [Google Scholar]
- Kalicki, T.; Kozłowski, R.; Przepióra, P.; Szwed, M. The geochemical diversity of lacustrine sediments of the Suchedniów water reservoir (Świętokrzyskie voivodeship, Poland)—Preliminary results. Acta Geobalcanica 2021, 7, 159–165. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Qu, H.; Zhao, W.; Duan, L.; Zhang, Y.; Zhou, Y.; Yu, G. The Influence of Nanoplastics on the Toxic Effects, Bioaccumulation, Biodegradation and Enantioselectivity of Ibuprofen in Freshwater Algae Chlorella Pyrenoidosa. Environ. Pollut. 2020, 263, 114593. [Google Scholar] [CrossRef]
- Przybylska, J.; Kozłowski, R. Pierwiastki śladowe w wodach wybranych rzek Gór Świętokrzyskich (Trace elements in water from selected rivers in the Świętokrzyskie Mountains). Monit. Sr. Przyr. 2018, 20, 61–69. [Google Scholar]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Int. Sch. Res. Not. Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Nawrot, N.; Wojciechowska, E.; Mohsin, M.; Kuittinen, S.; Pappinen, A.; Rezania, S. Trace Metal Contamination of Bottom Sediments: A Review of Assessment Measures and Geochemical Background Determination Methods. Minerals 2021, 11, 872. [Google Scholar] [CrossRef]
- Kulbat, E.; Sokołowska, A. Methods of Assessment of Metal Contamination in Bottom Sediments (Case Study: Straszyn Lake, Poland). Arch. Environ. Contam. Toxicol. 2019, 77, 605–618. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A. Macrophytes as passive bioindicators of trace element pollution in the aquatic environment. Wiley Interdiscip. Rev. Water 2022, 10, e1630. [Google Scholar] [CrossRef]
- Kluska, M.; Jabłońska, J. Variability and Heavy Metal Pollution Levels in Water and Bottom Sediments of the Liwiec and Muchawka Rivers (Poland). Water 2023, 15, 2833. [Google Scholar] [CrossRef]
- Germ, M.; Golob, A.; Zelnik, I.; Klink, A.; Polechońska, L. Contents of Metals in Sediments and Macrophytes Differed between the Locations in an Alpine Lake Revealing Human Impacts—A Case Study of Lake Bohinj (Slovenia). Water 2023, 15, 1254. [Google Scholar] [CrossRef]
- Polechońska, L.; Klink, A.; Dambiec, M.; Rudecki, A. Evaluation of Ceratophyllum Demersum as the Accumulative Bioindicator for Trace Metals. Ecol. Indic. 2018, 93, 274–281. [Google Scholar] [CrossRef]
- Ali, M.B.; Tripathi, R.D.; Rai, U.N.; Pal, A.; Singh, S.P. Physico-chemical characteristics and pollution level of lake Nainital (U.P., India): Role of macrophytes and phytoplankton in biomonitoring and phytoremediation of toxic metal ions. Chemosphere 1999, 39, 2171–2182. [Google Scholar] [CrossRef]
- Shah, A.B.; Rai, U.N.; Singh, R.P. Correlations between Some Hazardous Inorganic Pollutants in the Gomti River and Their Accumulation in Selected Macrophytes under Aquatic Ecosystem. Bull. Environ. Contam. Toxicol. 2015, 94, 783–790. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements; Polish Scientific Publishing Company: Warsaw, Poland, 1999; Volume 2, ISBN 0849315751. [Google Scholar]
- Bonanno, G.; Borg, J.A.; Di Martino, V. Levels of heavy metals in wetland and marine vascular plants and their biomonitoring potential: A comparative assessment. Sci. Total Environ. 2017, 576, 796–806. [Google Scholar] [CrossRef]
- Nour, H.E.; El-Sorogy, A.S.; Abdel-Wahab, M.; Almadani, S.; Alfaifi, H.; Youssef, M. Assessment of sediment quality using different pollution indicators and statistical analyses, Hurghada area, Red Sea coast. Egypt. Mar. Pollut. Bull. 2018, 133, 808–813. [Google Scholar] [CrossRef]
- Oladele, A.H.; Umaru, J.; Andy, N. Assessment of metals in sediments and Typha latifolia of Zobe Reservoir, Nigeria. J. Energy Nat. Resour. Manag. (JENRM) 2022, 8, 63–68. [Google Scholar] [CrossRef]
- Klink, A.; Macioł, A.; Wisłocka, M.; Krawczyk, J. Metal accumulation and distribution in the organs of Typha latifolia L. (cattail) and their potential use in bioindication. Limnologica 2013, 43, 164–168. [Google Scholar] [CrossRef]
- Świercz, A.; Tomczyk-Wydrych, I.; Bąk, Ł. Quality of Bottom Sediments of Sołtmany Lake (Masurian Lake District, Poland) in the Light of Geochemical and Ecotoxicological Criteria—Case Study. Water 2022, 14, 2045. [Google Scholar] [CrossRef]
- Sojka, M.; Jaskuła, J.; Barabach, J.; Ptak, M.; Zhu, S. Heavy metals in lake surface sediments in protected areas in Poland: Concentration, pollution, ecological risk, sources and spatial distribution. Sci Rep. 2022, 12, 15006. [Google Scholar] [CrossRef] [PubMed]
