Uptake, Efflux, and Sequestration of Mercury in the Asian Clam, Corbicula fluminea, at Environmentally Relevant Concentrations, and the Implications for Mercury Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism Collection and Care
2.2. Experimental Setup
2.3. Dosing and Sampling
2.4. Hg Analysis
2.5. Statistical Analysis
2.6. Toxicokinetic Modeling
- (1)
- The efflux rate parameter was fitted first only using the depuration data, assuming an exponential decline in internal Hg concentrations when the Corbicula were moved to clean water. The initial value of this phase was set by the concentration of each treatment level at the end of the uptake phase (Day 5) and we fitted the depuration data to Equation (1); in clean water, the concentration of Hg in the water was 0 (CW = 0 ng Hg/L), so Equation (1) simplifies to an exponential decline function at a rate ke.
- (2)
- During the exposure and uptake phase, the aqueous concentrations of Hg decreased with time due to sorption onto the tank walls and uptake into the Corbicula, so a constant value of CW was not assumed in each treatment tank. To account for this decline and better estimate the value of ku, an exponential decay function was fit to the measured aqueous Hg concentrations as the input for CW (Equation (2)):
- (3)
- Once this equation was fitted, the value of ku was fitted to Equation (1) using measured CW Hg aqueous concentrations with estimated values connecting these data points assuming the fit exponential decay function (Equation (2)). The empirically measured and simulated data points were combined for the CW input such that there was a data point every 200 min. The value of ke was held constant at the value estimated using the depuration data (Step 1 above), assuming efflux was occurring simultaneously with uptake during the 5 day uptake period.
3. Results and Discussion
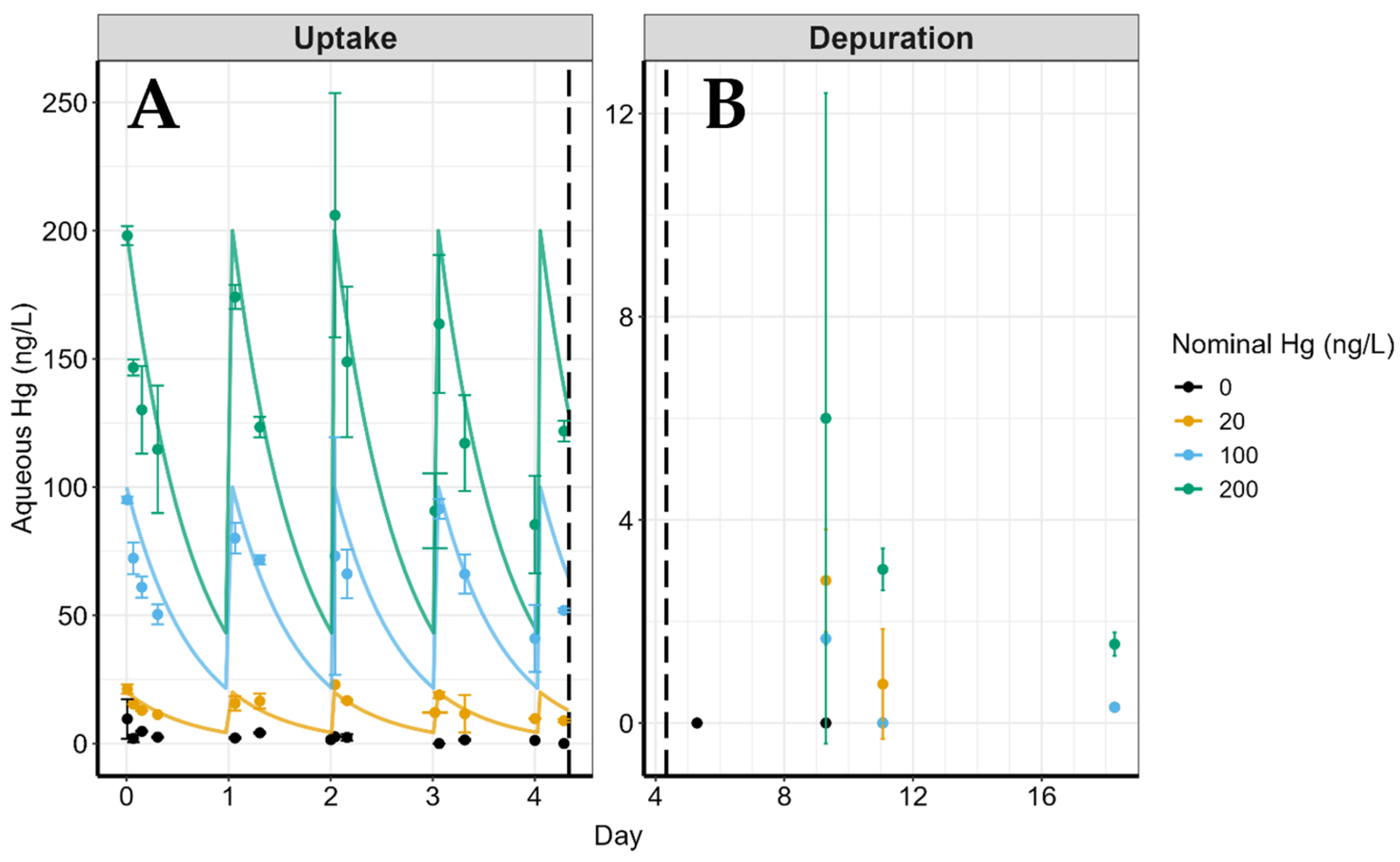
3.1. Aqueous Concentrations
3.2. Effect Sizes and Statistical Significance
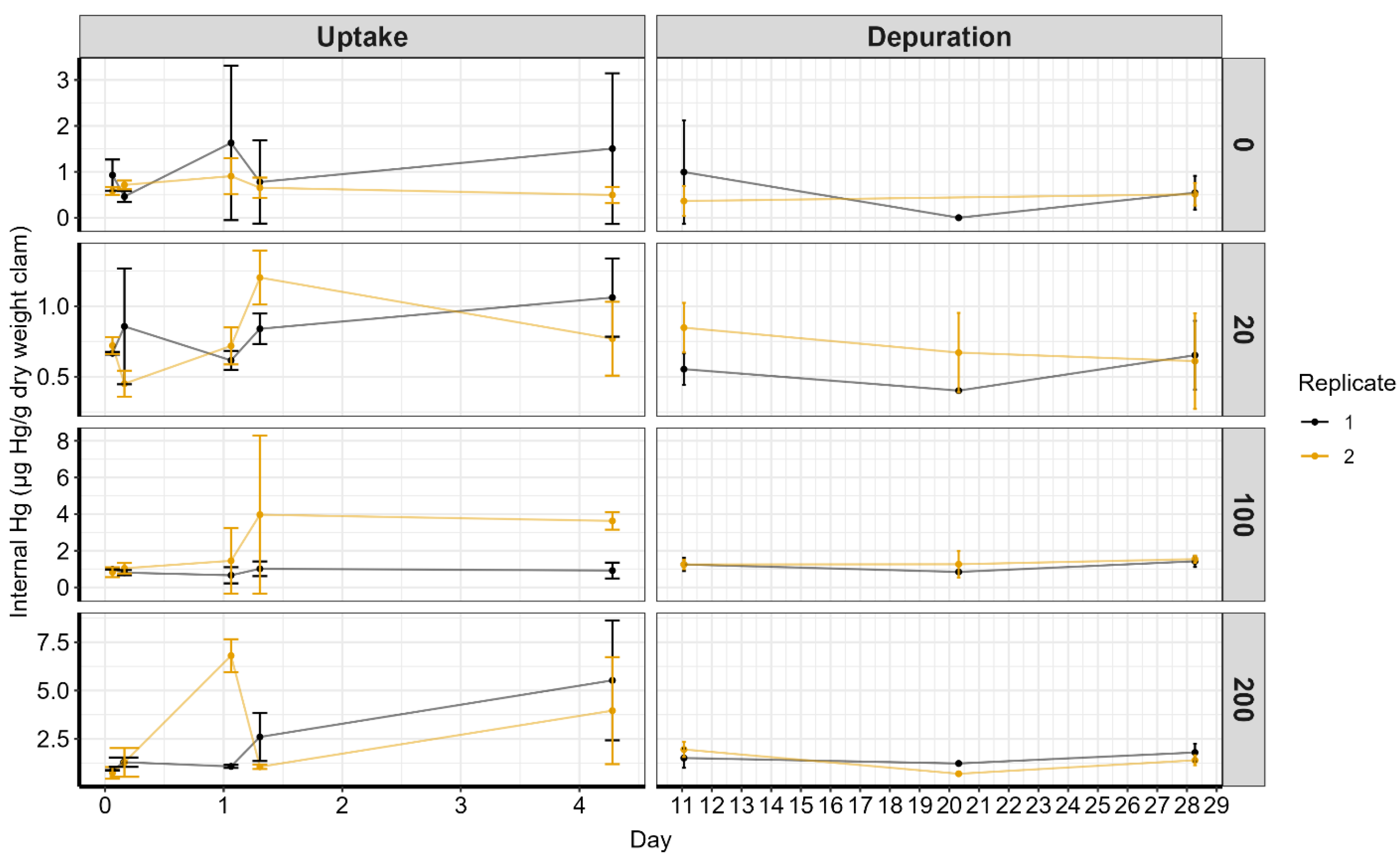
3.3. Bioconcentration
3.4. Bioconcentration Factors
3.5. Concentration and Biomagnification Variability
3.6. Toxicokinetic Model
