Tree-Based Machine Learning and Nelder–Mead Optimization for Optimized Cr(VI) Removal with Indian Gooseberry Seed Powder
Abstract
1. Introduction
1.1. Motivation
1.2. The Literature
1.3. Research Gaps
- The literature indicates that the traditional statistical approach of BBDs in the RSM for optimizing Cr(VI) removal from wastewater struggle to capture nonlinear relationships between the process parameters and removal efficiency.
- In addition, the focus of conventional experimental approaches for Cr(VI) removal using bio-absorption materials, which are lacking in terms of identifying optimal combinations of process parameters, may lead to increased experimentation time and cost.
- Furthermore, previous research by Krishna et al. [14], which explored the use of Indian gooseberry seed powder as an adsorbent for Cr(VI) removal, had a gap in the application of ML models for analyzing and optimizing this process. Similarly, the existing literature also does not fully capture the intricate interactions between various process parameters (such as the initial Cr(VI) concentration, pH level, and adsorbent dosage) and their impact on removal efficiency.
1.4. Novelty
1.5. Major Contributions
- Better prediction of efficiency in removing Cr(VI): ML models outperform conservative BBD approaches in realizing sophisticated nonlinear connections. Consequently, this permits more exact estimations of Cr(VI) removal efficiency using different process parameters.
- Improved maximization through optimization: The proposed approach employs ML-based Nelder–Mead optimization for maximizing Cr(VI) removal, and it reduces the experimentation time and treatment cost and allows efficient processing of larger wastewater volumes.
- Integration of ML models with optimization: The combination of ML models with optimization is a novel approach which has not been previously reported in the literature. Moreover, it offers a new direction for exploring this bio-absorption material.
1.6. Organization
2. Materials and Methods
2.1. Summary of Experimental Investigations
2.2. Dataset
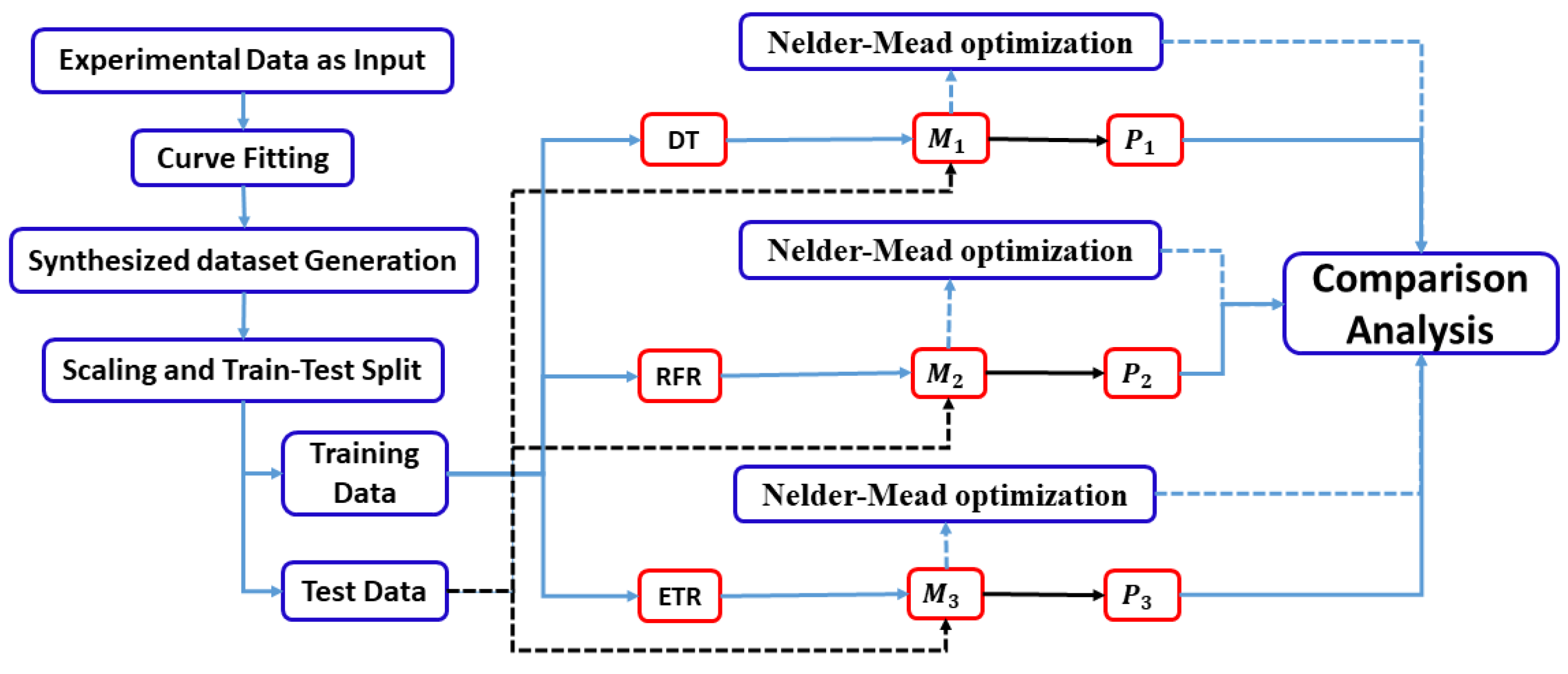
3. Proposed Model
3.1. Curve Fitting
3.2. Synthesized Dataset
| Algorithm 1: Nelder–Mead optimization for maximum Cr(VI) removal. |
|
3.3. Scaling and Splitting
3.4. Model Building and Training
