Mitigation of Fluoride Contamination in Drinking Water Supply Sources by Adsorption Using Bone Char: Effects of Mineral and Organic Matrix
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Study Area
2.2. Determination of Some Parameters (Ions, pH, and Dissolved Organic Carbon)
- Ions: Analyses for main and trace ions in the sampled water, which included Chloride (Cl−), Sulfate (SO42−), Nitrate (NO3−), Nitrite (NO2−), Fluoride (F−), Phosphate (PO43−), Bromide (Br−), Sodium (Na+), Potassium (K+), Calcium (Ca2+), Magnesium (Mg2+), Aluminum (Al3+), and Ferrous (Fe2+), were performed by means of ion chromatography (Metrohm IC, ECO IC combined with 863 Autosampler, MetroSep A supp 19-150/4.0 column, Swiss).
- pH: It was determined using pH meter (Mettler Toledo FEP20).
- Dissolved Organic Carbon (DOC): It was measured in accordance with the procedures of LCK 385 on Total Organic Carbon after the filtration of respective samples using filter paper (0.45 μm). Briefly, the water sample was filtered to remove solid particles. Next, the sample was acidified using sulfuric acid (1 M) to a pH below 2 to convert inorganic carbon and CO2 to ensure that it is removed during analysis. Next, 2 mL of the sample was placed in pre-dosed reagents (sodium persulfate and phosphoric acid) from the cuvette kit. The cuvette was then heated to 100 °C for 2 h to oxidize the organic carbon to CO2. After that, the cuvette was allowed to cool at room temperature. After cooling, the cuvette was purged with air for approximately 10 min to remove any CO2 generated from inorganic carbon. Finally, the cuvette was placed in a Hach spectrophotometer (DR 2800) and the DOC concentration (mg/L) was read in after selecting the LCK 385 method.
2.3. Preparation of Bone Char (BC)
2.4. Instrumental Characterization of Bone Char
2.5. Adsorption Experiments
3. Results and Discussion
3.1. Parameter Value of Sampled Groundwater
3.2. Characterization of the Prepared Bone Char (BC)
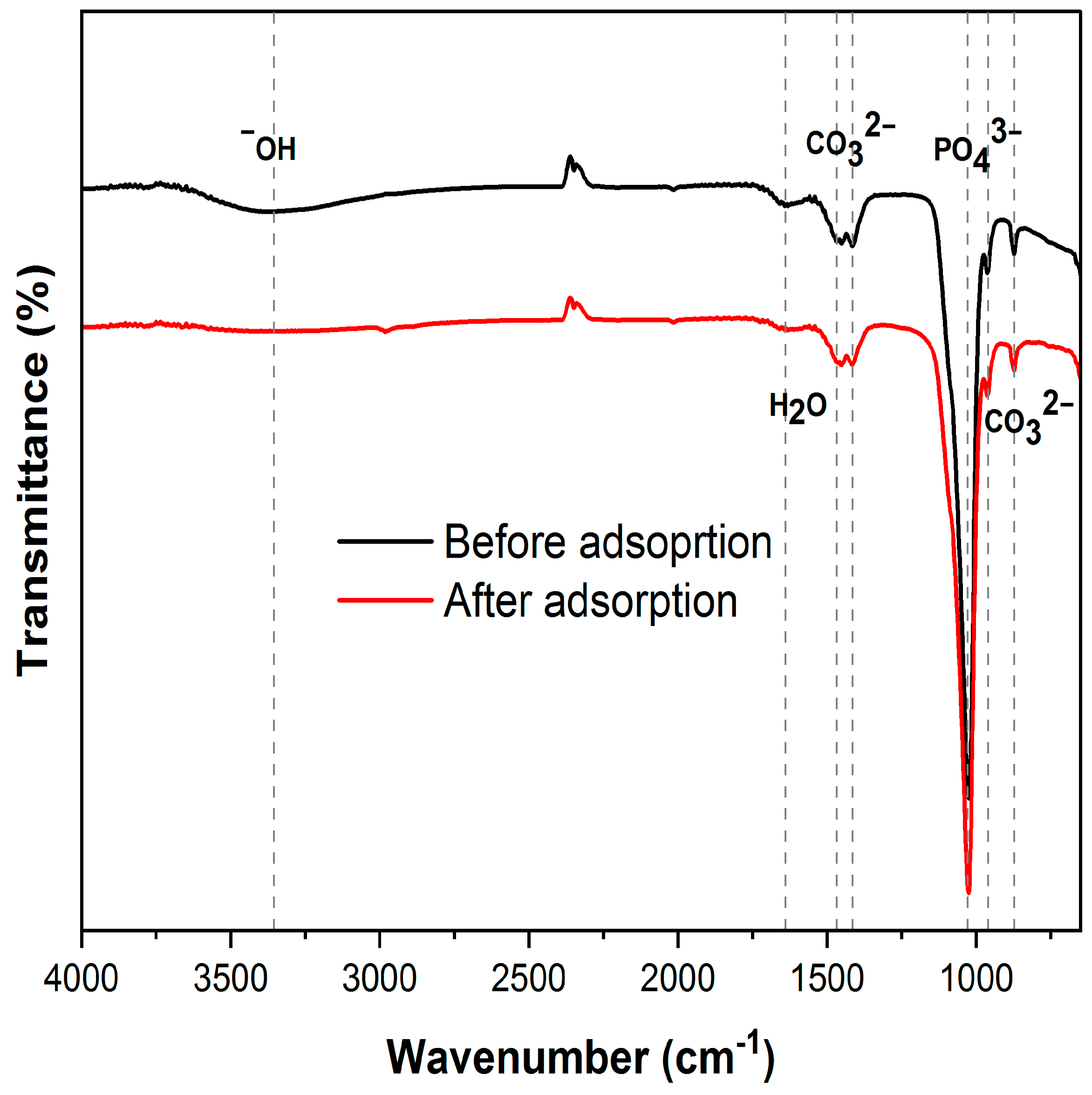
3.2.1. XRD and FTIR Analysis
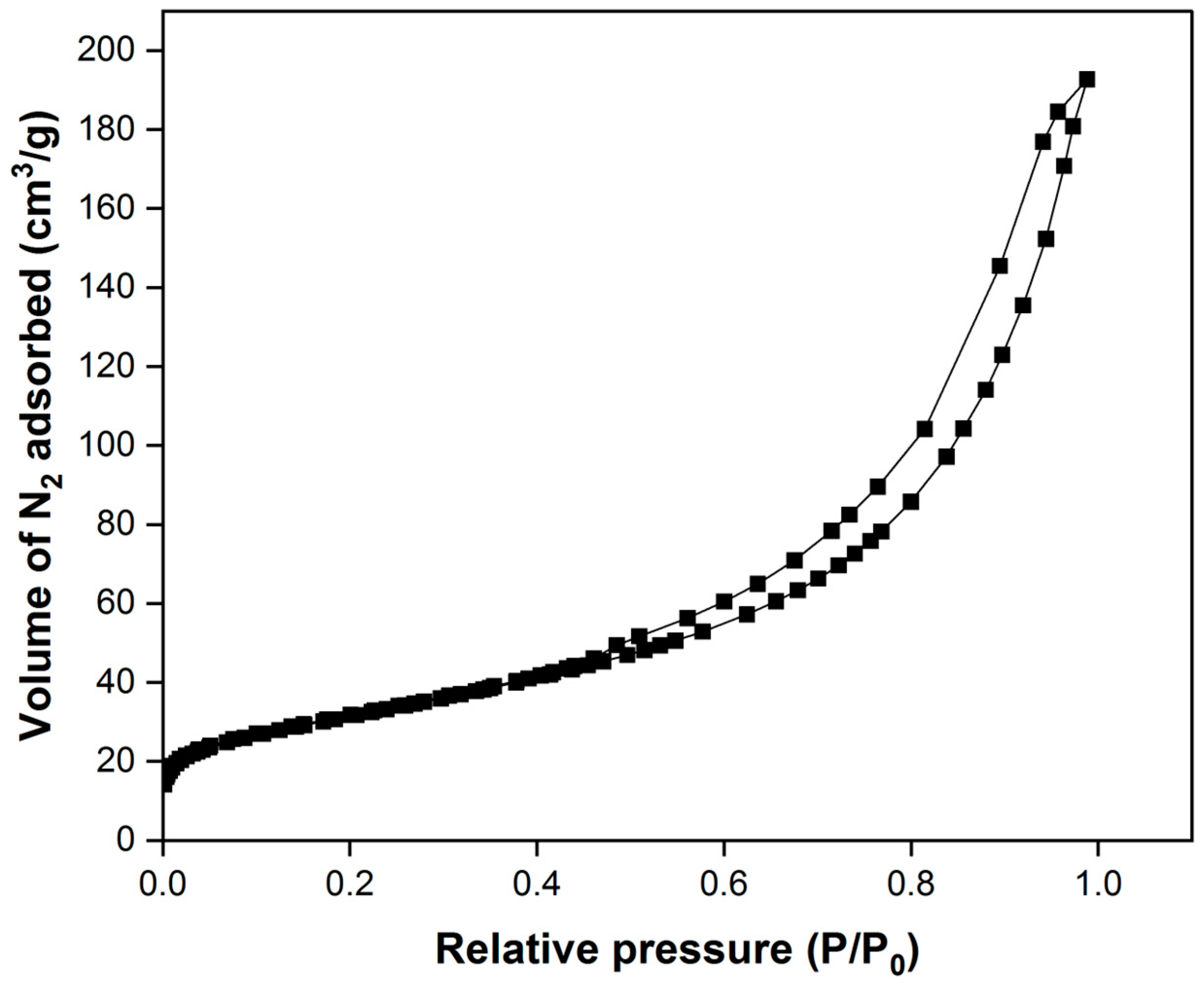
3.2.2. SEM Analysis and BET
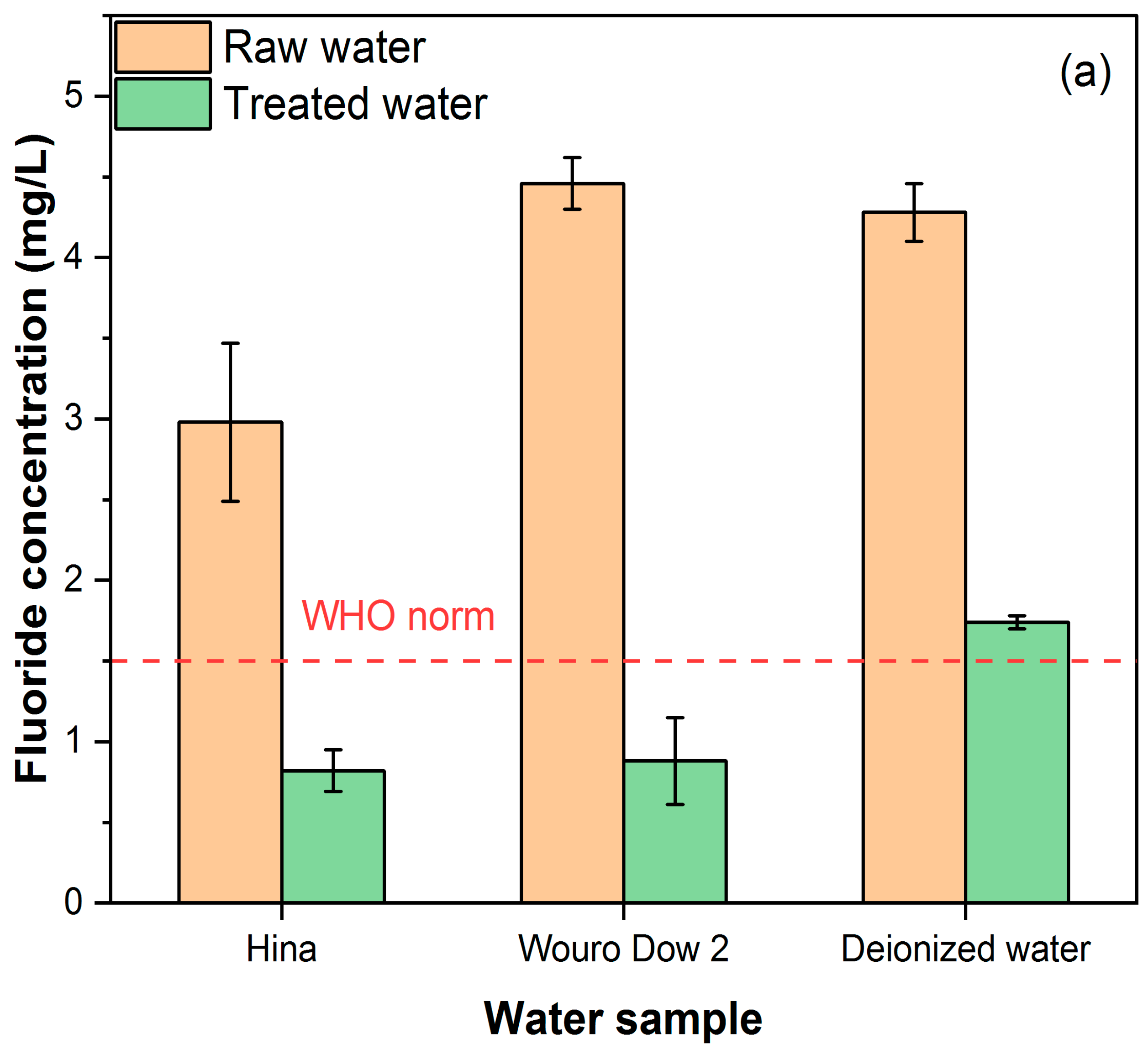
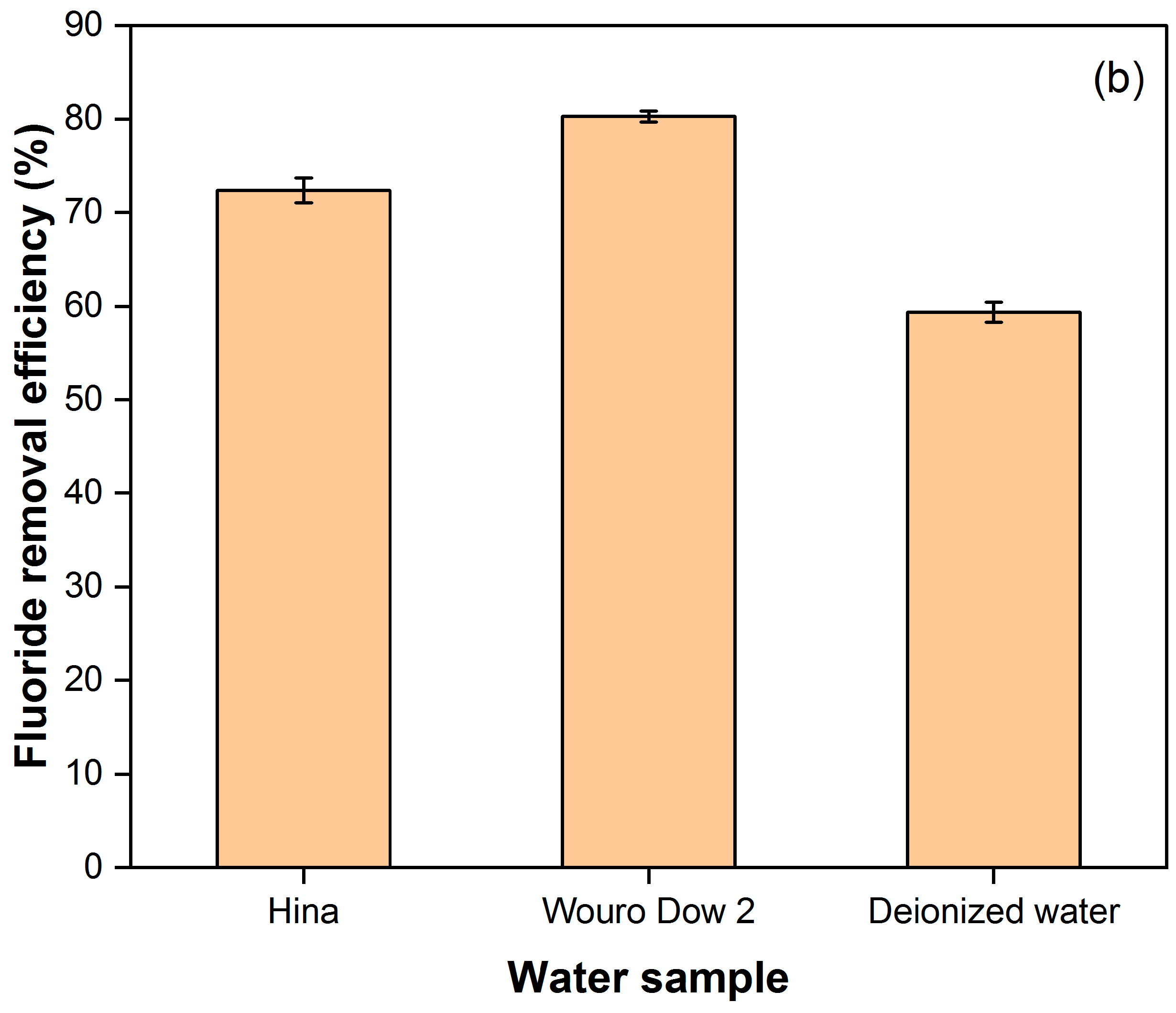
3.3. Fluoride Removal Efficiency of BC from Sampled Water
3.4. Effect of Coexisting Anions and Cations