3.7. Implications for Metal Sequestration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaughn, C.C. Ecosystem services provided by freshwater mussels. Hydrobiologia 2018, 810, 15–27. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Spooner, D.E. Scale-dependent associations between native freshwater mussels and invasive Corbicula. Hydrobiologia 2006, 568, 331–339. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Taylor, C.M. Impoundments and the decline of freshwater mussels: A case study of an extinction gradient. Conserv. Biol. 1999, 13, 912–920. [Google Scholar] [CrossRef]
- Strayer, D.L.; Downing, J.A.; Haag, W.R.; King, T.L.; Layzer, J.B.; Newton, T.J.; Nichols, J.S. Changing perspectives on pearly mussels, North America’s most imperiled animals. BioScience 2004, 54, 429–439. [Google Scholar] [CrossRef]
- Strayer, D.L.; Malcom, H.M. Causes of recruitment failure in freshwater mussel populations in southeastern New York. Ecol. Appl. 2012, 22, 1780–1790. [Google Scholar] [CrossRef]
- Nalepa, T.; Gardner, W.; Malczyk, J. Phosphorus cycling by mussels (Unionidae: Bivalvia) in Lake St. Clair. Hydrobiologia 1991, 219, 239–250. [Google Scholar] [CrossRef]
- Geeza, T.J.; Gillikin, D.P.; McDevitt, B.; Van Sice, K.; Warner, N.R. Accumulation of Marcellus Formation oil and gas wastewater metals in freshwater mussel shells. Environ. Sci. Technol. 2018, 52, 10883–10892. [Google Scholar] [CrossRef]
- Strayer, D.L. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance; Univ of California Press: Berkeley, CA, USA, 2008; Volume 1. [Google Scholar]
- Poole, K.E.; Downing, J.A. Relationship of declining mussel biodiversity to stream-reach and watershed characteristics in an agricultural landscape. J. N. Am. Benthol. Soc. 2004, 23, 114–125. [Google Scholar] [CrossRef]
- Morowski, D.; James, L.J.; Hunter, R.D. Freshwater Mussels in the Clinton River, Southeastern Michigan: An Assessment of Community Status. Mich. Acad. 2009, 39, 131. [Google Scholar]
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Ann. Limnol.-Int. J. Limnol. 2008, 44, 85–94. [Google Scholar] [CrossRef]
- Ferreira-Rodríguez, N.; Iglesias, J.; Pardo, I. Corbicula fluminea affecting supporting ecosystem services through nutrient and biogenic matter incorporation in invaded estuaries. Fund. Appl. Limnol. 2019, 192, 269–280. [Google Scholar] [CrossRef]
- McDowell, W.G.; Byers, J.E. High abundance of an invasive species gives it an outsized ecological role. Freshw. Biol. 2019, 64, 577–586. [Google Scholar] [CrossRef]
