Application of Immobilized Microorganism Gel Beads in Black-Odor Water with High Nitrogen and Phosphorus Removal Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Compound Strains and Amplification Preparation
2.2. Response Surface Methodology Optimization Experiments
2.3. Preparation of Microbial Immobilized Gel Beads
2.4. Performance Optimization of Immobilized Gel Beads
2.5. Characterization of Immobilized Microorganism Materials
2.6. Tolerance Analysis
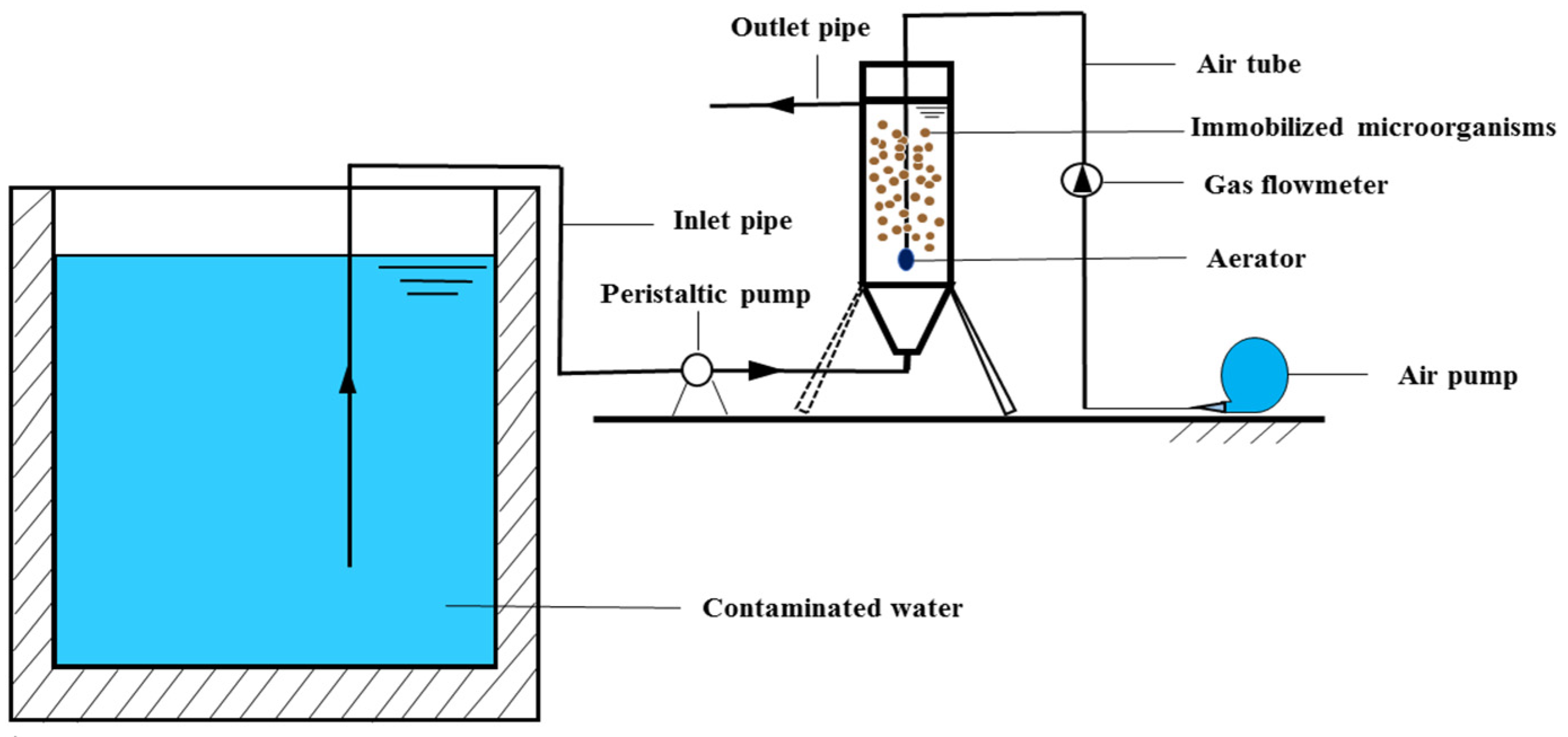
2.7. Water Purification Effect of Immobilization Materials
3. Results and Discussion
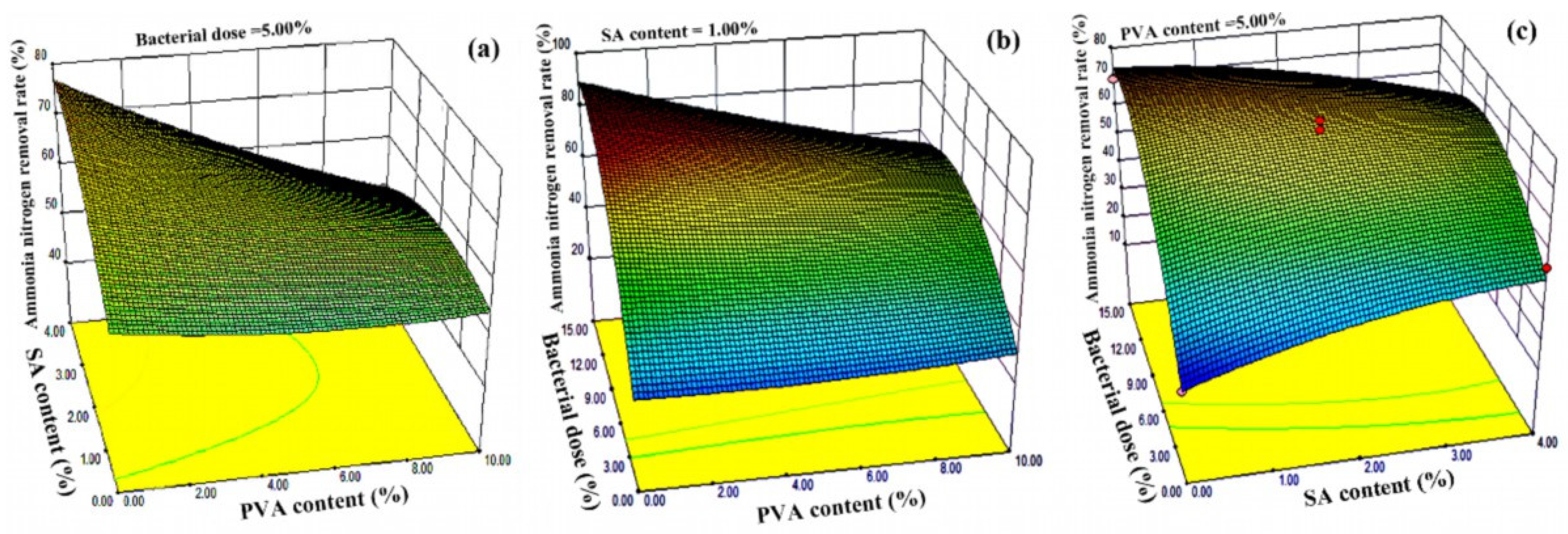
3.1. Optimization of Immobilized Microorganism Gel Beads by Response Surface
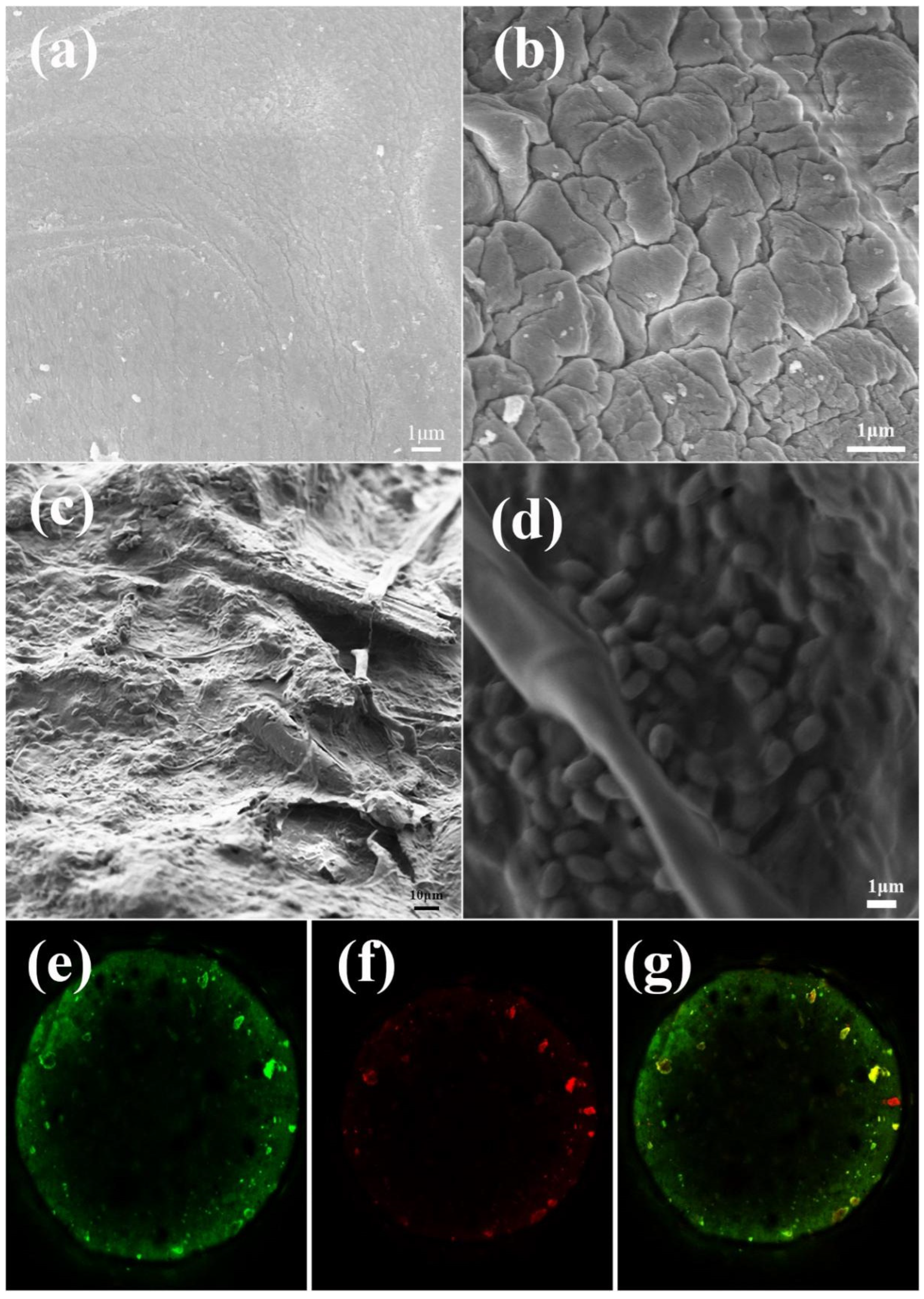
3.2. Characterization of Immobilized Microorganism Gel Beads
3.3. The Optimization Design of Immobilized Microorganism Gel Beads
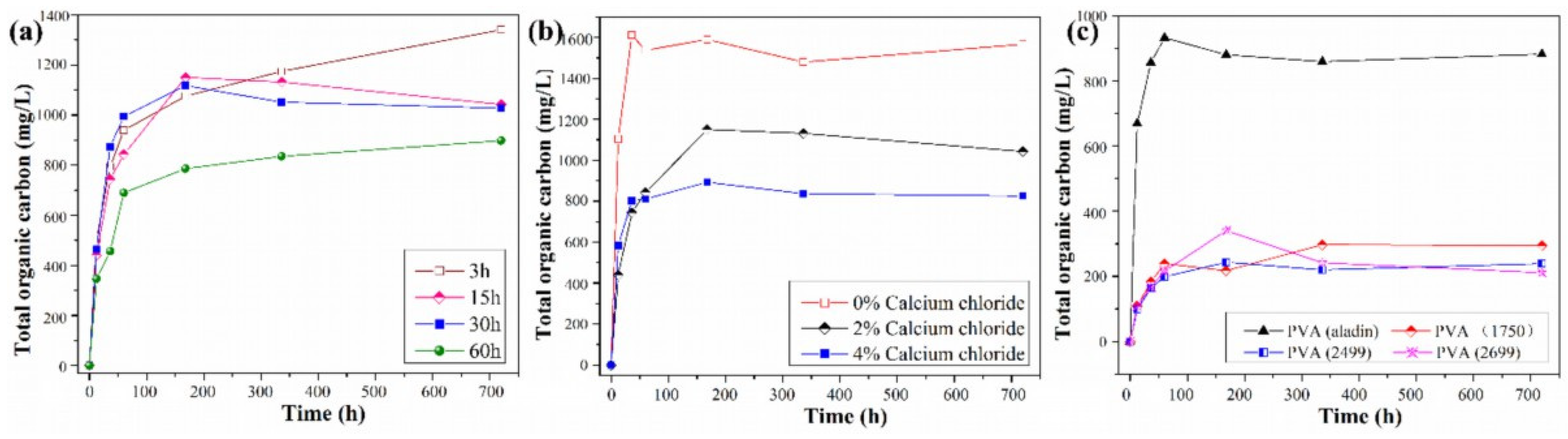
3.3.1. The Dissolution of Organic Matter from the Gel Beads
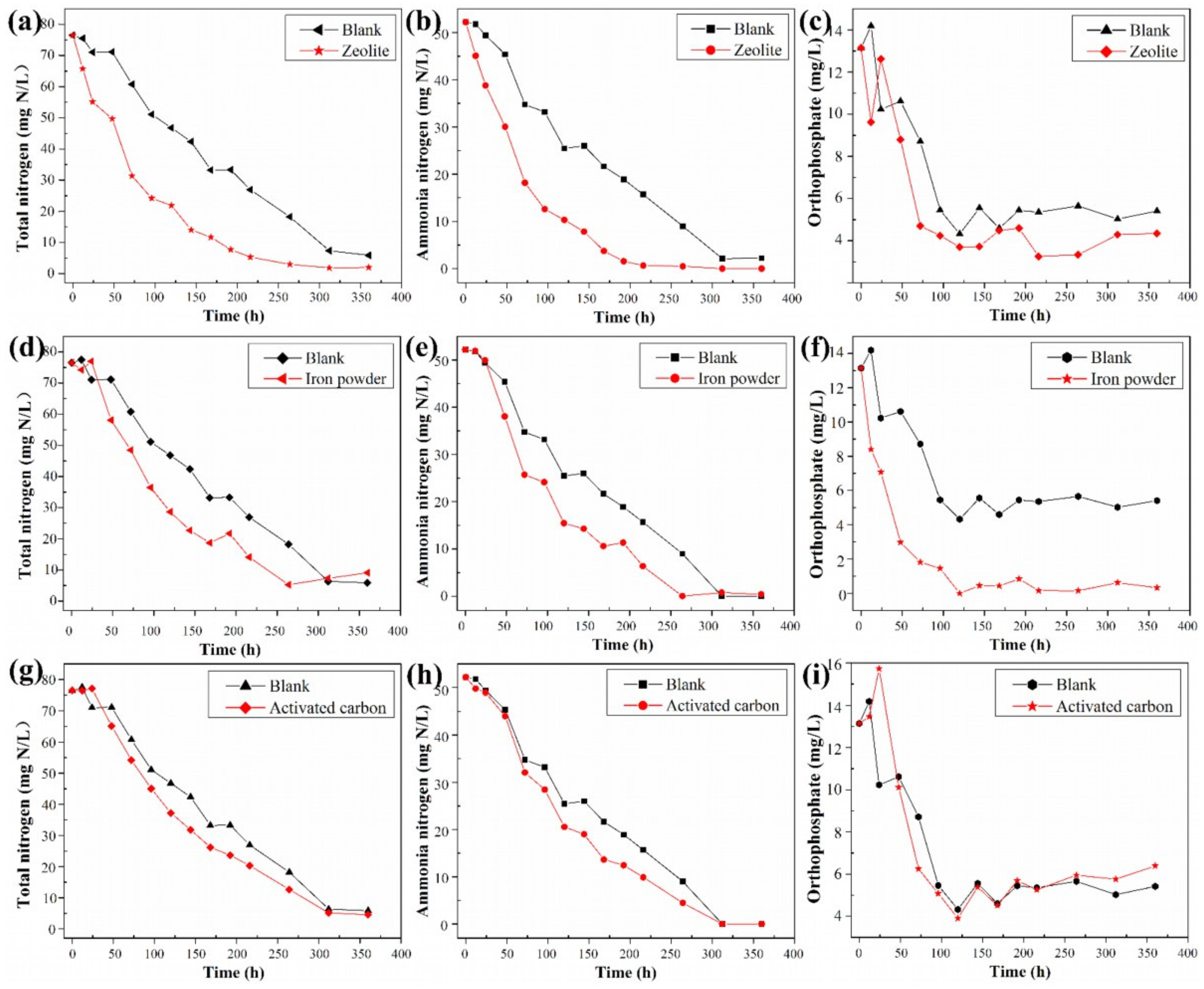
3.3.2. Loading Inorganic Particles to Improve Properties
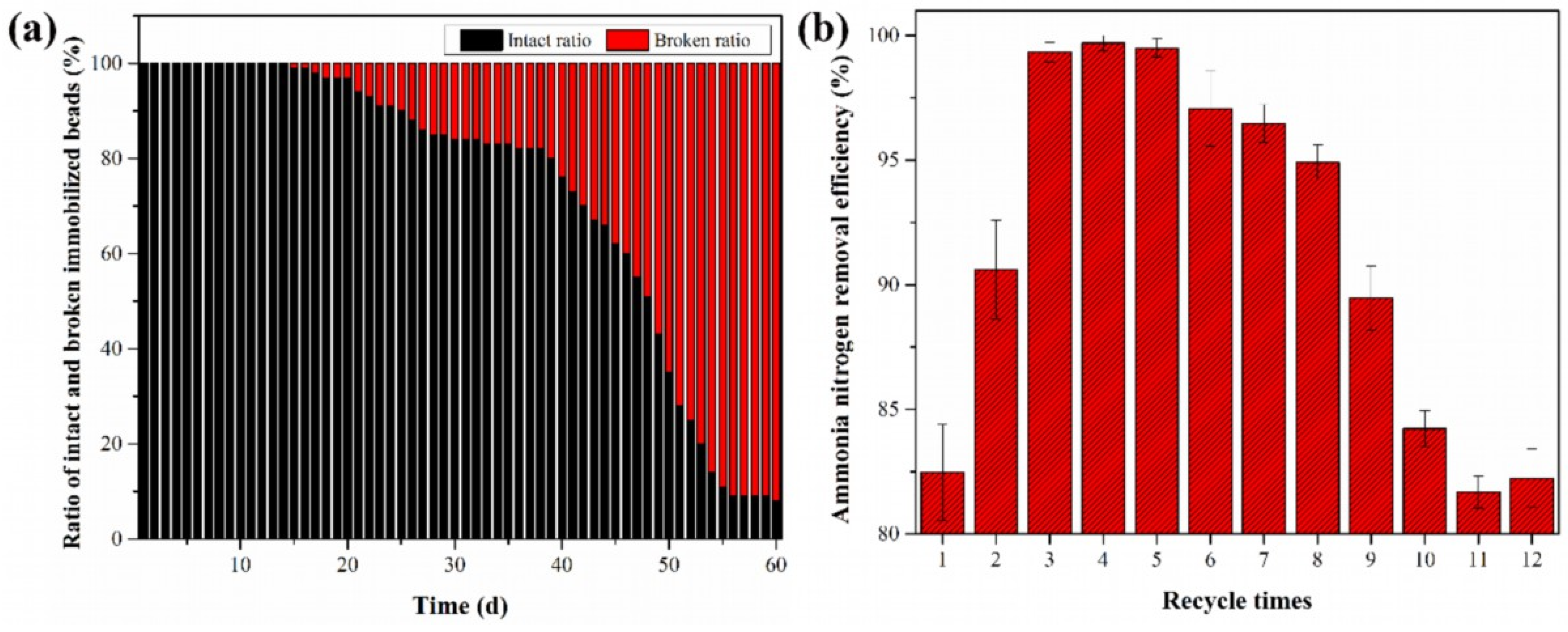
3.4. Tolerance Analysis of the Gel Beads
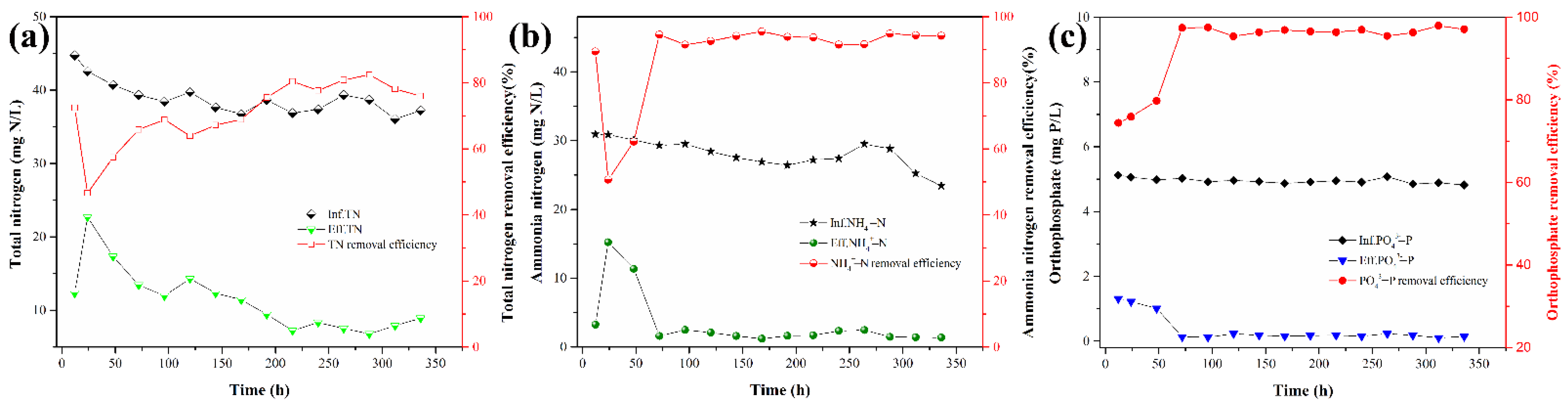
3.5. The Performance of Nitrogen and Phosphorus Removal in Black-Odor Water
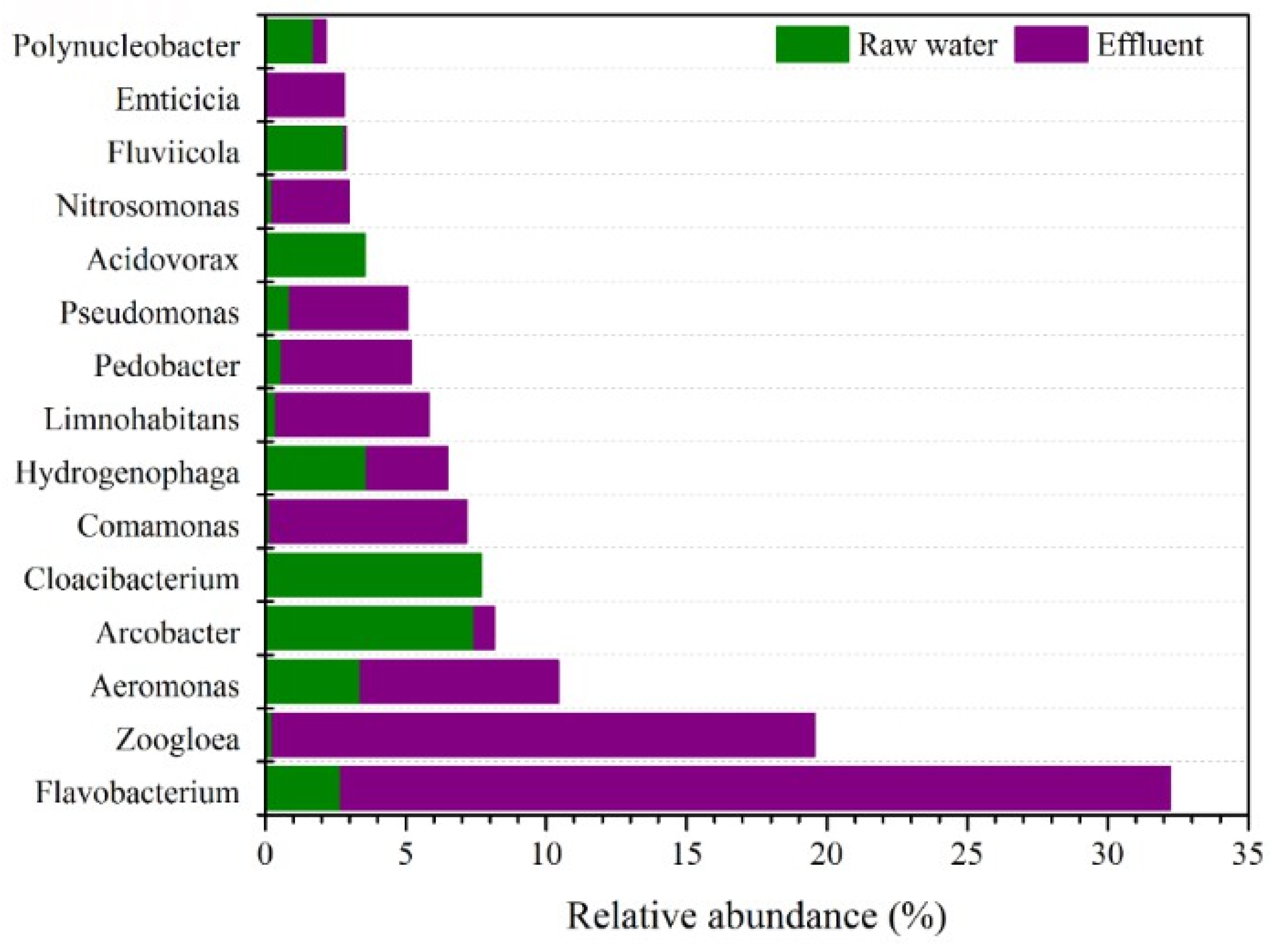
3.6. Microbial Colony Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.B.; Wang, L.; Zhang, X.; He, M.H.; Wang, D. Effects of different types of anthropogenic disturbances and natural wetlands on water quality and microbial communities in a typical black-odor river. Ecol. Indic. 2022, 136, 108613. [Google Scholar] [CrossRef]
- Yin, H.B.; Yang, P.; Kong, M. Effects of nitrate dosing on the migration of reduced sulfur in black odorous river sediment and the influencing factors. Chem. Eng. J. 2019, 371, 516–523. [Google Scholar] [CrossRef]
- Xie, H.W.; Wang, M.Y.; Zeng, H.Y.; Yu, M.R.; Wu, Z.J.; Chen, S.H.; Zhao, S.T.; Zheng, J.; Deng, D. Improvement of Black-Odor Water by Pichia Strain GW1 under Optimized NH3-N Degradation Conditions. Biomed. Res. Int. 2020, 1, 1537873. [Google Scholar] [CrossRef]
