Antibiotic Resistance and Aquatic Systems: Importance in Public Health
Abstract
1. Introduction
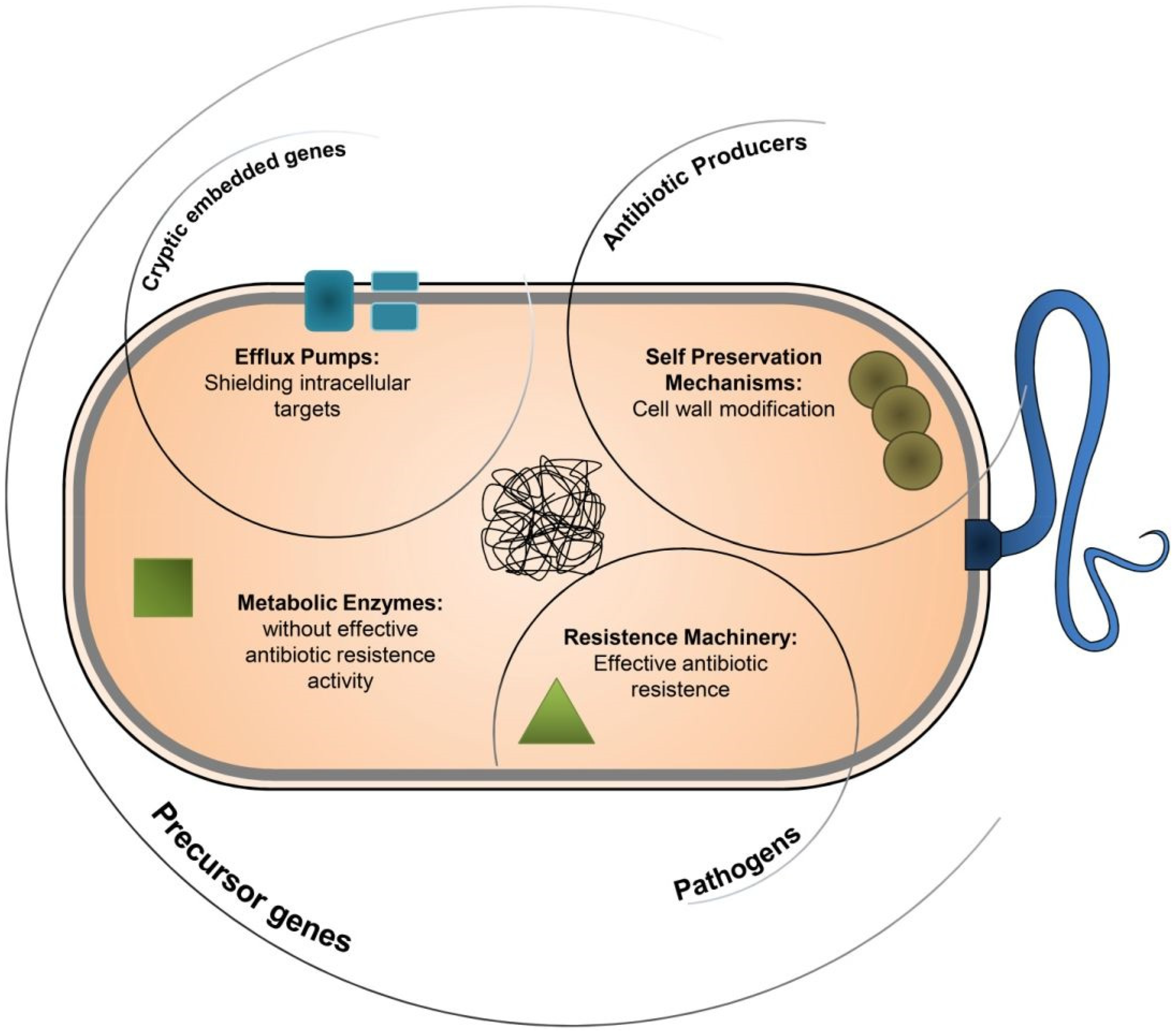
2. Antimicrobial Resistance in Water Bodies
| Class | Antimicrobial Agent | Mechanism of Action | Resistance Mechanism |
|---|---|---|---|
| Aminoglycosides | Gentamicin, Kanamycin, Streptomycin | Inhibition of protein synthesis | Efflux, enzymatic inactivation, mutated target |
| Amphenicols | Chloramphenicol | Inhibition of protein synthesis | Efflux |
| Macrolides | Clarithromycin, Erythromycin | Inhibition of protein synthesis | Efflux, mutated target |
| Tetracyclines | Tetracycline, Doxycycline | Inhibition of protein synthesis | Efflux |
| Beta-Lactams * | Penicillin, Aztreonam, Cefotaxime | Inhibition of cell-wall synthesis | Enzymatic inactivation, mutated target |
| Glycopeptides | Vancomycin, Bleomycin | Inhibition of cell-wall synthesis | Cell wall modification, efflux |
| Quinolones | Nalidixic Acid, Ciprofloxacin | Inhibition of nucleic acids synthesis | Efflux, mutated target |
| Sulfonamides | Sulfamethoxazole | Inhibition of folate synthesis pathway | Alternative enzymes, mutated target |
| Lipopeptides | Daptomycin | Cell membrane depolarization | Cell membrane modification, mutations |
| Amino-acid derivates | Polymyxin B | Cell membrane permeabilization | Cell membrane modification |
3. Antimicrobial Resistance in Freshwater and Wastewater
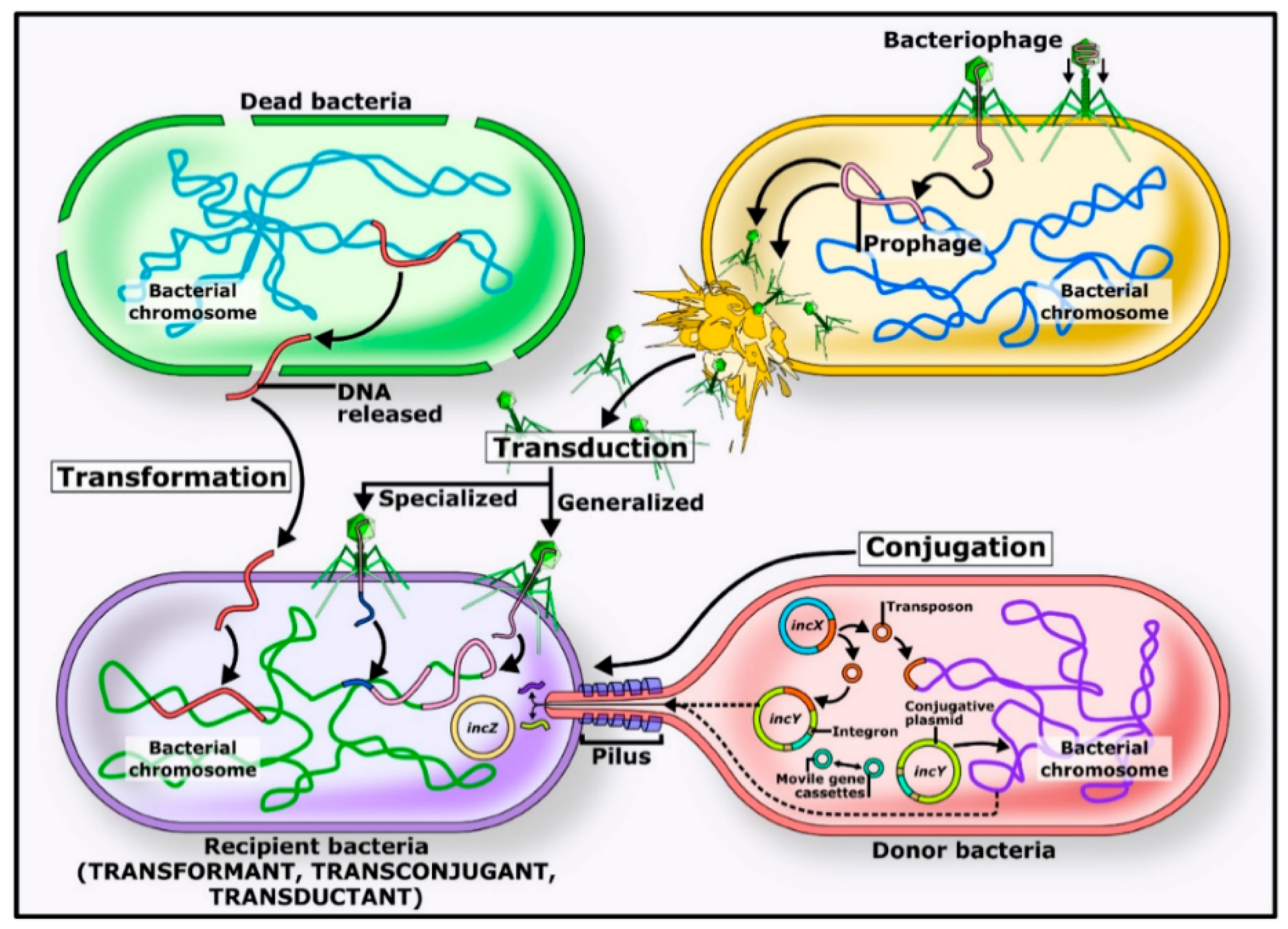
4. Antimicrobial Resistance in Marine Environments
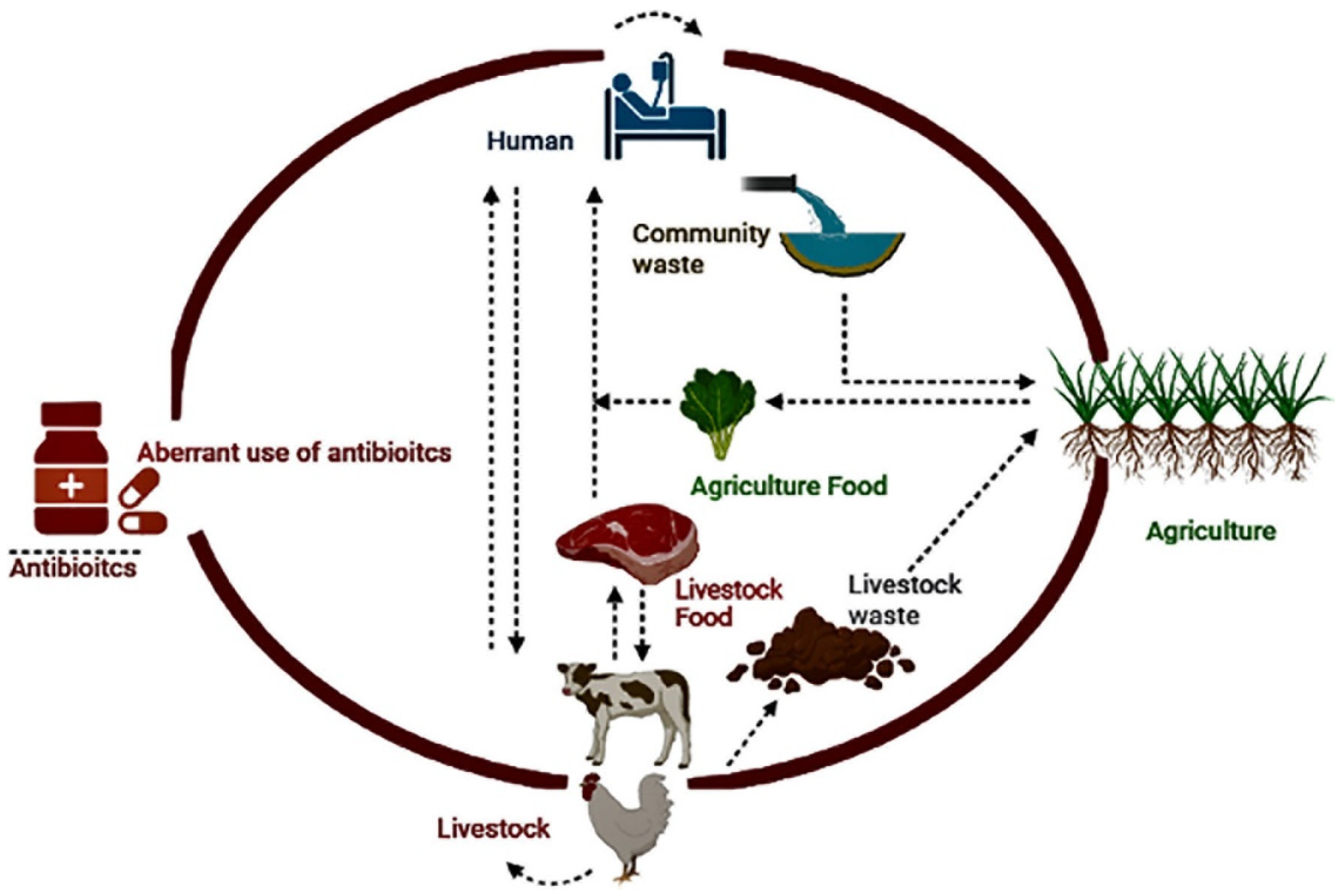
5. Antimicrobial Resistance in Water and Public Health
6. Discussion
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hossain, M.A.R.; Ahmed, M.; Ojea, E.; Fernandes, J.A. Impacts and Responses to Environmental Change in Coastal Livelihoods of South-West Bangladesh. Sci. Total Environ. 2018, 637–638, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Vilca, F.Z.; Angeles, W.G. Occurrence of Antibiotics Residues in the Marine Environment. Examines Mar. Biol. Ocean 2018, 2, 12–14. [Google Scholar] [CrossRef]
- Irfan, S.; Alatawi, A.M.M. Aquatic Ecosystem and Biodiversity: A Review. Open J. Ecol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Hassan, B.; Qadri, H.; Ali, M.N.; Khan, N.A.; Yatoo, A.M. Impact of Climate Change on Freshwater Ecosystem and its Sustainable Management. In Fresh Water Pollution Dynamics and Remediation; Springer: Singapore, 2020; pp. 105–121. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Ward, M.J. Sources of antimicrobial resistance. Science 2013, 341, 1460–1461. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and Fate of Antibiotics, Antibiotic Resistant Genes (ARGs) and Antibiotic Resistant Bacteria (ARB) in Municipal Wastewater Treatment Plant: An Overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Amarasiri, M.; Kitajima, M.; Nguyen, T.H.; Okabe, S.; Sano, D. Bacteriophage Removal Efficiency as a Validation and Operational Monitoring Tool for Virus Reduction in Wastewater Reclamation: Review. Water Res. 2017, 121, 258–269. [Google Scholar] [CrossRef]