- Sałata, A.; Bąk, Ł.Z.; Chmielowski, K.; Rabajczyk, A. Metal pollution of sediments in small water reservoirs in the Kielce Highland (South Eastern Poland). Arch. Environ. Prot. 2019, 45, 12–21. [Google Scholar] [CrossRef]
- Tarnawski, M.; Baran, A.; Koniarz, T.; Wyrębek, M.; Grela, J.; Piszczek, M.; Koroluk, A. The possibilities of the environmental use of bottom sediments from the silted inlet zone of the Rożnów Reservoir. Geol. Geophys. Environ. 2017, 43, 335–344. [Google Scholar] [CrossRef]
- Lee, P.K.; Kang, M.J.; Yu, S.; Ko, K.S.; Ha, K.; Shin, S.C.; Park, J.H. Enrichment and geochemical mobility of heavy metals in bottom sediment of the Hoedong reservoir, Korea and their source apportionment. Chemosphere 2017, 184, 74–85. [Google Scholar] [CrossRef]
- Baran, A.; Tarnawski, M.; Koniarz, T. Spatial distribution of trace elements and ecotoxicity of bottom sediments in Rybnik reservoir, Silesian-Poland. Environ. Sci. Pollut. Res. Int. 2016, 23, 17255–17268. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; He, J.; Lü, C.; Ren, L.; Fan, Q.; Wang, J.; Xie, Z. Distribution characteristics and potential ecological risk assessment of heavy metals (Cu, Pb, Zn, Cd) in water and sediments from Lake Dalinouer, China. Ecotoxicol. Environ. Saf. 2013, 93, 135–144. [Google Scholar] [CrossRef]
- Skorbiłowicz, E.; Skorbiłowicz, M.; Malinowska, D. Accumulation of heavy metals in organs of aqueous plants and its association with bottom sediments in Bug river (Poland). J. Ecol. Eng. 2016, 17, 295–303. [Google Scholar] [CrossRef]
- Chabukdhara, M.; Nema, A.K. Assessment of heavy metal contamination in Hindon River sediments: A chemometric and geochemical approach. Chemosphere 2012, 87, 945–953. [Google Scholar] [CrossRef]
- Krivokapić, M. Study on the Evaluation of (Heavy) Metals in Water and Sediment of Skadar Lake (Montenegro), with BCF Assessment and Translocation Ability (TA) by Trapa natans and a Review of SDGs. Water 2021, 13, 876. [Google Scholar] [CrossRef]
- Keser, G.; Topak, Y.; Sevgiler, Y. Concentrations of some heavy metal and macroelements in sediment, water, macrophyte species, and leech (Hirudo sulukii n. sp.) from the Kara Lake, Adiyaman, Turkey. Environ. Monit. Assess. 2020, 192, 75. [Google Scholar] [CrossRef]
- Flefel, H.; Nokhrin, D.; Donnik, I. Determine heavy metals in water, aquatic plants, and sediment in water systems. E3S Web of Conf. 2020, 222, 02028. [Google Scholar] [CrossRef]
- Kastratovic, V.; Jacimovic, Z.; Bigović, M.; Djurovic, D.; Krivokapic, S. The distribution and accumulation of chromium in the water, sediment and macrophytes of Skadar lake. Kragujev. J. Sci. 2016, 38, 125–134. [Google Scholar] [CrossRef]
- Richling, A.; Solon, J.; Macias, A.; Balon, J.; Borzyszkowski, J.; Kistowski, M. (Eds.) Regionalna Geografia Fizyczna Polski: Praca Zbiorowa; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2021. [Google Scholar]
- Decyzja Starosty Powiatowego w Kielcach znak: RO-II.6341.117.2013.JM z dn. 29.08.2013 o udzieleniu pozwolenia wodnoprawnego na piętrzenie i retencjonowanie wód rzeki Czarna Nida dla potrzeb istniejącego zbiornika wodnego Borków, gm. Daleszyce oraz o zatwierdzenie instrukcji gospodarowania wodą dla ww. zbiornika (Decision of the District Head in Kielce, reference number: RO-II.6341.117.2013.JM, of August 29, 2013, on granting a water-law permit for damming and retaining the waters of the Czarna Nida River for the needs of the existing Borków water reservoir, Daleszyce commune and on approving the water management instructions for the reservoir).
- Bąk, Ł. Zamulenie i osady małych zbiorników retencyjnych na Wyżynie Kieleckiej (Silting and Bottom Sediments of Small Water Reservoirs in the Kielce Upland); Monografie, Studia, Rozprawy nr M96; Wydawnictwo Politechniki Świętokrzyskiej: Kielce, Poland, 2017. [Google Scholar]
- Przepióra, P. Subatlantyckie przemiany zlewni Kamionki na Wyżynie Kieleckiej (Subatlantic Transformations of the Kamionka Catchment in the Kielce Upland). In Geografia i Geoarcheologia Tom I; Kalicki, T., Ed.; Monografie; Ośrodek Badań Europy Środkowo–Wschodniej: Białystok, Poland, 2021; Available online: https://www.researchgate.net/profile/Pawel-Przepiora/publication/351249183_Subatlantyckie_przemiany_zlewni_Kamionki_na_Wyzynie_Kieleckiej_The_Subatlantic_Transformations_of_the_Kamionka_Catchment_in_the_Kielce_Upland/links/63f3c64b0cf1030a563a062f/Subatlantyckie-przemiany-zlewni-Kamionki-na-Wyzynie-Kieleckiej-The-Subatlantic-Transformations-of-the-Kamionka-Catchment-in-the-Kielce-Upland.pdf (accessed on 22 October 2024).
