1. Introduction
With the development of industrialization and urbanization in Chinese coastal areas, sewage and wastewater containing all kinds of heavy metals eventually discharge into the sea through outfall pipes, groundwater and other ways, which leads to the continuous increase of heavy metal content in the marine environment [
1]. Among these kinds of heavy metal pollution, copper pollution is a representative and serious category, which is mainly because the pollution sources are various, including copper mining and biological oxidation [
2], metal smelting and processing, machinery manufacturing, etc. The commonly used copper pollution treatment methods, such as the reverse osmosis membrane method, although effective, are expensive and difficult to promote, so it is also difficult for the departments concerned to control this type of pollution [
3]. In the coastal waters of China, for example, the concentration of heavy metal ions, especially copper ions in the Beibu Gulf, has been gradually increasing in the past 20 years [
4,
5]. In the East China sea, the concentration of copper ions in nearshore sediments has reached 23.7 mg/kg (dw), which is a really high level [
6]. In other countries, such as Moreton Bay, Australia, the average concentration of copper ions will increase to more than twice as much in the next century, causing enormous harm to biology and the ecological environment [
7]. Marine copper pollution has become a widely concerning environmental issue, and the ecological risks and pollution monitoring of heavy metal pollution, especially copper pollution, have become a global problem [
8,
9].
Plastic has the characteristics of low price, light weight, biological inertia and so on. Since its invention, it has been widely used in commerce and industry, resulting in the accumulation of plastic waste in the environment [
10,
11]. It is estimated that tens of millions of tons of plastic waste were discharged into the coastal area in the last 20 years [
12], and it is expected that the amount of plastic waste entering the marine environment will increase to nearly 100 million tons by 2025 [
13]. In the natural environment, plastic waste gradually decreases under the combined action of various factors. Plastic waste is usually divided into macro-, meso-, micro-, and nanoplastics. Microplastics are plastic particles with a particle size of less than 5 mm, while nanoplastics have a particle size of less than 1 μm. Both are collectively referred to as micro-nano plastics in the fields of environmental science and materials science [
12]. Besseling et al. [
14] mentioned in a review that the concentration of microplastics in the marine environment will reach at 129.4 pieces/L in 2100 in the context of business as usual. After being discharged into water and ingested by organisms, it will accumulate through the food chain, which is not only harmful to aquatic organisms, but also negative to human health [
15]. Compared with other microplastics, the abundance of polystyrene (PS) microplastics in the water environment is higher; therefore, researchers have paid more attention to the toxic effect of PS plastics [
16,
17].
Skeletonema costatum is a kind of plankton widely distributed in the ocean and coastal zone. As one of the most abundant members of the diatom genus, it has strong carbon fixation ability, is the main producer of organic carbon in the ocean, and has an important influence on the global carbon cycle [
18]. In addition, it also has the characteristics of strong stress resistance, rapid growth, and easy cultivation. Therefore, it is often regarded as the research object of algae biology and toxicology [
19,
20]. Given the significant harm of copper ions and PS microplastics to the marine environment, it is of great significance to study their effects on the normal life activities of
Skeletonema costatum.
2. Background
As two important pollutants in the water environment, heavy metal and microplastic pollution have gradually become the focus of environmental science and toxicology research [
12,
15,
21]. In recent years, many scholars have conducted research on the effects of copper ions or microplastics on algae. For example, cadmium ions can cause a decrease in chlorophyll content and increase of reactive oxygen species in
Dunaliella salina [
22]; Copper ions can cause a slowdown in the growth rate of
Skeletonema marinoi and an increase in the activity of stress resistant enzymes of
S. costatum [
23,
24]. Mir et al. [
25] generally believe that excessive concentrations of copper ions have a negative impact on the growth of algae cells.
However, the impact of microplastics on the growth of microalgae is relatively complex. Mao et al. [
26] have found that PS microplastics exhibit an effect of first inhibiting and then promoting the growth of
Chlorella sp. during its growth process; Long et al. [
27] have also found that polystyrene microplastics have no significant effect on the growth of
Tisochrysis lutea and
Heterocapsa triquetra; Wang et al. [
28] found that polystyrene microplastics have a significant inhibitory effect on the growth of
Chaetoceros curvisetus.
As for the combined toxic effects of heavy metals and microplastics on algae, it is still under continuous exploration. Xie et al. [
29] found that PVC microplastics have no significant inhibitory effect on the growth of
Chlorella vulgaris when coexisting with zinc ions. Lin et al. [
30] also found that the combined effect of polyacrylonitrile microplastics and copper ions was not significantly different from the effect of copper ions alone on
Chlorella vulgaris. But some studies have also found that polystyrene microplastics, when coexisting with copper ions, can have a negative impact on the life activities of
Pseudokirchnerlla subcapitata [
31]. It can be seen that the combined toxicity of microplastics and heavy metals to algae is more complex.
