The Degradation of Rhodamine B by an Electro-Fenton Reactor Constructed with Gas Diffusion Electrode and Heterogeneous CuFeO@C Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation Processes of Modified r-GO Composites and GDEs
2.3. Preparation Process of CuFeO@C Particles
2.4. Electro-Fenton Reactor Setup with GDE Cathodes and Catalytic CuFeO@C Particles
2.5. Characterization
3. Results and Discussion
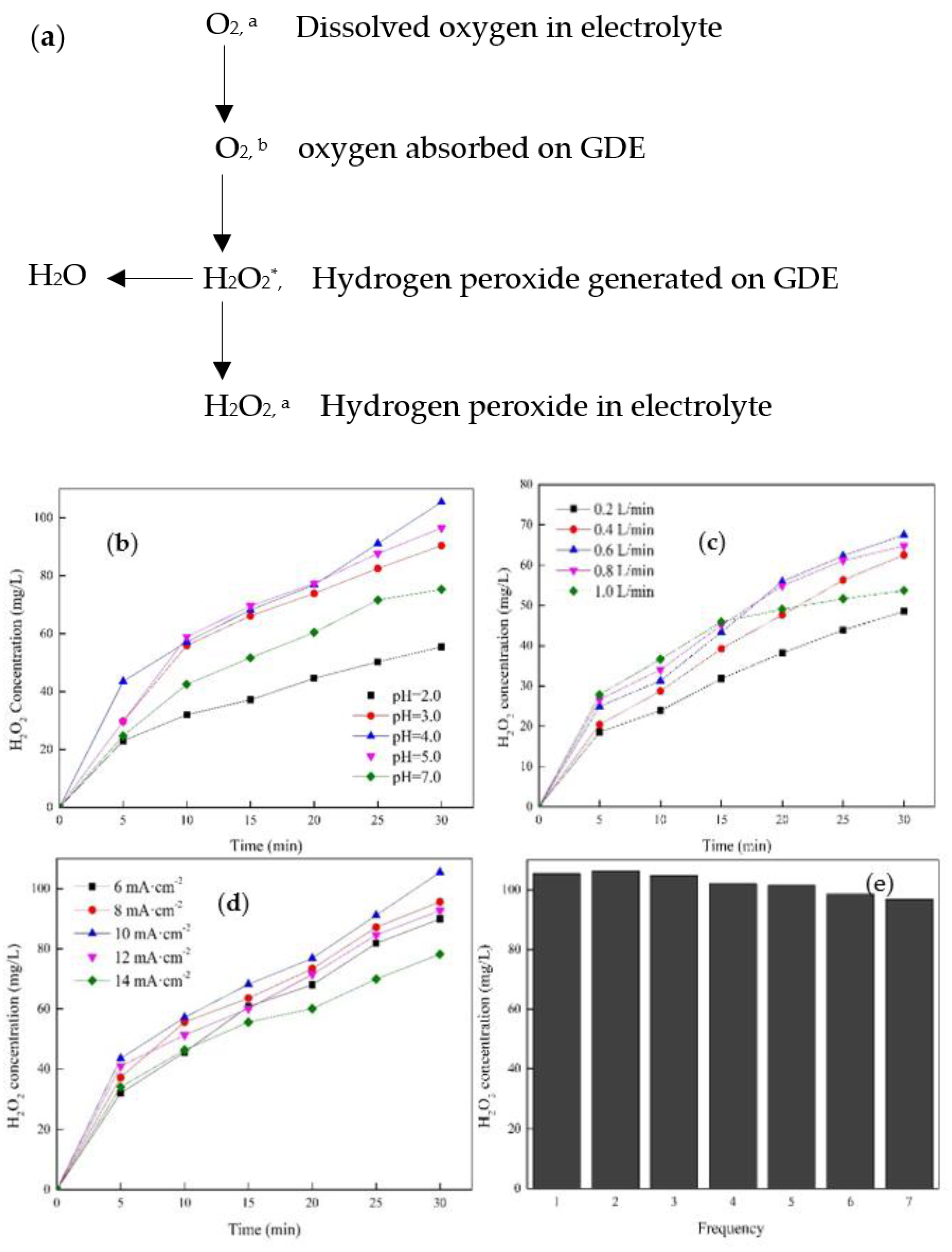
3.1. Characteristics of GDE and Its Performance on H2O2 Production
3.1.1. Specific Surface Area Analysis
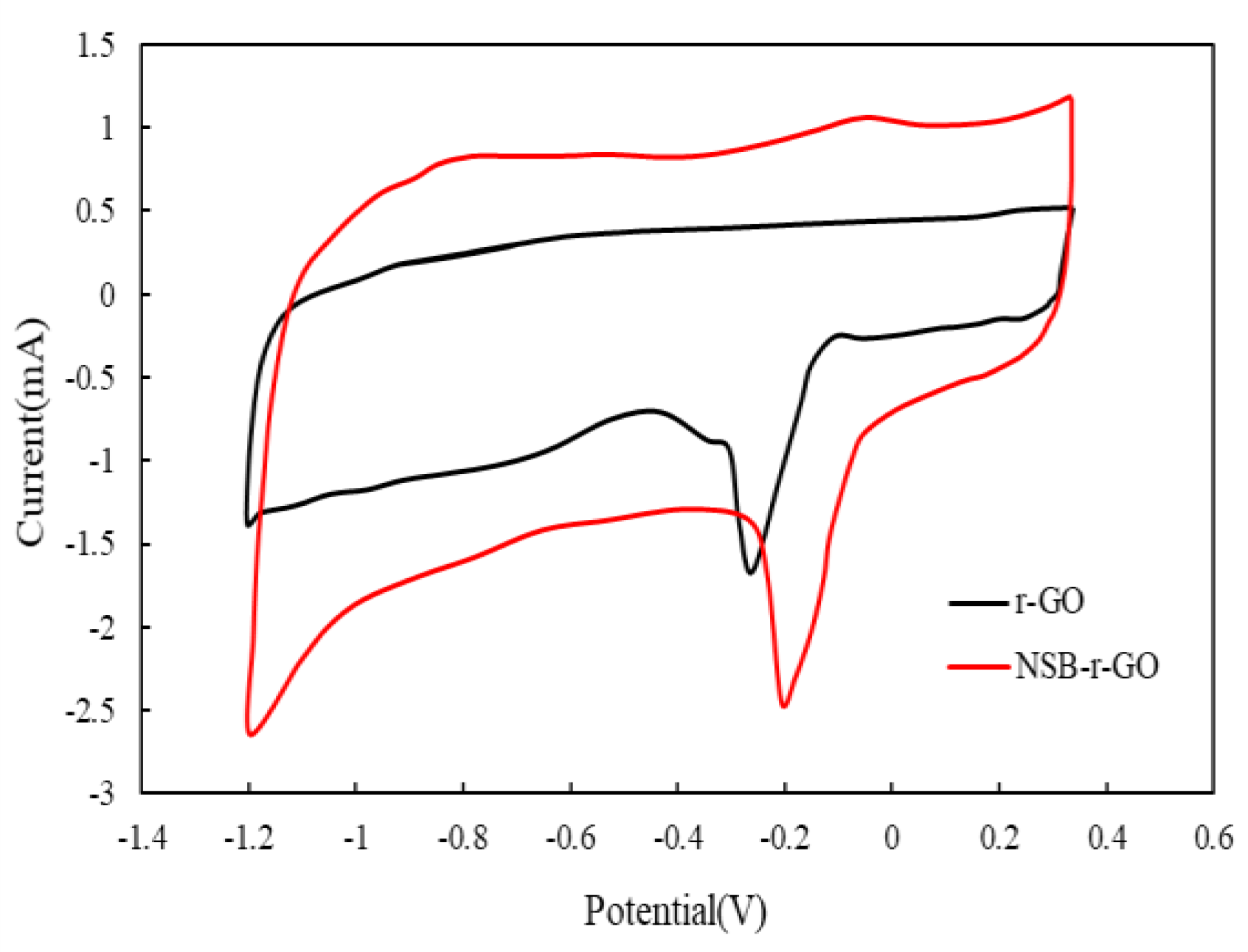
3.1.2. Cycle Voltammetry Curve
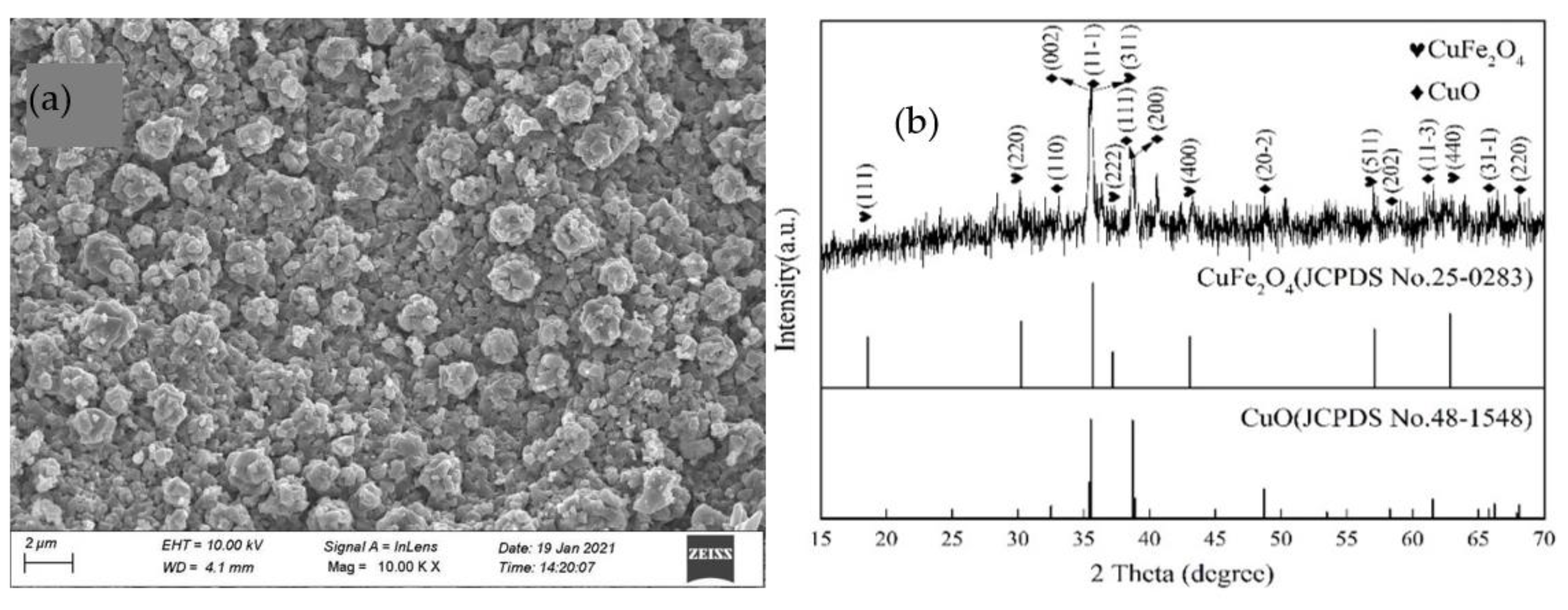
3.1.3. Morphologies and Element Analysis
3.1.4. Effects of pH, O2 Flow Rate, Current Density, and Reusage on the Production of H2O2
3.2. Characteristics of CuFeO@C Particles
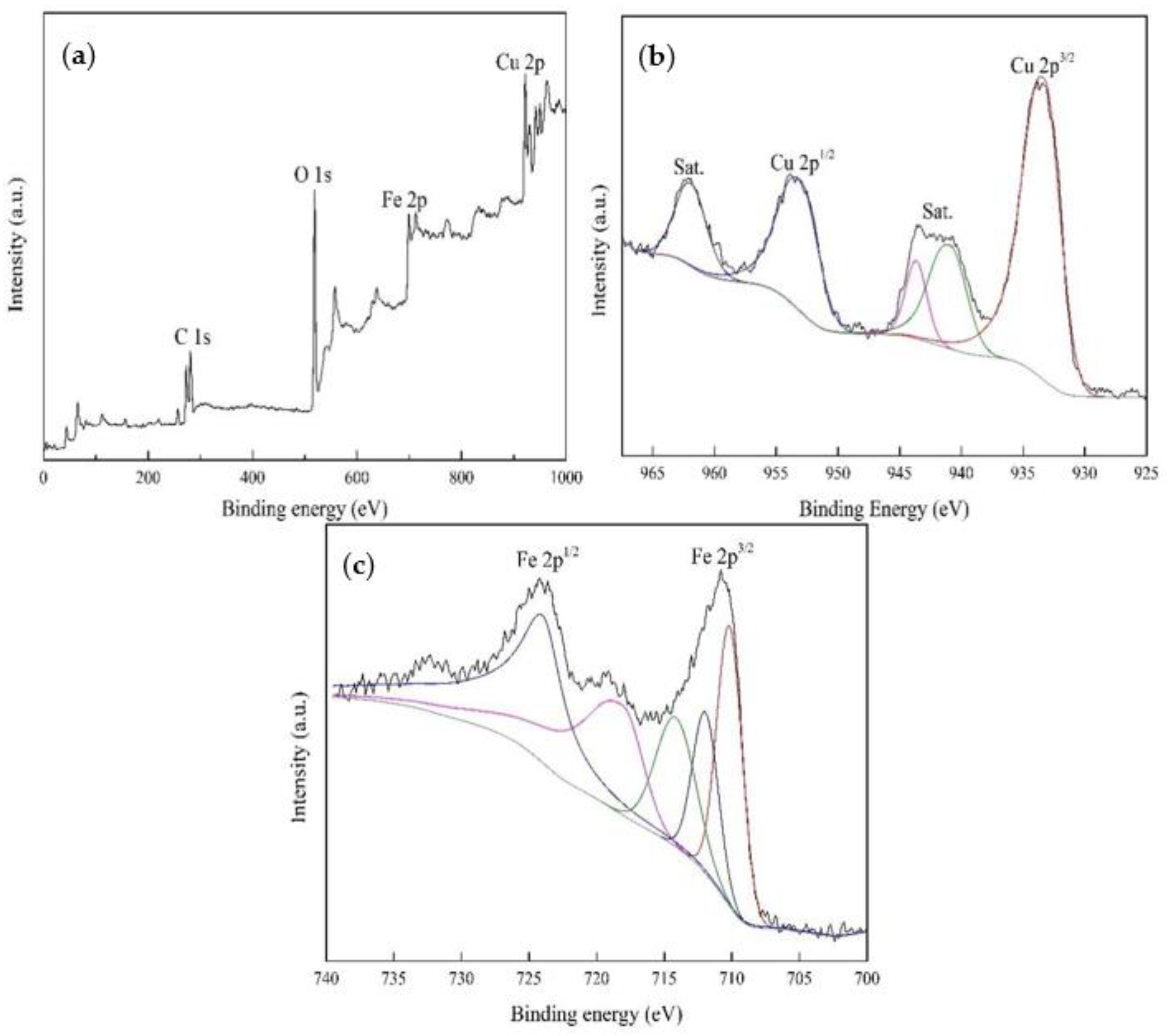
3.2.1. Surface Characterization of Heterogeneous CuFeO@C Particles
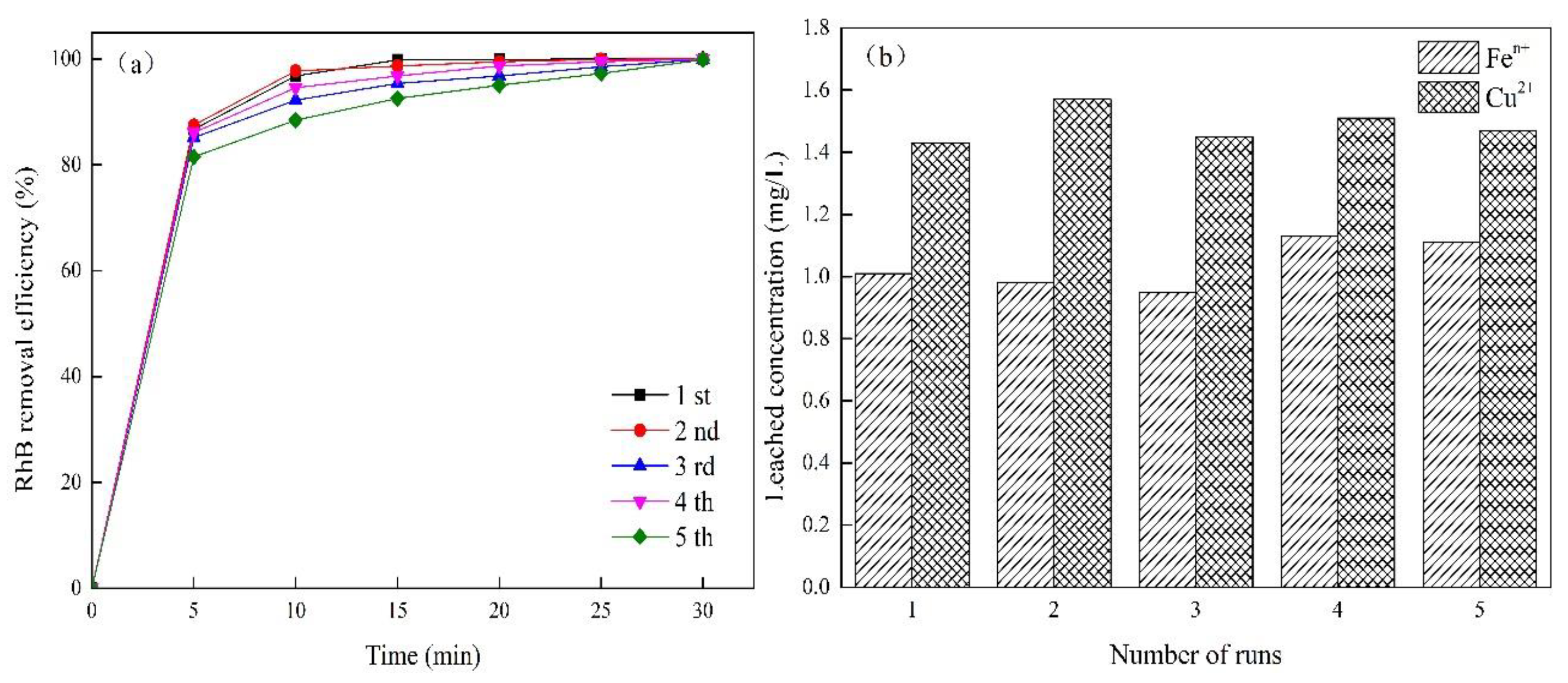
3.2.2. Reuse of CuFeO@C Particles
3.3. Degradation of Rh B by Electro-Fenton Process