- McDowell, W.; McDowell, W.; Byers, J. Mass mortality of a dominant invasive species in response to an extreme climate event: Implications for ecosystem function. Limnol. Oceanogr. 2017, 62, 177–188. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, J.-H.; Kong, D.-S.; Hwang, S.-J. Water quality improvement with the application of filter-feeding bivalve (Corbicula leana Prime) in a eutrophic lake. Korean J. Ecol. Environ. 2004, 37, 332–343. [Google Scholar]
- Ismail, N.S.; Muüller, C.E.; Morgan, R.R.; Luthy, R.G. Uptake of contaminants of emerging concern by the bivalves Anodonta californiensis and Corbicula fluminea. Environ. Sci. Technol. 2014, 48, 9211–9219. [Google Scholar] [CrossRef]
- Mathews, T.J.; Mayes, M.A.; Brooks, S.C.; Johs, A.; Nair, S.; Muller, K.A.; Rodriguez, L.G.; Derolph, C.R.; Hills, A.D.; Carter, E.; et al. Mercury Remediation Technology Development for Lower East Fort Poplar Creek-FY2019 Update; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2019. [Google Scholar]
- Strayer, D.L. Effects of alien species on freshwater mollusks in North America. J. N. Am. Benthol. Soc. 1999, 18, 74–98. [Google Scholar] [CrossRef]
- Pouil, S.; Hills, A.; Stevenson, L.; Mathews, T.J. Allometric relationships in the filtration rates of the Asian clam Corbicula fluminea fed two phytoplankton species. Aquat. Ecol. 2021, 55, 915–923. [Google Scholar] [CrossRef]
- Hills, A.; Pouil, S.; Hua, D.; Mathews, T.J. Clearance rates of freshwater bivalves Corbicula fluminea and Utterbackia imbecillis in the presence and absence of light. Aquat. Ecol. 2020, 54, 1059–1066. [Google Scholar] [CrossRef]
- Welker, M.; Walz, N. Can mussels control the plankton in rivers?—A planktological approach applying a Lagrangian sampling strategy. Limnol. Oceanogr. 1998, 43, 753–762. [Google Scholar] [CrossRef]
- Howard, J.K.; Cuffey, K.M. The functional role of native freshwater mussels in the fluvial benthic environment. Freshw. Biol. 2006, 51, 460–474. [Google Scholar] [CrossRef]
- Hakenkamp, C.C.; Palmer, M.A. Introduced bivalves in freshwater ecosystems: The impact of Corbicula on organic matter dynamics in a sandy stream. Oecologia 1999, 119, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, K.S.; Thorp, J.H.; Summers, R.B.; Guelda, D.L. Effects of an exotic bivalve mollusc on benthic invertebrates and food quality in the Ohio River. Hydrobiologia 2001, 462, 169–172. [Google Scholar] [CrossRef]
