Biodegradation of Cyanide Using Soda Lake-Derived Alkaliphilic Microbial Consortia
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Sample Site and Sample Collection
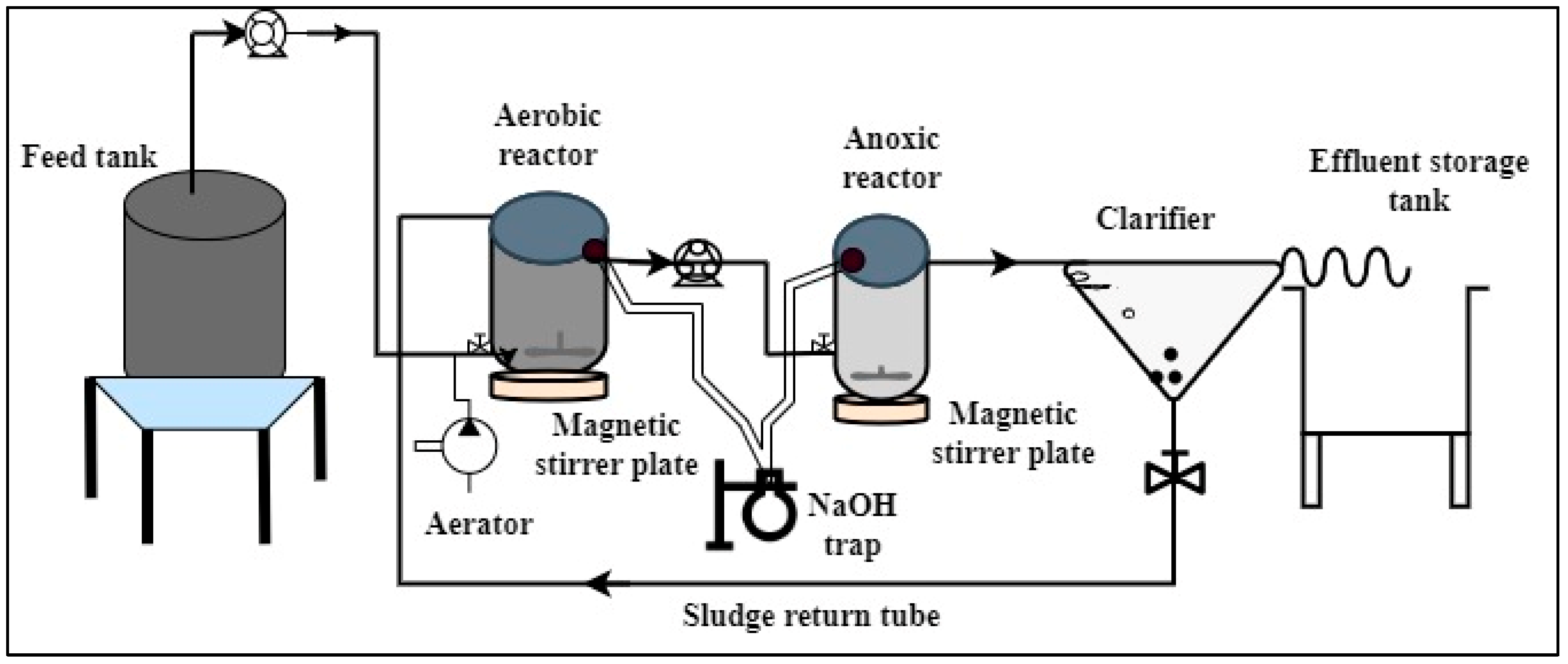
2.2. Experimental Setup and Formulation of Synthetic Wastewater
2.3. Inoculum Preparation and Operation of Reactors
2.4. Measurement of Residual Cyanide Concentration
2.5. Measurement of Other Nitrogenous Compounds
2.6. Statistical Analysis
3. Results
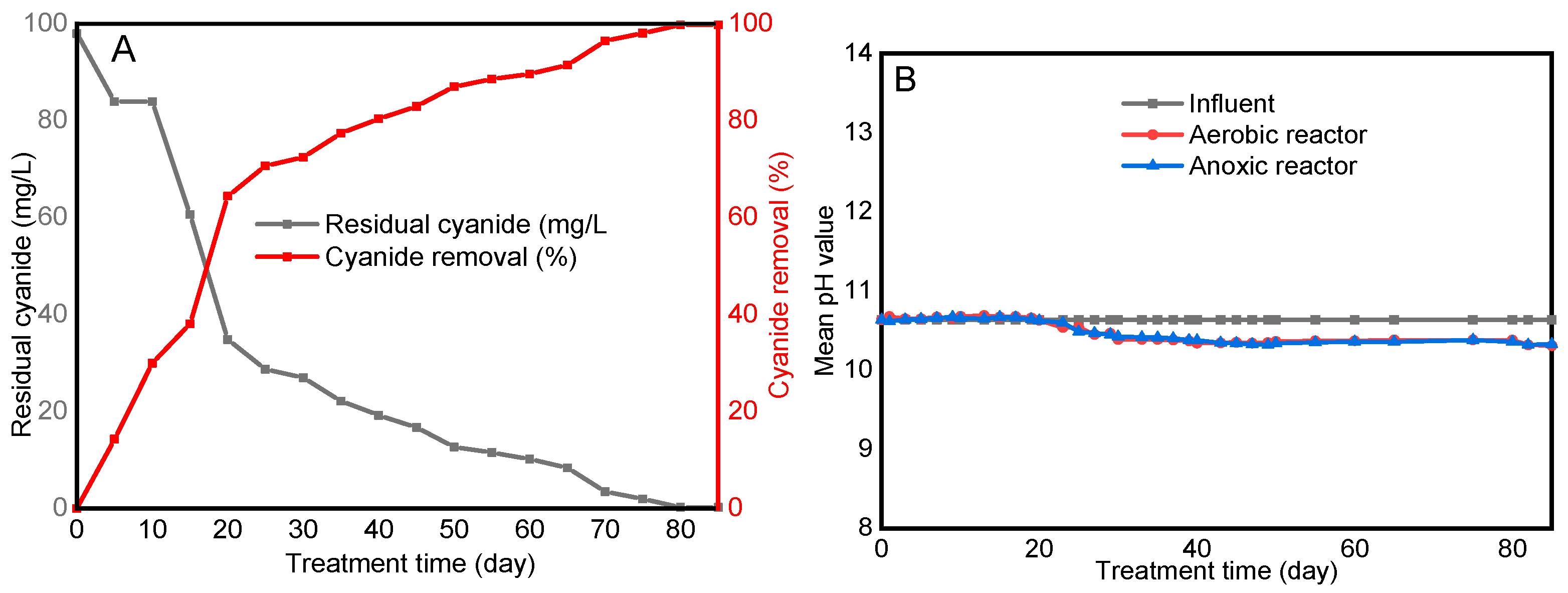
3.1. Establishment of Cyanide-Degrading Alkaliphilic Microbial Consortia
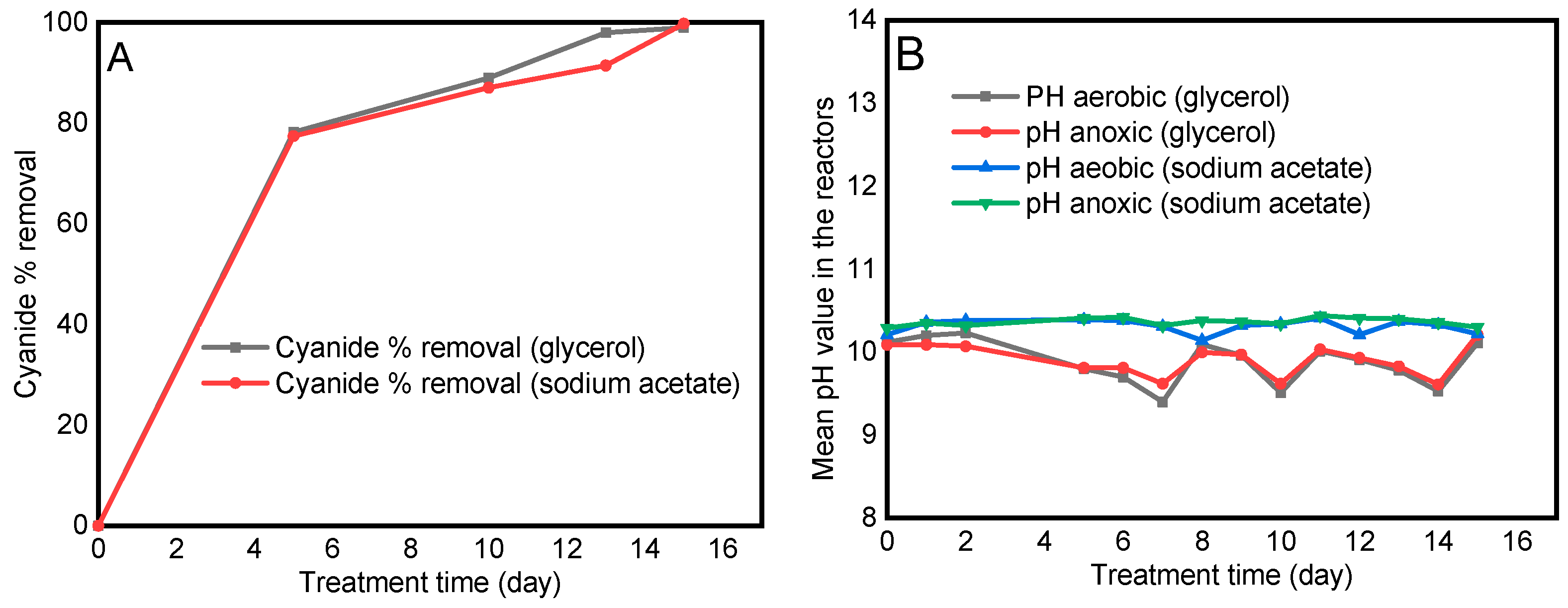
3.2. Cyanide Degradation Performance of the Treatment System
3.3. The pH of the Solution and the Feed
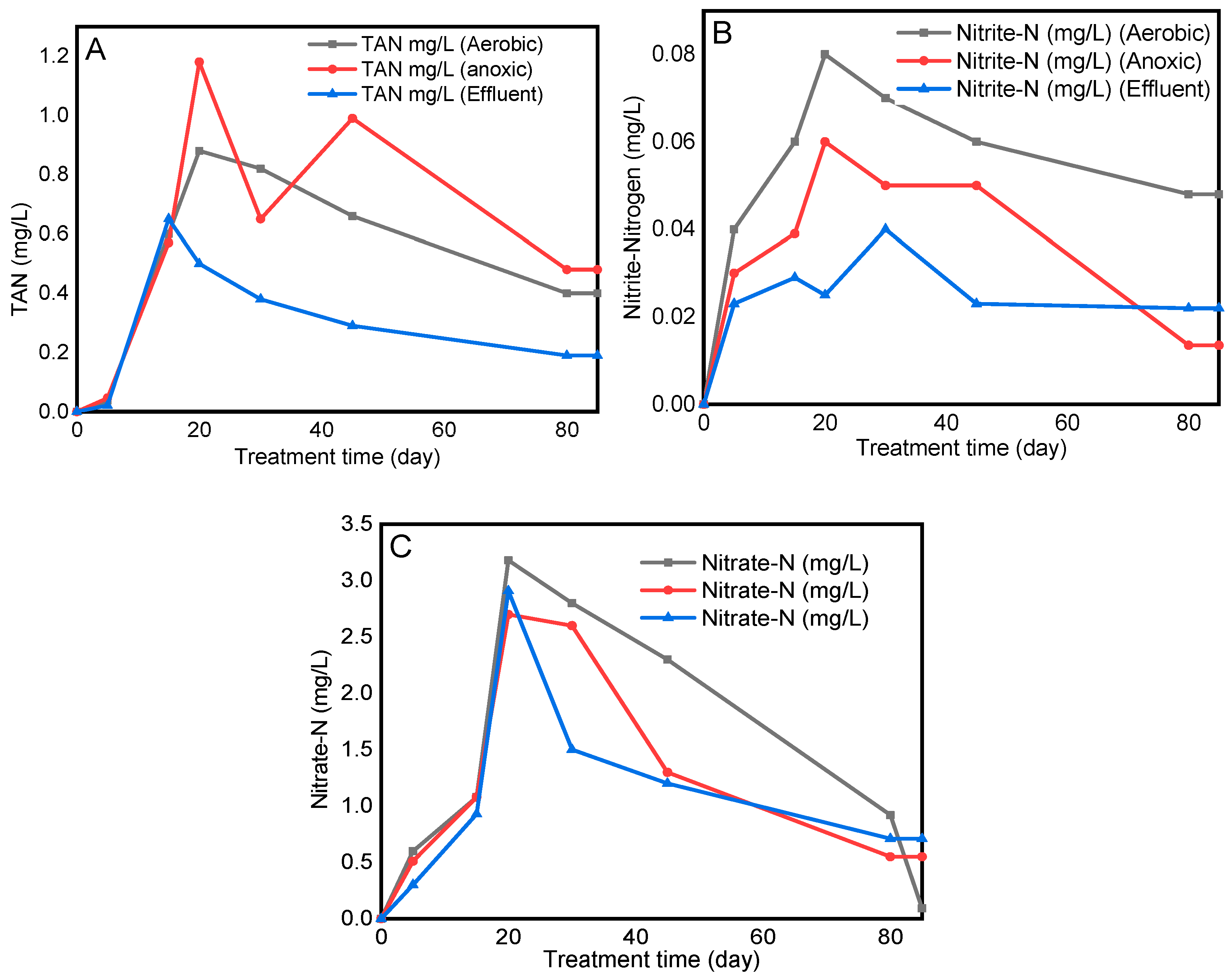
3.4. Production of Nitrogenous Compounds from Cyanide Biodegradation
3.5. Optimization of the Integrated System and the Established Consortia
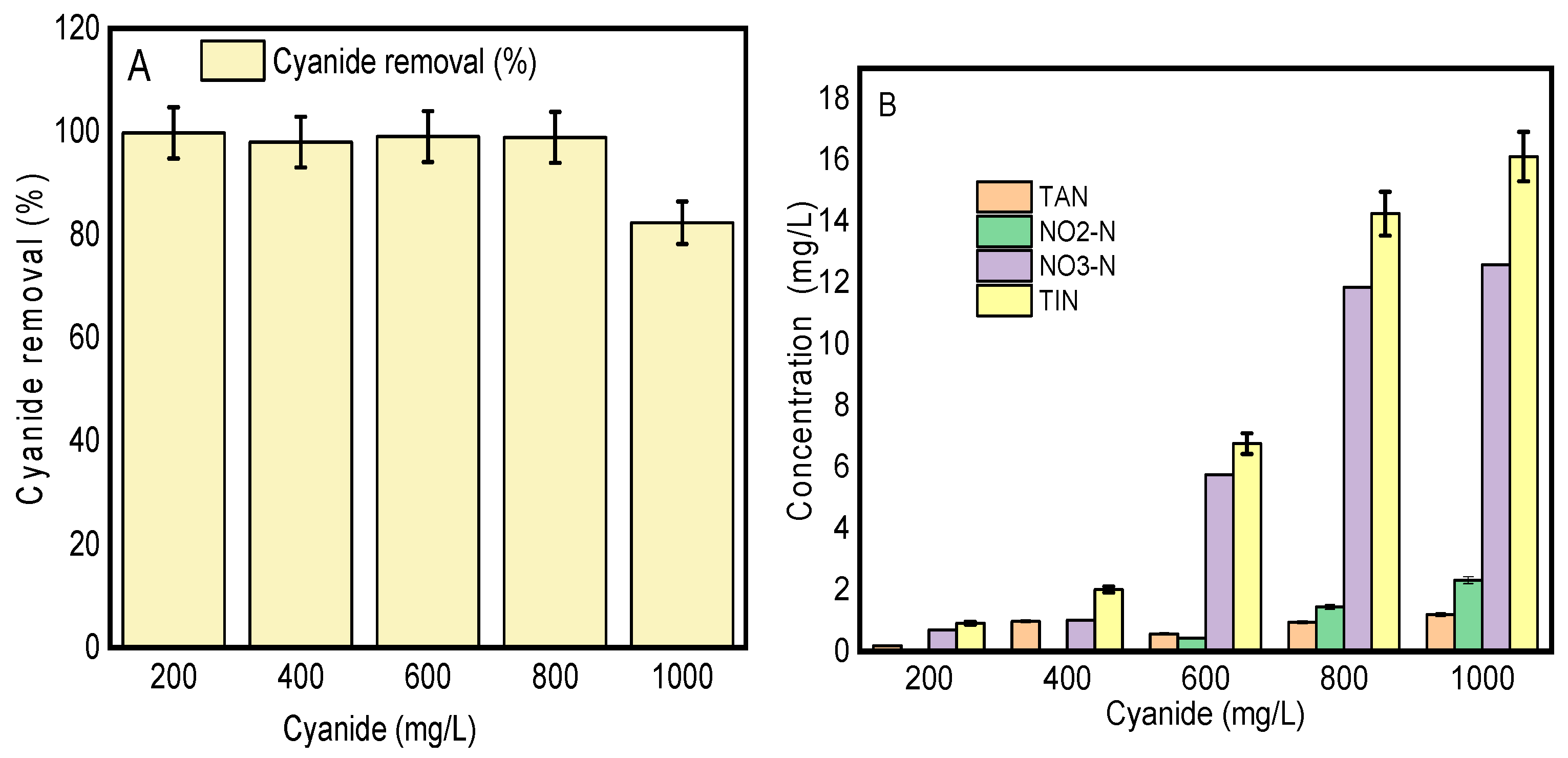
3.5.1. The Degradation Efficiency of the Consortia for Different Cyanide Loads
3.5.2. The Effects of Carbon Sources on Cyanide Biodegradation
3.5.3. Effect of Hydraulic Retention Time
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on remediation of cyanide containing industrial wastes using biological systems with special reference to enzymatic degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef]
- Alvillo-Rivera, A.; Garrido-Hoyos, S.; Buitrón, G.; Thangarasu-Sarasvathi, P.; Rosano-Ortega, G. Biological treatment for the degradation of cyanide: A review. J. Mater. Res. Technol. 2021, 12, 1418–1433. [Google Scholar] [CrossRef]
- Tu, Y.; Han, P.; Wei, L.; Zhang, X.; Yu, B.; Qian, P.; Ye, S. Removal of cyanide adsorbed on pyrite by H2O2 oxidation under alkaline conditions. J. Environ. Sci. 2019, 78, 287–292. [Google Scholar] [CrossRef]