- Amaya, E.; Reyes, D.; Paniagua, M.; Calderón, S.; Rashid, M.-U.; Colque, P.; Kühn, I.; Möllby, R.; Weintraub, A.; Nord, C. Antibiotic Resistance Patterns of Escherichia coli Isolates from Different Aquatic Environmental Sources in Leon, Nicaragua. Clin. Microbiol. Infect. 2012, 18, E347–E354. [Google Scholar] [CrossRef]
- World Health Organization. National Action Plan on Antimicrobial Resistance (NAP-AMR) 2017–2021. 2017. Available online: https://www.who.int/publications/m/item/india-national-action-plan-on-antimicrobial-resistance-(nap-amr)-2017-2021 (accessed on 15 November 2021).
- United Nations Environment Programme. Antibiotic Resistance: A Global Threat. Stockholm: United Nations. 2022. Available online: https://www.unep.org/explore-topics/chemicals-waste/what-we-do/emerging-issues/antimicrobial-resistance-global-threat#:∼:text=The%20key%20reasons%20contributing%20to,human%20and%20veterinary%20healthcare%20settings (accessed on 1 April 2022).
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathi, V.R.; Garg, S.K. Antibiotic Resistance and Genetic Diversity in Water-Borne Enterobacteriaceae Isolates from Recreational and Drinking Water Sources. Int. J. Environ. Sci. Technol. 2013, 10, 789–798. [Google Scholar] [CrossRef]
- Abdel Rahim, K.A.A.; Hassanein, A.M.; Abd El Azeiz, H.A.E.H. Prevalence, Plasmids and Antibiotic Resistance Correlation of Enteric Bacteria in Different Drinking Water Resources in Sohag, Egypt. Jundishapur J. Microbiol. 2015, 8, e18648. [Google Scholar] [CrossRef]
- Jabbar Ibrahim, I.A.; Kareem Hameed, T.A. Isolation, Characterization and Antimicrobial Resistance Patterns of Lactose-Fermenter Enterobacteriaceae Isolates from Clinical and Environmental Samples. Open J. Med. Microbiol. 2015, 5, 169–176. [Google Scholar] [CrossRef]
- Guzman-Otazo, J.; Gonzales-Siles, L.; Poma, V.; Bengtsson-Palme, J.; Thorell, K.; Flach, C.-F.; Iñiguez, V.; Sjöling, Å. Diarrheal Bacterial Pathogens and Multi-Resistant Enterobacteria in the Choqueyapu River in La Paz, Bolivia. PLoS ONE 2019, 14, e0210735. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Conventional Infection Prevention and Control Practices in Post-Antibiotic Era: A Perspective. J. Sci. Res. 2020, 64, 167–174. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic resistance: One Health One World outlook. Front. Cell Infect Microbiol. 2021, 11, 771510. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.F.; Vaz-Moreira, I.; Gonzalez-Pajuelo, M.; Nunes, O.C.; Manaia, C.M. Antimicrobial Resistance Patterns in Enterobacteriaceae Isolated from an Urban Wastewater Treatment Plant. FEMS Microbiol. Ecol. 2007, 60, 166–176. [Google Scholar] [CrossRef]