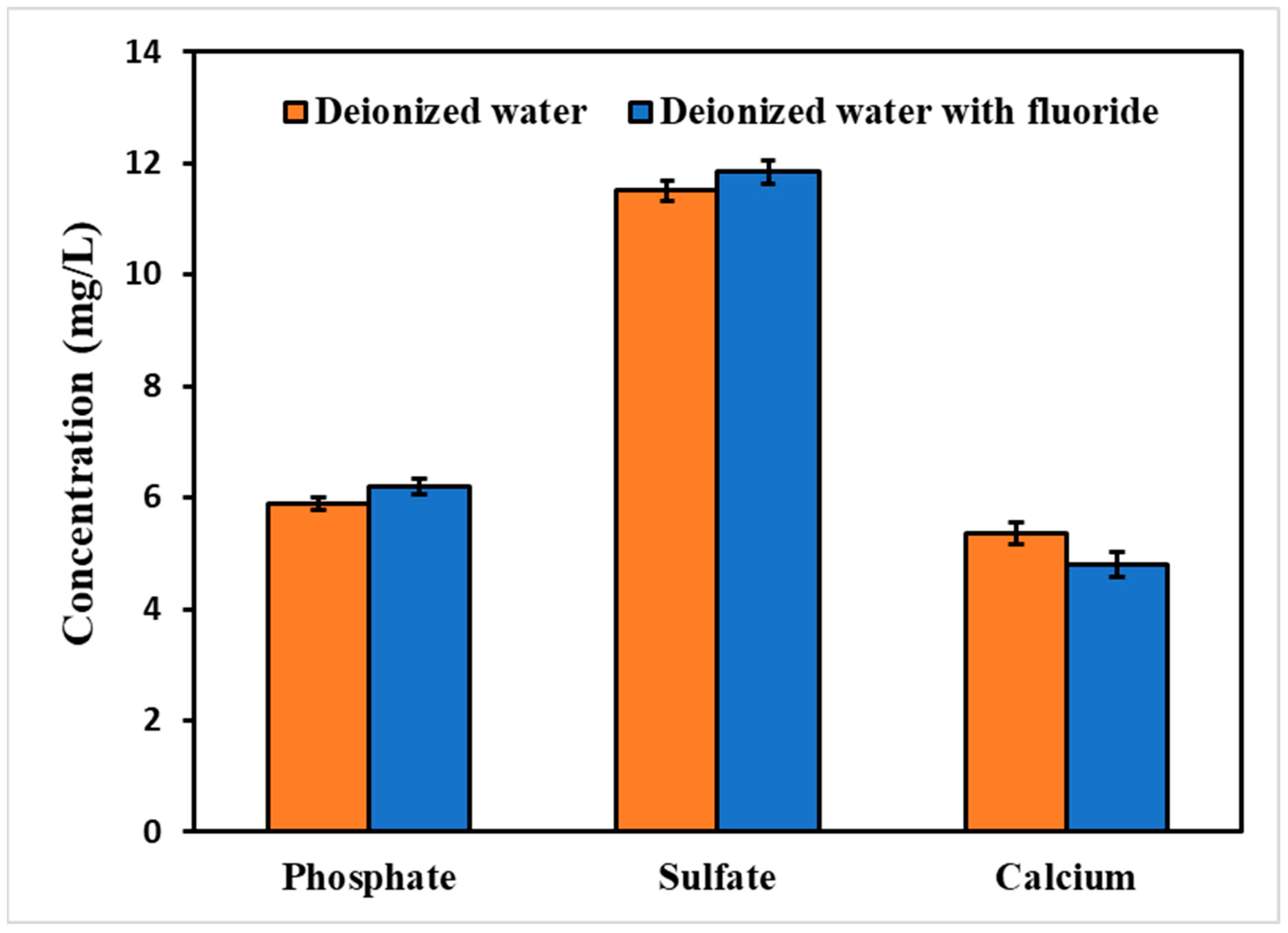
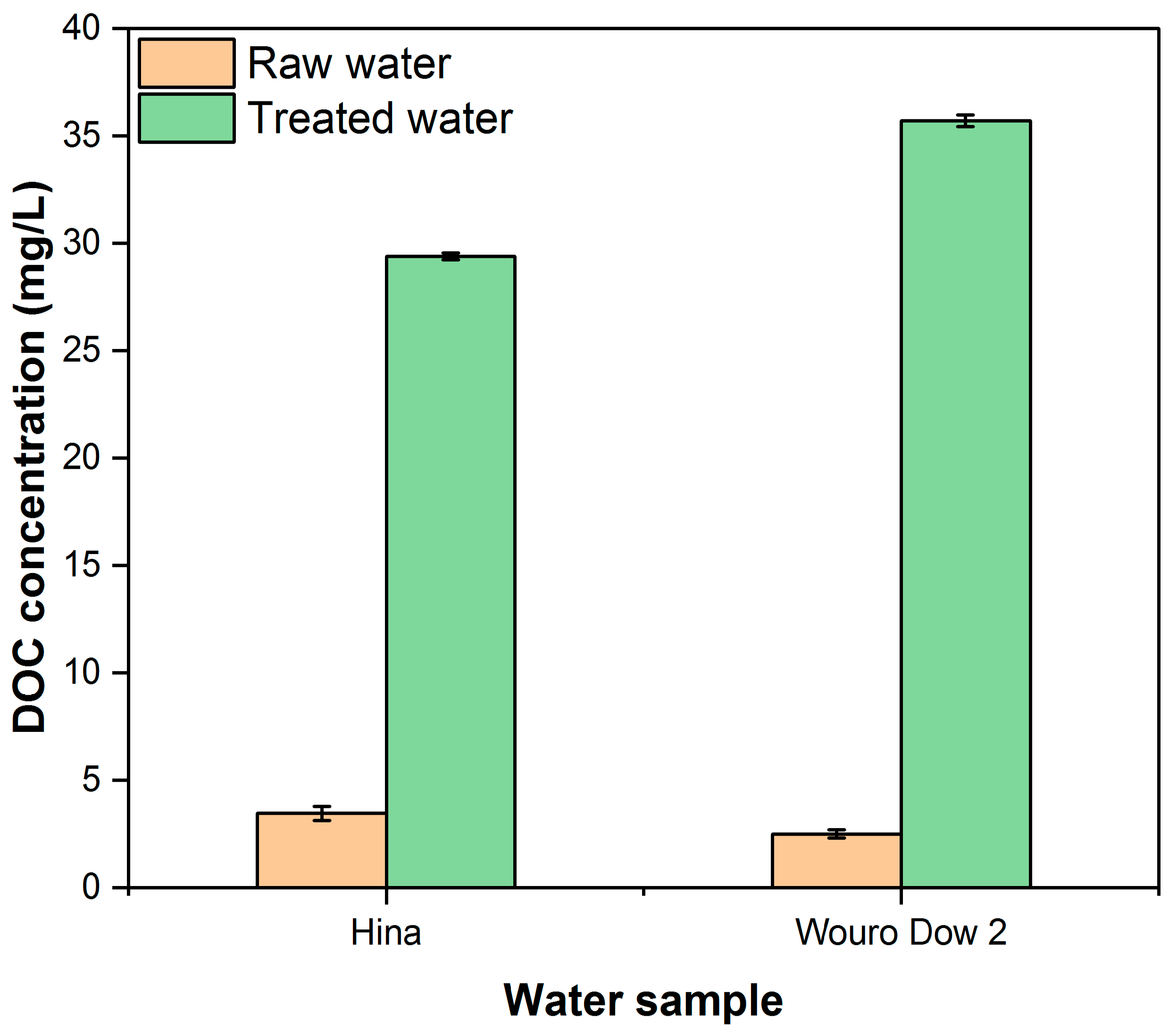
3.5. Effect of Dissolved Organic Carbon (DOC)
3.6. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Premathilaka, R.W.; Liyanagedera, N.D. Fluoride in drinking water and nanotechnological approaches for eliminating excess fluoride. J. Nanotechnol. 2019, 2019, 2192383. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking—Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2011.
- Wang, J.; Xu, W.; Chen, L.; Jia, Y.; Wang, L.; Huang, X.J.; Liu, J. Excellent fluoride removal performance by CeO2–ZrO2 nanocages in water environment. J. Chem. Eng. 2013, 231, 198–205. [Google Scholar] [CrossRef]
- Malago, J.; Makoba, E.; Muzuka, A.N. Fluoride levels in surface and groundwater in Africa: A review. Am. J. Water Sci. Eng. 2017, 3, 1–17. [Google Scholar] [CrossRef]
- Kut, K.M.K.; Sarswat, A.; Srivastava, A.; Pittman, C.U., Jr.; Mohan, D. A review of fluoride in African groundwater and local remediation methods. Groundwater Sustain. Dev. 2016, 2, 190–212. [Google Scholar] [CrossRef]
- Onipe, T.; Edokpayi, J.N.; Odiyo, J.O. A review on the potential sources and health implications of fluoride in groundwater of Sub-Saharan Africa. Environ. Sci. Health Part A 2020, 55, 1078–1093. [Google Scholar] [CrossRef]
- Fantong, W.Y.; Satake, H.; Ayonghe, S.N.; Suh, E.C.; Adelana, S.M.; Fantong, E.B.S.; Banseka, H.S.; Gwanfogbe, C.D.; Woincham, L.N.; Uehara, Y. Geochemical provenance and spatial distribution of fluoride in groundwater of Mayo Tsanaga River Basin, Far North Region, Cameroon: Implications for incidence of fluorosis and optimal consumption dose. Environ. Geochem. Health 2010, 32, 147–163. [Google Scholar] [CrossRef]
- Fantong, W.Y.; Jokam Nenkam, T.L.; Nbendah, P.; Kimbi, S.B.; Fru, E.C.; Kamtchueng, B.T.; Takoundjou, A.F.; Tejiobou, A.R.; Ngueutchoua, G.; Kringel, R. Compositions and mobility of major, δ D, δ 18 O, trace, and REEs patterns in water sources at Benue River Basin—Cameroon: Implications for recharge mechanisms, geo-environmental controls, and public health. Environ. Geochem. Health 2020, 42, 2975–3013. [Google Scholar] [CrossRef]