- Wang, J.; Faraji, F.; Ramsay, J.; Ghahreman, A. A review of biocyanidation as a sustainable route for gold recovery from primary and secondary low-grade resources. J. Clean. Prod. 2021, 296, 126457. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef]
- Razanamahandry, L.C.; Onwordi, C.T.; Saban, W.; Bashir, A.K.H.; Mekuto, L.; Malenga, E.; Manikandan, E.; Fosso-Kankeu, E.; Maaza, M.; Ntwampe, S.K.O. Performance of various cyanide degrading bacteria on the biodegradation of free cyanide in water. J. Hazard. Mater. 2019, 380, 120900. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Bhalla, T.C. Packed bed reactor for degradation of simulated cyanide-containing wastewater. 3 Biotech 2015, 5, 641–646. [Google Scholar] [CrossRef][Green Version]
- Gupta, P.; Sreekrishnan, T.; Shaikh, Z. Application of hybrid anaerobic reactor: Treatment of increasing cyanide containing effluents and microbial composition identification. J. Environ. Manag. 2018, 226, 448–456. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Bhalla, T.C. Alkaline active cyanide dihydratase of Flavobacterium indicum MTCC 6936: Growth optimization, purification, characterization and in silico analysis. Int. J. Biol. Macromol. 2018, 116, 591–598. [Google Scholar] [CrossRef]
- Ofori-Sarpong, G.; Adam, A.-S.; Amankwah, R.K. Detoxification of Cyanide Wastewater by Cyanotrophic Organisms: The case of Phanerochaete chrysosporium. Ghana Min. J. 2020, 20, 34–44. [Google Scholar] [CrossRef]
- Anning, C.; Asare, M.O.; Junxiang, W.; Yao, G.; Xianjun, L. Effects of physicochemical properties of Au cyanidation tailings on cyanide microbial degradation. J. Environ. Sci. Health-Part A Toxic/Hazard. Subst. Environ. Eng. 2021, 56, 413–433. [Google Scholar] [CrossRef]