- Tesfaye, H.; Alemayehu, H.; Desta, A.F.; Eguale, T. Antimicrobial Susceptibility Profile of Selected Enterobacteriaceae in Wastewater Samples from Health Facilities, Abattoir, Downstream Rivers and a WWTP in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control. 2019, 8, 134. [Google Scholar] [CrossRef]
- Adegoke, A.A.; Madu, C.E.; Aiyegoro, O.A.; Stenström, T.A.; Okoh, A.I. Antibiogram and Beta-Lactamase Genes Among Cefotaxime Resistant E. coli from Wastewater Treatment Plant. Antimicrob. Resist. Infect. Control. 2020, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Obayiuwana, A.; Ibekwe, A.M. Antibiotic Resistance Genes Occurrence in Wastewaters from Selected Pharmaceutical Facilities in Nigeria. Water 2020, 12, 1897. [Google Scholar] [CrossRef]
- Praveenkumarreddy, Y.; Akiba, M.; Guruge, K.S.; Balakrishna, K.; Vandana, K.E.; Kumar, V. Occurrence of Antimicrobial-Resistant Escherichia coli in Sewage Treatment Plants of South India. J. Water Sanit Hyg. Dev. 2020, 10, 48–55. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial Resistance: A Global Multifaceted Phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and Economic Impact of Antibiotic Resistance in Developing Countries: A Systematic Review and Meta-Analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding Human Health Risks Caused by Antibiotic Resistant Bacteria (ARB) and Antibiotic Resistance Genes (ARG) in Water Environments: Current Knowledge and Questions to Be Answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Serwecinska, L. Antimicrobials and Antibiotic-Resistant Bacteria. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Michael, C.A.; Dominey-Howes, D.; Labbate, M. The Antimicrobial Resistance Crisis: Causes, Consequences, and Management. Front. Public Health 2014, 2, 145. [Google Scholar] [CrossRef]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic Resistance in Foodborne Bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbio. 2011, 2, 203. [Google Scholar] [CrossRef]
- Samreen; Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental Antimicrobial Resistance and its Drivers: A Potential Threat to Public Health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef]
- Kunhikannan, S.; Thomas, C.J.; Franks, A.E.; Mahadevaiah, S.; Kumar, S.; Petrovski, S. Environmental Hotspots for Antibiotic Resistance Genes. Microbiologyopen 2021, 10, e1197. [Google Scholar] [CrossRef] [PubMed]
- Amábile-Cuevas, C.F. Antibiotic Resistance from, and to the Environment. AIMS Environ. Sci. 2021, 8, 18–35. [Google Scholar] [CrossRef]
- Sriram, A.; Kalanxhi, E.; Kapoor, G.; Craig, J.; Balasubramanian, R.; Brar, S.; Criscuolo, N.; Hamilton, A.; Klein, E.; Tseng, K.; et al. The State of the World’s Antibiotics in 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers; Center for Disease Dynamics, Economics & Policy: Washington, DC, USA, 2021; Available online: https://cddep.org/blog/posts/the-state-of-the-worlds-antibiotics-report-in-2021/ (accessed on 20 November 2021).