- Roya, B.; Piero, F.; Mentore, V. Best management practices for minimizing undesired effects of thermal remediation and soil washing on soil properties. A review. Environ. Sci. Pollut. Res. 2023, 30, 103480–103495. [Google Scholar] [CrossRef]
- Chen, G.N.; Pan, L.S.; Sun, Z.; Xiong, J.H.; Zhu, H.X.; Wang, S.F.; Song, H.N.; Lin, H.F.; Chen, Y.L.; Liang, J.X. Removal of Nitrogen and Phosphorus from Black-Odor Water by Different Submerged Plants. J. Biobased Mater. Bioenergy 2020, 14, 524–530. [Google Scholar] [CrossRef]
- Wang, L.G.; Han, Y.Q.; Yu, H.H.; Fan, S.F.; Liu, C.H. Submerged Vegetation and Water Quality Degeneration from Serious Flooding in Liangzi Lake, China. Front. Plant Sci. 2019, 10, 1504. [Google Scholar] [CrossRef] [PubMed]
- Meinert, B.; Knox, L. Good Odor Treatment Makes Good Neighbors—Piloting and Design of DC Water’s Main & O Street Pumping Stations’ Odor Control. Proc. Water Environ. Fed. 2017, 4, 5572–5589. [Google Scholar] [CrossRef]
- Monge-Amaya, O.; Barragan, M.T.C.; Almendariz-Tapia, F.J. Microbial Biomass in Batch and Continuous System. In Biomass Now-Sustainable Growth and Use; IntechOpen Limited: London, UK, 2013. [Google Scholar] [CrossRef]
- Lan, H.X.; Qi, S.X.; Yang, D.; Wang, X.Z.; Zhang, P.M.; Zhang, H.; Sun, Y.H. Treatment of White Water with Combined Predominant Bacteria and Immobilized Enzyme. BioResources 2020, 15, 4016–4025. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell. Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Malusa, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 357–369. [Google Scholar] [CrossRef]
- Baransi-Karkaby, K.; Hassanin, M.; Muhsein, S.; Massalha, N.; Sabbah, I. Innovativeex-situbiological biogas upgrading using immobilized biomethanation bioreactor (IBBR). Water Sci. Technol. 2020, 81, 1319–1328. [Google Scholar] [CrossRef]
- Magri, A.; Vanotti, M.B.; Szogi, A.A. Anammox sludge immobilized in polyvinyl alcohol (PVA) cryogel carriers. Bioresour. Technol. 2012, 114, 231–240. [Google Scholar] [CrossRef]