- Bird, G.; Brewer, P.A.; Macklin, M.G.; Balteanu, D.; Driga, B.; Serban, M.; Zaharia, S. The solid state partitioning of contaminant metals and As in river channel sediments of the mining affected Tisa drainage basin, northwestern Romania and eastern Hungary. Appl. Geochem. 2003, 18, 1583–1595. [Google Scholar] [CrossRef]
- Selebalo, I.M.; Scholes, M.C.; Clifford-Holmes, J.K. A Systemic Analysis of the Environmental Impacts of Gold Mining within the Blyde River Catchment, a Strategic Water Area of South Africa. Water 2021, 13, 301. [Google Scholar] [CrossRef]
- Baxter, J.; Cummings, S.P. The current and future applications of microorganism in the bioremediation of cyanide contamination. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2006, 90, 1–17. [Google Scholar] [CrossRef]
- Vuono, D.C.; Vanneste, J.; Figueroa, L.A.; Hammer, V.; Aguilar-Huaylla, F.N.; Malone, A.; Smith, N.M.; Garcia-Chevesich, P.A.; Bolaños-Sosa, H.G.; Alejo-Zapata, F.D.; et al. Photocatalytic Advanced Oxidation Processes for Neutralizing Free Cyanide in Gold Processing Effluents in Arequipa, Southern Peru. Sustainability 2021, 13, 9873. [Google Scholar] [CrossRef]
- Kaku, D.U.; Cao, Y.; Al-Masnay, Y.A.; Nizeyimana, J.C. An Integrated Approach to Assess the Environmental Impacts of Large-Scale Gold Mining: The Nzema-Gold Mines in the Ellembelle District of Ghana as a Case Study. Int. J. Environ. Res. Public Health 2021, 18, 7044. [Google Scholar] [CrossRef]
- Quiroz, C.J.C.; Quispe, G.d.L.F.; Mamani, M.C.; Choque, G.J.M.; Sacari, E.J.S. Cyanide Bioremediation by Bacillus subtilis under Alkaline Conditions. Water 2023, 15, 3645. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.; Akcil, A. An integrated biological approach for treatment of cyanidation wastewater. Sci. Total. Environ. 2016, 571, 711–720. [Google Scholar] [CrossRef]