- von Wintersdorff, C.J.H.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F.G. Dissemination of Antimicrobial Resistance in Microbial Ecosystems through Horizontal Gene Transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, M.; Caudell, M.A.; Mair, C.; Davis, M.A.; Matthews, L.; Quinlan, R.J.; Quinlan, M.B.; Lyimo, B.; Buza, J.; Keyyu, J.; et al. Antimicrobial Resistant Enteric Bacteria Are Widely Distributed Amongst People, Animals and the Environment in Tanzania. Nat. Commun. 2020, 11, 13995. [Google Scholar] [CrossRef]
- Adzitey, F. Incidence and Antimicrobial Susceptibility of Escherichia coli Isolated from Beef (Meat Muscle, Liver and Kidney) Samples in Wa Abattoir, Ghana. Cogent Food Agric. 2020, 6, 1718269. [Google Scholar] [CrossRef]
- Forsberg, K.J.; Reyes, A.; Wang, B.; Selleck, E.M.; Sommer, M.O.A.; Dantas, G. The Shared Antibiotic Resistome of Soil Bacteria and Human Pathogens. Science 2012, 337, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, X.; Li, J.; Lv, N.; Liu, F.; Wu, J.; Lin, I.Y.C.; Wu, N.; Weimer, B.C.; Gao, G.F.; et al. The Bacterial Mobile Resistome Transfer Network Connecting the Animal and Human Microbiomes. Appl. Environ. Microbiol. 2016, 82, 6672–6681. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.A.; Roshdy, H.; Samir, A.H.; Hamed, E.A. Antibiotic Resistance and Extended-Spectrum β-lactamase in Escherichia coli Isolates from Imported 1-Day-Old Chicks, Ducklings, and turkey Poults. Vet. World 2020, 13, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Liu, H.; Lan, T.; Dong, L.; Hu, H.; Zhao, S.; Zhang, Y.; Zheng, N.; Wang, J. Antibiotic Resistance Patterns of Pseudomonas spp. Isolated from Raw Milk Revealed by Whole Genome Sequencing. Front. Microbiol. 2020, 11, 1005. [Google Scholar] [CrossRef]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196–220. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A Review of the Occurrence of Pharmaceuticals and Personal Care Products in Indian Water Bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic Resistance Is Ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Pruden, A.; Virta, M.; Zhang, T. Editorial: Antibiotic Resistance in Aquatic Systems. Front. Microbiol. 2017, 8, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Wellington, E.M.; Boxall, A.B.; Cross, P.; Feil, E.J.; Gaze, W.H.; Hawkey, P.M.; Johnson-Rollings, A.S.; Jones, D.L.; Lee, N.M.; Otten, W.; et al. The Role of the Natural Environment in the Emergence of Antibiotic Resistance in Gram-Negative Bacteria. Lancet Infect. Dis. 2013, 13, 155–165. [Google Scholar] [CrossRef]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and Colonisation by Antibiotic-Resistant E. coli in UK Coastal Water Users: Environmental Surveillance, Exposure Assessment, and Epidemiological Study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Søraas, A.; Sundsfjord, A.; Sandven, I.; Brunborg, C.; Jenum, P.A. Risk Factors for Community-Acquired Urinary Tract Infections Caused by ESBL-Producing Enterobacteriaceae -A Case-Control Study in a Low Prevalence Country. PLoS ONE 2013, 8, e69581-7. [Google Scholar] [CrossRef]
- Leonard, A.F.C.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human Recreational Exposure to Antibiotic Resistant Bacteria in Coastal Bathing Waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef]
- O’Flaherty, E.; Borrego, C.M.; Balcázar, J.L.; Cummins, E. Human Exposure Assessment to Antibiotic-Resistant Escherichia coli through Drinking Water. Sci. Total Environ. 2018, 616–617, 1356–1364. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W.; Xu, L.; Strong, P.J.; Chen, H. Antibiotic-resistant Genes and Antibiotic-Resistant Bacteria in the Effluent of Urban Residential Areas, Hospitals, and a Municipal Wastewater Treatment Plant System. Environ. Sci. Pollut. Res. 2015, 22, 4587–4596. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of Antibiotics and Antibiotic Resistance Genes in Hospital and Urban Wastewaters and Their Impact on the Receiving River. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- McGowan, E. Comment on “Antibiotic Resistance Genes as Emerging Contaminants: Studies in Northern Colorado”. Environ. Sci. Technol. 2007, 41, 2651–2652. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; DePristo, M.A.; Collins, J.J. Sublethal Antibiotic Treatment Leds to Multidrug Resistance via Radical-Induced Mutagenesis. Mol. Cell 2010, 37, 311–320. [Google Scholar] [CrossRef]
- Ben, W.; Wang, J.; Cao, R.; Yang, M.; Zhang, Y.; Qiang, Z. Distribution of Antibiotic Resistance in the Effluents of Ten Municipal Wastewater Treatment Plants in China and the Effect of Treatment Processes. Chemosphere 2017, 172, 392–398. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human Health Consequences of Use of Antimicrobial Agents in Aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Hinchliffe, S.; Butcher, A.; Rahman, M.M. The AMR Problem: Demanding Economies, Biological Margins, and Co-producing Alternative Strategies. Palgrave Commun. 2018, 4, 142. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance. World Health Organization [Internet]. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 15 November 2021).
