Abstract
In this paper, a lab-scale reactor designed to simulate the operations of the North Water Saline Wastewater Treatment Plant (SWWTP) located in Delfzijl, The Netherlands, was constructed and assessed. Unlike conventional municipal wastewater treatment facilities, this industrial plant deals with wastewater containing stubborn chemicals that are difficult to break down, along with a high ratio of chemical oxygen demand (COD) to nitrogen and elevated sodium chloride levels. Furthermore, its treatment process diverges from standard industrial setups by employing an aerobic process preceding the anaerobic phase. The proposed lab-scale reactors were proven stable and effective in mimicking the conditions of the studied industrial SWWTP, particularly in the presence of abundant glycerol, a factor not explored in similar lab-scale models. Throughout the experiment, the removal of COD (specifically glycerol) and nitrogen were monitored, alongside changes in the microbial community within both reactors. The data enabled us to examine the proliferation of microbial populations within the sludge. The results indicated the complete removal of glycerol and ammonia from the system, with some residual nitrate detected in the effluent. The soluble COD decreased in the first reactor (R1) to approximately 50% of the influent and reduced further to less than 100 mg/L in the second reactor (R2), while nitrogen was majorly removed in the R1. By the experiment’s conclusion, Actinomycetales was identified as the dominant order in the anaerobic reactor (sometimes even exceeding 70% of the population), which is known for its utilization of glycerol as a carbon source and its tolerance to high salt concentrations in the influent. Conversely, the aerobic reactor was predominantly inhabited by the order Flavobacteriales, which correlates with ammonia concentration.
1. Introduction
Wastewater is a big part of human activities’ excess and needs to be treated before it is environmentally safe to be released into the natural water resources [1]. If it is released while untreated, it will pollute the water resources and result in hazardous ecological, environmental, and economic impacts. Wastewater mainly comes from two major sources: human sewage systems and process waste from industries. In the late nineteenth century, the use of chemicals to treat wastewater was the norm. It was not until the early twentieth century that the biological treatment of wastewater was first introduced, and it has become the basis of wastewater treatment worldwide. One of the biological treatment of wastewater uses activated sludge, which consists of a high concentration of microorganisms living in microbial communities, mainly consisting of bacteria, protozoa, and archaea. These microbial communities consume organic compounds, ammonia, and phosphate for their metabolism and growth. These compounds are incorporated into the microbial cells, thereby reducing the concentration in the wastewater. The cells are separated from the water through the sedimentation of the activated sludge in sedimentation tanks. The clarified wastewater is released safely into natural water resources, such as rivers or the sea.
Although the operation of the treatment process seems to be straightforward, the underlying biological–chemical process is complex and difficult to monitor [2]. Consequently, process control relies mainly on the physical process variables that can be measured. There is no monitoring or control of microbial activities. It is already known that the dynamical variation in WWTP is mainly because of the time-varying composition of the microbial communities in the reactors, which is mainly influenced by the variation in influent characteristics [3]. The influent can vary in the flow rate, chemical composition, pH, and temperature, which in turn affect the population dynamics and metabolic process in the activated sludge.
Different WWTPs of municipal wastewater, in which pollutants can be relatively easily and almost completely removed, that treat industrial wastewater face much greater challenges. Industrial wastewater, which is the focus of this paper, often contains recalcitrant chemicals that are difficult for activated sludge to degrade. These complex and persistent pollutants require more advanced and targeted treatment processes, as they do not break down as easily as the organic matter typically found in municipal wastewater. The presence of such stubborn pollutants necessitates the use of specialized microbial communities or supplementary treatment technologies to achieve effective degradation.
Additionally, industrial wastewater often contains harmful chemicals and exhibits more extreme properties, such as very low or high pH levels and high salt concentrations. These harsh conditions can directly impact the performance of the activated sludge, inhibiting the activity of the microorganisms responsible for breaking down contaminants. For example, high salinity can cause osmotic stress to bacteria, while extreme pH levels can denature enzymes and disrupt cellular processes. These factors make it crucial to develop and optimize treatment strategies that can withstand and adapt to the challenging conditions presented by industrial wastewater, ensuring the effective operation of WWTPs in such demanding environments.