- PN-EN 15933:2013-02E; Osady ściekowe, uzdatnione bioodpady oraz gleba–Oznaczanie pH [Soil, treated bio-waste and sewage sludge–Determination of pH]. Polski Komitet Normalizacyjny: Warszawa, Poland, 2013.
- Folk, R.L.; Ward, W.C. A Study in the Significance of Grain-Size Parameters. J. Sediment. Petrol. 1957, 27, 3–26. [Google Scholar] [CrossRef]
- Müller, G. Index of geoaccumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Bojakowska, I. Criteria for evaluation of water sediments pollution. Pol. Geol. Rev. 2001, 49, 213–219. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z. Geochemical background—An environmental perspective. Mineralogia 2012, 42, 7–17. [Google Scholar] [CrossRef]
- Sojka, M.; Kałuża, T.; Siepak, M.; Strzeliński, P. Zawartość metali ciężkich w osadach dennych śródleśnych zbiorników wodnych. Sylwan 2019, 163, 694–704. [Google Scholar]
- Kozłowski, R.; Przybylska, J.; Szwed, M. Chemizm małych rzek wyżynnych na przykładzie Gór Świętokrzyskich. Proc. ECOpole 2016, 10, 667–675. [Google Scholar]
- Ciupa, T. Wpływ zagospodarowania terenu, w tym urbanizacji, na stężenie głównych jonów w wodach rzeki Silnicy i Sufragańca (Kielce). Ochr Środow I Zasob Nat. 2009, 38, 44–53. [Google Scholar]
- Górski, J.; Bąk, Ł.; Szeląg, B. Jakość wody i osadów dennych zbiornika wodnego Lubianka w Starachowicach. Proc. ECOpole 2016, 10, 635–643. [Google Scholar]
- Bąk, Ł.; Górski, J.; Sałata, A.; Gawdzik, J.; Muszyńska, J.; Górska, K. Ecological risk assessment and spatial distribution of heavy metals in surface sediments. A case study of two small storage reservoirs, SE Poland. Desalination Water Treat. 2020, 186, 206–215. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2001. [Google Scholar]
- Otte, M.L.; Jacob, D.L. Chemical fingerprinting of plants from contrasting wetlands–salt marsh, geothermal and mining impacted. Phyton 2005, 45, 303–316. [Google Scholar]