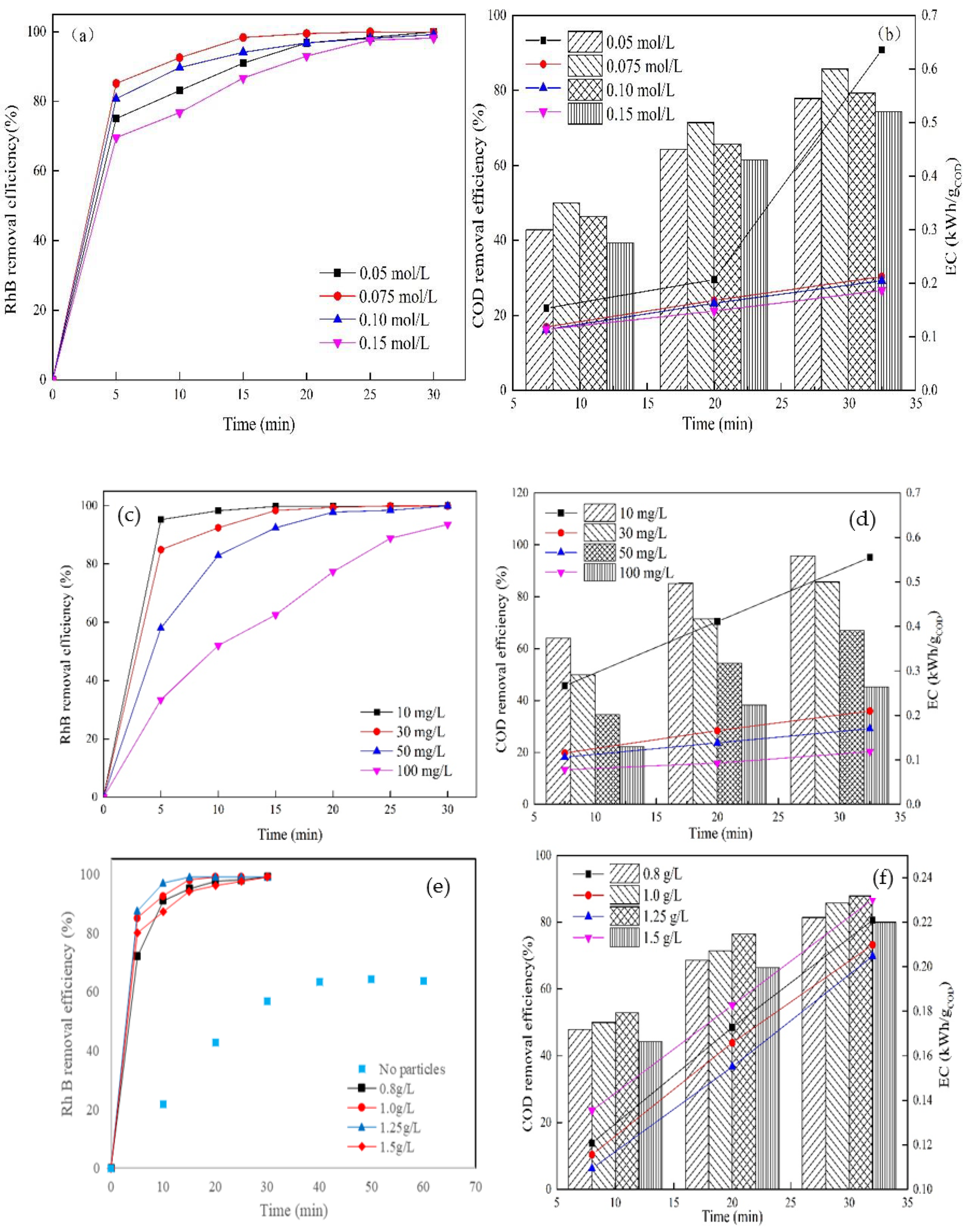
3.3.1. Effects of Electrolytes
3.3.2. Initial Rh B Concentrations
3.3.3. CuFeO@C Particle Concentrations
3.3.4. Simulated Wastewater Containing Anions and Other Organic Pollutants
3.3.5. Pathway of RhB Degradation by Electro-Fenton Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J.; Ren, X.; Ye, S.; Peng, B.; Feng, H. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye using Tectona grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Monash, P.; Velu, S.; Banat, F.; Naushad, M.; Arthanareeswaran, G.; Show, P.L. Effective treatment of dye polluted wastewater using nanoporous CaCl2 modified polyethersulfone membrane. Process Saf. Environ. Prot. 2019, 124, 266–278. [Google Scholar] [CrossRef]
- Asadollahfardi, G.; Zangooei, H.; Motamedi, V.; Davoodi, M. Selection of coagulant using jar test and analytic hierarchy process: A case study of Mazandaran textile wastewater. Adv. Environ. Res. 2018, 7, 1–11. [Google Scholar]