External carbon sources are often fundamental to achieving a high cleaning efficiency in sewage treatment. Glycerol showed significant potential as an external carbon source and energy source for microbial growth in industrial microbiology. It has been shown that complex microbial networks are in reactors with glycerol [4]. Previous studies reported glycerol as a suitable carbon source in wastewater treatment and useful for reducing biomass production. It is also used to remove nitrogen from wastewater during municipal wastewater treatment in two independent activated SBR-type sludge reactors [5]. In another study, it was shown that the use of glycerol resulted in good denitrification and phosphorus removal [6].
The microbial utilization of glycerol as a renewable raw material for biomanufacturing holds great promises [7]. Glycerol, a byproduct of biodiesel production, presents an abundant and inexpensive feedstock for microbial processes. Harnessing this resource can contribute to more sustainable industrial practices, reducing reliance on fossil fuels and minimizing environmental impact.
Advancements in genetic engineering have enabled us to take advantage of this opportunity by developing microbial production hosts that can convert glycerol into a wide range of valuable chemicals. By modifying the metabolic pathways of microorganisms, scientists can optimize the conversion efficiency and specificity for desired products. This opens up possibilities for producing various bulk and fine chemicals through more eco-friendly and cost-effective methods.
Numerous studies have explored the bioconversion of glycerol into valuable chemicals. For instance, dihydroxyacetone, 1,3-propanediol, succinate, and ethanol are among the many chemicals that can be produced through microbial fermentation of glycerol [8]. Each of these chemicals has significant industrial applications, ranging from pharmaceuticals to biodegradable plastics, underscoring the potential of glycerol as a versatile and sustainable feedstock in biomanufacturing.
The metabolism of glycerol in microorganisms mainly occurs by two distinct and parallel pathways, oxidative and reductive [9]. The oxidative metabolism of glycerol involves several key steps that convert glycerol into intermediates that can enter central metabolic pathways such as glycolysis and the citric acid cycle (TCA cycle) while the reductive metabolism of glycerol, typically occurring under anaerobic conditions, involves its conversion into compounds such as 1,3-propanediol (1,3-PDO) through a series of enzymatic reactions. The members of the Enterobacteriaceae family, such as Klebsiella, Citrobacter, and Clostridia and some acetic acid bacteria, e.g., Acetobacter and Gluconobacter, carry out glycerol degradation using both oxidative and reductive pathways [10].
This paper present a novel approach to treating industrial wastewater with a high carbon-to-nitrogen (C/N) ratio using a saltwater treatment plant. Unlike conventional wastewater treatment plants, which struggle to manage saline wastewater effectively, this study demonstrates that a saltwater treatment plant can successfully address this challenge. This research focuses on lab-scale reactors that model an industrial SWWTP, specifically the North Water SWWTP, which is unique in treating influent with a high C/N ratio in a saltwater environment. Through monitoring the microbial community within these reactors, the impact of glycerol on nitrogen removal in the system can be investigated. The use of lab-scale reactors provided valuable insights into the microbial activities within the WWTP processes, which are crucial for developing a dynamic model of the system. The microbial community dynamics later can be used and coupled with the parameter-varying of the dynamic model (see [11]). These findings offer a potential solution for industries dealing with saline effluents and contribute to the advancement of new strategies for saline wastewater treatment.
2. Materials and Methods
2.1. North Water’s Wastewater Treatment Plant
In this study, the system used was the one from North Water’s Saline Wastewater Treatment Plant (SWWTP) in Delfzijl, The Netherlands (DD: 53.30856189250916, 6.9687649567922945). This industrial SWWTP (the plant is also known by its Dutch acronym “ZAWZI”) treats collective industrial wastewater from chemical industries that are operated in a chemical park in Delfzijl and its neighborhood. The processed clean water is then released into the surface water. The treatment process involves several stages, including primary sedimentation, biological treatment, and advanced filtration, ensuring that the discharged water meets stringent environmental standards. This system plays a crucial role in maintaining the ecological balance of the surrounding aquatic environment, while also supporting the sustainability goals of the local chemical industry. General operational data of this SWWTP are given in Table 1.

Table 1.
North Water’s SWWTP purification data.