3.5. Model Testing
- Root Mean Squared Error (RMSE): Also called the root mean square deviation (RMSD) or root mean squared error on prediction (RMSE), the square root of summation of the squared residuals divided by the total number of instances, as reported in Equation (6), is known as the RMSE:
- –Score: Also called the coefficient of determination, this specifies the variance or score of a model based on given test data and indicates how much of the variance in the dependent features is explained by an independent feature. Equations (7) and (8) show the mathematical formulas for the score’s calculation, where a continuous value between 0 and 1 indicates model score and a model score near one indicates that the model performance is good with minimal error:
- Relative Root Mean Squared Error (RRMSE): The RRMSE is calculated as stated in Equation (9). The model performance is expressed as a percentage. A model with a value < 10% is said to be excellent, while it is good if it is between 10% and 20%, fair if it is between 20% and 30%, and poor if it is above 30%:
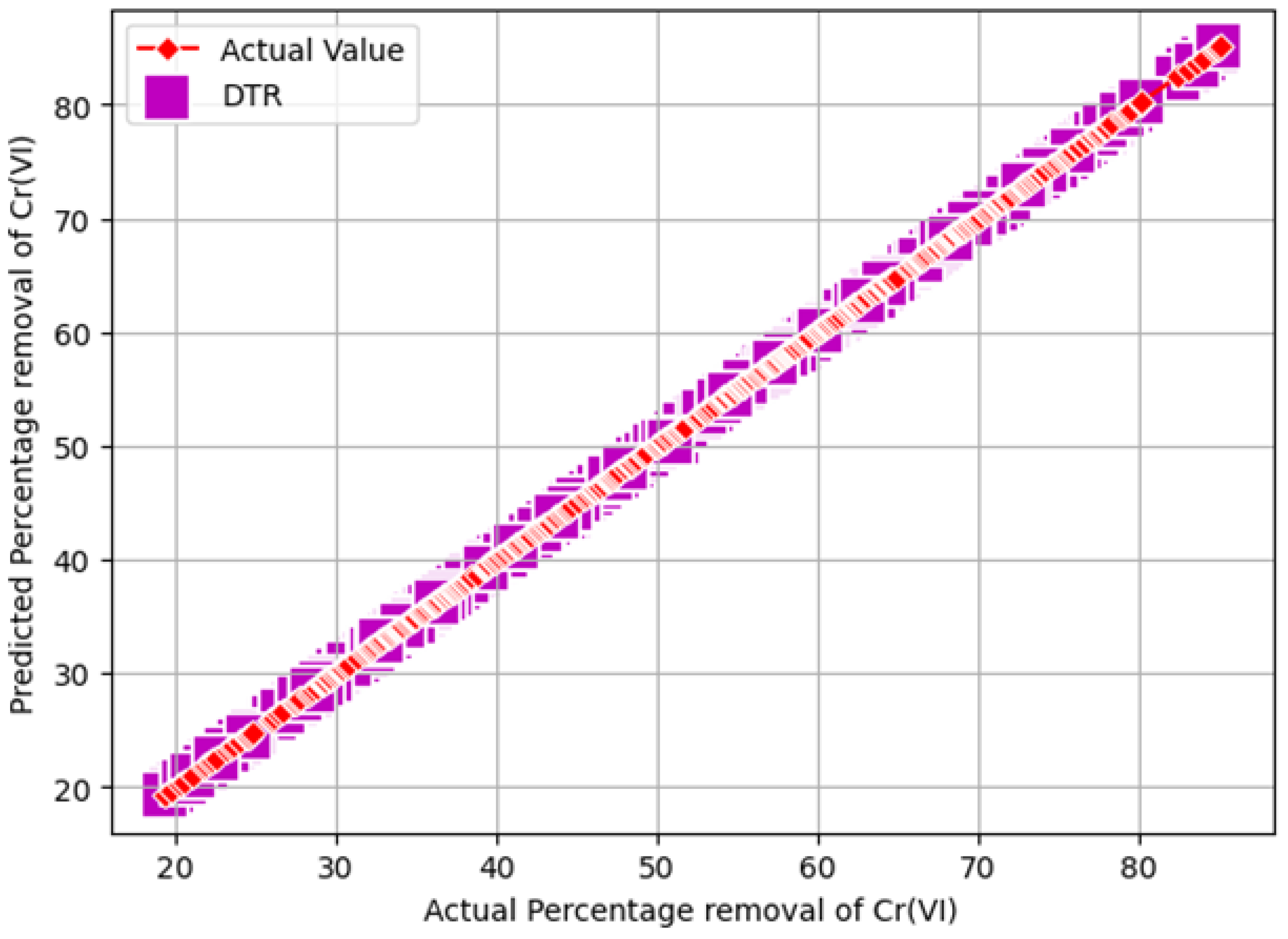
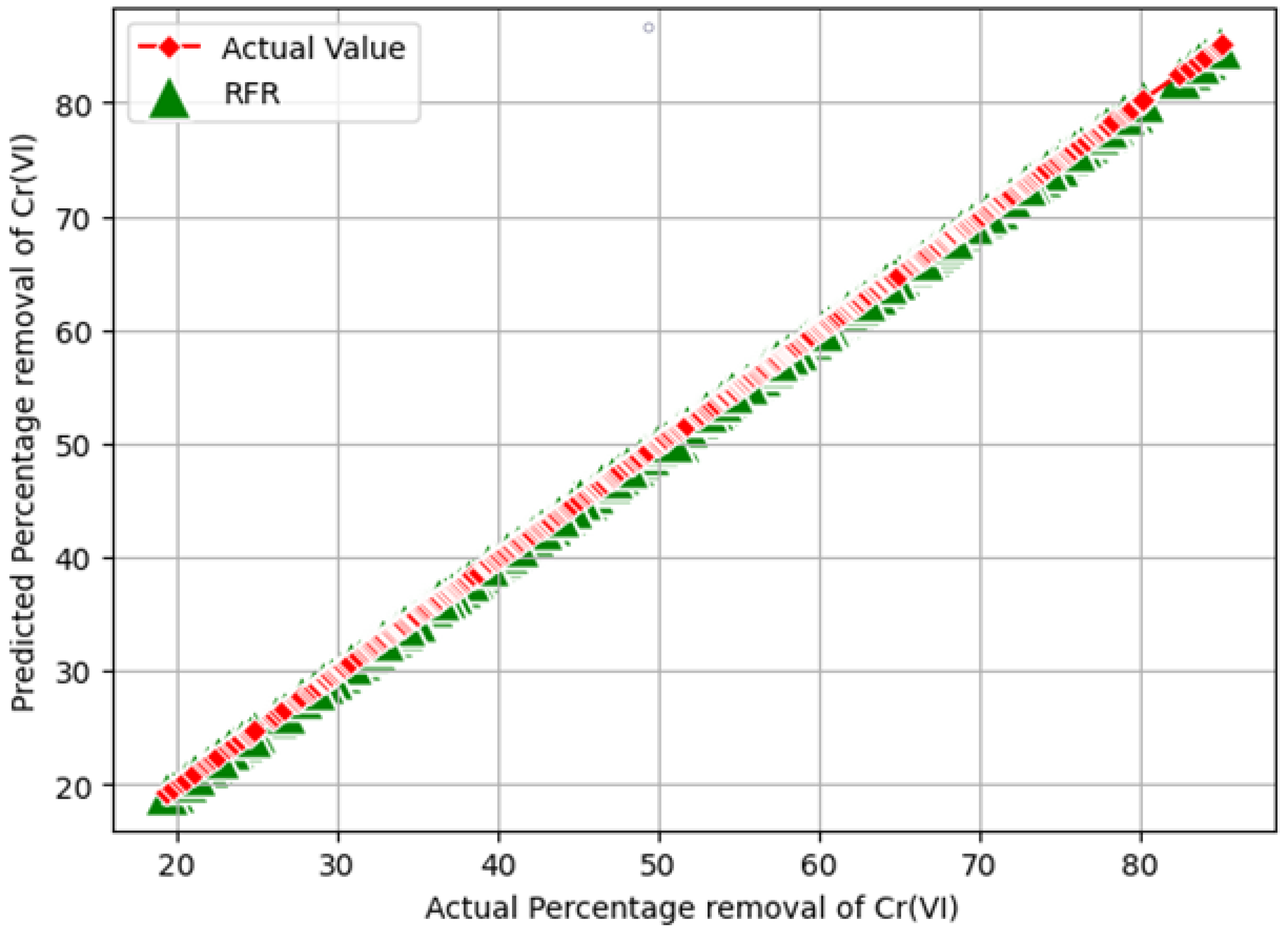
- Chromium (VI) removal percentage: In the traditional approach, chromium (VI) removal efficiency [14,31] is measured as shown in Equation (10). However, in this work, the synthesized dataset with 2000 instances was built using original experimental data (shown in Table 1) by varying the initial chromium (VI) concentration (20–100 mg/L), pH level (1–5), and Indian gooseberry powder dosage (2–10 g/L). The synthesized data samples were given as testing data to all trained ML models to determine the optimal values which removed the highest percentage of chromium (VI) from synthetic wastewater for the three independent features (“initial concentration of Cr(VI)”, “pH”, and “Adsorbent dosage”). During the prediction procedure, comparison analysis of the three ML models through six evolution metrics—the MAE, MSE, RMSE, –Score, and RRMSE—is presented in Table 5 and the optimal values for the maximum percentage of chromium (VI) removal are presented in Table 6.
3.6. Nelder–Mead Optimization
4. Results and Discussions