- West, N.J.; Obernosterer, I.; Zemb, O.; Lebaron, P. Major Differences of Bacterial Diversity and Activity inside and outside of a Natural Iron-Fertilized Phytoplankton Bloom in the Southern Ocean. Environ. Microbiol. 2008, 10, 738–756. [Google Scholar] [CrossRef]
- Zou, M.; Keelara, S.; Thakur, S. Molecular Characterization of Salmonella enterica Serotype Enteritidis Isolates from Humans by Antimicrobial Resistance, Virulence Genes, and Pulsed-Field Gel Electrophoresis. Foodborne Pathog. Dis. 2012, 9, 232–238. [Google Scholar] [CrossRef]
- Oh, E.-G.; Son, K.-T.; Yu, H.; Lee, T.-S.; Lee, H.-J.; Shin, S.; Kwon, J.-Y.; Park, K.; Kim, J. Antimicrobial Resistance of Vibrio parahaemolyticus and Vibrio alginolyticus Strains Isolated from Farmed Fish in Korea from 2005 through 2007. J. Food Prot. 2011, 74, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, C.; Shu, C.; Liu, L.; Geng, J.; Hu, S.; Feng, J. Marine Sediment Bacteria Harbor Antibiotic Resistance Genes Highly Similar to Those Found in Human Pathogens. Microb. Ecol. 2013, 65, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ellabaan, M.M.H.; Charusanti, P.; Munck, C.; Blin, K.; Tong, Y.; Weber, T.; Sommer, M.O.A.; Lee, S.Y. Dissemination of Antibiotic Resistance Genes from Antibiotic Producers to Pathogens. Nat. Commun. 2017, 8, 15784–15787. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal Transfer of Antibiotic Resistance Genes in Clinical Environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Editorial: Horizontal Gene Transfer Mediated Bacterial Antibiotic Resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Chen, B.; Nie, X.; Shi, Z.; Huang, X.; Li, X. The Distribution and Partitioning of Common Antibiotics in Water and Sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar] [CrossRef]
- Coutinho, F.H.; Pinto, L.H.; Vieira, R.P.; Martins, O.B.; Salloto, G.R.; de Oliveira Santoro, D.; Clementino, M.M.; Cardoso, A.M. Antibiotic resistance in aquatic environments of Rio de Janeiro, Brazil. Perspect. Water Pollut. 2013, 10, 54638. [Google Scholar]
- Ma, Y.; Li, M.; Wu, M.; Li, Z.; Liu, X. Occurrences and Regional Distributions of 20 Antibiotics in Water Bodies during Groundwater Recharge. Sci. Total Environ. 2015, 518–519, 498–506. [Google Scholar] [CrossRef]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Pharmaceutical Residues in Water and Sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere 2015, 134, 133–140. [Google Scholar] [CrossRef]
- Deng, W.; Li, N.; Zheng, H.; Lin, H. Occurrence and Risk Assessment of Antibiotics in River Water in Hong Kong. Ecotoxicol. Environ. Saf. 2016, 125, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.M.; Tun, H.M.; Poole, J.; Patidar, R.; Li, R.; Mi, R.; Amarawansha, G.E.A.; Fernando, W.G.D.; Khafipour, E.; Farenhorst, A.; et al. Detection of Antibiotic Resistance Genes in Source and Drinking Water Samples from a First Nations Community in Canada. Appl. Environ. Microbiol. 2016, 82, 4767–4775. [Google Scholar] [CrossRef] [PubMed]
- Madikizela, L.M.; Tavengwa, N.T.; Chimuka, L. Status of Pharmaceuticals in African Water Bodies: Occurrence, Removal and Analytical Methods. J. Environ. Manage. 2017, 193, 211–220. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic Pollution in Surface Fresh Waters: Occurrence and Effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Mindlin, S.Z.; Soina, V.S.; Petrova, M.A.; Gorlenko, Z.M. Isolation of Antibiotic Resistance Bacterial Strains from Eastern Siberia Permafrost Sediments. Russ. J. Genet. 2008, 44, 27–34. [Google Scholar] [CrossRef]
- Lobova, T.I.; Feil, E.J.; Popova, L.Y. Multiple Antibiotic Resistance of Heterotrophic Bacteria Isolated from Siberian Lakes Subjected to Differing Degrees of Anthropogenic Impact. Microb. Drug Resist. 2011, 17, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.I.; Cattanach, M. Antibiotic resistance of native and fecal bacteria isolated from rivers, reservoirs, and sewage treatment facilities in Victoria, southeastern Australia. Lett. Appl. Microbiol. 1999, 28, 164–168. [Google Scholar] [CrossRef]
- Gallert, C.; Fund, K.; Winter, J. Antibiotic resistance in raw and biological treated sewage and groundwater below leaking sewers. Appl. Environ. Biotechnol. 2005, 69, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Dib, J.R.; Weiss, A.; Neumann, A.; Ordoñez, O.; Estévez, M.C.; Farías, M.E. Isolation of bacteria from remote high-altitude Andean lakes able to grow in the presence of antibiotics. Recent Pat. Antiinfect. Drug Discov. 2009, 4, 66–76. [Google Scholar] [CrossRef]
- Jara, D.; Bello-Toledo, H.; Domínguez, M.; Cigarroa, C.; Fernández, P.; Vergara, L.; Quezada-Aguiluz, M.; Opazo-Capurro, A.; Lima, C.A.; González-Rocha, G. Antibiotic Resistance in Bacterial Isolates from Freshwater Samples in Fildes Peninsula, King George Island, Antarctica. Sci. Rep. 2020, 10, 3145. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Fiorentino, A.; Anselmo, A. Advanced Treatment of Urban Wastewater by UV Radiation: Effect on Antibiotics and Antibiotic-Resistant E. coli Strains. Chemosphere 2013, 92, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; El Ghany, M.A. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef]
- Hiller, C.X.; Hübner, U.; Fajnorova, S.; Schwartz, T.; Drewes, J.E. Antibiotic Microbial Resistance (AMR) Removal Efficiencies by Conventional and Advanced Wastewater Treatment Processes: A Review. Sci. Total Environ. 2019, 685, 596–608. [Google Scholar] [CrossRef]
- Woolhouse, M.; Ward, M.; Van Bunnik, B.; Farrar, J. Antimicrobial Resistance in Humans, Livestock and the Wider Environment. Phil. Trans. R. Soc. B 2015, 370, 20140083. [Google Scholar] [CrossRef]
- Bouki, C.; Venieri, D.; Diamadopoulos, E. Detection and Fate of Antibiotic Resistant Bacteria in Wastewater Treatment Plants: A Review. Ecotoxicol. Environ. Saf. 2013, 91, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hocquet, D.; Muller, A.; Bertrand, X. What Happens in Hospitals Does Not Stay in Hospitals: Antibiotic-Resistant Bacteria in Hospital Wastewater Systems. J. Hosp. Infect. 2016, 93, 395–402. [Google Scholar] [CrossRef]