- Habuda-Stanić, M.; Ravančić, M.E.; Flanagan, A. A review on adsorption of fluoride from aqueous solution. Materials 2014, 7, 6317–6366. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, V.; Khan, S.A.; Kumar, A. A review of emerging adsorbents and current demand for defluoridation of water: Bright future in water sustainability. Environ. Int. 2018, 111, 80–108. [Google Scholar] [CrossRef]
- Alkurdi, S.S.A.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; Marchuk, A. Inorganic arsenic species removal from water using bone char: A detailed study on adsorption kinetic and isotherm models using error functions analysis. J. Hazard. Mater. 2021, 405, 124112. [Google Scholar] [CrossRef]
- Jia, P.; Tan, H.; Liu, K.; Gao, W. Enhanced photocatalytic performance of ZnO/bone char composites. Mater. Lett. 2017, 205, 233–235. [Google Scholar] [CrossRef]
- Ranjbar, N.; Hashemi, S.; Ramavandi, B.; Ravanipour, M. Chromium(VI) removal by bone char–ZnO composite: Parameters optimization by response surface methodology and modeling. Environ. Prog. Sustain. Energy 2018, 37, 1684–1695. [Google Scholar] [CrossRef]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Hamawand, I. Bone char as a green sorbent for removing health threatening fluoride from drinking water. Environ. Int. 2019, 127, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Schäfer, A.I. Factors affecting fluoride and natural organic matter (NOM) removal from natural waters in Tanzania by nanofiltration/reverse osmosis. Sci. Total Environ. 2015, 527, 520–529. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Khiadani, M.; Foroughi, M.; Zolghadr Nasab, H. Defluoridation of water using activated alumina in presence of natural organic matter via response surface methodology. Environ. Sci. Pollut. Res. 2016, 23, 887–897. [Google Scholar] [CrossRef]
- Pagano, T.; Bida, M.; Kenny, J.A. Trends in levels of allochthonous dissolved organic carbon in natural water: A review of potential mechanisms under a changing climate. Water 2014, 6, 2862–2897. [Google Scholar] [CrossRef]
- Wongrueng, A.; Rakruam, P.; Siri, A.; Siyasukh, A. Synthesis of porous pig bone char as adsorbent for removal of DBP precursors from surface water. Water Sci. Technol. 2019, 79, 857–865. [Google Scholar] [CrossRef]
- Brunson, L.R.; Sabatini, D.A. Practical considerations, column studies and natural organic material competition for fluoride removal with bone char and aluminum amended materials in the Main Ethiopian Rift Valley. Sci. Total Environ. 2014, 488, 580–587. [Google Scholar] [CrossRef]