- Cabello, P.; Luque-Almagro, V.M.; Olaya-Abril, A.; Sáez, L.P.; Moreno-Vivián, C.; Roldán, M.D. Assimilation of cyanide and cyano-derivatives by Pseudomonas pseudoalcaligenes CECT5344: From omic approaches to biotechnological applications. FEMS Microbiol. Lett. 2018, 365, fny032. [Google Scholar] [CrossRef]
- Razanamahandry, L.C.; Mekuto, L.; Bazie, V.; Andrianisa, H.A.; Karoui, H.; Meibom, K.; Frutschi, M. Free Cyanide Degradation Kinetics of Cyanide Degrading Bacteria. In Proceedings of the 10th International Conference on Advance in Science, Engineering, Technology & Healthcare, Cape Town, South Africa, 19–20 November 2018. [Google Scholar]
- Olaya-Abril, A.; Pérez, M.D.; Cabello, P.; Martignetti, D.; Sáez, L.P.; Luque-Almagro, V.M.; Moreno-Vivián, C.; Roldán, M.D. Role of the Dihydrodipicolinate Synthase DapA1 on Iron Homeostasis During Cyanide Assimilation by the Alkaliphilic Bacterium Pseudomonas pseudoalcaligenes CECT5344. Front. Microbiol. 2020, 11, 28. [Google Scholar] [CrossRef]
- Alvarado-Lopez, M.J.; Garrido-Hoyos, S.E.; Raynal-Gutierrez, M.E.; El-Kassis, E.G.; Marrugo-Negrete, J.L.; Rosano-Ortega, G. Native Cyanide Degrading Bacterial Consortium and its Potential for Gold Mine Tailings Tertiary Biotechnological Treatment. Chem. Eng. Trans. 2022, 94, 1441–1446. [Google Scholar] [CrossRef]
- Jiang, W.; Lu, Y.; Feng, Z.; Yu, H.; Ma, P.; Zhu, J.; Wang, Y.; Sun, J. Biodegradation of Cyanide by a New Isolated Aerococcus viridans and Optimization of Degradation Conditions by Response Surface Methodology. Sustainability 2022, 14, 15560. [Google Scholar] [CrossRef]
- Antunes, A.; de la Haba, R.R.; Jebbar, M.; Hedlund, B.P. Editorial: Community series-extremophiles: Microbial genomics and taxogenomics, volume II. Front. Microbiol. 2024, 15, 1371210. [Google Scholar] [CrossRef]