- Greger, M.; Kautsky, L. Use of macrophytes for mapping bioavailable heavy metals in shallow coastal areas, Stockholm, Sweden. Appl. Geochem. 1993, 8, 37–43. [Google Scholar] [CrossRef]
- Regulation of the Minister of Infrastructure of June 25, 2021 on the Classification of Ecological Status, Ecological Potential and Chemical Status and the Method of Classifying the Status of Surface Water Bodies, as Well as Environmental Quality Standards for Priority Substances (Journal of Laws 2021 item 1475). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20210001475 (accessed on 22 October 2024).
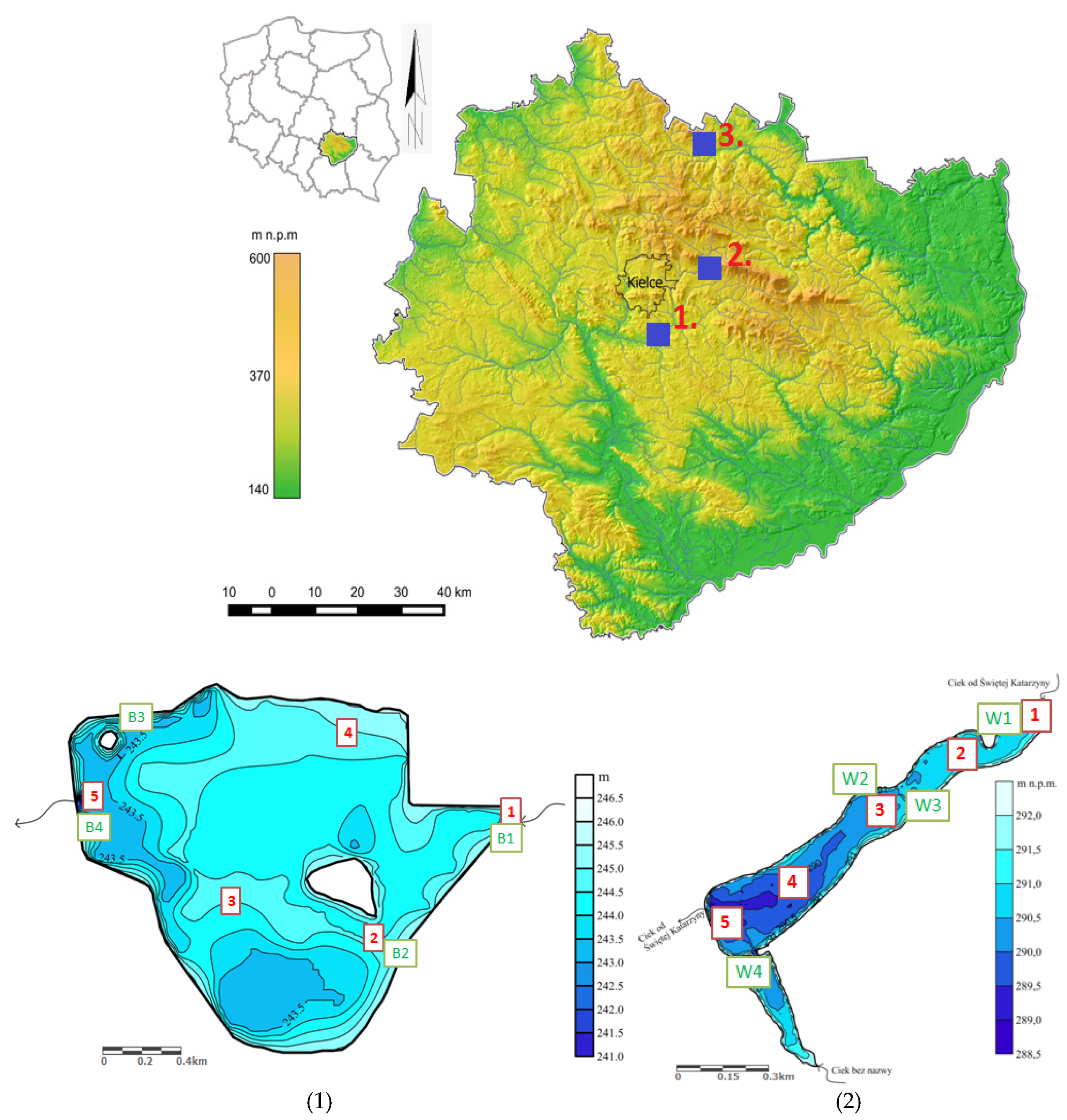
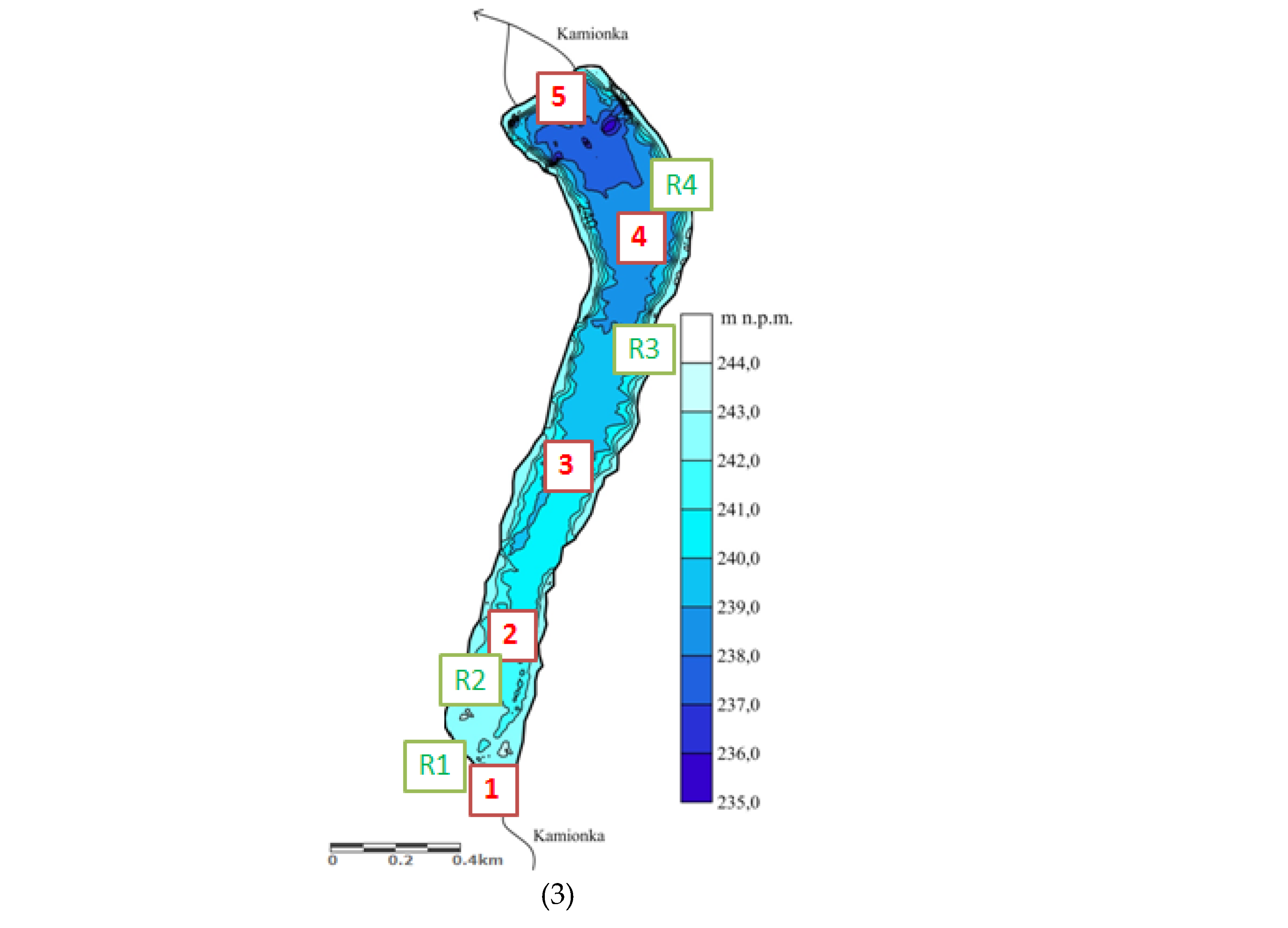




| Reservoir | Administrative Location | Year of Construction of the Dam Reservoir | Reservoir Area [ha] | Depth [m] | Catchment Area | Catchment Development [%] | Sources of Pollution Located Above Structures |
|---|---|---|---|---|---|---|---|
| Borków | Kielce district, Daleszyce commune | 1971 | 36 35.7 | avg. 2 m; max. 4 m | Zlewnia Belnianki (Czarna Nida) | agricultural areas (57.63%), forest areas (37.21%), built-up areas (5.12%) [7] | Sewage treatment plant in Daleszyce (Czarna Nida receiver); Daleszyce sewage treatment plant (Kakonianka receiver), Bieliny sewage treatment plant (Belnianka receiver), main source of chemical pressures: development of urban areas: transport, tourism, urban runoff; agriculture, industrial sewage discharge points. |
| Wilków | Kielce district, Bodzentyn commune | 2004 | 10.4 | avg. 1.57 m; max. 3.0 m | Dopływ ze Św. Katarzyny, (Ciek od Psar, Ciek od Zagórza) | forest areas (44.3%); agricultural areas (44.2%), grasslands (7.9%), loose construction (2.5%), industrial/commercial zones (1.1%) [37] | Sewage treatment plant in Św. Katarzyna (Lubrzanka receiver), agriculture. |