- Al-Alwani, M.A.M.; Ludin, N.A.; Mohamad, A.; Kadhum, A.H.; Mukhlus, A. Application of dyes extracted from Alternanthera dentata leaves and Musa acuminata bracts as natural sensitizers for dye-sensitized solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Bassyouni, D.G.; Hamad, H.A.; El-Ashtoukhy, E.S.Z.; Amin, N.K.; Abd El-Latif, M.M. Comparative performance of anodic oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187. [Google Scholar] [CrossRef]
- Li, N.; An, J.; Zhou, L.; Li, T.; Li, J.; Feng, C.; Wang, X. A novel carbon black graphite hybrid air-cathode for efficient hydrogen peroxide production in bioelectrochemical systems. J. Power Sources 2016, 306, 495–502. [Google Scholar] [CrossRef]
- Zhou, W.; Meng, X.; Gao, J.; Alshawabkeh, A.N. Hydrogen peroxide generation from O2 electroreduction for environmental remediation: A state-of-the-art review. Chemosphere 2019, 225, 588–607. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, H.; Liu, Y.; Yan, C.; Hong, S.; Masa, J.; Robertson, A.W.; Liu, S.; Qiu, J.; Sun, Z. Ultrasound-Assisted Nitrogen and Boron Co-doping of Graphene Oxide for Efficient Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 3434–3442. [Google Scholar] [CrossRef]