- McLeod, P.B.; Luoma, S.N.; Luthy, R.G. Biodynamic modeling of PCB uptake by Macoma balthica and Corbicula fluminea from sediment amended with activated carbon. Environ. Sci. Technol. 2008, 42, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Inza, B.; Ribeyre, F.; Boudou, A. Dynamics of cadmium and mercury compounds (inorganic mercury or methylmercury): Uptake and depuration in Corbicula fluminea. Effects of temperature and pH. Aquat. Toxicol. 1998, 43, 273–285. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Xu, N.; Pan, B.; Ni, J. Pharmaceuticals and personal care products (PPCPs) in water, sediment and freshwater mollusks of the Dongting Lake downstream the Three Gorges Dam. Chemosphere 2022, 301, 134721. [Google Scholar] [CrossRef]
- Croteau, M.-N.; Luoma, S.N.; Topping, B.R.; Lopez, C.B. Stable metal isotopes reveal copper accumulation and loss dynamics in the freshwater bivalve Corbicula. Environ. Sci. Technol. 2004, 38, 5002–5009. [Google Scholar] [CrossRef]
- Rosa, I.C.; Costa, R.; Gonçalves, F.; Pereira, J.L. Bioremediation of metal-rich effluents: Could the invasive bivalve Corbicula fluminea work as a biofilter? J. Environ. Qual. 2014, 43, 1536–1545. [Google Scholar] [CrossRef]
- Baudrimont, M.; Metivaud, J.; Maury-Brachet, R.; Ribeyre, F.; Boudou, A. Bioaccumulation and metallothionein response in the Asiatic clam (Corbicula fluminea) after experimental exposure to cadmium and inorganic mercury. Environ. Toxicol. Chem. Int. J. 1997, 16, 2096–2105. [Google Scholar] [CrossRef]
- Neufeld, D.S. Mercury accumulation in caged Corbicula: Rate of uptake and seasonal variation. Environ. Monit. Assess. 2010, 168, 385–396. [Google Scholar] [CrossRef]
- Regnell, O.; Watras, C.J. Microbial mercury methylation in aquatic environments: A critical review of published field and laboratory studies. Environ. Sci. Technol. 2018, 53, 4–19. [Google Scholar] [CrossRef]
- Johs, A.; Eller, V.A.; Mehlhorn, T.L.; Brooks, S.C.; Harper, D.P.; Mayes, M.A.; Pierce, E.M.; Peterson, M.J. Dissolved organic matter reduces the effectiveness of sorbents for mercury removal. Sci. Total Environ. 2019, 690, 410–416. [Google Scholar] [CrossRef]
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef] [PubMed]