- Sawangjang, B.; Induvesa, P.; Wongrueng, A.; Pumas, C.; Wattanachira, S.; Rakruam, P.; Punyapalakul, P.; Takizawa, S.; Khan, E. Evaluation of fluoride adsorption mechanism and capacity of different types of bone char. Int. J. Environ. Res. Public Health 2021, 18, 6878. [Google Scholar] [CrossRef]
- Chen, J.; Wu, H.; Qian, H.; Gao, J. Assessing nitrate and fluoride contaminants in drinking water and their health risk of rural residents living in a semiarid region of Northwest China. Expo. Health 2017, 9, 183–195. [Google Scholar] [CrossRef]
- Rahman, M.M.; Bodrud-Doza, M.; Siddiqua, M.T.; Zahid, A.; Islam, A.R.M.T. Spatiotemporal distribution of fluoride in drinking water and associated probabilistic human health risk appraisal in the coastal region, Bangladesh. Sci. Total Environ. 2020, 724, 138316. [Google Scholar] [CrossRef] [PubMed]
- Oyetade, J.A.; Machunda, R.L.; Hilonga, A. Investigation of functional performance of treatment systems for textile wastewater in selected textile industries in Tanzania. Water Sci. Technol. 2023, 87, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Bidu, J.M.; Van der Bruggen, B.; Rwiza, M.J.; Njau, K.N. Current status of textile wastewater management practices and effluent characteristics in Tanzania. Water Sci. Technol. 2021, 83, 2363–2376. [Google Scholar] [CrossRef] [PubMed]
- Mouttoucomarassamy, S.; Virk, H.S.; Dharmalingam, S.N. Evaluation and health risk assessment of arsenic and potentially toxic elements pollution in groundwater of Majha Belt, Punjab, India. Environ. Geochem. Health 2024, 46, 208. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.K.; Kim, J.Y.; Shin, G.; Choi, Y. Effect of pyrolysis conditions on characteristics and fluoride adsorptive performance of bone char derived from bone residue. J. Water Process. Eng. 2020, 37, 101499. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Bonilla-Petriciolet, A.; Aguayo-Villarreal, I.A.; Hernandez-Montoya, V.; Moreno-Virgen, M.R.; Tovar-Gómez, V.; Montes-Morán, M.A. Optimization of pyrolysis conditions and adsorption properties of bone char for fluoride removal from water. J. Anal. Appl. Pyrolysis 2013, 104, 10–18. [Google Scholar] [CrossRef]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; McKnight, S. Effect of pyrolysis conditions on bone char characterization and its ability for arsenic and fluoride removal. Environ. Pollut. 