- Lu, K.Q.; Yuan, L.; Xin, X.; Xu, Y.-J. Hybridization of graphene oxide with commercial graphene for constructing 3D metal-free aerogel with enhanced photocatalysis. Appl. Catal. B Environ. 2018, 226, 16–22. [Google Scholar] [CrossRef]
- Jiang, Y.; Ni, P.; Chen, C.; Lu, Y.; Yang, P.; Kong, B.; Fisher, A.; Wang, X. Selective Electrochemical H2O2 Production through Two Electron Oxygen Electrochemistry. Adv. Energy Mater. 2018, 8, 1801909. [Google Scholar] [CrossRef]
- Sun, Z.H.; Yan, Z.Q.; Yue, K.C.; Li, A.; Qian, L. Novel high-performance electromagnetic absorber based on Nitrogen/Boron co-doped reduced graphene oxide. Compos. Part B Eng. 2020, 196, 108132. [Google Scholar] [CrossRef]
- Liu, J.L.; Zhu, Y.R.; Chen, X.H.; Yi, W. Nitrogen, sulfur and phosphorus tri-doped holey graphene oxide as a novel electrode material for application in supercapacitor. J. Alloys Compd. 2020, 815, 152328. [Google Scholar] [CrossRef]
- Meki, K.; Wang, Z.; Jing, B.; Qiu, S.; Deng, F. Enhanced electrochemical O2 reduction to H2O2 through resilient hybrid catalysts modified with DMSO in electro-Fenton. Sep. Purif. Technol. 2024, 346, 127490. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, C.; Liu, L.; Jiang, X.; Liu, C.; Liu, F.; Sun, J.; Wang, Y. Progress in copper-based supported heterogeneous electro-Fenton catalysts. Chem. Eng. J. 2024, 486, 150217. [Google Scholar] [CrossRef]
- Labiadh, L.; Ammar, S.; Kamali, A.R. Oxidation/mineralization of AO7 by electro-Fenton process using chalcopyrite as the heterogeneous source of iron and copper catalysts with enhanced degradation activity and reusability. J. Electroanal. Chem. 2019, 853, 10–32. [Google Scholar] [CrossRef]
- Zahrani, A.A.; Ayati, B. Using heterogeneous Fe-ZSM-5 nanocatalyst to improve the electro Fenton process for acid blue 25 removal in a novel reactor with orbiting electrodes. J. Electroanal. Chem. 2020, 873, 114456. [Google Scholar] [CrossRef]