It is worth noting that the interaction between heavy metals and microplastics, especially the adsorption–desorption effect, has a significant impact on the toxicity of pollutants to organisms [
32,
33]. Bao et al. [
34] found that both PA and PET plastic particles in aquatic environments have strong adsorption effects on copper ions, and this adsorption effect is related to the number of oxygen-containing functional groups on the surface of microplastics; PA microplastics with more functional groups have a stronger adsorption effect on copper than PET, in theory. Xue et al. [
35] found that a certain concentration of aged PS plastic particles can achieve a theoretical high adsorption capacity of 15 mg/kg for divalent mercury ions, which would greatly affect their combined toxicity to aquatic organisms. However, there is little research on the combined stress of copper ions and PS microplastics on algal cells, especially for key species like
S. costatum.
In conclusion, the combined toxic effects of copper ions and microplastics on algae need to be deeply analyzed, and the effect of their adsorption on their biological toxicity also needs to be further explored. This study undertakes a biological analysis of the effect of copper ions and microplastics on growth, thus providing a basis for algal biology and research into the ecological effects of pollutants, thus revealing the interaction between pollutants, microplastics and heavy metals on the coastal sea ecosystem.
3. Materials and Methods
To investigate the effect of different concentrations of microplastics on copper ion toxicity, this experiment used a culture medium containing 0.05 mg/L copper ions and 1 μm particle size PS microspheres with a certain concentration gradient to conduct cultivation experiments and pollutant adsorption experiments on ribbed algae. By observing and statistically measuring the growth of algal cells and the adsorption of copper ions, corresponding experimental results were obtained.
3.1. Algal Origin and Cultivation
The algae selected for this study were stored in the Key Laboratory of Marine Environment and Ecology, Ministry of Education, Ocean University of China. As a routine procedure, we use glass culture containers. According to Jin et al. [
36], glass containers have a certain adsorption effect on Cu
2+, but its rate is slow, and the adsorption effect on copper ions within 96 h can be ignored. Also, it does not introduce plastic interference. With sterilized f/2 medium, algal cells of different treatments were cultured in a 20 °C constant temperature light incubator (GXZ-310B, Ningbo Jiangnan Instrument Factory, Ningbo, China). The light-dark cycle was 12 h:12 h, and the light intensity was 3000 lx.
3.2. Experimental Reagents
The copper salt used in this experiment was CuSO4·5H2O (Sinopharm Holdings, analytically pure), 0.1 mol/L copper sulfate reserve solution was prepared before use and added to the culture medium after dilution. Microplastics were monodisperse polystyrene (Polystyrene, PS) microballs (Jiangsu Zhichuan Technology Company, Jiangsu, China) with particle size of 1 μm and solid content of 2.5%, which are also used after dilution.
3.3. Algal Cell Growth Experiment Design
According to Chinese seawater quality standards, when the concentration of copper ions reaches 0.05 mg/L, it exceeds the limit values of the four types of water quality standards, indicating that the seawater is polluted to a certain extent by copper ions [
37]. As mentioned in the first part of this article, according to the density of PS particles [
14], we believe that when there are 1–10 mg microplastic particles per liter of seawater, the water has already been severely polluted. Therefore, the copper ion concentration for the copper ion pollution group is set to 0.05 mg/L, and the PS microsphere concentration is set to: 2.50 mg/L, 5.00 mg/L, 7.50 mg/L, and 10.00 mg/L. The experimental groups and their corresponding pollutant concentrations are detailed in
Table 1.
The initial cell concentration was 2 × 105 cells/mL, the culture volume was 100 mL, and 3 parallel samples were set up for 96 h. After the start of the experiment, algal cell concentration was measured every 24 h and growth curves drawn.
3.4. The Adsorption Experiment
To study the adsorption effect of microplastics on copper ions, mixed contamination groups I to IV (containing 0.05 mg/L copper ions, PS microsphere concentration was 2.50 mg/L, 5.00 mg/L, 7.50 mg/L and 10.00 mg/L, respectively). No algal cells were added to the culture system of the adsorption experiment, and the remaining conditions were exactly the same as those in the algal cell growth experiment. In the chart shown in this article, they are referred to as mixed groups I–IV (NC) to distinguish them from the mixed group in algal cell culture experiments.