- Bello-López, J.M.; Cabrero-Martínez, O.A.; Ibáñez-Cervantes, G.; Hernández-Cortez, C.; Pelcastre-Rodríguez, L.I.; Gonzalez-Avila, L.U.; Castro-Escarpulli, G. Horizontal Gene Transfer and Its Association with Antibiotic Resistance in the Genus Aeromonas spp. Microorganisms 2019, 7, 363. [Google Scholar] [CrossRef] [PubMed]
- Treangen, T.J.; Rocha, E.P.C. Horizontal Transfer, Not Duplication, Drives the Expansion of Protein Families in Prokaryotes. PLoS Genet. 2011, 7, e1001284. [Google Scholar] [CrossRef]
- Stecher, B.; Denzler, R.; Maier, L.; Bernet, F.; Sanders, M.J.; Pickard, D.J.; Barthel, M.; Westendorf, A.M.; Krogfelt, K.A.; Walker, A.W.; et al. Gut Inflammation Can Boost Horizontal Gene Transfer between Pathogenic and Commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA 2012, 109, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial Resistance and Virulence: A Successful or Deleterious Association in the Bacterial World? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.A.; Cryer, T.L.; Lafi, S.Q.; Basha, E.A.; Good, L.; Tarazi, Y.H. Identification of Escherichia coli from Broiler Chickens in Jordan, Their Antimicrobial Resistance, Gene Characterization and the Associated Risk Factors. BMC Vet. Res. 2019, 15, 159–216. [Google Scholar] [CrossRef]
- Galhano, B.S.P.B.; Ferrari, R.G.R.; Panzenhagen, P.; de Jesus, A.C.S.; Conte-Junior, C.A. Antimicrobial Resistance Gene Detection Methods for Bacteria in Animal-Based Foods: A Brief Review of Highlights and Advantages. Microorganisms 2021, 9, 923. [Google Scholar] [CrossRef]
- Koch, N.; Islam, N.F.; Sonowal, S.; Prasad, R.; Sarma, H. Environmental Antibiotics and Resistance Genes as Emerging Contaminants: Methods of Detection and Bioremediation. Curr. Res. Microb. Sci. 2021, 2, 100027. [Google Scholar] [CrossRef]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of Antibiotic Resistance Genes in the Environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef]
- Grenni, P. Antimicrobial Resistance in Rivers: A Review of the Genes Detected and New Challenges. Enviro Tox. Chem. 2022, 41, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Salyers, A.; Gupta, A.; Wang, Y. Human Intestinal Bacteria as Reservoirs for Antibiotic Resistance Genes. Trends Microbiol. 2004, 12, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.K.; Touno, E.; Higashiyama, Y.; Sasamoto, M.; Soma, M.; Yoshida, N.; Ito, A.; Umita, T. Determination of Tylosin Excretion from Sheep to Assess Tylosin Spread to Agricultural fields by Manure Application. Sci. Total Environ. 2018, 633, 399–404. [Google Scholar] [CrossRef]
- Hendriksen, R.S.; Munk, P.; Njage, P.; Van Bunnik, B.; McNally, L.; Lukjancenko, O.; Röder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global Monitoring of Antimicrobial Resistance Based on Metagenomics Analyses of Urban Sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- Anderson, M.A.; Whitlock, J.E.; Harwood, V.J. Diversity and Distribution of Escherichia coli Genotypes and Antibiotic Resistance Phenotypes in Feces of Humans, Cattle, and Horses. Appl. Environ. Microbiol. 2006, 72, 6914–6922. [Google Scholar] [CrossRef]
- Hajrulai-Musliu, Z.; Uzunov, R.; Krluku, M.; Jovanov, S.; Stojkovski, V.; Arapcheska, M.; Musliu, D.; Sasanya, J.J. Determination of Multi-Class Antimicrobial Residues and Antimicrobial Resistance in Cow Milk and Feces Samples during Withdrawal Period. Animals 2023, 13, 3603. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, J.H.; Shin, J.; Jang, H.M.; Kim, S.; Song, M.S.; Kim, Y.M. Quantitative and Qualitative Changes in Antibiotic Resistance Genes after Passing through Treatment Processes in Municipal Wastewater Treatment Plants. Sci. Total Environ. 2017, 605–606, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Hatosy, S.M.; Martiny, A.C. The Ocean as a Global Reservoir of Antibiotic Resistance Genes. Appl. Environ. Microbiol. 2015, 81, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current Status of the Use of Antibiotics and the Antimicrobial Resistance in the Chilean salmon Farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef]
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati; Nakada, N.; Murata, A.; Suzuki, T.; Suzuki, S.; et al. Ubiquitous Occurrence of Sulfonamides in Tropical Asian Waters. Sci. Total Environ. 2013, 452–453, 108–115. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158-9. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; Available online: https://apps.who.int/iris/handle/10665/112642 (accessed on 12 November 2021).
- World Health Organization. Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 1 January 2020).
- George, A. Antimicrobial Resistance (AMR) in the Food Chain: Trade, One Health and Codex. Trop. Med. Infect. Dis. 2019, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Baekkeskov, E.; Rubin, O.; Munkholm, L.; Zaman, W. Antimicrobial resistance as a global health crisis. In Oxford Research Encyclopedia of Politics; Stern, E., Ed.; Oxford University Press: Oxford, UK, 2020; pp. 1–24. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe 2018; ECDC: Stockholm, Sweden, 2019. [Google Scholar]
- Graham, D.W.; Bergeron, G.; Bourassa, M.W.; Dickson, J.; Gomes, F.; Howe, A.; Kahn, L.H.; Morley, P.S.; Scott, H.M.; Simjee, S.; et al. Complexities in Understanding Antimicrobial Resistance across Domesticated Animal, Human, and Environmental Systems. Ann. N. Y. Acad. Sci. 2019, 1441, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Sharma, M. Antimicrobial Resistance in the Environment: The Indian Scenario. Indian J. Med. Res. 2019, 149, 119–128. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013; CDC: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf (accessed on 1 January 2020).
- The World Bank. Antimicrobial Resistance (AMR). The World Bank [Internet]. 2021. Available online: https://www.worldbank.org/en/topic/health/brief/antimicrobial-resistance-amr (accessed on 12 November 2021).