- Khandelwal, M.; Li, Y.; Hur, S.H.; Chung, J.S. Surface modification of co-doped reduced graphene oxide through alkanolamine functionalization for enhanced electrochemical performance. New J. Chem. 2018, 42, 1105–1114. [Google Scholar] [CrossRef]
- Mannan, M.A.; Hirano, Y.; Quitain, A.T.; Koinuma, M.; Kida, T. Graphene Oxide to, B. N Co-doped Graphene through Tris-dimethylaminoborane Complex by Hydrothermal Implantation. Am. J. Mater. Sci. 2019, 9, 22–28. [Google Scholar]
- Prakash, D.; Manivannan, S. N, B co-doped and Crumpled Graphene Oxide Pseudocapacitive Electrode for High Energy Supercapacitor. Surf. Interfaces 2021, 23, 101025. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, M.; Oturan, N.; Li, Y.; Oturan, M.A. Electrocatalytic destruction of pharmaceutical imatinib by electro-Fenton process with graphene-based cathode. Electrochim. Acta 2019, 305, 285–294. [Google Scholar] [CrossRef]
- Xia, Y.; Shang, H.; Zhang, Q.; Zhou, Y.; Hu, X. Electrogeneration of hydrogen peroxide using phosphorus-doped carbon nanotubes gas diffusion electrodes and its application in electro-Fenton. J. Electroanal. Chem. 2019, 840, 400–408. [Google Scholar] [CrossRef]