2020, 262, 114221. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Choi, Y. Synthesis of bone char from cattle bones and its application for fluoride removal from the contaminated water. Groundwater Sustain. Dev. 2019, 8, 324–331. [Google Scholar] [CrossRef]
- Smolen, D.; Chudoba, T.; Malka, I.; Kedzierska, A.; Lojkowski, W.; Swieszkowski, W.; Kurzydlowski, K.J.; Kolodziejczyk-Mierzynska, M.; Lewandowska-Szumiel, M. Highly biocompatible, nanocrystalline hydroxyapatite synthesized in a solvothermal process driven by high energy density microwave radiation. Int. J. Nanomed. 2013, 8, 653–668. [Google Scholar] [CrossRef]
- Irfan, M.; Suprajaa, P.; Baraneedharan, P.; Reddy, B. A comparative study of nanohydroxyapetite obtained from natural shells and wet chemical process. J. Mater. Sci. Surf. Eng. 2020, 7, 938–943. [Google Scholar]
- Patel, S.; Han, J.; Qiu, W.; Gao, W. Synthesis and characterisation of mesoporous bone char obtained by pyrolysis of animal bones, for environmental application. J. Environ. Chem. Eng. 2015, 3, 2368–2377. [Google Scholar] [CrossRef]
- Obada, D.O.; Osseni, S.A.; Sina, H.; Oyedeji, A.N.; Salami, K.A.; Okafor, E.; Csaki, S.; Abolade, S.A.; Akande, A.; Dauda, M. Hydroxyapatite materials-synthesis routes, mechanical behavior, theoretical insights, and artificial intelligence models: A review. J. Aust. Ceram. Soc. 2023, 59, 565–596. [Google Scholar] [CrossRef]
- Cruz-Briano, S.A.; Medellín-Castillo, N.A.; Torres-Dosal, A.; Leyva-Ramos, R.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Díaz-Flores, P.E.; Reyes-López, S.Y.; Ocampo-Pérez, R. Bone char from an invasive aquatic specie as a green adsorbent for fluoride removal in drinking water. Water Air Soil Pollut. 2021, 232, 346. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Cruz-Briano, S.A.; Leyva-Ramos, R.; Moreno-Piraján, J.C.; Torres-Dosal, A.; Giraldo-Gutiérrez, L.; Labrada-Delgado, G.J.; Pérez, R.O.; Rodriguez-Estupiñan, J.P.; Reyes Lopez, S.Y.; et al. Use of bone char prepared from an invasive species, pleco fish (Pterygoplichthys spp.), to remove fluoride and Cadmium(II) in water. J. Environ. Manag. 2020, 256, 109956. [Google Scholar] [CrossRef] [PubMed]