- Ahmad, M.; Liu, S.T.; Mahmood, N.; Mahmood, A.; Ali, M.; Zheng, M.S.; Ni, J.R. Synergic Adsorption-Biodegradation by an Advanced Carrier for Enhanced Removal of High-Strength Nitrogen and Refractory Organics. ACS Appl. Mater. Interfaces 2017, 15, 13188–13200. [Google Scholar] [CrossRef]
- Wong, E.T.; Chan, K.H.; Idris, A. Kinetic and equilibrium investigation of Cu(II) removal by Co(II)-doped iron oxide nanoparticle-immobilized in PVA-alginate recyclable adsorbent under dark and photo condition. Chem. Eng. J. 2015, 268, 311–324. [Google Scholar] [CrossRef]
- Jeong, D.; Cho, K.; Lee, C.H.; Lee, S.; Bae, H. Integration of forward osmosis process and continuous airlift nitrifying bioreactor containing PVA/alginate-immobilized cells. Chem. Eng. J. 2016, 306, 1212–1222. [Google Scholar] [CrossRef]
- Jang, J.; Lee, D.S. Enhanced adsorption of cesium on PVA-alginate encapsulated Prussian blue-graphene oxide hydrogel beads in a fixed-bed column system. Bioresour. Technol. 2016, 218, 294–300. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Lei, C.; Khan, E.; Chen, S.S.; Tsang, D.C.W.; Ok, Y.S.; Lin, D.H.; Feng, Y.J.; Li, X.D. Aging effects on chemical transformation and metal(loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment. Sci. Total Environ. 2018, 615, 498–507. [Google Scholar] [CrossRef]
- Yi, H.; Li, M.F.; Huo, X.Q.; Zeng, G.M.; Lai, C.; Huang, D.L.; An, Z.W.; Qin, L.; Liu, X.G.; Li, B.S.; et al. Recent development of advanced biotechnology for wastewater treatment. Crit. Rev. Biotechnol. 2019, 40, 99–118. [Google Scholar] [CrossRef]
- Genisheva, Z.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Evaluating the potential of wine-making residues and corn cobs as support materials for cell immobilization for ethanol production. Ind. Crops Prod. 2011, 34, 979–985. [Google Scholar] [CrossRef]