Table 2 shows the average value of the influent for North Water’s SWWTP. These average values are based on the measurements that were performed in week 40 in 2014 until week 10 in 2017.

Table 2.
Average composition of the influent at North Water’s SWWTP.
A general schematic of the biological treatment process of North Water’s SWWTP is shown in Figure 1. The influent coming from various sources is collected in the influent tank before it is pumped into the inner part of a 2-zone treatment tank, where an anaerobic biological process takes place and an aerobic process in the outer part of the treatment tank. In the clarifier tank, the activated sludge is separated from the water and is partly fed back into the treatment tank, while the treated water is then discharged into the Wadden Sea.
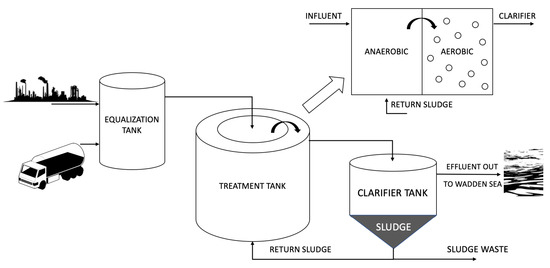
Figure 1.
Schematic of North Water’s saline wastewater treatment plant.
The setup of an anaerobic process followed by an aerobic one is not common for a standard WWTP. This reverse setup is possible in North Water’s WWTP since the influent has a high chemical oxygen demand (COD) such that it contains sufficient carbon and energy in the wastewater for the biological removal process of + in the first reactor. The further removal of contaminants takes place in the outer reactor part, where industrial waste is further broken down by biological processes.
2.2. Reactor Set-Up
The experiment was conducted based on North Water’s SWWTP, resulting in the experimental setup using a two-reactor system, as shown in Figure 2. The first reactor (R1) is anaerobic, and the second reactor (R2) is aerobic, with each reactor having a working volume of 500 mL. The operating conditions during the experimental period were maintained at room temperature, with a pH of 7, an influent flow rate of 0.2 mL/min, corresponding to a hydraulic retention time (HRT) of 2 days, and a dilution rate of 0.9 day−1. To avoid excessive microbial growth in the influent vessels, the influent was separated into two different vessels: one for carbon and one for nitrogen sources.
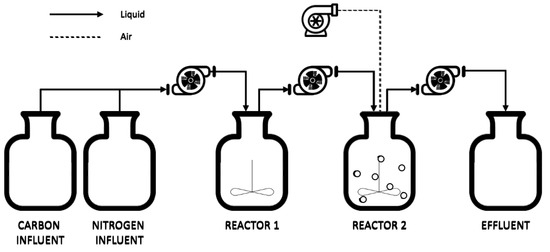
Figure 2.
Schematic of the experiment setup based on North Water’s SWWTP.
In this setup, as illustrated in Figure 2, the influent from the two vessels was pumped into the first reactor, where the anaerobic biological process took place. Subsequently, the effluent from the first reactor was pumped into the second reactor, where aerobic processes occurred. Finally, the treated water was pumped into the effluent vessel. A notable deviation from North Water’s SWWTP operation was that the return sludge from the effluent did not go back into the first reactor, which was a slight modification in the experimental approach. The return sludge was used to maintain the right concentration of biomass in the reactor. Especially in the winter, when the sludge was not sufficiently active or when an influent with different compositions was treated, a decrease in biomass was observed in the full-scale reactor. There was no decrease in biomass concentration in the laboratory reactor because the temperature in the lab was around 20 °C, and the influent had a constant composition.
2.3. Experimental Start-Up and Operation
The experiment was divided into three sequential phases that ran continuously. The first part was the initialization phase, where sludge and influent from North Water were used to start up the reactors in the laboratory. This phase lasted for four weeks, during which the system was carefully monitored to ensure stable conditions and proper functioning. The aim was to establish a baseline performance and acclimate the microbial communities to the operational environment.
Following the initialization phase, the second phase began with the replacement of the influent with a synthetic influent. This period lasted for eight weeks and was crucial for testing the reactors’ response to a controlled and consistent feed. The synthetic influent allowed for the precise manipulation of nutrient concentrations and provided a stable environment to study the reactors’ performance and microbial dynamics under defined conditions. Regular sampling was conducted during this period to measure chemical oxygen demand (COD), ammonia, nitrate, and microbial composition, ensuring the detailed tracking of the reactors’ performance and microbial community changes.