- Andreote, A.P.D.; Dini-Andreote, F.; Rigonato, J.; Machineski, G.S.; Souza, B.C.E.; Barbiero, L.; Rezende-Filho, A.T.; Fiore, M.F. Contrasting the Genetic Patterns of Microbial Communities in Soda Lakes with and without Cyanobacterial Bloom. Front. Microbiol. 2018, 9, 244. [Google Scholar] [CrossRef]
- Jeilu, O.; Alexandersson, E.; Johansson, E.; Simachew, A.; Gessesse, A. A novel GH3-β-glucosidase from soda lake metagenomic libraries with desirable properties for biomass degradation. Sci. Rep. 2024, 14, 10012. [Google Scholar] [CrossRef]
- Jeilu, O.; Gessesse, A.; Simachew, A.; Johansson, E.; Alexandersson, E. Prokaryotic and eukaryotic microbial diversity from three soda lakes in the East African Rift Valley determined by amplicon sequencing. Front. Microbiol. 2022, 13, 999876. [Google Scholar] [CrossRef]
- Ali, Y.; Simachew, A.; Gessesse, A. Diversity of Culturable Alkaliphilic Nitrogen-Fixing Bacteria from a Soda Lake in the East African Rift Valley. Microorganisms 2022, 10, 1760. [Google Scholar] [CrossRef]
- Simachew, A.; Lanzén, A.; Gessesse, A.; Øvreås, L. Prokaryotic Community Diversity Along an Increasing Salt Gradient in a Soda Ash Concentration Pond. Microb. Ecol. 2016, 71, 326–338. [Google Scholar] [CrossRef]
- Shin, D.; Park, J.; Park, H.; Lee, J.-C.; Kim, M.-S.; Lee, J. Key Microbes and Metabolic Potentials Contributing to Cyanide Biodegradation in Stirred-Tank Bioreactors Treating Gold Mining Effluent. Miner. Process. Extr. Met. Rev. 2020, 41, 85–95. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.; Utomi, C.E.; Mobo, M.; Mudumbi, J.B.; Ngongang, M.M.; Akinpelu, E.A. Performance of a continuously stirred tank bioreactor system connected in series for the biodegradation of thiocyanate and free cyanide. J. Environ. Chem. Eng. 2017, 5, 1936–1945. [Google Scholar] [CrossRef]