- Schroeder, A.; Souza, D.H.; Fernandes, M.; Rodrigues, E.B.; Trevisan, V.; Skoronski, E. Application of glycerol as carbon source for continuous drinking water denitrification using microorganism from natural biomass. J. Environ. Manag. 2020, 256, 109964. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Bayat, Z.; Hassanshahian, M.; Cappello, S. Immobilization of Microbes for Bioremediation of Crude Oil Polluted Environments: A Mini Review. Open Microbiol. J. 2015, 9, 48–54. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; El-Naas, M. Immobilization of Pseudomonas putida in PVA gel particles for the biodegradation of phenol at high concentrations. Biochem. Eng. J. 2011, 56, 46–50. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Cassidy, M.B.; Lee, H.; Trevors, J.T. Environmental applications of immobilized microbial cells: A review. J. Ind. Microbiol. Biotechnol. 1996, 16, 79–101. [Google Scholar] [CrossRef]
- Kumar, T.; Mandlimath, T.R.; Sangeetha, P.; Revathi, S.K.; Kumar, S.K.A. Nanoscale materials as sorbents for nitrate and phosphate removal from water. Environ. Chem. Lett. 2017, 16, 389–400. [Google Scholar] [CrossRef]
- Zhu, Q.; Hou, D. Preparation of PVA Carrier for Microorganism Immobilization and Its Application in Removing Ammonia Nitrogen from Wastewater. Environ. Sci. Manag. 2018, 43, 116–120. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yaneth, A.B.; Rogelio, E.; Blenda, R.; Victoria, B.; Jesús, G.R. Kinetics of a Fixed Bed Reactor with Immobilized Microorganisms for the Removal of Organic Matter and Phosphorous. Water Environ. Res. 2020, 92, 1956–1965. [Google Scholar] [CrossRef]
- Quan, L.M.; Khanh, D.P.; Hira, D.; Fujii, T.; Furukawa, K. Reject water treatment by improvement of whole cell anammox entrapment using polyvinyl alcohol/alginate gel. Biodegradation 2011, 22, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Kiran, M.G.; Pakshirajan, K.; Das, G. Heavy metal removal from aqueous solution using sodium alginate immobilized sulfate reducing bacteria: Mechanism and process optimization. J. Environ. Manag. 