Validation of the Optimization Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salavatifar, M.; Khosravi-Darani, K. Investigation of the simulated microgravity impact on heavy metal biosorption by Saccharomyces cerevisiae. Food Sci. Nutr. 2024, 12, 3642–3652. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Veerendra, G.T.N.; Manoj, A.V.P.; Padavala, S.S.A.B. Removal of chlorides and hardness from contaminated water by using various biosorbents: A comprehensive review. Water-Energy Nexus 2024, 7, 39–76. [Google Scholar] [CrossRef]
- Li, Z.; Yu, D.; Wang, X.; Liu, X.; Xu, Z.; Wang, Y. A novel strategy of tannery sludge disposal–converting into biochar and reusing for Cr (VI) removal from tannery wastewater. J. Environ. Sci. 2024, 138, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Amjad, S.; Javed, A. Optimum conditions for growth and copper (II) removal from leachate by Chlorella vulgaris, Spirogyra ellipsospora and Ulva lactuca. Bioremediat. J. 2024. [Google Scholar] [CrossRef]
- Iddya, A.; Elezi, G.; Hembade, S.V.; Whitelegge, J.P.; Schwabe, K.; Jassby, D. Integrated Electrochemical Treatment Process for Hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (RDX), Hexavalent Chromium, and Ammonia Using Electroactive Membranes. Ind. Eng. Chem. Res. 2024, 63, 1941–1952. [Google Scholar] [CrossRef]
- Musielak, M.; Serda, M.; Gagor, A.; Talik, E.; Sitko, R. Ultratrace determination and speciation of hexavalent chromium by EDXRF and TXRF using dispersive micro-solid phase extraction and tetraethylenepentamine graphene oxide. Spectrochim. Acta Part B At. Spectrosc. 2024, 213, 106863. [Google Scholar] [CrossRef]
- Kundu, S.; Layek, M.; Mondal, S.; Mitra, M.; Karmakar, P.; Rahaman, S.M.; Mahali, K.; Acharjee, A.; Saha, B. Insights into the micellar catalysed efficient oxidation of 2-and 3-pentanol by cerium (iv) in a greener medium of SDS and STS. New J. Chem. 2024, 48, 3804–3812. [Google Scholar] [CrossRef]
- Aryal, M. An analysis of drinking water quality parameters to achieve sustainable development goals in rural and urban areas of Besisahar, Lamjung, Nepal. World Water Policy 2024, 10, 297–323. [Google Scholar] [CrossRef]