- Medellin-Castillo, N.A.; Padilla-Ortega, E.; Tovar-García, L.D.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Carrasco-Marín, F.; Berber-Mendoza, M.S. Removal of fluoride from aqueous solution using acid and thermally treated bone char. Adsorption 2016, 22, 951–961. [Google Scholar] [CrossRef]
- Azeem, M.; Shaheen, S.M.; Ali, A.; Jeyasundar, P.G.; Latif, A.; Abdelrahman, H.; Li, R.; Almazroui, M.; Niazi, N.K.; Sarmah, A.K.; et al. Removal of potentially toxic elements from contaminated soil and water using bone char compared to plant-and bone-derived biochars: A review. J. Hazard Mater. 2022, 427, 128131. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Silvestre-Albero, J.; Aguayo-Villarreal, I.A.; Mendoza-Castillo, D.I.; Bonilla-Petriciolet, A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater. 2015, 209, 38–44. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, K.S.; He, J.Y.; Xu, W.H.; Huang, X.J.; Liu, J.H. Enhanced fluoride removal from water by sulfate-doped hydroxyapatite hierarchical hollow microspheres. Chem. Eng. 2016, 285, 616–624. [Google Scholar] [CrossRef]
- Xiang, B.; Tang, J.; Feng, X.; Zhu, Y.; Li, Y.; Tan, T. Preparation of aluminium-hydroxide-modified diatomite and its fluoride adsorption mechanism. Sci. Rep. 2023, 13, 3871. [Google Scholar] [CrossRef]
- Herath, H.A.S.; Kawakami, T.; Tafu, M. The extremely high adsorption capacity of fluoride by chicken bone char (CBC) in defluoridation of drinking water in relation to its finer particle size for better human health. Healthcare 2018, 6, 123. [Google Scholar] [CrossRef]
- Kariuki, S.M.; Ngari, M.S.; Mavura, W.J.; Ollengo, M.S.; Ongoma, P.O. Effect of essential mineral ions from aqueous media on adsorption of fluoride by bone char. J. Environ. Sci. Toxicol. Food Technol. 2015, 9, 9–17. [Google Scholar]
- Kawasaki, N.; Ogata, F.; Tominaga, H.; Yamaguchi, I. Removal of fluoride ion by bone char produced from animal biomass. J. Oleo Sci. 2009, 58, 529–535. [Google Scholar] [CrossRef] [PubMed]
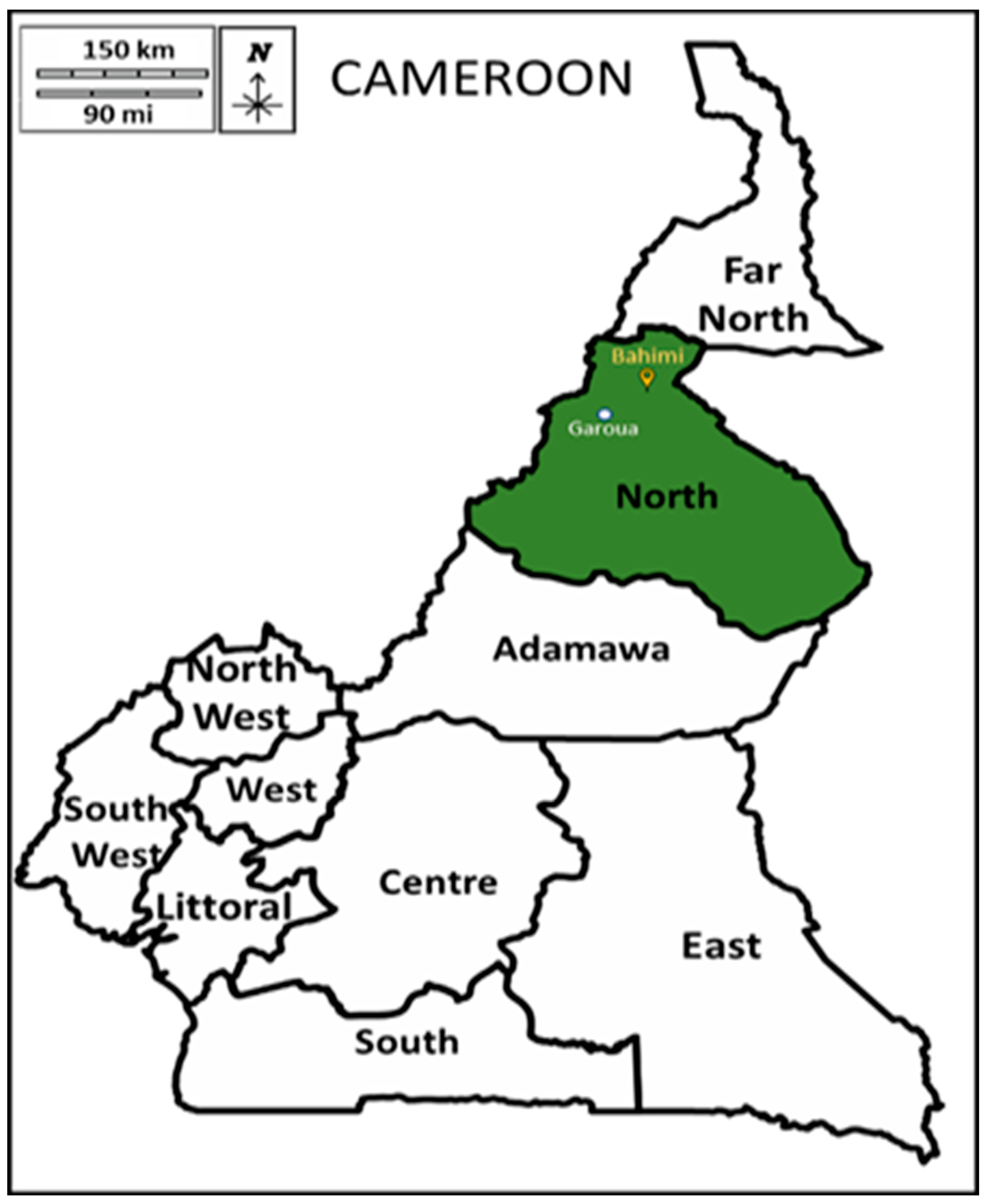
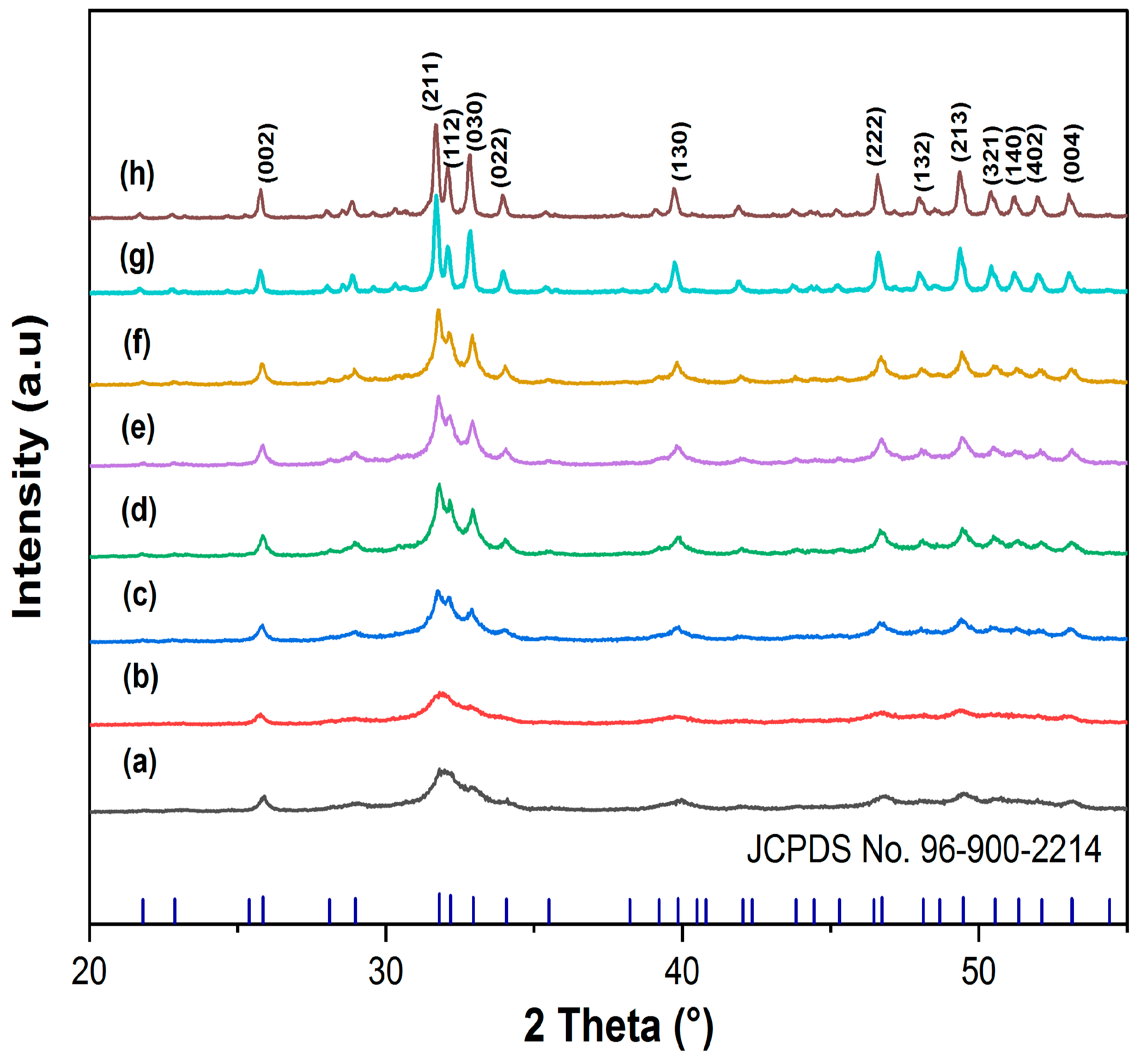
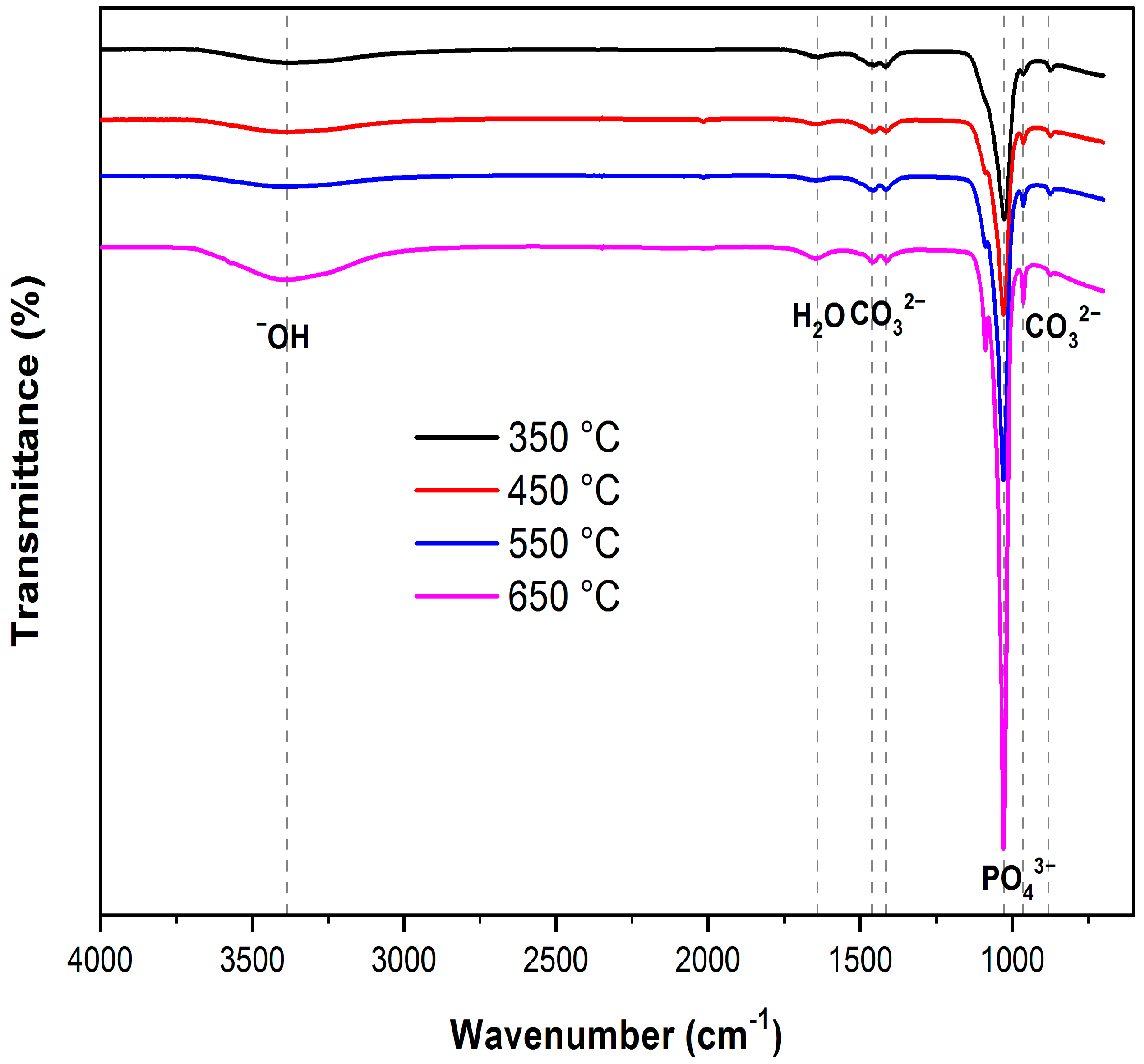
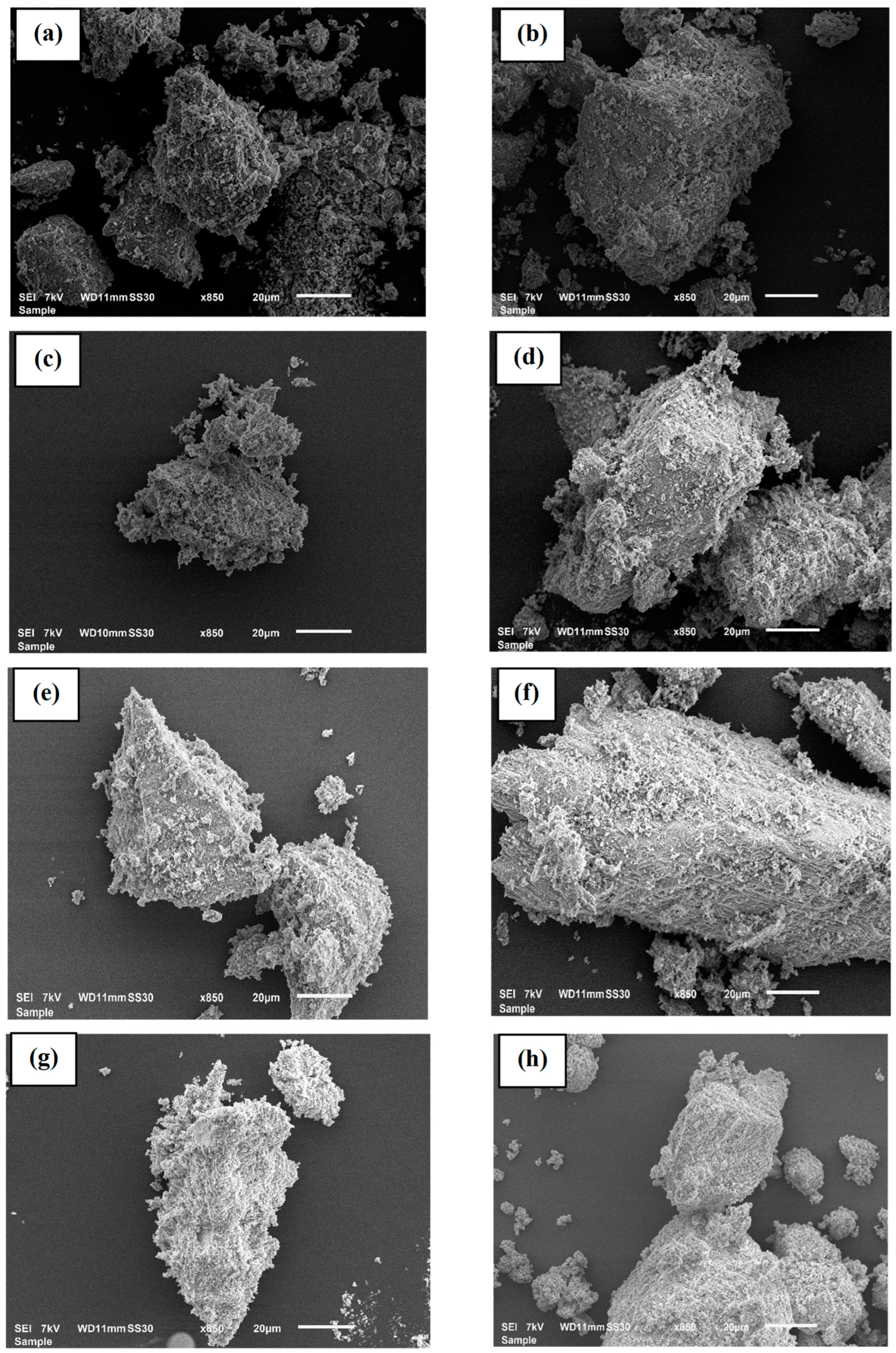
| Samples No | Location | Source of Water | GPS |
|---|---|---|---|