- Bilger, H.E.; Wolf, H.H. Method for the Coloremetric Determination of the Cyande Concentration of Aoueous Solutions. U.S. Patent US4871681A, 3 October 1989. [Google Scholar]
- Guadalima, M.P.G.; Monteros, D.A.N. Evaluation of the rotational speed and carbon source on the biological removal of free cyanide present on gold mine wastewater, using a rotating biological contactor. J. Water Process. Eng. 2018, 23, 84–90. [Google Scholar] [CrossRef]
- Qureshi, N.; Annous, B.A.; Ezeji, T.C.; Karcher, P.; Maddox, I.S. Biofilm reactors for industrial bioconversion processes: Employing potential of enhanced reaction rates. Microb. Cell Factories 2005, 4, 24. [Google Scholar] [CrossRef]
- Clemente, S.M.; Olivera, C.C.; Benites-Alfaro, E. Microbial Biofilm as a Methodology for Treatment of Cyanide-Contaminated Water. Chem. Eng. Trans. 2022, 93, 151–156. [Google Scholar] [CrossRef]
- Kao, C.; Liu, J.; Lou, H.; Lin, C.; Chen, S. Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 2003, 50, 1055–1061. [Google Scholar] [CrossRef]
- Mirizadeh, S.; Yaghmaei, S.; Nejad, Z.G. Biodegradation of cyanide by a new isolated strain under alkaline conditions and optimization by response surface methodology (RSM). J. Environ. Health Sci. Eng. 2014, 12, 85. [Google Scholar] [CrossRef]
- Botz, M.M.; Mudder, T.I.; Akcil, A.U. Cyanide treatment: Physical, chemical and biological processes. Dev. Miner. Process. 2005, 15, 672–702. [Google Scholar] [CrossRef]
- Ogato, T.; Kifle, D.; Fetahi, T.; Sitotaw, B. Evaluation of growth and biomass production of Arthrospira (Spirulina) fusiformis in laboratory cultures using waters from the Ethiopian soda lakes Chitu and Shala. J. Appl. Phycol. 2014, 26, 2273–2282. [Google Scholar] [CrossRef]
- Maniyam, M.N.; Sjahrir, F.; Ibrahim, A.L.; Cass, A.E.G. Biodegradation of cyanide by Rhodococcus UKMP-5M. Biologia 2013, 68, 177–185. [Google Scholar] [CrossRef]