3.5. Sample Processing
Samples were taken every 24 h after the start of the experiment. The algal cell counting sample was temporarily stored in a 2mL centrifuge tube and fixed with Luge reagent (Beijing Solai Biotechnology Co., Ltd., Beijing, China) before counting. As for the free copper ion sample, 10mL of culture medium was taken from each culture bottle in the adsorption experiment, and PS microspheres were filtered through a 0.45 μm filter membrane (Jinteng Technology, Tianjin, China). The filtrate was refrigerated at 4 °C. After all experiments were completed, ICP-MS was used to detect free copper ions in the samples.
3.6. Measurement of the Experimental Parameters
The algal cell density was counted by cell counting plate: 100 μL algal fluid was applied to the cell counting plate, and counted under a microscope (BX 51, Olympus, Tokyo, Japan) to discover the algal cell density in the sample (unit: 105 cells/mL). The content of free copper ions in the adsorption experiment was determined by inductively coupled high-frequency plasma mass spectrometry (305X, PerkinElmer, Waltham, MA, USA). The specific method is as follows: prepare the copper ion standard solution, open the ICP-MS instrument switch, complete the ventilation and ignition operations in turn, and enter the sample through the injection tube to draw the standard curve. The sample solution is then analyzed by the same method to obtain the concentration of copper ions not adsorbed in the sample.
3.7. Experimental Data Processing
For algal cell growth and adsorption experimental detection data, differential significance analysis was performed using a t-test and plot using Origin 2024.
In this experiment, the adsorption rate of PS microspheres for copper ions (
ADR) and the alleviation rate of PS microspheres for copper ion toxicity (
ALR) was also calculated as follows:
ICOC: Initial concentration of copper ions
COCA: Concentration of free copper ions after absorbtion
MCC: Mixed pollution group algal cell concentration
CICC: Copper ion group algal cell concentration
CNCC: Control group algal cell concentration
The correlation between the two sets of data is judged on the ADR (X) and the ALR (Y) after the data correlation analysis. To make the relationship between the two more intuitive, each group of data was drawn into a scatter plot, and the model was selected for fitting according to the analysis results.
4. Results and Discussion
4.1. The Adsorption of Copper Ions by Microplastics of Different Concentrations
Figure 1 shows the change of free copper ion concentration in the adsorption experiment. According to the figure, the adsorption effect of PS microspheres on copper ions was extremely significant (
p < 0.05). In the first 24 h of the fastest adsorption rate, the higher the PS concentration, the stronger the adsorption of copper ions (
p < 0.05). After the 48th hour of the experiment, there was no significant difference in the culture system (
p > 0.05).
The adsorption kinetics of copper ions affected by microplastics basically coincides with the characteristics of the first-order reaction dynamics. With time, the adsorption rate is first fast and then slow, until equilibrium is finally reached [
38,
39]. When the concentration of microplastics in the solution is high, the collision probability with copper ions is greater, and the adsorption capacity is larger, so the adsorption rate gradually increases with the concentration of microplastics at the beginning of the reaction.
4.2. Effects of Copper Ions and Microplastics on Algal Cell Growth
According to
Figure 2, 0.05 mg/L copper ions produced an extremely significant inhibitory effect on the growth of algal cells (
p < 0.01). From this, it can be seen that 0.05 mg/L copper ions have a significant toxic effect on algal cells, which can rapidly decrease the growth rate of algal cells. However, the four concentrations of PS microspheres set in the experiment had no significant effect on algal cells in the first 48 h of the cultivation process (
p > 0.05). After the last 48 h of growth (
p < 0.05), the algae cells in the control group were still in the logarithmic phase and the growth rate of cell density was kept at a higher growth rate at 3.0 × 10
5 cells/day. However, the growth rate of the experimental group containing PS microspheres slowed from the original 3.0 × 10
5 cells/day~4.0 × 10
5 cells/day to less than 1.5 × 10
5 cells/day, even reaching a plateau.
It can be seen that both copper ions and PS microspheres inhibit algal cell growth, but the acute toxic effect of the copper ions in the experimental concentration on algal cells is significant, while the inhibition of PS microspheres on growth was reflected in the last 48 h of the experiment. However, when these two pollutants coexist, although they still inhibited the growth of algal cells, the inhibition of algal cell growth was weakened compared with the presence of only copper ions, which was significantly reflected in the experimental group containing low and medium concentrations of PS microspheres (2.50–7.50 mg/L) (p < 0.05). This indicates that the PS microspheres can reduce the toxic effect of copper ions on algal cells in this experiment. However, in the latter half of the incubation time, the inhibitory effect of the mixed contamination on the algal cell growth became more obvious compared to the first 48 h.