- Brigham, M.E.; Wentz, D.A.; Aiken, G.R.; Krabbenhoft, D.P. Mercury cycling in stream ecosystems. 1. Water column chemistry and transport. Environ. Sci. Technol. 2009, 43, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Pirrone, N.; Mahaffey, K.R. Dynamics of Mercury Pollution on Regional and Global Scales: Atmospheric Processes and Human Exposures around the World; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Mathews, T.J.; Southworth, G.R.; Peterson, M.J.; Roy, W.K.; Ketelle, R.H.; Valentine, C.S.; Gregory, S.M. Decreasing aqueous mercury concentrations to achieve safe levels in fish: Examining the water-fish relationship in two point-source contaminated streams. Sci. Total Environ. 2013, 443, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.; Maiti, S.K. Sources, toxicity, and remediation of mercury: An essence review. Environ. Monit. Assess. 2019, 191, 566. [Google Scholar] [CrossRef]
- Kidd, K.; Hesslein, R.; Fudge, R.; Hallard, K. The influence of trophic level as measured by δ 15 N on mercury concentrations in freshwater organisms. Water Air Soil Pollut. 1995, 80, 1011–1015. [Google Scholar] [CrossRef]
- Ebinghaus, R.; Turner, R.R.; de Lacerda, L.D.; Vasiliev, O.; Salomons, W. Mercury Contaminated Sites: Characterization, Risk Assessment and Remediation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Southworth, G.; Turner, R.; Peterson, M.; Bogle, M. Form of mercury in stream fish exposed to high concentrations of dissolved inorganic mercury. Chemosphere 1995, 30, 779–787. [Google Scholar] [CrossRef]
- Scheuhammer, A.M.; Meyer, M.W.; Sandheinrich, M.B.; Murray, M.W. Effects of environmental methylmercury on the health of wild birds, mammals, and fish. AMBIO J. Hum. Environ. 2007, 36, 12–19. [Google Scholar] [CrossRef]
- Lindqvist, O.; Johansson, K.; Bringmark, L.; Timm, B.; Aastrup, M.; Andersson, A.; Hovsenius, G.; Håkanson, L.; Iverfeldt, Å.; Meili, M. Mercury in the Swedish environment—Recent research on causes, consequences and corrective methods. Water Air Soil Pollut. 1991, 55, xi-261. [Google Scholar] [CrossRef]
- Bloom, N.S. On the chemical form of mercury in edible fish and marine invertebrate tissue. Can. J. Fish. Aquat. Sci. 1992, 49, 1010–1017. [Google Scholar] [CrossRef]