| Rejów | Skarżysko district, Skarżysko-Kamienna commune | 1939 | 30 | avg. 1.5 m; max. 7 m | Kamionka | forest areas (59%); agricultural areas (31.2%), meadows (4.8%), other (5%) [24]. | Sewage treatment plant in Kamionki (Kamionka receiver); Sewage treatment plant in Suchedniów (Kamionka receiver); industrial, municipal and domestic sewage discharge points. |
| Igeo Value | Class | Pollution Degree |
|---|---|---|
| <0 | 0 | Unpolluted |
| 0–1 | 1 | Unpolluted to moderately polluted |
| 1–2 | 2 | Moderately polluted |
| 2–3 | 3 | Moderately to highly polluted |
| 3–4 | 4 | Highly polluted |
| 4–5 | 5 | Highly to very highly polluted |
| >5 | 6 | Very highly polluted |
| CF | Class | Pollution Degree |
|---|---|---|
| <1 | 1 | Low contamination |
| 1 ≤ CF < 3 | 2 | Moderate contamination |
| 3 ≤ CF < 6 | 3 | Considerable contamination |
| ≤6 | 4 | Very high contamination |
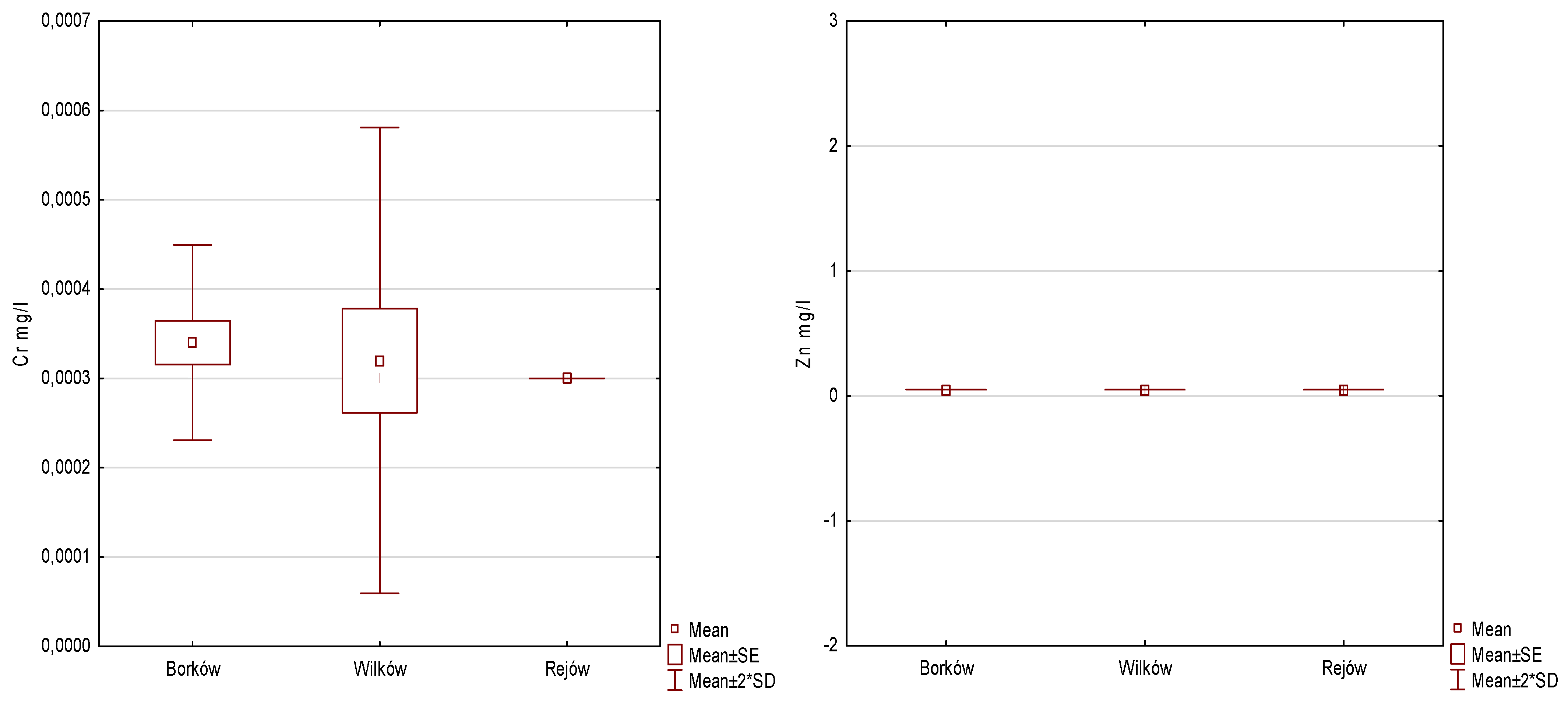
| PTEs [mg/L] | Borków (1) | Wilków (2) | Rejów (3) | Anova | Post Hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | df | p | ||
| Cr | 0.0003 | 0.0000 | 0.0003 | 0.0001 | 0.0003 | 0.0000 | 1.004 | 2 | 0.605 | - |
| Zn | 0.0500 | 0.0000 | 0.0500 | 0.0000 | 0.0500 | 0.0000 | 0.000 | 2 | 1.000 | - |
| Cd | 0.0001 | 0.0000 | 0.0002 | 0.0001 | 0.0001 | 0.0000 | 4.286 | 2 | 0.117 | - |
| Mn | 0.0050 | 0.0000 | 0.1400 | 0.0000 | 0.0112 | 0.0091 | 12.614 | 2 | 0.002 | 1 < 2, 1 < 3, 2 > 3 |
| Cu | 0.0027 | 0.0007 | 0.0028 | 0.0004 | 0.0020 | 0.0005 | 6.098 | 2 | 0.047 | 1 > 3, 2 > 3 |
| Ni | 0.0018 | 0.0018 | 0.0014 | 0.0005 | 0.0010 | 0.0000 | 2.041 | 2 | 0.360 | - |
| Pb | 0.0016 | 0.0009 | 0.0020 | 0.0000 | 0.0020 | 0.0000 | 2.500 | 2 | 0.287 | - |
| Fe | 0.2638 | 0.0450 | 0.4030 | 0.1761 | 0.1474 | 0.0692 | 6.980 | 2 | 0.031 | 2 > 1, 2 > 3, 1 > 3 |
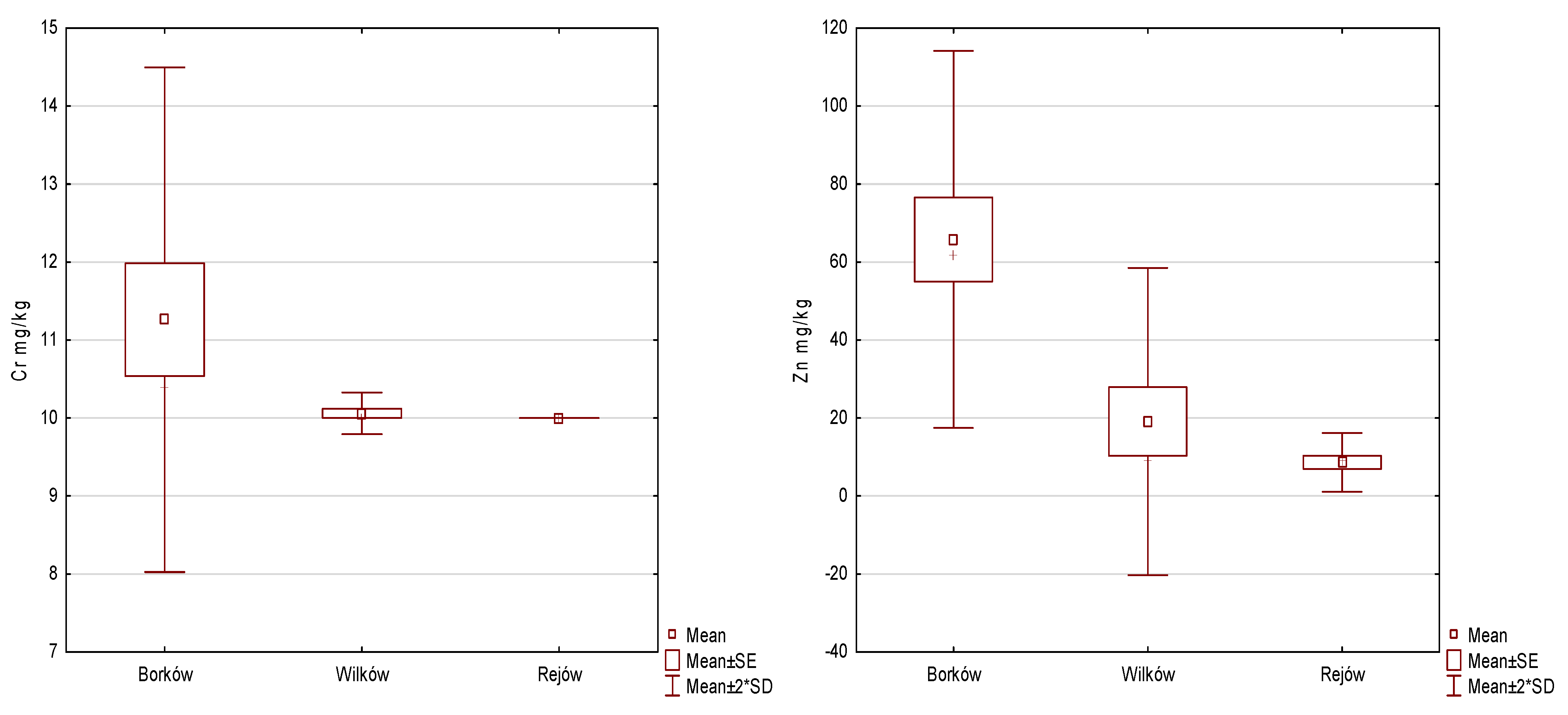
| Metal [mg/kg] | Borków (1) | Wilków (2) | Rejów (3) | Anova | Post Hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | df | p | ||