| 1 | Hina | Borehole water | 9.5026050, 13.7682890 |
| 2 | Wouro Dow 1 | Well water | 9.5039300, 13.7675970 |
| 3 | Wouro Dow 2 | Well water | 9.5031810, 13.7672480 |
| 4 | Djaouro Sali | Surface water | 9.5038220, 13.7718340 |
| Parameters | Water Sample | ||||
|---|---|---|---|---|---|
| Hina | Wouro Dow 1 | Wouro Dow 2 | Djaouro Sali | WHO Standards (2011) | |
| pH | 7.8 ± 0.2 | 7.4 ± 0.3 | 8.2 ± 0.1 | 8.12 ± 0.09 | 6.5–8.5 |
| Cl (mg/L) | 15.2 ± 0.4 | 34.0 ± 0.1 | 14.4 ± 0.2 | 38.87 ± 0.05 | 250.0 |
| SO4 (mg/L) | 9.2 ± 0.1 | 23.0 ± 0.3 | 9.8 ± 0.1 | 31.5 ± 0.2 | 250.0 |
| NO3 (mg/L) | 55.7 ± 0.2 | 102.6 ± 0.2 | 57.6 ± 0.6 | 105.7 ± 0.4 | 50.0 |
| NO2 (mg/L) | 1.23 ± 0.07 | 1.00 ± 0.02 | 1.22 ± 0.05 | 0.88 ± 0.03 | 3.0 |
| F (mg/L) | 3.0 ± 0.5 | 3.3 ± 0.2 | 4.5 ± 0.2 | 2.3 ± 0.1 | 1.5 |
| PO4 (mg/L) | BDL | BDL | BDL | BDL | - |
| Br (mg/L) | BDL | BDL | BDL | BDL | - |
| K (mg/L) | 2.04 ± 0.09 | 2.2 ± 0.1 | 1.39 ± 0.06 | 1.7 ± 0.1 | 12.0 |
| Na (mg/L) | 25.2 ± 0.1 | 32.9 ± 0.1 | 29.4 ± 0.2 | 39.7 ± 0.3 | 200.0 |
| Ca (mg/L) | 52.5 ± 0.3 | 60.1 ± 0.2 | 40.93 ± 0.03 | 42.7 ± 0.4 | 75.0 |
| Mg (mg/L) | 21.2 ± 0.5 | 13.6 ± 0.4 | 23.7 ± 0.3 | 14.0 ± 0.2 | 50.0 |
| Al (mg/L) | BDL | BDL | BDL | BDL | - |
| Fe (mg/L) | BDL | BDL | BDL | BDL | - |
| DOC (mg/L) | 3.5 ± 0.3 | 4.3 ± 0.2 | 2.5 ± 0.2 | 5.0 ± 0.2 | - |
| T | Resistance Time (h) | SBET (m2/g) | Vp (cm3/g) | Dp (nm) |
|---|---|---|---|---|
| 350 | 1 | 112.3 ± 0.3 | 0.26 ± 0.03 | 9.3 ± 0.3 |
| 2 | 110.0 ± 0.4 | 0.29 ± 0.06 | 10.7 ± 0.3 | |
| 450 | 1 | 98.6 ± 0.3 | 0.30 ± 0.02 | 12.2 ± 0.6 |
| 2 | 83.2 ± 0.2 | 0.29 ± 0.03 | 13.9 ± 0.1 | |
| 550 | 1 | 69.6 ± 0.2 | 0.23 ± 0.03 | 13.5 ± 0.2 |
| 2 | 54.8 ± 0.4 | 0.26 ± 0.02 | 18.8 ± 0.3 | |
| 650 | 1 | 9.5 ± 0.3 | 0.06 ± 0.03 | 26.7 ± 0.4 |
| 2 | 4.4 ± 0.3 | 0.04 ± 0.02 | 39.9 ± 0.4 |
| Water Source | Deionized Water | Hina Water Sample | Wouro Dow 2 Water Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|
| [X] (mg/L) | Raw | Treated | Remark | Raw | Treated | Remark | Raw | Treated | Remark |
| F− | 4.3 ± 0.2 | 1.74 ± 0.04 | Adsorbed | 3.0 ± 0.5 | 0.8 ± 0.1 | Adsorbed | 4.5 ± 0.2 | 0.9 ± 0.3 | adsorbed |
| Cl− | BDL | 10.92 ± 0.03 | Released | 15.2 ± 0.4 | 23.93 ± 0.08 | Released | 14.4 ± 0.4 | 23.5 ± 0.2 | released |