A series of studies have shown that when the concentration of copper ions in the culture system exceeds the tolerance range of algal cells, the cells will accumulate copper ions, leading to damage of a variety of organelles in cells, degeneration and inactivation of proteins, and inhibition of various enzymes involved in biochemical reactions from their normal roles, thus rapidly inhibiting the life activities of algal cells [
40,
41,
42,
43]. The presence of microplastics will promote the algal cells to produce reactive oxygen species, damage the cell membrane and the chloroplast membrane, and affect the photosynthesis of the algal cells [
44]. In addition, the diameter of the cell is about 6–7 μm, while the diameter of PS microspheres used in this experiment is 1 μm. PS particles may also adsorb on algal cells and interfere with the absorption of nutrient salts by algal cells, thus inhibiting their growth [
45]. When PS microspheres coexist with copper ions, the interaction of the two would make the mixture less toxic to algal cells than when copper ions are present alone.
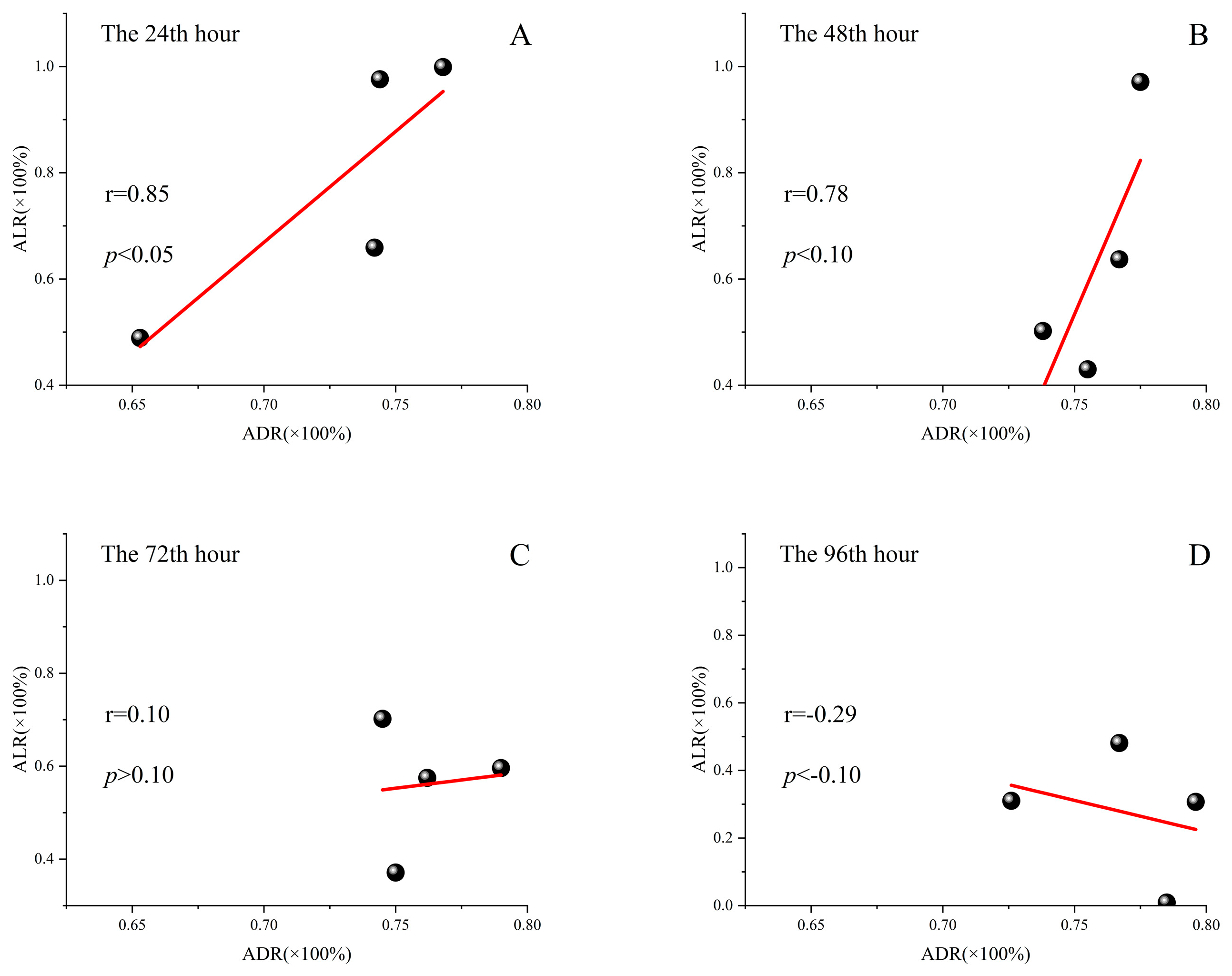
4.3. The Adsorption Rate of Microplastics to Copper Ions and the Remission Rate of Copper Ion Toxicity
After the calculation (the results is in
Table 2) and correlation analysis, we found that the adsorption rate and the remission rate were positively correlated, but the degree of correlation varied according to the culture stages. This is confirmed by a linear fit (
Figure 3). However, by investigating the data points of the copper ion adsorption rate and toxicity remission rate in the last 48 h of the culture stage, it was found that the PS adsorption of copper ions by each mixed pollution group no longer had a significant mitigation effect on the inhibition of cell growth. This is probably because the algae cell growth of the experimental group containing only the PS microspheres was significantly inhibited in the last 48 h as compared to the first 48 h. The negative effect of PS microspheres on algal cell growth in culture began to appear later, which could offset the mitigation of PS microsphere to copper ion toxicity.