- Vallenas-Arévalo, A.T.; Rosario, C.G.A.; Espinosa, D.C.R.; Tenório, J.A.S. Bacterial degradation of free cyanide in alkaline medium using bacillus licheniformis strain. Miner. Met. Mater. Ser. Part 2018, F6, 367–373. [Google Scholar] [CrossRef]
- Ingvorsen, K.; Højer-Pedersen, B.; Godtfredsen, S.E. Novel cyanide-hydrolyzing enzyme from Alcaligenes xylosoxidans subsp. denitrificans. Appl. Environ. Microbiol. 1991, 57, 1783–1789. [Google Scholar] [CrossRef]
- Mpongwana, N.; Ntwampe, S.K.O.; Mekuto, L.; Akinpelu, E.A.; Dyantyi, S.; Mpentshu, Y. Isolation of high-salinity-tolerant bacterial strains, Enterobacter sp., Serratia sp., Yersinia sp., for nitrification and aerobic denitrification under cyanogenic conditions. Water Sci. Technol. 2016, 73, 2168–2175. [Google Scholar] [CrossRef]
- Kandasamy, S.; Dananjeyan, B.; Krishnamurthy, K.; Benckiser, G. Aerobic cyanide degradation by bacterial isolates from cassava factory wastewater. Braz. J. Microbiol. 2015, 46, 659–666. [Google Scholar] [CrossRef]
- Müller, T.; Walter, B.; Wirtz, A.; Burkovski, A. Ammonium Toxicity in Bacteria. Curr. Microbiol. 2006, 52, 400–406. [Google Scholar] [CrossRef]
- Mekuto, L.; Kim, Y.M.; Ntwampe, S.K.O.; Mewa-Ngongang, M.; Mudumbi, J.B.N.; Dlangamandla, N.; Itoba-Tombo, E.F.; Akinpelu, E.A. Heterotrophic nitrification-aerobic denitrification potential of cyanide and thiocyanate degrading microbial communities under cyanogenic conditions. Environ. Eng. Res. 2019, 24, 254–262. [Google Scholar] [CrossRef]
- US/ETH/99/068/Ehiopia; EEPA, Provisional Standards for Industrial Pollution Control in Ethiopia. Prepared under the Ecologically Sustainable Industrial Development (ESID) Project; EPA/UNIDO: Addis Ababa, Ethiopia, 2003.