- Ismail, U.M.; Onaizi, S.A.; Vohra, M.S. Aqueous Pb (II) removal using ZIF-60: Adsorption studies, response surface methodology and machine learning predictions. Nanomaterials 2023, 13, 1402. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.J.S.; Chan, Y.J.; Arumugasamy, S.K.; Yazdi, S.K.; Lim, J.W. Optimisation and performance evaluation of response surface methodology (RSM), artificial neural network (ANN) and adaptive neuro-fuzzy inference system (ANFIS) in the prediction of biogas production from palm oil mill effluent (POME). Energy 2023, 266, 126449. [Google Scholar] [CrossRef]
- Sharma, P.; Sahoo, B.B.; Said, Z.; Hadiyanto, H.; Nguyen, X.P.; Nižetić, S.; Huang, Z.; Hoang, A.T.; Li, C. Application of machine learning and Box-Behnken design in optimizing engine characteristics operated with a dual-fuel mode of algal biodiesel and waste-derived biogas. Int. J. Hydrogen Energy 2023, 48, 6738–6760. [Google Scholar] [CrossRef]
- Yaro, N.S.A.; Sutanto, M.H.; Habib, N.Z.; Napiah, M.; Usman, A.; Al-Sabaeei, A.M.; Rafiq, W. Mixture design-based performance optimization via response surface methodology and moisture durability study for palm oil clinker fine modified bitumen asphalt mixtures. Int. J. Pavement Res. Technol. 2024, 17, 123–150. [Google Scholar] [CrossRef]
- Uddin, M.K.; Rao, R.A.K.; Mouli, K.V.C. The artificial neural network and Box-Behnken design for Cu2+ removal by the pottery sludge from water samples: Equilibrium, kinetic and thermodynamic studies. J. Mol. Liq. 2018, 266, 617–627. [Google Scholar] [CrossRef]
- Krishna, D.; Padma, D.; Kavya Srithi, P.; Siva Prasad, P. Removal of chromium from aqueous solution by Indian Gooseberry Seed Powder as adsorbent. J. Future Eng. Technol. 2014, 9, 24–31. [Google Scholar] [CrossRef]
- Qing, Y.; Gao, W.; Long, Y.; Kang, Y.; Xu, C. Functionalized Titanium-Based MOF for Cr (VI) Removal from Wastewater. Inorg. Chem. 2023, 62, 6909–6919. [Google Scholar] [CrossRef]
- Misganaw, A.; Akenaw, B.; Getu, S. Determination of the level of chromium (III) and comparison of chemical precipitating agents to recover and reuse it from tannery waste water. Desalin. Water Treat. 2024, 37, 100150. [Google Scholar] [CrossRef]
- Hu, G.; He, Y.; Zhu, K.F.; Zhang, Z.; Lou, W.; Zhang, K.N.; Chen, Y.G.; Wang, Q. Experimental study on injection of ferrous sulphate for remediation of a clayey soil contaminated with hexavalent chromium. Environ. Earth Sci. 2023, 82, 185. [Google Scholar] [CrossRef]