| Cr | 11.26 | 1.62 | 10.06 | 0.13 | 10.00 | 0.00 | 5.139 | 2 | 0.077 | - |
| Zn | 65.82 | 24.18 | 19.10 | 19.72 | 8.62 | 3.77 | 8.555 | 2 | 0.014 | 1 > 2, 1 > 3 |
| Cd | 1.12 | 0.18 | 1.02 | 0.04 | 1.00 | 0.00 | 2.647 | 2 | 0.266 | - |
| Co | 5.00 | 0.00 | 5.00 | 0.00 | 5.00 | 0.00 | 0.000 | 2 | 1.000 | - |
| Mn | 170.60 | 56.00 | 140.88 | 50.34 | 52.52 | 14.66 | 9.893 | 2 | 0.007 | 1 > 3, 2 > 3 |
| Cu | 6.72 | 1.77 | 5.38 | 0.85 | 5.00 | 0.00 | 6.911 | 2 | 0.032 | 1 > 3 |
| Ni | 6.16 | 1.77 | 5.42 | 0.94 | 5.00 | 0.00 | 2.299 | 2 | 0.317 | - |
| Pb | 8.66 | 2.69 | 5.74 | 1.65 | 5.00 | 0.00 | 10.411 | 2 | 0.005 | 1 > 2, 1 > 3 |
| Fe | 5456.60 | 1516.06 | 2780.00 | 1955.05 | 1844.80 | 1406.41 | 8.060 | 2 | 0.018 | 1 > 2, 1 > 3 |
| Metal [mg/kg] | Borków (1) | Wilków (2) | Rejów (3) | Anova | Post Hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | df | p | ||
| Cr | 25.48 | 14.21 | 27.33 | 3.63 | 76.05 | 79.37 | 1.660 | 2 | 0.436 | - |
| Zn | 69.13 | 21.44 | 73.85 | 14.12 | 67.40 | 38.35 | 0.808 | 2 | 0.668 | - |
| Cd | 3.08 | 3.31 | 2.65 | 0.66 | 1.25 | 0.50 | 5.126 | 2 | 0.077 | - |
| Co | 5.00 | 0.00 | 5.00 | 0.00 | 5.00 | 0.00 | 0.000 | 2 | 1.000 | - |
| Mn | 1501.75 | 1269.70 | 2307.75 | 1470.72 | 1287.75 | 1521.24 | 2.000 | 2 | 0.368 | - |
| Cu | 8.03 | 0.90 | 11.03 | 1.62 | 11.85 | 7.25 | 4.699 | 2 | 0.095 | - |
| Ni | 13.58 | 5.60 | 17.00 | 2.10 | 29.23 | 19.35 | 2.192 | 2 | 0.334 | - |
| Pb | 6.25 | 1.48 | 10.85 | 3.94 | 5.00 | 0.00 | 5.309 | 2 | 0.070 | - |
| Fe | 11,089.5 | 11,687.97 | 10,793.25 | 4259.45 | 5004.50 | 4223.45 | 3.500 | 2 | 0.174 | - |
| Metal [mg/kg] | Water (1) | Bottom Sediment (2) | Macrophytes (3) | Anova | Post Hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | df | p | ||
| Cr | 0.0003 | 0.0000 | 11.26 | 1.62 | 25.48 | 14.21 | 11.752 | 2 | 0.003 | 3 > 1, 3 > 2 |
| Zn | 0.0500 | 0.0000 | 65.82 | 24.18 | 69.13 | 21.44 | 9.414 | 2 | 0.009 | 2 > 1, 3 > 1 |