After eight weeks, the third phase commenced with a change in the composition of the synthetic influent. This adjustment aimed to assess the reactors’ adaptability and resilience to variations in influent composition. By altering the nutrient ratios, the experiment sought to understand the impact of different feed compositions on the treatment process and microbial communities. Samples continued to be taken at regular intervals to monitor COD, ammonia, nitrate levels, and microbial composition, providing comprehensive data on the reactors’ response to the new conditions.
The composition of the synthetic wastewater that was used throughout the experimental period can be seen in Table 3. As shown in Figure 2, the influent was separated into two different solutions: carbon influent and nitrogen influent. The carbon influent comprised a solution of glycerol, methanol, and magnesium sulfate, providing essential carbon sources for microbial growth. The nitrogen influent contained the remaining components, supplying the necessary nutrients for the microbial processes. This separation allowed for precise control over the nutrient inputs and facilitated the study of their individual and combined effects on the reactors’ performance.

Table 3.
Synthetic influent’s composition.
2.4. Chemical Analysis
The chemical composition and the pH of both reactors were determined twice a week. This frequent monitoring is essential for ensuring the stability and efficiency of the reactors, allowing for the prompt detection of any irregularities or changes in the system. Regular checks help maintain optimal conditions for the biological and chemical processes taking place within the reactors.
The chemical oxygen demand (COD) was measured using LCK314 (Hach, Düsseldorf, Germany), a method that quantifies the amount of organic matter present in the water. This measurement is crucial for assessing the efficiency of the treatment process in breaking down organic pollutants. Similarly, ammonia levels were measured using LCK303 (Hach, Germany). Monitoring ammonia is vital as it indicates the presence of nitrogenous waste, which can be harmful if not properly managed. The nitrate concentration was determined using LCK339 (Hach, Germany), providing insights into the nitrogen cycle and the effectiveness of nitrification and denitrification processes within the reactors.
The pH was measured using a laboratory standard pH meter, ensuring accurate and consistent readings. Maintaining the appropriate pH level is critical for the health and activity of the microbial communities involved in the treatment process. The pH can influence the rate of biochemical reactions and the solubility of various compounds, making its regular monitoring a key aspect of reactor management.
2.5. High-Throughput 16S-rDNA Gene Sequencing and Analysis
The frozen samples, stored at (−20 °C), were thawed at room temperature prior to DNA extraction. This step is crucial to ensure the integrity of the samples and the quality of the DNA to be extracted. Once thawed, the total DNA was extracted using the FastDNA® Spin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA) following the manufacturer’s protocol. This kit is specifically designed to efficiently lyse cells and purify DNA from various soil samples, ensuring a high yield and purity of the extracted DNA.
The extracted genomic DNA was then used as the template in the PCR reactions. Specifically, a part of the 16S-rDNA genes was amplified using the primers 338F (5′-ACT CCT ACG GGA GGC AG-3′) and 805R (5′>-GAC TAC CAG GGT ATC TAA TCC-3′). These primers target a conserved region of the bacterial 16S ribosomal RNA gene, making them ideal for profiling microbial communities. The amplified DNA was then barcoded prior to the sequencing procedure using the Illumina Nexseq 1000 platform, which allows for the high-throughput sequencing and multiplexing of samples.
Consequently, the diversity profiles of the microbial communities were obtained by plotting the number of 16S-rDNA sequences as a percentage in a stacked bar chart. This visualization method effectively represents the relative abundance of different microbial taxa within the samples, providing insights into the composition and diversity of the microbial communities present. Such detailed profiling is essential for understanding the microbial dynamics and their potential roles in both reactors.
3. Results and Discussion
3.1. pH, COD, and Nitrogen
The results of the measurements that were taken for around eleven weeks for the second and third phase of the experiment, where the influent were synthetic influents, are shown in Figure 3. The yellow lines is where the phase 3 started and the change in the influent composition started. The soluble COD decreased in R1 to approximately 50% of the influent and reduced further to less than 100 mg/L in R2, which is a positive outcome, as it falls below the EU regulatory limit for COD discharge from wastewater treatment plants. The reason of the decomposition discrepancy of COD in both reactors is that the anaerobic reactor consumed more COD due to their lower metabolic efficiency and the conversion of a significant portion of the organic matter into methane while also producing less biomass. This represents a more complete reduction in COD compared to the end products of the aerobic reactor.