- El-Gawad, H.A.; Hassan, G.K.; Aboelghait, K.M.; Mahmoud, W.H.; Mohamed, R.; Afify, A.A. Removal of chromium from tannery industry wastewater using iron-based electrocoagulation process: Experimental; kinetics; isotherm and economical studies. Sci. Rep. 2023, 13, 19597. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, L.H.; Zhuang, Y.F. Effect of electrolyte, potential gradient and treatment time on remediation of hexavalent chromium contaminated soil by electrokinetic remediation and adsorption. Environ. Earth Sci. 2023, 82, 40. [Google Scholar] [CrossRef]
- Mendil, J.; Alalou, A.; Mazouz, H.; Al-Dahhan, M.H. Review of Emulsion Liquid Membrane for Heavy Metals Recovery from Wastewater/water: Stability, Efficiency, and Optimization. Chem. Eng.-Process.-Process. Intensif. 2023, 196, 109647. [Google Scholar] [CrossRef]
- Meena, G.; Rawal, N. Artificial Neural Network Modeling for Adsorption Efficiency of Cr (VI) Ion from Aqueous Solution Using Waste Tire Activated Carbon. Nat. Environ. Pollut. Technol. 2023, 22, 1481–1491. [Google Scholar] [CrossRef]
- Shih, Y.J.; Hsieh, H.L.; Hsu, C.H. Electrochemical Fe (III) mediation for reducing hexavalent chromium Cr (VI) on templated copper-nickel foam electrode. J. Clean. Prod. 2023, 384, 135596. [Google Scholar] [CrossRef]
- Nighojkar, A.; Zimmermann, K.; Ateia, M.; Barbeau, B.; Mohseni, M.; Krishnamurthy, S.; Dixit, F.; Kandasubramanian, B. Application of neural network in metal adsorption using biomaterials (BMs): A review. Environ. Sci. Adv. 2023, 2, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Uthayakumar, H.; Radhakrishnan, P.; Shanmugam, K.; Kushwaha, O.S. Growth of MWCNTs from Azadirachta indica oil for optimization of chromium (VI) removal efficiency using machine learning approach. Environ. Sci. Pollut. Res. 2022, 29, 34841–34860. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Ansari, M.H.; Bandyopadhyay, S.; Selvam, K.; David, S.S. Maximization of Cr Removal in Continuous Counter-current Liquid-Solid Fluidized Bed: A Machine Learning Approach. J. Phys. Conf. Ser. 2021, 1979, 012009. [Google Scholar] [CrossRef]