- Håkanson, L.; Nilsson, Å.; Andersson, T. Mercury in fish in Swedish lakes. Environ. Pollut. 1988, 49, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Turner, A.; Brown, M.T. Bioaccumulation of metals by Fucus ceranoides in estuaries of South West England. Mar. Pollut. Bull. 2011, 62, 2557–2562. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Monteiro, R.J.; Duarte, A.d.C.; Pardal, M.; Pereira, E. Study on bioaccumulation and biosorption of mercury by living marine macroalgae: Prospecting for a new remediation biotechnology applied to saline waters. Chem. Eng. J. 2015, 281, 759–770. [Google Scholar] [CrossRef]
- Kannan, K.; Smith, R.G., Jr.; Lee, R.; Windom, H.; Heitmuller, P.; Macauley, J.; Summers, J. Distribution of total mercury and methyl mercury in water, sediment, and fish from south Florida estuaries. Arch. Environ. Contam. Toxicol. 1998, 34, 109–118. [Google Scholar] [CrossRef]
- Gray, J.E.; Theodorakos, P.M.; Bailey, E.A.; Turner, R.R. Distribution, speciation, and transport of mercury in stream-sediment, stream-water, and fish collected near abandoned mercury mines in southwestern Alaska, USA. Sci. Total Environ. 2000, 260, 21–33. [Google Scholar] [CrossRef]
- Arini, A.; Pierron, F.; Mornet, S.; Baudrimont, M. Bioaccumulation dynamics and gene regulation in a freshwater bivalve after aqueous and dietary exposures to gold nanoparticles and ionic gold. Environ. Sci. Pollut. Res. 2020, 27, 3637–3650. [Google Scholar] [CrossRef]
- Ponis, E.; Robert, R.; Parisi, G. Nutritional value of fresh and concentrated algal diets for larval and juvenile Pacific oysters (Crassostrea gigas). Aquaculture 2003, 221, 491–505. [Google Scholar] [CrossRef]
- Jing, W.; Lang, L.; Lin, Z.; Liu, N.; Wang, L. Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere 2019, 219, 321–327. [Google Scholar] [CrossRef]
- Metian, M.; Warnau, M.; Cosson, R.P.; Oberhänsli, F.; Bustamante, P. Bioaccumulation and detoxification processes of Hg in the king scallop Pecten maximus: Field and laboratory investigations. Aquat. Toxicol. 2008, 90, 204–213. [Google Scholar] [CrossRef]
- Zhang, J.; Chao, J.; Tang, Y.; Wan, P.; Yang, X.J.; Wong, C.; Bruce, M.; Hu, Q. Quantification of Trace Mercury in Water: Solving the Problem of Adsorption, Sample Preservation, and Cross-Contamination. Glob. Chall. 2020, 4, 1900061. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Method 1631, Revision E: Mercury in Water by Oxidation, Purge and Trap, and Cold Vapor Atomic Fluorescence Spectrometry. 2002. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_1631e_2002.pdf (accessed on 18 July 2022).