- Koren, D.W.; Gould, W.D.; Bédard, P. Biological removal of ammonia and nitrate from simulated mine and mill effluents. Hydrometallurgy 2000, 56, 127–144. [Google Scholar] [CrossRef]
- Kim, Y.M.; Cho, H.U.; Lee, D.S.; Park, C.; Park, D.; Park, J.M. Response of nitrifying bacterial communities to the increased thiocyanate concentration in pre-denitrification process. Bioresour. Technol. 2011, 102, 913–922. [Google Scholar] [CrossRef]
- Zhao, R.; Babbin, A.R.; Roerdink, D.L.; Thorseth, I.H.; Jørgensen, S.L. Nitrite accumulation and anammox bacterial niche partitioning in Arctic Mid-Ocean Ridge sediments. ISME Commun. 2023, 3, 1–14. [Google Scholar] [CrossRef]
- Felföldi, T.; Nagymáté, Z.; Székely, A.J.; Jurecska, L.; Márialigeti, K. Biological treatment of coke plant effluents: From a microbiological perspective. Biol. Futur. 2020, 71, 359–370. [Google Scholar] [CrossRef]
- Dhamole, P.B.; D’souza, S.F.; Lele, S.S. A Review on Alternative Carbon Sources for Biological Treatment of Nitrate Waste. J. Inst. Eng. Ser. E 2015, 96, 63–73. [Google Scholar] [CrossRef]
- Raghunandan, K.; Mchunu, S.; Kumar, A.; Kumar, K.S.; Govender, A.; Permaul, K.; Singh, S. Biodegradation of glycerol using bacterial isolates from soil under aerobic conditions. J. Environ. Sci. Health Part A 2014, 49, 85–92. [Google Scholar] [CrossRef]
- Adjei, M.D.; Ohta, Y. Factors affecting the biodegradation of cyanide by Burkholderia cepacia strain C-3. J. Biosci. Bioeng. 2000, 89, 274–277. [Google Scholar] [CrossRef]
- Bell, L.N.; Labuza, T.P. Compositional Influence on the pH of Reduced-Moisture Solutions. J. Food Sci. 1992, 57, 732–734. [Google Scholar] [CrossRef]
- Villemur, R.; Juteau, P.; Bougie, V.; Ménard, J.; Déziel, E. Development of four-stage moving bed biofilm reactor train with a pre-denitrification configuration for the removal of thiocyanate and cyanate. Bioresour. Technol. 2015, 181, 254–262. [Google Scholar] [CrossRef]



| Salt Solution (g/L) | Amount (mg/L) |
|---|---|
| K2HPO4·2H2O | 3 |
| Na2HPO4·2H2O | 7 |
| MgSO4·7H2O | 0.3 |
| NaCl | 0.25 |
| CaCl2·2H2O | 0.02 |
| Na2CO3 | 10 |
| CH3COONa | 0.25 |
| NaCN | (0.2, 0.4, 0.6, 0.8, 1) * |
| MnSO4·4H2O | 0.01 |
| FeCl3·6H2O | 0.045 |
| ZnSO4·7H2O | 0.01 |
| CuSO4·H2O | 0.002 |
| CoCl2·6H2O | 0.003 |
| NiCl2·6H2O | 0.003 |
| NaMoO4·2H2O | 0.02 |
| NaCN (mg/L) | Carbon Source | pH | Treatment Period (Day) |
|---|---|---|---|
| 200 | CH3COO-Na | 10.32–10.69 | 0–85 |
| 400 | C2H3NaO2 | 10.01–10.4 | 85–107 |
| 600 | C2H3NaO2 | 9.71–10.31 | 107–127 |
| 800 | C2H3NaO2 | 9.66–9.98 | 127–145 |
| 1000 | C2H3NaO2 | 9.76–9.99 | 145–170 |
| 200 | C3H8O3 | 9.73–9.99 | 170–190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belay, G.; Suarez, C.; Paul, C.J.; Simachew, A. Biodegradation of Cyanide Using Soda Lake-Derived Alkaliphilic Microbial Consortia. Water 2024, 16, 2956. https://doi.org/10.3390/w16202956
Belay G, Suarez C, Paul CJ, Simachew A. Biodegradation of Cyanide Using Soda Lake-Derived Alkaliphilic Microbial Consortia. Water. 2024; 16(20):2956. https://doi.org/10.3390/w16202956
Chicago/Turabian StyleBelay, Getnet, Carolina Suarez, Catherin J. Paul, and Addis Simachew. 2024. "Biodegradation of Cyanide Using Soda Lake-Derived Alkaliphilic Microbial Consortia" Water 16, no. 20: 2956. https://doi.org/10.3390/w16202956
APA StyleBelay, G., Suarez, C., Paul, C. J., & Simachew, A. (2024). Biodegradation of Cyanide Using Soda Lake-Derived Alkaliphilic Microbial Consortia. Water, 16(20), 2956. https://doi.org/10.3390/w16202956