But overall, the more adsorption of copper ions by PS microspheres, the less the inhibitory effect of these two pollutants on algal cell growth. At this point, we can conclude that the adsorption of copper ions by PS microspheres is an important reason for the weaker inhibitory effect on algal cell growth in the presence of both pollutants compared to the presence of copper ions alone.
4.4. Summary and Further Discussion
We found the effects of copper ions and PS microplastics on the growth of S. costatum at experimental concentrations. As an important group of planktonic organisms in coastal waters, this study can be used to evaluate the impact of these two pollutants on nearshore ecosystems; in addition, the interaction between PS microplastics and copper ions also affects the toxic effect of copper ions on algal cells, providing inspiration and reference for further exploration of pollutant interactions.
Some readers may ask why there are no algae cells in the culture system during adsorption experiments. As mentioned earlier, algae cells absorb copper ions, which affects adsorption. However, according to the experimental results, in the presence of microplastics, especially during the first 48 h of cultivation, the toxic effect of copper ions on algal cells is minimal. In other words, under these conditions, the absorption of copper ions by algal cells can be ignored.
It is worth mentioning that the study by Zhu et al. [
46] on the interaction between copper ions and microplastics on the toxicity of pollutants is highly similar to this experiment. They investigated the toxicity of copper nanoparticles and PVC on ribbed algae. It was found that adsorption of copper ions on mPVC and aggregation between copper nanoparticles and mPVC are the main reasons for the impact on toxicity of nanocopper with added microplastics. This is highly consistent with our research results.
But what sets us apart is that the copper ions in the culture medium used in our laboratory are completely derived from the ionization of copper salts, while Zhu et al. used nano-copper particles, the copper ions of which in water are not entirely generated by dissolution ionization. More importantly, we use PS, while Zhu et al. used PVC as the MPs. Brennecke et al. [
47] showed that under certain conditions, PVC has a stronger adsorption capacity for copper ions than PS. Therefore, even though Zhu et al. confirmed the adsorption effect of PVC on copper ions, the adsorption of PS on copper ions still needed to be explored. Our study still has innovation and scientific significance.
5. Conclusion
The following conclusions can be drawn from this experiment:
(1) 0.05 mg/L of copper ions significantly inhibited algal cell growth.
(2) PS microspheres had no obvious effect on algal cells in the first 48 h of the experiment. In the late culture period, PS microspheres will cause a decrease in algal cell density, and when the concentration of PS microsphere is too high, they will advance the plateau of algal cell growth, which is different from copper ion contamination.
(3) The coexistence of PS microspheres and copper ions can alleviate their toxicity on algal cells to some extent. The reason is that the PS microspheres have an adsorption effect on copper ions in the early culture period.
In summary, the adsorption of copper ions by PS microspheres is an important reason why the pre-growth condition of algae cells is better under mixed pollution of PS and copper ions than under pollution solely by copper ions. In the later stage of cultivation, the negative impact of microplastics on algal cell growth began to emerge, offsetting its weakening effect on copper ion toxicity.
6. Prospect
Firstly, the physicochemical properties of microplastics vary with different types, particle sizes, and formation processes, resulting in different inhibitory effects on algal cell growth and adsorption effects on heavy metal ions. However, the feasibility of comprehensively exploring the effects of heavy metal–microplastic combined exposure on phytoplankton by replacing experimental materials is not strong. Our research team believes that in the future, we should start from the adsorption–desorption behavior between microplastics and heavy metal ions, as well as the inhibitory mechanisms of these two types of pollutants on the life activities of phytoplankton, to explore general conclusions or quantitative and semi-quantitative models, in order to have a deeper understanding of the interactions between heavy metals, microplastics, and phytoplankton.