- Southworth, G.R.; Peterson, M.J.; Bogle, M.A. Bioaccumulation factors for mercury in stream fish. Environ. Pract. 2004, 6, 135–143. [Google Scholar] [CrossRef]
- Nakagawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N.; Rainbow, P.S. Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ. Sci. Technol. 2005, 39, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Luoma, S.N.; Rainbow, P.S. Metal Contamination in Aquatic Environments: Science and Lateral Management; Cambridge University Press: Cambridge, UK, 2008; Volume 126. [Google Scholar]
- Jager, T. Robust likelihood-based approach for automated optimization and uncertainty analysis of toxicokinetic-toxicodynamic models. Integr. Environ. Assess. Manag. 2021, 17, 388–397. [Google Scholar] [CrossRef]
- Oliveira, P.; Lopes-Lima, M.; Machado, J.; Guilhermino, L. Comparative sensitivity of European native (Anodonta anatina) and exotic (Corbicula fluminea) bivalves to mercury. Estuar. Coast. Shelf Sci. 2015, 167, 191–198. [Google Scholar] [CrossRef]
- Cohen, H. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Gagnon, C.; Fisher, N.S. Bioavailability of sediment-bound methyl and inorganic mercury to a marine bivalve. Environ. Sci. Technol. 1997, 31, 993–998. [Google Scholar] [CrossRef]
- Pan, K.; Wang, W.-X. Mercury accumulation in marine bivalves: Influences of biodynamics and feeding niche. Environ. Pollut. 2011, 159, 2500–2506. [Google Scholar] [CrossRef]
- Wang, W.-X.; Wong, R.S.; Wang, J.; Yen, Y.-F. Influences of different selenium species on the uptake and assimilation of Hg (II) and methylmercury by diatoms and green mussels. Aquat. Toxicol. 2004, 68, 39–50. [Google Scholar] [CrossRef]
- Tessier, L.; Vaillancourt, G.; Pazdernik, L. Comparative study of the cadmium and mercury kinetics between the short-lived gastropod Viviparus georgianus (Lea) and pelecypod Elliptio complanata (Lightfoot), under laboratory conditions. Environ. Pollut 1994, 85, 271–282. [Google Scholar] [CrossRef]
- Tessier, L.; Vaillancourt, G.; Pazdernik, L. Temperature effects on cadmium and mercury kinetics in freshwater molluscs under laboratory conditions. Arch. Environ. Contam. Toxicol. 1994, 26, 179–184. [Google Scholar] [CrossRef]
- Cunningham, P.; Tripp, M. Factors affecting the accumulation and removal of mercury from tissues of the American oyster Crassostrea virginica. Mar. Biol. 1975, 31, 311–319. [Google Scholar] [CrossRef]
- Valenti, T.W.; Cherry, D.S.; Neves, R.J.; Schmerfeld, J. Acute and chronic toxicity of mercury to early life stages of the rainbow mussel, Villosa iris (Bivalvia: Unionidae). Environ. Toxicol. Chem. Int. J. 2005, 24, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Ituarte, C.F. Growth dynamics in a natural population of Corbicula fluminea (Bivalvia Sphaeriacea) at Punta Atalaya, Río de la Plata, Argentina. Stud. Neotrop. Fauna Environ. 1985, 20, 217–225. [Google Scholar] [CrossRef]
- Ortmann, C.; Grieshaber, M.K. Energy metabolism and valve closure behaviour in the Asian clam Corbicula fluminea. J. Exp. Biol. 2003, 206, 4167–4178. [Google Scholar] [CrossRef]
- Doherty, F.G.; Cherry, D.S.; Cairns, J. Valve closure responses of the Asiatic clam Corbicula fluminea exposed to cadmium and zinc. Hydrobiologia 1987, 153, 159–167. [Google Scholar] [CrossRef]
- Tran, D.; Fournier, E.; Durrieu, G.; Massabuau, J.C. Inorganic mercury detection by valve closure response in the freshwater clam Corbicula fluminea: Integration of time and water metal concentration changes. Environ. Toxicol. Chem. Int. J. 2007, 26, 1545–1551. [Google Scholar] [CrossRef]
- Denton, G.; Burdon-Jones, C. Influence of temperature and salinity on the uptake, distribution and depuration of mercury, cadmium and lead by the black-lip oyster Saccostrea echinata. Mar. Biol. 1981, 64, 317–326. [Google Scholar] [CrossRef]