2018, 218, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Z.; Zhang, F.F.; Luo, L.; Li, Y.J.; Zhang, H.M.; Cheng, L.; Cao, Y.X. The immobilization of Pseudomonas flava WD-3 and its application in SBR for sewage treatment. J. Environ. Sci. 2016, 36, 1639–1647. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, Z.L.; Megharaj, M.; Naidu, R. Biodegradation of TNT using Bacillus mycoides immobilized in PVA–sodium alginate–kaolin. Appl. Clay Sci. 2013, 84, 336–342. [Google Scholar] [CrossRef]
- Hong, M.; Wang, D.; Li, Y.Q.; Wang, A.M.; Zhuang, H. Experiment on the Remediation Chlorobenzene-contaminated Groundwater by Using Immobilized Microorganisms. Technol. Rev. 2012, 30, 21–24. [Google Scholar] [CrossRef]
- Tauro, F. Particle tracers and image analysis for surface flow observations. Wiley Interdiscip. Rev.-Water 2015, 3, 25–39. [Google Scholar] [CrossRef]
- Dave, R.; Madamwar, D. Esterification in organic solvents by lipase immobilized in polymer of PVA-alginate-boric acid. Process Biochem. 2006, 41, 951–955. [Google Scholar] [CrossRef]
- Min, X.B.; Chai, L.Y.; Zhang, C.F.; Takasaki, Y.; Okura, T. Control of metal toxicity, effluent COD and regeneration of gel beads by immobilized sulfate-reducing bacteria. Chemosphere 2008, 72, 1086–1091. [Google Scholar] [CrossRef]
- Aljerf, L. Advanced highly polluted rainwater treatment process. J. Environ. Sci. Manag. 2018, 12, 50–58. [Google Scholar] [CrossRef]
- Uemoto, H.; Morita, M. Nitrogen removal with a dual bag system capable of simultaneous nitrification and denitrification. Biochem. Eng. J. 2010, 52, 104–109. [Google Scholar] [CrossRef]
- Li, L.T. Study on the Sewage Treatment Efficiency of SBBR Immobilized Microorganisms on Sodium Alginate Gel Beads; Sichuan University: Chengdu, China, 2021. [Google Scholar]
- Zoratto, N.; Di Lisa, D.; de Rutte, J.; Sakib, M.N.; Silva, A.; Tamayol, A.; Di Carlo, D.; Khademhosseini, A.; Sheikhi, A. In situ forming microporous gelatin methacryloyl hydrogel scaffolds from thermostable microgels for tissue engineering. Bioeng. Transl. Med. 2020, 5, e10180. [Google Scholar] [CrossRef]