- Petran, A.; Radu, T.; Culic, B.; Turcu, R. Tailoring the properties of magnetite nanoparticles clusters by coating with double inorganic layers. Appl. Surf. Sci. 2016, 390, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Yan, J.F.; Yao, G.; Zhang, Y.; Li, X.; Lai, B. Improving the degradation of atrazine in the three-dimensional (3D) electrochemical process using CuFe2O4 as both particle electrode and catalyst for persulfate activation. Chem. Eng. J. 2019, 361, 1317–1332. [Google Scholar] [CrossRef]
- Peng, M.; He, J.; An, J.; Xie, T.; Zhao, T.; Li, G. Establishment of a reagent-free three-dimensional electro-Fenton system for high H2O2 production and efficient degradation of Roxarsone. Chem. Eng. J. 2023, 477, 146963. [Google Scholar] [CrossRef]







| Samples | Specific Surface Area (m2·g−1) | Pore Volume (cm3·g−1) | Pore Size (nm) |
|---|---|---|---|
| r-GO composites | 23.793 | 0.105 | 3.805 |
| NSB-r-GO composites | 46.491 | 0.098 | 3.805 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Gu, S.; Jia, X.; Su, X.; Li, Y.; Zhang, Y.; Du, Y.; Ding, Y. The Degradation of Rhodamine B by an Electro-Fenton Reactor Constructed with Gas Diffusion Electrode and Heterogeneous CuFeO@C Particles. Water 2024, 16, 2906. https://doi.org/10.3390/w16202906
Li S, Gu S, Jia X, Su X, Li Y, Zhang Y, Du Y, Ding Y. The Degradation of Rhodamine B by an Electro-Fenton Reactor Constructed with Gas Diffusion Electrode and Heterogeneous CuFeO@C Particles. Water. 2024; 16(20):2906. https://doi.org/10.3390/w16202906
Chicago/Turabian StyleLi, Shuo, Siyang Gu, Xiaotong Jia, Xin Su, Yifan Li, Yang Zhang, Yunmei Du, and Yuanhong Ding. 2024. "The Degradation of Rhodamine B by an Electro-Fenton Reactor Constructed with Gas Diffusion Electrode and Heterogeneous CuFeO@C Particles" Water 16, no. 20: 2906. https://doi.org/10.3390/w16202906
APA StyleLi, S., Gu, S., Jia, X., Su, X., Li, Y., Zhang, Y., Du, Y., & Ding, Y. (2024). The Degradation of Rhodamine B by an Electro-Fenton Reactor Constructed with Gas Diffusion Electrode and Heterogeneous CuFeO@C Particles. Water, 16(20), 2906. https://doi.org/10.3390/w16202906