- Gifford, S.; Dunstan, R.; O’Connor, W.; Roberts, T.; Toia, R. Pearl aquaculture—Profitable environmental remediation? Sci. Total Environ. 2004, 319, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Voice, T.C.; Weber, W.J. Sorbent concentration effects in liquid/solid partitioning. Environ. Sci. Technol. 1985, 19, 789–796. [Google Scholar] [CrossRef]
- Dearden, J.C.; Bresnen, G.M. The measurement of partition coefficients. Quant. Struct. -Act. Relatsh. 1988, 7, 133–144. [Google Scholar] [CrossRef]
- Basen, T.; Martin-Creuzburg, D.; Rothhaupt, K.-O. Role of essential lipids in determining food quality for the invasive freshwater clam Corbicula fluminea. J. N. Am. Benthol. Soc. 2011, 30, 653–664. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Kiørboe, T.; Møhlenberg, F.; Drabaek, I.; Madsen, P.P. Accumulation, elimination and chemical speciation of mercury in the bivalves Mytilus edulis and Macoma balthica. Mar. Biol. 1985, 86, 55–62. [Google Scholar] [CrossRef]


| Parameter | Description | Units | Parameter Estimate | Confidence Interval |
|---|---|---|---|---|
| λ | Exponential decay rate of aqueous Hg adsorbing to sides of container | min−1 | 1.09 × 10−3 | [8.14 × 10−4–1.37 × 10−3] |
| ku | Uptake rate | L·g dry weight clam−1·min−1 | 7.04 × 10−3 | [5.21 × 10−3–8.17 × 10−3] |
| ke | Efflux rate | min−1 | 5.27 × 10−5 | [3.55 × 10−5–8.31 × 10−5] |
| Uptake on Day 5 | Depuration after 2 Weeks | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Avg. Hg (Aq) (ng/L) | Hg (µg/g) | Std. Dev | BCF (L/g) | Cohen’s D | CI Cohen’s D | Hg (µg/g) | Std. Dev | Cohen’s D | CI Cohen’s D |
| Control 2 | 2.09 | 0.5 | 0.18 | 0.51 | 0.243 | |||||
| Control 1 | 2.62 | 1.51 | 1.64 | 0.87 | [−0.15, 5.02] | 0.545 | 0.367 | 0.11 | [−0.71, 1.87] | |
| 20 ng/L (Rep 1) | 14.93 | 1.06 | 0.28 | 71.1 | 2.43 | [−0.91, 3.37] | 0.653 | 0.244 | 0.58 | [−0.99, 1.66] |
| 20 ng/L (Rep 2) | 14.31 | 0.77 | 0.26 | 53.8 | 1.23 | [−0.87, 3.43] | 0.611 | 0.339 | 0.33 | [1.25, 5.25] * |
| 100 ng/L (Rep 1) | 68.45 | 0.92 | 0.43 | 13.4 | 1.28 | [2.35, 14.97] * | 1.424 | 0.306 | 3.25 | [2.15, 7.21] * |
| 100 ng/L (Rep 2) | 72.7 | 3.63 | 0.48 | 49.9 | 8.66 | [−0.23, 4.81] | 1.539 | 1.539 | 4.68 | [1.36, 5.19] * |
| 200 ng/L (Rep 1) | 140 | 5.52 | 3.1 | 39.5 | 2.29 | [−0.54, 4.08] | 1.795 | 0.459 | 3.28 | [1.31, 5.75] * |
| 200 ng/L (Rep 2) | 140.1 | 3.96 | 2.77 | 28.3 | 1.77 | [−1.18, 2.92] | 1.392 | 0.256 | 3.53 | [−1.16, 1.37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geeza, T.J.; Stevenson, L.M.; Mathews, T.J. Uptake, Efflux, and Sequestration of Mercury in the Asian Clam, Corbicula fluminea, at Environmentally Relevant Concentrations, and the Implications for Mercury Remediation. Water 2024, 16, 2931. https://doi.org/10.3390/w16202931
Geeza TJ, Stevenson LM, Mathews TJ. Uptake, Efflux, and Sequestration of Mercury in the Asian Clam, Corbicula fluminea, at Environmentally Relevant Concentrations, and the Implications for Mercury Remediation. Water. 2024; 16(20):2931. https://doi.org/10.3390/w16202931
Chicago/Turabian StyleGeeza, Thomas Jeremy, Louise Mote Stevenson, and Teresa Joan Mathews. 2024. "Uptake, Efflux, and Sequestration of Mercury in the Asian Clam, Corbicula fluminea, at Environmentally Relevant Concentrations, and the Implications for Mercury Remediation" Water 16, no. 20: 2931. https://doi.org/10.3390/w16202931
APA StyleGeeza, T. J., Stevenson, L. M., & Mathews, T. J. (2024). Uptake, Efflux, and Sequestration of Mercury in the Asian Clam, Corbicula fluminea, at Environmentally Relevant Concentrations, and the Implications for Mercury Remediation. Water, 16(20), 2931. https://doi.org/10.3390/w16202931