| SO42− | BDL | 11.8 ± 0.2 | Released | 9.2 ± 0.1 | 15.9 ± 0.2 | Released | 9.8 ± 0.2 | 16.08 ± 0.04 | released |
| NO2− | BDL | BDL | - | 1.23 ± 0.07 | 1.2 ± 0.2 | No change | 1.2 ± 0.2 | 1.2 ± 0.2 | No change |
| NO3− | BDL | BDL | - | 55.7 ± 0.2 | 55.6 ± 0.4 | No change | 57.6 ± 0.6 | 57.8 ± 0.2 | No change |
| PO43− | BDL | 6.3 ± 0.2 | Released | BDL | BDL | - | BDL | BDL | - |
| Water Source | Hina | Wouro Dow 2 | Remark | ||
|---|---|---|---|---|---|
| [X] (mg/L) | Raw Water | Treated Water | Raw Water | Treated Water | |
| K+ | 2.04 ± 0.09 | 3.18 ± 0.1 | 1.39 ± 0.06 | 2.8 ± 0.2 | Released |
| Na+ | 25.2 ± 0.1 | 39.4 ± 0.5 | 29.4 ± 0.2 | 45.3 ± 0.2 | Released |
| Ca2+ | 52.5 ± 0.3 | 41.3 ± 0.2 | 49.93 ± 0.03 | 36.2 ± 0.1 | Adsorbed |
| Mg2+ | 21.2 ± 0.5 | 20.3 ± 0.2 | 23.7 ± 0.4 | 21.6 ± 0.3 | Adsorbed |
| Element | Weight % | Weight % Sigma | Atomic % |
|---|---|---|---|
| C | 12.03 | 0.69 | 21.42 |
| O | 35.55 | 0.71 | 47.52 |
| Na | 0.86 | 0.15 | 0.80 |
| Mg | 0.73 | 0.14 | 0.64 |
| P | 15.88 | 0.50 | 10.96 |
| Ca | 34.94 | 0.86 | 18.65 |
| Total | 100.00 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raoul Ibrahim, M.; Oyetade, J.A.; Dalhatou, S.; Nikiforov, A.; Leys, C.; Hilonga, A. Mitigation of Fluoride Contamination in Drinking Water Supply Sources by Adsorption Using Bone Char: Effects of Mineral and Organic Matrix. Water 2024, 16, 2991. https://doi.org/10.3390/w16202991
Raoul Ibrahim M, Oyetade JA, Dalhatou S, Nikiforov A, Leys C, Hilonga A. Mitigation of Fluoride Contamination in Drinking Water Supply Sources by Adsorption Using Bone Char: Effects of Mineral and Organic Matrix. Water. 2024; 16(20):2991. https://doi.org/10.3390/w16202991
Chicago/Turabian StyleRaoul Ibrahim, Mohamed, Joshua Akinropo Oyetade, Sadou Dalhatou, Anton Nikiforov, Christophe Leys, and Askwar Hilonga. 2024. "Mitigation of Fluoride Contamination in Drinking Water Supply Sources by Adsorption Using Bone Char: Effects of Mineral and Organic Matrix" Water 16, no. 20: 2991. https://doi.org/10.3390/w16202991
APA StyleRaoul Ibrahim, M., Oyetade, J. A., Dalhatou, S., Nikiforov, A., Leys, C., & Hilonga, A. (2024). Mitigation of Fluoride Contamination in Drinking Water Supply Sources by Adsorption Using Bone Char: Effects of Mineral and Organic Matrix. Water, 16(20), 2991. https://doi.org/10.3390/w16202991