- Zafar, M.; Aggarwal, A.; Rene, E.R.; Barbusiński, K.; Mahanty, B.; Behera, S.K. Data-driven machine learning intelligent tools for predicting chromium removal in an adsorption system. Processes 2022, 10, 447. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Z.; You, S.; Komárek, M.; Alessi, D.S.; Yuan, X.; Palansooriya, K.N.; Ok, Y.S.; Tsang, D.C. Machine learning exploration of the direct and indirect roles of Fe impregnation on Cr (VI) removal by engineered biochar. Chem. Eng. J. 2022, 428, 131967. [Google Scholar] [CrossRef]
- Kabir, M.M.; Ferdousi; Sultana, F.S.; Rahman, M.M.; Uddin, M.K. Chromium (VI) removal efficacy from aqueous solution by modified tea wastes-polyvinyl alcohol (TW-PVA) composite adsorbent. Desalin. Water Treat. 2019, 174, 311–323. [Google Scholar] [CrossRef]
- Rao, R.A.K.; Ikram, S.; Uddin, M.K. Removal of Cr (VI) from aqueous solution on seeds of Artimisia absinthium (novel plant material). Desalin. Water Treat. 2015, 54, 3358–3371. [Google Scholar] [CrossRef]
- Ali Khan Rao, R.; Rehman, F.; Kashifuddin, M. Removal of Cr (VI) from electroplating wastewater using fruit peel of leechi (Litchi chinensis). Desalin. Water Treat. 2012, 49, 136–146. [Google Scholar] [CrossRef]
- Hafsa, N.; Rushd, S.; Al-Yaari, M.; Rahman, M. A generalized method for modeling the adsorption of heavy metals with machine learning algorithms. Water 2020, 12, 3490. [Google Scholar] [CrossRef]


| Sno | Initial Concentration of Cr(VI) | pH | Adsorbent Dosage (g/L) | Percentage Removal of Cr(VI) | Sno | Initial Concentration of Cr(VI) | pH | Adsorbent Dosage (g/L) | Percentage Removal of Cr(VI) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 2 | 8 | 73.55 | 29 | 100 | 1 | 6 | 59.35 |
| 2 | 20 | 2 | 6 | 65.09 | 30 | 100 | 2 | 6 | 60.94 |
| 3 | 60 | 2 | 8 | 71.53 | 31 | 20 | 1 | 6 | 63.47 |
| 4 | 100 | 2 | 8 | 69.44 | 32 | 20 | 2 | 4 | 61.48 |
| 5 | 20 | 2 | 10 | 72.47 | 33 | 60 | 1 | 6 | 61.46 |
| 6 | 80 | 2 | 8 | 70.32 | 34 | 40 | 2 | 6 | 64.27 |
| 7 | 80 | 1 | 8 | 68.71 | 35 | 20 | 1 | 8 | 71.98 |
| 8 | 20 | 1 | 10 | 70.85 | 36 | 80 | 1 | 6 | 60.24 |
| 9 | 80 | 3 | 10 | 66.45 | 37 | 80 | 2 | 6 | 61.83 |
| 10 | 100 | 1 | 8 | 67.85 | 38 | 20 | 3 | 6 | 62.33 |
| 11 | 100 | 3 | 8 | 64.93 | 39 | 20 | 4 | 8 | 65.51 |
| 12 | 40 | 1 | 8 | 71.14 | 40 | 20 | 5 | 8 | 60.88 |
| 13 | 40 | 2 | 10 | 71.68 | 41 | 20 | 1 | 2 | 51.23 |
| 14 | 60 | 1 | 10 | 68.83 | 42 | 100 | 3 | 6 | 58.17 |
| 15 | 40 | 3 | 8 | 68.25 | 43 | 20 | 2 | 2 | 52.82 |
| 16 | 80 | 2 | 10 | 69.25 | 44 | 20 | 4 | 2 | 47.82 |
| 17 | 80 | 1 | 10 | 67.62 | 45 | 80 | 3 | 6 | 59.05 |