- Sun, L.; Wang, J.X.; Liang, J.D.; Li, G.G. Boric Acid Cross-linked 3D Polyvinyl Alcohol Gel Beads by NaOH-Titration Method as a Suitable Biomass Immobilization Matrix. J. Polym. Environ. 2020, 28, 532–541. [Google Scholar] [CrossRef]
- Purnomo, A.S.; Hairunnisa, F.W.; Misdar Maria, V.P.; Rohmah, A.A.; Putra, S.R. Anionic dye removal by immobilized bacteria into alginate-polyvinyl alcohol-bentonite matrix. Heliyon 2024, 10, 11139. [Google Scholar] [CrossRef]
- Syiem, M.B.; Bhattacharjee, A. Structural and functional stability of regenerated cyanobacteria following immobilization. J. Appl. Phycol. 2015, 27, 743–753. [Google Scholar] [CrossRef]
- Patro, T.U.; Wagner, H.D. Influence of Graphene Oxide Incorporation and Chemical Cross-Linking on Structure and Mechanical Properties of Layer-by-Layer Assembled Poly(VinylAlcohol)-Laponite Free-Standing Films. J. Polym. Sci. Part B-Polym. Phys. 2016, 54, 2377–2387. [Google Scholar] [CrossRef]
- Ao, W.L.; Qiang, C.Z.; Hui, Y.; Gang, H.U.; Zhang, J. Conditions of Imbedded Immobilization for the Treatment of Methanol Wastewater by Immobilized Microorganism. J. Chongqing Univ. Nat. Sci. Ed. 2005, 28, 113–117. [Google Scholar] [CrossRef]
- Al-Mayah, A.M.R. Simulation of Enzyme Catalysis in Calcium Alginate Beads. Enzyme Res. 2012, 2012, 459190. [Google Scholar] [CrossRef]
- Soo, C.L.; Chen, C.A.; Bojo, O.; Hii, Y.S. Feasibility of Marine Microalgae Immobilization in Alginate Bead for Marine Water Treatment: Bead Stability, Cell Growth, and Ammonia Removal. Int. J. Polym. Sci. 2017, 2017, 6951212. [Google Scholar] [CrossRef]
- Banerjee, A.; Sarkar, P.; Banerjee, S. Application of statistical design of experiments for optimization of As(V) biosorption by immobilized bacterial biomass. Ecol. Eng. 2016, 86, 13–23. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C-Biomimetic Supramol. Syst. 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Pen, Y.; Chen, Y.M.; Liu, Y.G.; Lu, M.; Zeng, X.X. Polyvinyl alcohol sodium alginate fixation of copper green microcystis for phosphorus adsorption. J. Environ. Eng. 2013, 3, 2563–2568. [Google Scholar] [CrossRef]