| Cd | 0.0001 | 0.0000 | 1.12 | 0.18 | 3.08 | 3.31 | 10.468 | 2 | 0.005 | 2 > 1,3 > 1 |
| Co | - | - | 5.00 | 0.00 | 5.00 | 0.00 | 0.000 | 1 | 1.000 | - |
| Mn | 0.0050 | 0.0000 | 170.60 | 56.00 | 1501.75 | 1269.70 | 10.732 | 2 | 0.005 | 2 > 1, 3 > 1 |
| Cu | 0.0027 | 0.0007 | 6.72 | 1.77 | 8.03 | 0.90 | 9.685 | 2 | 0.008 | 2 > 1, 3 > 1 |
| Ni | 0.0018 | 0.0018 | 6.16 | 1.77 | 13.58 | 5.60 | 11.435 | 2 | 0.003 | 2 > 1, 3 > 1 |
| Pb | 0.0016 | 0.0009 | 8.66 | 2.69 | 6.25 | 1.48 | 10.036 | 2 | 0.007 | 2 > 1, 3 > 1 |
| Fe | 0.2638 | 0.0450 | 5456.60 | 1516.06 | 11,089.5 | 11,687.97 | 9.103 | 2 | 0.011 | 2 > 1, 3 > 1 |
| Metal [mg/L; mg/kg] | Water (1) | Bottom Sediments (2) | Macrophytes (3) | Anova | Post Hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | df | p | ||
| Cr | 0.0003 | 0.0001 | 10.06 | 0.13 | 27.33 | 3.63 | 11.858 | 2 | 0.003 | 2 > 1, 3 > 1, 3 > 2 |
| Zn | 0.0500 | 0.0000 | 19.10 | 19.72 | 73.85 | 14.12 | 12.103 | 2 | 0.002 | 2 > 1, 3 > 1, 3 > 2 |
| Cd | 0.0002 | 0.0001 | 1.02 | 0.04 | 2.65 | 0.66 | 11.939 | 2 | 0.003 | 2 > 1, 3 > 1, 3 > 2 |
| Co | - | - | 5.00 | 0.00 | 5.00 | 0.00 | 0.000 | 1 | 1.000 | - |
| Mn | 0.1400 | 0.0000 | 140.88 | 50.34 | 2307.75 | 1470.72 | 12.103 | 2 | 0.002 | 2 > 1, 3 > 1, 3 > 2 |
| Cu | 0.0028 | 0.0004 | 5.38 | 0.85 | 11.03 | 1.62 | 11.831 | 2 | 0.003 | 2 > 1, 3 > 1, 3 > 2 |
| Ni | 0.0014 | 0.0005 | 5.42 | 0.94 | 17.00 | 2.10 | 11.966 | 2 | 0.003 | 2 > 1, 3 > 1, 3 > 2 |
| Pb | 0.0020 | 0.0000 | 5.74 | 1.65 | 10.85 | 3.94 | 11.249 | 2 | 0.004 | 2 > 1, 3 > 1, 3 > 2 |
| Fe | 0.4030 | 0.1761 | 2780.00 | 1955.05 | 10,793.25 | 4259.45 | 11.083 | 2 | 0.004 | 2 > 1, 3 > 1, 3 > 2 |
| Metal [mg/kg] | Water (1) | Sediment (2) | Macrophytes (3) | Anova | Post Hoc | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | F | df | p | ||
| Cr | 0.0003 | 0.0000 | 10.00 | 0.00 | 76.05 | 79.37 | 11.883 | 2 | 0.003 | 3 > 1 |