In addition, this study also provides ideas for preventing and controlling heavy metal pollution in the ocean. In addition to reducing pollutant emissions at the source, high-performance and environmentally friendly adsorption materials can also be developed, which can be deployed in areas with severe heavy metal pollution to achieve remediation goals.
Author Contributions
Topic proposal, experimental design, experimental operation, data analysis: H.L.; experimental guidance: Y.Z., X.Z. and L.D.; writing: H.L.; review and revision: Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Students Research and Development Program of Ocean University of China (OUC-SRDP), Project No. 202310423423X.
Data Availability Statement
Data will be made available on reasonable request.
Acknowledgments
This study is an extension project of OUC-SRDP (202310423423X), and part of the experimental funding is supported by OUC-SRDP. The research direction was conceived by the author of this article based on the research results of the other four students, Zongxue Jing, Jinlai Hua, Hongmei Tan, and Yonghui Fang. We express our sincere gratitude to them here.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Filimonova, V.; Gonçalves, F.; Marques, C.J.; Troch, D.M.; Goncaloves, M.M.A. Fatty acid profiling as bioindicator of chemical stress in marine organisms: A review. Ecol. Indic. 2016, 67, 657–672. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Luo, W.; Wang, X.; Liao, R.; Yu, S.; Hong, M.; Zhao, C.; Yang, B.; Liu, X.; et al. Inhibition of humic acid on copper pollution caused by chalcopyrite biooxidation. Sci. Total Environ. 2022, 851, 158200. [Google Scholar] [CrossRef] [PubMed]
- Abdulrab, A.M.A.; Sonu, S.; Monu, S.; Roman, K.; Naresh, A.K.; Brajesh, K.; Ahmad, U.; Sotirios, B.; Tapan, M.K. Microbial strategies for copper pollution remediation: Mechanistic insights and recent advances. Environ. Pollut. 2024, 346, 123588. [Google Scholar]
- Wang, Y.; Hu, Y.; Liu, Y.; Chen, Q.; Xu, J.; Zhang, F.; Mao, J.; Shi, Q.; He, C.; Cai, R.; et al. Heavy metal induced shifts in microbial community composition and interactions with dissolved organic matter in coastal sediments. Sci. Total Environ. 2024, 927, 172003. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, Z.; Feng, A.; Guo, D.; Zhang, R.; Xia, P.; Yan, W.; Zhou, X. Recent history of metal contamination in the Fangcheng Bay (Beibu Gulf, South China) utilizing spatially-distributed sediment cores: Responding to local urbanization and industrialization. Mar. Pollut. Bull. 2020, 158, 111418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sun, X.; Xu, J. Heavy metal pollution in the East China Sea: A review. Mar. Pollut. Bull. 2020, 159, 111473. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.; Chaloupka, M.; Sanò, M.; Rodger, T. Modelling the effects of ‘coastal’ acidification on copper speciation. Ecol. Model. 2011, 222, 3559–3567. [Google Scholar] [CrossRef]
- Gao, Y.; Qiao, Y.; Xu, Y.; Zhu, L.; Feng, J. Assessment of the transfer of heavy metals in seawater, sediment, biota samples and determination the baseline tissue concentrations of metals in marine organisms. Environ. Sci. Pollut. Res. 2021, 28, 28764–28776. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Dong, F.; Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 2020, 159, 111505. [Google Scholar] [CrossRef]
- Javier, R.; Gonzalo, Z.; Luis, O.; José, P.L.; Isabel, S. A novel ‘sea-thermal’, synergistic co-valorisation approach for biofuels production from unavoidable food waste (almond hulls) and plastic residues (disposable face masks). Chem. Eng. J. 2022, 449, 137810. [Google Scholar]
- Gluth, A.; Xu, Z.; Fifield, L.S.; Yang, B. Advancing biological processing for valorization of plastic wastes. Renew. Sustain. Energy Rev. 2022, 170, 112966. [Google Scholar] [CrossRef]