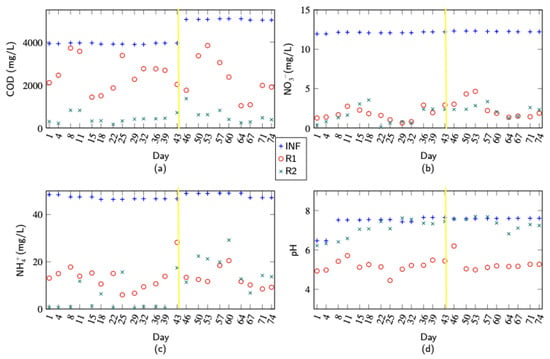
Figure 3.
Lab measurement results for chemical composition and pH were taken from 05/07 until 16/09. (a) COD. (b) Nitrate. (c) Ammonia. (d) pH.
Ammonium was removed in R1 to 20% of the initial concentration in the influent during the whole experiment. In R2, most of the ammonia was further removed to below the detection limit in the first phase. In the second phase, when the COD content in the influent was increased by 20%, not all ammonia was removed in R2, and no significant changes in ammonia removal were observed in R1. Some acids were produced in R1, as can be concluded from the decrease in pH. In R2, the acids were consumed by the microorganisms, and the pH increased to the initial pH of the influent.
In R1, more than 90% of the incoming nitrate was removed, indicating the high efficiency of the anaerobic process in the first reactor. This substantial reduction occurred exclusively in R1, with no further nitrate removal observed in the subsequent R2. The efficiency of nitrate removal in R1 remained consistent even after the chemical oxygen demand (COD) was increased in the second phase of the experiment, suggesting that the system’s capacity for nitrate removal was robust against changes in organic load. In the anaerobic environment of R1, nitrate most likely served as the primary electron acceptor for microbial processes, facilitating its significant reduction. Conversely, in the aerated conditions of R2, oxygen became the preferred electron acceptor, leading to the stabilization of nitrate concentration without further reduction (Figure 3b). This shift in electron acceptor preference highlights the distinct roles and operational conditions of the two reactors, with R1 focusing on anaerobic nitrate reduction and R2 primarily supporting aerobic processes.
Figure 3 also shows some fluctuation happening in the value of the measured chemical components, which was clearly visible in both COD and . One possible reason for these fluctuations is that the microbial community in the reactors is still in development. As the microbial community matures and becomes more stable, it is expected that these fluctuations will decrease, leading to more consistent and predictable performance in terms of chemical component levels.
3.2. Microbial Community
The composition of the microbial community in the two-reactor setup, R1 and R2, was analyzed using NGS of the 467 bp PCR product. DNA was isolated from bacteria that were present in the reactors at different time points during the feeding with the artificial wastewater. The PCR products were barcoded, pooled, and sequenced on the NEXTgen 1000. The general characteristics of the sequencing data can be seen in the supplementary materials. The sequences were used to identify which taxonomic order, and their relative abundance were present in the reactors at the different time points. The sequencing data are shown in Figure 4.
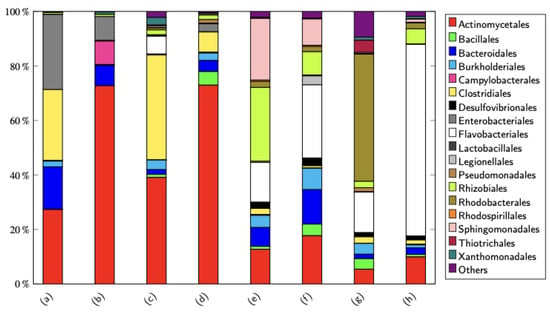
Figure 4.
Stacked bar chart of microbial communities showing the populations in order for a few samples, where (a–d) are R1 samples while (e–h) are R2 samples. Samples (a,e) were taken on day 8, samples (b,f) were taken on day 15, samples (c,g) were taken on day 43, and samples (d,h) were taken on day 53.