- Llor, C.; Bjerrum, L. Antimicrobial Resistance: Risk Associated with Antibiotic Overuse and Initiatives to Reduce the Problem. Ther. Adv. Drug. Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- Shrestha, P.; Cooper, B.S.; Coast, J.; Oppong, R.; Thuy, N.D.T.; Phodha, T.; Celhay, O.; Guerin, P.J.; Wertheim, H.; Lubell, Y. Enumerating the Economic Cost of Antimicrobial Resistance Per Antibiotic Consumed to Inform the Evaluation of Interventions Affecting Their Use. Antimicrob. Resist. Infect. Control. 2018, 7, 98–99. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance (AR/AMR); U.S. Department of Health & Human Services: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/drugresistance/food.html (accessed on 22 November 2021).
- Bassetti, M.; Peghin, M.; Vena, A.; Giacobbe, D.R. Treatment of Infections Due to MDR Gram-Negative Bacteria. Front. Med. 2019, 6, 74. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An Evaluation of Multidrug-Resistant Escherichia coli Isolates in Urinary Tract Infections from Aguascalientes, Mexico: Cross-Sectional Study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef]
- Annavajhala, M.K.; Gomez-Simmonds, A.; Uhlemann, A.C. Multidrug-resistant Enterobacter cloacae Complex Emerging as a Global, Diversifying Threat. Front. Microbiol. 2019, 10, 44. [Google Scholar] [CrossRef]
- Friedrich, M.J. Health Care Attacks Continue in 23 Countries Around the World. JAMA 2017, 318, 231. [Google Scholar] [CrossRef]
- Salvatore, P.P.; Kendall, E.A.; Seabrook, D.; Brown, J.; Durham, G.H.; Dowdy, D.W. Projecting the Impact of Variable MDR-TB Transmission Efficiency on Long-Term Epidemic Trends in South Africa and Vietnam. Sci. Rep. 2019, 9, 18099. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic Resistance: What Is So Special about Multidrug-Resistant Gram-Negative Bacteria? GMS Hyg. Infect. Control. 2017, 12, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, E.; Korzeniewska, A.; Harnisz, M. Antibiotic Resistant Escherichia coli in Hospital and Municipal Sewage and Their Emission to the Environment. Ecotoxicol. Environ. Saf. 2013, 91, 96–102. [Google Scholar] [CrossRef]
- Razavi, M.; Marathe, N.P.; Gillings, M.R.; Flach, C.-F.; Kristiansson, E.; Joakim Larsson, D.G. Discovery of the Fourth mobile Sulfonamide Resistance Gene. Microbiome 2017, 5, 160. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The Human Microbiome: At the Interface of Health and Disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2016, 6, 1543. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human Health Risk Assessment of Antibiotic Resistance Associated with Antibiotic Residues in the Environment: A Review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 15 November 2021).
- World Bank. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017; Available online: https://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf (accessed on 18 November 2021).
- Baquero, F.; Martínez, J.L.; Cantón, R. Antibiotics and Antibiotic Resistance in Water Environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Schar, D.; Klein, E.Y.; Laxminarayan, R.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Aquaculture. Sci. Rep. 2020, 10, 21878. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; Van Boeckel, T.P. Twenty-year Trends in Antimicrobial Resistance from Aquaculture and Fisheries in Asia. Nat. Commun. 2021, 12, 6. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, J.; Li, J.; Cheng, Z.; Chaemfa, C.; Liu, D.; Zheng, Q.; Song, M.; Luo, C.; Zhang, G. Occurrence and Risks of Antibiotics in the Coastal Aquatic Environment of the Yellow Sea, North China. Sci. Total Environ. 2013, 450–451, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Binh, V.N.; Dang, N.; Anh, N.T.K.; Ky, L.X.; Thai, P.K. Antibiotics in the Aquatic Environment of Vietnam: Sources, Concentrations, Risk and Control Strategy. Chemosphere 2018, 197, 438–450. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef]
- Liyanage, G.Y.; Illango, A.; Manage, P.M. Prevalence and Quantitative Analysis of Antibiotic Resistance Genes (ARGs) in Surface and Groundwater in Meandering Part of the Kelani River Basin in Sri Lanka. Water Air Soil Pollut. 2021, 232, 351. [Google Scholar] [CrossRef]
- Ali, O.S.; Hozayen, W.G.; Almutairi, A.S.; Edris, S.A.; Abulfaraj, A.A.; Ouf, A.A.; Mahmoud, H.M. Metagenomic Analysis Reveals the Fate of Antibiotic Resistance Genes in a Full-Scale Wastewater Treatment Plant in Egypt. Sustainability 2021, 13, 11131–11219. [Google Scholar] [CrossRef]
- Buriánková, I.; Kuchta, P.; Molíková, A.; Sovová, K.; Výravský, D.; Rulík, M.; Novák, D.; Lochman, J.; Vítězová, M. Antibiotic Resistance in Wastewater and its Impact on a Receiving River: A Case Study of WWTP Brno-Modřice, Czech Republic. Water 2021, 13, 2309. [Google Scholar] [CrossRef]
- Guo, X.; Tang, N.; Lei, H.; Fang, Q.; Liu, L.; Zhou, Q.; Song, C. Metagenomic Analysis of Antibiotic Resistance Genes in Untreated Wastewater from Three Different Hospitals. Front. Microbiol. 2021, 12, 709051. [Google Scholar] [CrossRef]
- Markkanen, M.A.; Haukka, K.; Pärnänen, K.M.M.; Dougnon, V.T.; Bonkoungou, I.J.O.; Garba, Z.; Tinto, H.; Sarekoski, A.; Karkman, A.; Kantele, A.; et al. Metagenomic Analysis of Antimicrobial Resistance Genes in Wastewaters in Benin and Burkina Faso Indicates a Serious Health Risk from Untreated Hospital Wastewaters in Low-Income Countries. medRxiv 2021, 1–24. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, G. Investigation of the Prevalence of Antibiotic Resistance Genes According to the Wastewater Treatment Scale Using Metagenomic Analysis. Antibiotics 2021, 10, 188–213. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, Y.; Chan, C.-L.; On, H.; Wai, H.K.-F.; Shekhawat, S.S.; Gupta, A.B.; Varshney, A.K.; Chuanchuen, R.; Zhou, X.; et al. Metagenomic Survey Reveals More Diverse and Abundant Antibiotic Resistance Genes in Municipal Wastewater than Hospital Wastewater. Front. Microbiol. 2021, 12, 712843. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Vu, H.P.; Nguyen, L.N.; Wang, Q.; Djordjevic, S.P.; Donner, E.; Yin, H.; Nghiem, L.D. Monitoring Antibiotic Resistance Genes in Wastewater Treatment: Current Strategies and Future Challenges. Sci. Total Environ. 2021, 783, 146964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, F.; Zeng, Z.; Xu, M.; Sun, F.; Yang, L.; Bi, X.; Lin, Y.; Gao, Y.; Hao, H.; et al. Advances in Metagenomics and Its Application in Environmental Microorganisms. Front. Microbiol. 2021, 12, 766364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buschmann, A.H.; Tomova, A.; López, A.; Maldonado, M.A.; Henríquez, L.A.; Ivanova, L.; Moy, F.; Godfrey, H.P.; Cabello, F.C. Salmon Aquaculture and Antimicrobial Resistance in the marine Environment. PLoS ONE 2012, 7, e42724-28. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.D.; Knox, N.C.; Ronholm, J.; Pagotto, F.; Reimer, A. Metagenomics: The Next Culture-independent Game Changer. Front. Microbiol. 2017, 8, 1069. [Google Scholar] [CrossRef]