- Williams, A.T.; Nelson, R. The past, present, and future of plastic pollution. Mar. Pollut. Bull. 2022, 176, 113429. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perrymen, M.; Andrady, A.; Narayan, R.; Lavenderlaw, K. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Besseling, E.; Redondo-Hasselerhard, P.; Foekema, E.M.; Koelmans, A.A. Quantifying ecological risks of aquatic micro- and nanoplastic. Crit. Rev. Environ. Sci. Technol. 2019, 49, 32–80. [Google Scholar] [CrossRef]
- De Felice, B.; Sabatini, V.; Antenucci, S.; Gattoni, G.; Santo, N.; Bacchetta, R.; Ortenzi, A.M.; Parolini, M. Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 2019, 231, 423–431. [Google Scholar] [CrossRef]
- Nora, E.; Joaquim, R.; Jordi, S.; Jaume, F.; Marta, S. Microplastics levels, size, morphology and composition in marine water, sediments and sand beaches. Case study of Tarragona coast (western Mediterranean). Sci. Total Environ. 2021, 786, 147453. [Google Scholar]
- Fan, F.Y.; Liu, T.; Qian, X.; Deng, L.; Rao, W.; Zhang, Q.; Zheng, J.; Gao, X. Metabolic impacts of polystyrene microplastics on the freshwater microalga Microcystis aeruginosa. Sci. Total Environ. 2022, 836, 155655. [Google Scholar] [CrossRef]
- Maria, S.; Emanuel, B.; Mahnoor, Z.; Kwantes, M.; Pohnert, G.; Steinbeck, C. Draft genome assembly and sequencing dataset of the marine diatom Skeletonema cf. costatum RCC75. Data Brief 2022, 41, 107931. [Google Scholar]
- Zhang, B.; Hu, S.; Sun, S.; Fang, T.; Yu, Y.; Sun, X.; Xu, N. Transcriptomic analysis provides insights into the algicidal mechanism of cocamidopropyl betaine against the red tide microalgae Skeletonema costatum. Mar. Environ. Res. 2023, 183, 105838. [Google Scholar] [CrossRef]
- Guo, X.; Han, T.; Tan, L.; Zhao, T.; Zhao, T.; Zhu, X.; Huang, W.; Lin, K.; Zhang, N.; Wang, J. The allelopathy and underlying mechanism of Skeletonema costatum on Karenia mikimotoi integrating transcriptomics profiling. Aquat. Toxicol. 2022, 242, 106042. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lu, L.; Zheng, M.; Zhang, X.; Tian, H.; Wang, W.; Ru, S. Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 2019, 254, 11302. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, M.; Bao, J.; Liu, J. Physiological, metabolomic, and transcriptomic analyses reveal the dynamic redox homeostasis upon extended exposure of Dunaliella salina GY-H13 cells to Cd. Ecotoxicol. Environ. Saf. 2021, 223, 112593. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, K.; Karthikeyan, P.; Ashokkumar, S.; AshokPrabu, V.; Sampathkumar, P. Effect of copper on growth and enzyme activities of marine diatom, Odontella mobiliensis. Bull. Environ. Contam. Toxicol. 2012, 88, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Andersson, B.; Godhe, A.; Filipsson, L.H.; Rengefors, K.; Berglund, O. Differences in metal tolerance among strains, populations, and species of marine diatoms–Importance of exponential growth for quantification. Aquat. Toxicol. 2020, 226, 105551. [Google Scholar] [CrossRef]
- Mir, H.D.; Rather, A.M. Kinetic and thermodynamic investigations of copper (II) biosorption by green algae Chara vulgaris obtained from the waters of Dal Lake in Srinagar (India). J. Water Process Eng. 2024, 58, 104850. [Google Scholar] [CrossRef]
- Mao, Y.; Ai, H.; Chen, Y.; Zhang, Z.; Zeng, P.; Kang, L.; Li, W.; Gu, w.; He, Q.; Li, H. Phytoplankton response to polystyrene microplastics: Perspective from an entire growth period. Chemosphere 2018, 208, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Paul-Pont, I.; Hégaret, H.; Mariceau, B.; Lambert, C.; Huvet, A.; Soudant, P. Interactions between polystyrene microplastics and marine phytoplankton lead to species-specific hetero-aggregation. Environ. Pollut. 2017, 228, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xi, J.; Tian, J.; Chen, R.; Yu, J.; Lai, J.; Wang, S.; Yang, G. Effects of microplastics on the growth and dimethylsulfide production in two diatoms. Period. Ocean. Univ. China 2022, 52, 97–106, (In Chinese with English Abstract). [Google Scholar]
- Xie, X.; Chen, L.; Shao, S.; Zhou, Y.; Wu, J.; Zhou, Q.; Luo, S. Growth inhibition and toxic effects of microplastics on Chlorella vulgaris. Algal Res. 2024, 78, 103378. [Google Scholar] [CrossRef]