The top five bacteria in Reactor 1 contained members of the order Actinomycetales, Enterobacteriales, Clostridiales, Bacteriodales, and Burkholderiales. Reactor 1 was run under anoxic conditions, and the top 5 of the order all contained members that can grow under oxygen limitation or under anaerobic conditions. As shown in Figure 4, in the second sample of R1, the Actinomycetales increased to 70% of the population, and the Clostridiales and Burkholderiales were not detected. At this time point, 10% of the population were members of Campylobacteriales. Actinomycetales remained the largest part of the population throughout the experiment, and Clostridiales reappeared in R1 in the third and fourth samples.
In the artificial wastewater, the major carbon source was glycerol (4 g/L) and a minor portion of methanol (0.16 g/L). Several species of the detected order are able to metabolize glycerol and methanol under oxygen-limited conditions. For example, E. coli, Citrobacter freundii, and Klebsiella pneumoniae from Enterobacteriales and also Clostridium pasteurianum and Clostridium butyricum from Clostridiales were previously shown to use glycerol as a carbon source [12]. In the aerobic reactor, R2 also contains microorganisms that are able to degrade, e.g., Streptomyces natalensis. Actinomycetales was previously shown to use glycerol as a carbon source [13]. In other research works [14,15], it was concluded that glycerol can be a good carbon source for the other Streptomyces species.
Some species of Bacillales and Rhodobacterales, e.g., Bacillus methanolicus and Paracoccus denitrificans, are able to consume methanol (see [16,17]). The microbial consortium is apparently not able to degrade all of the COD at the dilution rate and anoxic conditions that were used for R1 (see Figure 3a). The effluent of R1 is passed to R2. The volumes of R1 and R2 were the same, so the dilution rate for both reactors was the same.
The top 5 bacteria in R2 contained members of the order Flavobacteriales, Rhodobacterales, Rhizobiales, Actinomycetales, and Sphingomonadales. Because the presence of oxygen is higher in R2 than in R1, the COD can be further decomposed in the anaerobic reactor; see Figure 3a. Looking at Figure 4, it can be seen that in the population, Rhizobiales decreased from the second sample until the last sample. The opposite occurred with Flavobacteriales, and they became the major part of the microbial community in R2 in the last sample.
The high presence of Actinomycetales in both R1 and R2 can be explained because Actinomycetales can tolerate the high salt concentration in the influent (there was around 3% salt concentration in the media). Other salt-tolerant representatives of the order are also present in the two reactors, e.g., Bacillales, Enterobacteriales, Pseudomonadales, Lactobacillales, and Sphingomonadales (see [18,19]).
Another insight that can be gained from the sequencing results of both reactors is the correlation with the laboratory measurement results shown in Figure 3d. Specifically, the pH readings reveal that Reactor 1 consistently exhibits a lower pH value compared to Reactor 2. This difference can be attributed to the higher combined population of fermentative bacteria present in Reactor 1, including species from the orders Enterobacteriales, Clostridiales, and Lactobacillales. Fermentative bacteria are known to produce acids as metabolic byproducts, which leads to a decrease in pH. The substantial presence of these acid-producing microorganisms in Reactor 1 accounts for the observed lower pH levels compared to the more neutral conditions in Reactor 2. Additionally, by focusing on the lab measurement results corresponding to the same dates as the sequencing samples, further correlations between the lab measurements and the sequencing data can be observed, as seen in Figure 3.
The microbial community in R1 consists of a fair amount of fermentative microorganisms, e.g., Enterobacteriales, Clostridiales, and Lactobacillales. This is in agreement with the increase in acidity to pH 5 on average. Figure 3c also shows that at day 8, the concentration of nitrate and ammonia in the R2 is the lowest compared to days 15, 43, and 53. This happened because the concentration of Rhizobiales is the highest, just as shown in Figure 4. According to [20], Rhizobium, Mesorhizobium, and Tardiphaga, Rhizobiales used ammonium and nitrate as nitrogen sources. Another thing that can be seen in Figure 3c is the value of ammonia in the R2 at day 53, which shows the highest value compared to days 8, 15, and 43. This happened because the concentration of Flavobacteriales is the highest, just as shown in Figure 4. Based on the work of [21], the order Flavobacteriales was positively correlated with ammonia concentration.