- Zeng, J.; Pan, Y.; Yang, J.; Hou, M.; Zeng, Z.; Xiong, W. Metagenomic Insights into the Distribution of Antibiotic Resistome between the Gut-Associated Environments and the Pristine Environments. Environ. Int. 2019, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.H.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and Risk Assessment of Multiple Classes of Antibiotics in Urban Canals and Lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef]
- McInnes, R.S.; Uz-Zaman, H.; Alam, I.T.; Ho, S.F.S.; Moran, R.A.; Clemens, J.D.; Islam, S.; van Schaik, W. Metagenome-Wide Analysis of Rural and Urban Surface Waters and Sediments in Bangladesh Identifies Human Waste as a Driver of Antibiotic Resistance. mSystems 2021, 6, e0013721. [Google Scholar] [CrossRef]
- Czekalski, N.; Berthold, T.; Caucci, S.; Egli, A.; Bürgmann, H. Increased levels of multiresistant bacteria and resistance genes after wastewater treatment and their dissemination into Lake Geneva. Front. Microbiol. 2012, 3, 106. [Google Scholar] [CrossRef] [PubMed]
- Czekalski, N.; Imminger, S.; Salhi, E.; Veljkovic, M.; Kleffel, K.; Drissner, D.; Hammes, F.; Bürgmann, H.; von Gunten, U. Inactivation of antibiotic-resistant bacteria and resistance genes by ozone: From laboratory experiments to full-scale wastewater treatment. Environ. Sci. Technol. 2016, 50, 11862–11871. [Google Scholar] [CrossRef]
- Bourrouet, A.; Garcia, J.; Mujeriego, R.; Penuelas, G. Fecal bacteria and bacteriophage inactivation in a full-scale UV disinfection system used for wastewater reclamation. Water Sci. Technol. 2001, 43, 187–194. [Google Scholar] [CrossRef]
- Breazeal, M.R.; Novak, J.T.; Vikesland, P.J.; Pruden, A. Effect of wastewater colloids on membrane removal of antibiotic resistance genes. Water Res. 2013, 47, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hong, P. Removal of antibiotic-resistant bacteria and antibiotic-resistance genes affected by varying degrees of fouling on anaerobic microfiltration membranes. Environ. Sci. Technol. 2017, 51, 12200–12209. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.; Waldron, L.; Gillings, M.R. Potential impacts of aquatic pollutants: Subclinical antibiotic concentrations induce genome changes and promote antibiotic resistance. Front. Microbiol. 2015, 6, 803. [Google Scholar] [CrossRef]
- Courvalin, P. Transfer of antibiotic resistance genes between gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 1994, 38, 1447–1451. [Google Scholar] [CrossRef]
- Strange, J.E.S.; Leekitcharoenphon, P.; Møller, F.D.; Aarestrup, F.M. Metagenomics Analysis of Bacteriophages and Antimicrobial Resistance from Global Urban Sewage. Sci. Rep. 2021, 11, 1600. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.R.; Parekh, S.; Rathbone, J.; Del Mar, C.B.; Hoffmann, T.C. A Systematic Review of the Public’s Knowledge and Beliefs about Antibiotic Resistance. J. Antimicrob. Chemother. 2016, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Yaseri, M.; Faraji, M. Removal of Antibiotics from Aqueous Solutions by Nanoparticles: A Systematic Review and Meta-Analysis. Environ. Sci. Pollut. Res. 2019, 26, 8444–8458. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, A.; Kaur, R.; Singh, S.; Bhatti, M.S.; Umar, A.; Baskoutas, S.; Kansal, S.K. Cu-BTC Metal Organic Framework (MOF) Derived Cu-Doped TiO2 Nanoparticles and Their Use as Visible Light Active Photocatalyst for the Decomposition of Ofloxacin (OFX) Antibiotic and Antibacterial Activity. Adv. Powder Technol. 2021, 32, 1350–1361. [Google Scholar] [CrossRef]
- Singh, S.; Kishore, D.; Singh, R.K. Potential for Further Mismanagement of Fever during COVID-19 Pandemic: Possible Causes and Impacts. Front. Med. 2022, 9, 751929. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- World Economic Forum. Antimicrobial Resistance and Water: The Risks and Costs for Economies and Societies. 2021. Available online: http://www3.weforum.org/docs/WEF_Antimicrobial_Resistance_and_Water_2021.pdf (accessed on 10 February 2022).
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2018; Available online: https://apps.who.int/iris/bitstream/handle/10665/312266/9789241515528-eng.pdf (accessed on 15 November 2021).
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019.
- Léger, A.; Lambraki, I.; Graells, T.; Cousins, M.; Henriksson, P.J.G.; Harbarth, S.; Carson, C.; Majowicz, S.; Troell, M.; Parmley, E.J.; et al. AMR-intervene: A Social-Ecological Framework to Capture the Diversity of Actions to Tackle Antimicrobial Resistance from a One Health Perspective. J. Antimicrob. Chemother. 2021, 76, 1–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajqi Berisha, N.; Poceva Panovska, A.; Hajrulai-Musliu, Z. Antibiotic Resistance and Aquatic Systems: Importance in Public Health. Water 2024, 16, 2362. https://doi.org/10.3390/w16172362
Lajqi Berisha N, Poceva Panovska A, Hajrulai-Musliu Z. Antibiotic Resistance and Aquatic Systems: Importance in Public Health. Water. 2024; 16(17):2362. https://doi.org/10.3390/w16172362
Chicago/Turabian StyleLajqi Berisha, Njomza, Ana Poceva Panovska, and Zehra Hajrulai-Musliu. 2024. "Antibiotic Resistance and Aquatic Systems: Importance in Public Health" Water 16, no. 17: 2362. https://doi.org/10.3390/w16172362
APA StyleLajqi Berisha, N., Poceva Panovska, A., & Hajrulai-Musliu, Z. (2024). Antibiotic Resistance and Aquatic Systems: Importance in Public Health. Water, 16(17), 2362. https://doi.org/10.3390/w16172362