- Lv, X.B.; Li, R.Y. Pilot study on the denitrification effect of immobilized microorganisms on low-temperature river water. J. Environ. Sci. 2022, 42, 159–169. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, J.S.; Fang, F. Effect of pH on the microbial community structure of SBBR autotropHic nitrogen removal process. Adv. Mater. Res. 2012, 378–379, 428–432. [Google Scholar] [CrossRef]
- Li, Y.F.; Yao, J.B.; Hao, Y.; LI, J.; Wang, X.; Qin, Y.M. Experimental Study on Nitrogen and Phosphorus Removal by SBBR with Polyurethane foam as Microbial Immobilized Carrier. J. Environ. Eng. 2011, 5, 5. [Google Scholar]
- Kristensen, J.M.; Nierychlo, M.; Albertsen, M.; Nielsen, P.H. Bacteria from the Genus Arcobacter Are Abundant in Effluent from Wastewater Treatment Plants. Appl. Environ. Microbiol. 2020, 86, e03044-19. [Google Scholar] [CrossRef]
- Webb, A.L.; Taboada, E.N.; Selinger, L.B.; Boras, V.F.; Inglis, G.D. Efficacy of wastewater treatment on Arcobacter butzleri density and strain diversity. Water Res. 2016, 105, 291–296. [Google Scholar] [CrossRef]
- Ghosh, S.; Sar, P. Identification and characterization of metabolic properties of bacterial populations recovered from arsenic contaminated ground water of North East India (Assam). Water Res. 2013, 47, 6992–7005. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Ni, L.X.; Li, X.L.; Xu, C.; Chen, X.Q.; Li, S.Y. Mechanistic insight into the inhibitory effect of artemisinin sustained-release inhibitors with different particle sizes on microcystis aeruginosa. Environ. Sci. Pollut. Res. 2022, 29, 87545–87554. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010, 44, 2253–2266. [Google Scholar] [CrossRef]

| Independent Variables | Symbols | Levels | |||
|---|---|---|---|---|---|
| Coding Values | True Values | −1 | 0 | 1 | |
| PVA dose | Χ1 | χ1 | 0 | 5%(w/v) | 10%(w/v) |
| SA dose | Χ2 | χ2 | 0 | 2%(w/v) | 4%(w/v) |
| Bacterial dose | Χ3 | χ3 | 0 | 7.5%(w/v) | 15%(w/v) |