At the end of the experiment, as illustrated in Figure 4, it was observed that the order Actinomycetales dominated the anaerobic reactor, while the order Flavobacteriales was more prevalent in the aerobic reactor. This significant shift in microbial community composition underscores the remarkable adaptability of the microbial populations to the specific environmental conditions within each reactor. The distinct microbial distributions reflect how different microbial orders thrive under varying conditions, optimizing the degradation processes to suit the reactor’s environment.
The dominance of Actinomycetales in the anaerobic reactor indicates their crucial role in breaking down complex organic materials under oxygen-limited conditions. These bacteria are well suited to anaerobic environments, where they efficiently decompose recalcitrant compounds that require specialized metabolic pathways. On the other hand, the prevalence of Flavobacteriales in the aerobic reactor highlights their effectiveness in aerobic degradation processes. These microorganisms are adept at utilizing oxygen to metabolize organic matter, resulting in the efficient breakdown and conversion of pollutants. The observed microbial community structure emphasizes the importance of tailoring reactor conditions to foster specific microbial populations that enhance the overall treatment efficiency.
4. Conclusions
The proposed lab-scale SWWTP reactors have been shown to be stable and can be used to model the biological process in industrial SWWTPs. It has been used to show that glycerol can be used as the carbon source for the reactors and is able to help in the removal of nitrogen by the orders of Rhizobiales and Flavobacteriales, among others, which consume glycerol as a carbon source. The lab-scale reactors were able to remove glycerol and ammonia completely with a low amount of nitrate in the effluent. In analyzing the dynamics of microbial population in the two reactors R1 and R2, R1 consists of a fair amount of fermentative microorganisms, which is shown in the acidity value of pH 5 on average. In R1, the concentration of ammonia might be related to the amount of Clostridiales and Enterobacteriales. On the other hand, the abundance of Rhizobiales and Flavobacteriales in R2 affected the concentration of ammonia and nitrate. At the end of the experiment, it can also be seen that the order Actinomycetales dominates the anaerobic reactor while the order Flavobacteriales dominates the aerobic reactor.
Further work on this research is to use the knowledge of the microbial activities and also the chemical composition in the two reactors to create a dynamic model of the system, such as using the well-known Activated Sludge Model and coupling some of the microbial population to some of the parameters to obtain a parameter time-varying model based on the microbial dynamics.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/w16172517/s1. Table S1: General characteristics of the sequencing data.
Author Contributions
Conceptualization, M.A.P.N. and G.-J.W.E.; methodology, M.A.P.N. and G.-J.W.E.; validation, M.A.P.N., B.J. and G.-J.W.E.; formal analysis, M.A.P.N. and G.-J.W.E.; investigation, M.A.P.N.; resources, G.-J.W.E.; writing—original draft preparation, M.A.P.N.; writing—review and editing, B.J. and G.-J.W.E.; supervision, G.-J.W.E. All authors have read and agreed to the published version of the manuscript.