| Zn | 0.0500 | 0.0000 | 8.62 | 3.77 | 67.40 | 38.35 | 12.131 | 2 | 0.002 | 2 > 1, 3 > 2 |
| Cd | 0.0001 | 0.0000 | 1.00 | 0.00 | 1.25 | 0.50 | 11.875 | 2 | 0.003 | 2 > 1,3 > 1 |
| Co | - | - | 5.00 | 0.00 | 5.00 | 0.00 | 0.000 | 1 | 1.000 | - |
| Mn | 0.0112 | 0.0091 | 52.52 | 14.66 | 1287.75 | 1521.24 | 11.571 | 2 | 0.003 | 2 > 1, 3 > 1 |
| Cu | 0.0020 | 0.0005 | 5.00 | 0.00 | 11.85 | 7.25 | 12.103 | 2 | 0.002 | 3 > 1 |
| Ni | 0.0010 | 0.0000 | 5.00 | 0.00 | 29.23 | 19.35 | 12.687 | 2 | 0.002 | 2 > 1, 3 > 1, 3 > 2 |
| Pb | 0.0020 | 0.0000 | 5.00 | 0.00 | 5.00 | 0.00 | 13.000 | 2 | 0.002 | 2 > 1, 3 > 1 |
| Fe | 0.1474 | 0.0692 | 1844.80 | 1406.41 | 5004.50 | 4223.45 | 10.260 | 2 | 0.006 | 2 > 1, 3 > 1, 3 > 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świercz, A.; Tomczyk-Wydrych, I. Assessment of Water Status, Bottom Sediments, Macrophytes in the Light of Index Analysis and Geochemical Parameters of Selected Dam Reservoirs of Kielce Upland (Poland). Water 2024, 16, 3072. https://doi.org/10.3390/w16213072
Świercz A, Tomczyk-Wydrych I. Assessment of Water Status, Bottom Sediments, Macrophytes in the Light of Index Analysis and Geochemical Parameters of Selected Dam Reservoirs of Kielce Upland (Poland). Water. 2024; 16(21):3072. https://doi.org/10.3390/w16213072
Chicago/Turabian StyleŚwiercz, Anna, and Ilona Tomczyk-Wydrych. 2024. "Assessment of Water Status, Bottom Sediments, Macrophytes in the Light of Index Analysis and Geochemical Parameters of Selected Dam Reservoirs of Kielce Upland (Poland)" Water 16, no. 21: 3072. https://doi.org/10.3390/w16213072
APA StyleŚwiercz, A., & Tomczyk-Wydrych, I. (2024). Assessment of Water Status, Bottom Sediments, Macrophytes in the Light of Index Analysis and Geochemical Parameters of Selected Dam Reservoirs of Kielce Upland (Poland). Water, 16(21), 3072. https://doi.org/10.3390/w16213072