| Variation Sources | Square Sum | Degree of Freedom | Mean Square | F Value | Prob > F |
|---|---|---|---|---|---|
| Model | 5475.92 | 7 | 782.27 | 21.46 | <0.0001 |
| χ1 | 902.44 | 1 | 902.44 | 24.76 | 0.0008 |
| χ2 | 0.059 | 1 | 0.059 | 4.42 | 0.9687 |
| χ3 | 2061.42 | 1 | 2061.42 | 56.55 | <0.0001 |
| χ1χ2 | 142.67 | 1 | 142.67 | 3.91 | 0.0793 |
| χ1χ3 | 336.93 | 1 | 336.93 | 9.24 | 0.0140 |
| χ2χ3 | 466.55 | 1 | 466.55 | 12.80 | 0.0060 |
| χ32 | 1565.86 | 1 | 1565.86 | 42.95 | 0.0001 |
| Residual error | 328.09 | 9 | 36.45 | ||
| Lack of fit | 310.95 | 5 | 62.19 | 14.51 | 0.0113 |
| No. | PVA (%) | SA (%) | Bacterial Dose (%) | Removal Rate of NH3-N (%) |
|---|---|---|---|---|
| 1 | 5.00 | 1.00 | 6.00 | 63.5106 |
| 2 | 5.00 | 0.94 | 6.00 | 63.4415 |
| 3 | 5.12 | 1.00 | 6.00 | 63.3614 |
| 4 | 5.25 | 1.00 | 6.00 | 63.2201 |
| 5 | 5.00 | 0.72 | 6.00 | 63.1992 |
| 6 | 5.00 | 0.41 | 6.00 | 62.8455 |
| 7 | 5.00 | 1.00 | 5.83 | 62.8362 |
| 8 | 5.00 | 0.30 | 6.00 | 62.7271 |
| 9 | 5.00 | 0.19 | 6.00 | 62.6017 |
| 10 | 5.00 | 0.07 | 6.00 | 62.4639 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Liu, S.; Fang, X.; Yang, N. Application of Immobilized Microorganism Gel Beads in Black-Odor Water with High Nitrogen and Phosphorus Removal Performance. Water 2024, 16, 2534. https://doi.org/10.3390/w16172534
Zhao F, Liu S, Fang X, Yang N. Application of Immobilized Microorganism Gel Beads in Black-Odor Water with High Nitrogen and Phosphorus Removal Performance. Water. 2024; 16(17):2534. https://doi.org/10.3390/w16172534
Chicago/Turabian StyleZhao, Fengbin, Shumin Liu, Xin Fang, and Ning Yang. 2024. "Application of Immobilized Microorganism Gel Beads in Black-Odor Water with High Nitrogen and Phosphorus Removal Performance" Water 16, no. 17: 2534. https://doi.org/10.3390/w16172534
APA StyleZhao, F., Liu, S., Fang, X., & Yang, N. (2024). Application of Immobilized Microorganism Gel Beads in Black-Odor Water with High Nitrogen and Phosphorus Removal Performance. Water, 16(17), 2534. https://doi.org/10.3390/w16172534