Funding
M. A. Prawira Negara profoundly expresses gratitude to the Islamic Development Bank (IsDB) for the scholarship funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Henze, M.; Comeau, Y. Biological Wastewater Treatment; IWA Publishing: London, UK, 2008. [Google Scholar]
- Davies, P. The Biological Basis of Wastewater Treatment; Strathkelvin Instruments Ltd.: Motherwell, UK, 2005. [Google Scholar]
- Shi, L.; Liu, N.; Liu, G.; Fang, J. Bacterial Community Structure and Dynamic Changes in Different Functional Areas of a Piggery Wastewater Treatment System. Microorganisms 2021, 9, 2134. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, R.Z.; Wang, Y.; Chen, G.L.; Fu, Y.Y.; Yu, H.Q. Carbon source shaped microbial ecology, metabolism and performance in denitrification systems. Water Res. 2023, 243, 120330. [Google Scholar] [CrossRef] [PubMed]
- Smyk, J.; Ignatowicz, K. The influence of glycerin on nitrogen removal in wastewater treatment with activated sludge. In Proceedings of the International Conference on Advances in Energy Systems and Environmental Engineering (ASEE17), Wroclaw, Poland, 2–5 July 2017; E3S Web Conferences. Volume 22. [Google Scholar]
- Salamah, S.; Randall, A. Optimization of Hetrotrophic Denitrification Using Glycerol as a Sustainable External Carbon Substrate. Proceedings 2020, 48, 23. [Google Scholar]
- Keita, V.M.; Gonzalez-Villanueva, M.; Wong, T.S.; Tee, K.L. Microbial Utilization of Glycerol for Biomanufacturing. In Engineering of Microbial Biosynthetic Pathways; Singh, V., Singh, A., Bhargava, P., Joshi, M., Joshi, C., Eds.; Springer: Singapore, 2020. [Google Scholar]
- da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, S.S.; Gonzalez, R. Anaerobic fermentation of glycerol: A path to economic viability for the biofuels industry. Curr. Opin. Biotechnol. 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, V.K.; Qazi, G.N.; Kumar, A. Gluconobacter oxydans: Its biotechnological applications. J. Mol. Microbiol. Biotechnol. 2001, 3, 445–456. [Google Scholar] [PubMed]
- Negara, M.A.P.; Keesman, K.J.; Euverink, G.J.W.; Jayawardhana, B. NGS-Enriched Activated Sludge Modelling of an Industrial Wastewater Treatment Plant. 2023. Available online: https://www.biorxiv.org/content/10.1101/2023.01.23.523537v1 (accessed on 25 April 2024).
- Clomburg, J.M.; Gonzalez, R. Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trends Biotechnol. 2013, 31, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Recio, E.; Aparicio, J.F.; Rumbero, A.; Martín, J.F. Glycerol, ethylene glycol and propanediol elicit pimaricin biosynthesis in the PI-factor-defective strain Streptomyces natalensis npi287 and increase polyene production in several wild-type actinomycetes. Microbiology 2006, 152, 3147–3156. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Onken, U.; Sattler, I.; Zeeck, A. Influence of increased dissolved oxygen concentration on the formation of secondary metabolites by manumycin-producing Streptomyces parvulus. Appl. Microbiol. Biotechnol. 1994, 41, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Borodina, I.; Scholler, C.; Eliasson, A.; Nielsen, J. Metabolic network analysis of Streptomyces tenebrarius, a Streptomyces species with an active Entner–Doudoroff pathway. Appl. Environ. Microbiol. 2005, 71, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Verseveld, H.W.V.; Stouthamer, A.H. Electron-Transport Chain and Coupled Oxidative Phosphorylation in Methanol-Grown Paracoccus denitrificans. Arch. Microbiol. 1978, 118, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Krog, A.; Heggeset, T.M.B.; Muller, J.E.N.; Kupper, C.E.; Schneider, O.; Vorholt, J.A.; Ellingsen, T.E.; Brautaset, T. Methylotrophic Bacillus methanolicus Encodes Two Chromosomal and One Plasmid Born NAD+ Dependent Methanol Dehydrogenase Paralogs with Different Catalytic and Biochemical Properties. PLoS ONE 2013, 8, e59188. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Arora, N.K. Plant Growth-Promoting Rhizospheric Microbes for Remediation of Saline Soils. Phyto Rhizo Remediat. Microorg. Sustain. 2019, 9, 121–146. [Google Scholar]
- Kearl, J.; McNary, C.; Lowman, J.S.; Mei, C.; Aanderud, Z.T.; Smith, S.T.; West, J.; Colton, E.; Hamson, M.; Nielsen, B.L. Salt-Tolerant Halophyte Rhizosphere Bacteria Stimulate Growth of Alfalfa in Salty Soil. Front. Microbiol. 2019, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Kutvonen, H.; Rajala, P.; Carpén, L.; Bomberg, M. Nitrate and ammonia as nitrogen sources for deep subsurface microorganisms. Front. Microbiol. 2015, 6, 1079. [Google Scholar] [CrossRef] [PubMed]
- Muck, S.; Corte, D.D.; Clifford, E.L.; Bayer, B.; Herndl, G.J.; Sintes, E. Niche Differentiation of Aerobic and Anaerobic Ammonia Oxidizers in a High Latitude Deep Oxygen Minimum Zone. Front. Microbiol. 2019, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).