- Lin, W.; Su, F.; Lin, M.; Jin, M.; Li, Y.; Ding, K.; Chen, Q.; Qian, Q.; Sun, X. Effect of microplastics PAN polymer and/or Cu2+ pollution on the growth of Chlorella pyrenoidosa. Environ. Pollut. 2020, 265, 114985. [Google Scholar] [CrossRef]
- Kit, J.W.; Loy, W.C.; Yih, Y.K.; Sin, C.K. Influence of polystyrene microplastic and nanoplastic on copper toxicity in two freshwater microalgae. Environ. Sci. Pollut. Res. 2021, 28, 33649–33668. [Google Scholar]
- Gu, C.; Liu, W.; Zhang, Y.; Li, J.; Zhang, X.; Liu, X. Impact of High Salinity on the Adsorption Behaviors of Polystyrene and Polyamide Microplastics and Alternation of the Toxic Effect toward Synechococcus. Water Air Soil Pollution. 2024, 235, 429. [Google Scholar] [CrossRef]
- Adamu, H.; Haruna, A.; Zango, U.Z.; Garba, N.Z.; Musa, M.S.; IbrahimTafida, U.; Bello, U.; Danmallam, N.U.; Akinpelu, A.A.; Ibrahim, S.A.; et al. Microplastics and Co-pollutants in Soil and Marine Environments: Sorption and Desorption Dynamics in Unveiling Invisible Danger and Key to Ecotoxicological Risk Assessment. Chemosphere 2024, 362, 142630. [Google Scholar] [CrossRef] [PubMed]
- Ruiqi, B.; Dongdong, F.; Zhengquan, F.; Peng, X.; Peng, L. Aging of microplastics and their role as vector for copper in aqueous solution. Gondwana Res. 2022, 108, 81–90. [Google Scholar]
- Xue, B.; Lan, W.; Lin, H.; Feng, Q.; Wei, J.; Lu, Y. Studies of behavior and mechanism of Hg(II) adsorption-desorption onto aged polystyrene. Environ. Sci. Technol. 2022, 45, 31–37, (In Chinese with English Abstract). [Google Scholar]
- Jin, H.; Zhang, Y.; Ren, D.; Ma, D.; Zhang, J.; Su, Y. Effect of the adsorption of Cu2+, Cd2+ in food analysis container. Sci. Technol. Food Ind. 2013, 34, 69–72, (In Chinese with English Abstract). [Google Scholar]
- GB 3097-1997; Sea Water Quality Standards. Ministry of Ecology and Environment the People’s Republic of China: Beijing, China, 1997.
- Zhang, X.; Zheng, M.; Wang, L.; Lou, Y.; Shi, L.; Jiang, S. Sorption of three synthetic musks by microplastics. Mar. Pollut. Bull. 2018, 126, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lin, L.; Wang, X.; Feng, A.; Yi, A. Pb(II) uptake onto nylon microplastics: Interaction mechanism and adsorption performance. J. Hazard. Mater. 2020, 386, 121960. [Google Scholar] [CrossRef] [PubMed]
- Szivák, I.; Behra, R.; Sigg, L. Metal-induced reactive oxygen species production in Chlamydomonas reinhardtii (Chlorophyceae). J. Phycol. 2009, 45, 427–435. [Google Scholar] [CrossRef]
- Sathasivam, R.; Guo, R.Y.; Wang, H.; Lim, W.A.; Ki, J.S. Expressed sequence tag library of the marine green alga Tetraselmis suecica: A focus on stress-related genes for marine pollution. J. Appl. Phycol. 2018, 30, 2387–2402. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.S. Differential transcriptional responses of carotenoid biosynthesis genes in the marine green alga Tetraselmis suecica exposed to redox and non-redox active metals. Mol. Biol. Rep. 2019, 46, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, Y.; Xu, J.; Beardall, J. High copper and UVR synergistically reduce the photochemical activity in the marine diatom Skeletonema costatum. J. Photochem. Photobiol. B 2019, 192, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.; Yi, A.; Ip, J.; Mak, S.; Leung, K. Photosynthetic and transcriptional responses of the marine diatom Thalassiosira pseudonana to the combined effect of temperature stress and copper exposure. Mar. Pollut. Bull. 2017, 124, 938–945. [Google Scholar] [CrossRef]
- Xu, C.; Luo, Y.; Ling, C.; Ying, C.; Li, J. Effects of polystyrene microparticles on growth and physiological metabolism of microalgae. Acta Sci. Circumstantiae 2023, 43, 509–515, (In Chinese with English Abstract). [Google Scholar]
- Zhu, X.; Zhao, W.; Chen, X.; Zhao, T.; Tan, L.; Wang, J. Growth inhibition of the microalgae Skeletonema costatum under copper nanoparticles with microplastic exposure. Mar. Environ. Res. 2020, 158, 105005. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Cacador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).