| 18 | 100 | 2 | 10 | 68.37 | 46 | 20 | 1 | 6 | 63.47 |
| 19 | 100 | 1 | 10 | 66.74 | 47 | 60 | 3 | 6 | 60.26 |
| 20 | 80 | 3 | 8 | 65.82 | 48 | 20 | 4 | 6 | 59.09 |
| 21 | 20 | 3 | 10 | 69.71 | 49 | 20 | 5 | 6 | 55.32 |
| 22 | 60 | 3 | 10 | 67.66 | 50 | 40 | 2 | 8 | 72.74 |
| 23 | 100 | 3 | 10 | 65.54 | 51 | 40 | 1 | 6 | 62.65 |
| 24 | 60 | 2 | 8 | 71.53 | 52 | 60 | 3 | 8 | 67.02 |
| 25 | 80 | 2 | 8 | 70.32 | 53 | 20 | 3 | 8 | 69.07 |
| 26 | 20 | 2 | 8 | 73.55 | 54 | 60 | 1 | 8 | 69.9 |
| 27 | 60 | 2 | 10 | 70.48 | 55 | 60 | 2 | 6 | 63.05 |
| 28 | 100 | 2 | 8 | 69.44 | 56 | 40 | 3 | 8 | 68.81 |
| Initial Concentration of Cr(VI) | pH | Adsorbent Dosage (g/L) | Percentage Removal of Cr(VI) | |
|---|---|---|---|---|
| Count | 2000 | 2000 | 2000 | 2000 |
| Mean | 58.62 | 2.52 | 5.50 | 52.14 |
| Std | 22.98 | 1.14 | 2.31 | 16.15 |
| Min | 20.00 | 1.00 | 2.00 | 19.30 |
| 25% | 39.00 | 1.00 | 4.00 | 38.50 |
| 50% | 58.00 | 3.00 | 5.00 | 52.42 |
| 75% | 79.00 | 4.00 | 8.00 | 65.22 |
| Max | 100 | 5 | 10 | 73.55 |
| Feature Name | Starting Value | End Value | Number of Instances |
|---|---|---|---|
| Initial concentration of Cr(VI) | 20 | 100 | 2000 |
| pH | 1 | 5 | 2000 |
| Adsorbent dosage (g/L) | 2 | 10 | 2000 |
| Notations | Description |
|---|---|
| The instance of actual chromium VI removal percentage | |
| The instance of predicted chromium VI removal percentage | |
| The difference between the instance of and | |
| m | The number of samples or instances in the dataset |
| The average or mean of all chromium VI removal percentage values of a given dataset | |
| The MAE of the chromium removal percentage | |
| The MSE of the chromium removal percentage | |
| The RMSE of the chromium removal percentage | |
| The RMSE of the chromium removal percentage | |
| The Coefficient of determination of the chromium removal percentage | |
| Initial concentration of Cr(VI) | |
| Final concentration of Cr(VI) |
| Evolution Metrics | DTR | RFR | ETR |
|---|---|---|---|
| MAE | 0.06 | 0.06 | 0.01 |
| MSE | 0.01 | 0.01 | 0.00 |
| R2–Score | 0.999960 | 0.999968 | 0.99990 |
| RRMSE | 0.01 | 0.01 | 0.01 |
| Optimal Initial Concentration of Cr(VI) | Optimal pH | Optimal Adsorbent Dosage (g/L) | Obtained Cr(VI) Removal % | |
|---|---|---|---|---|
| DTR-Nelder–Mead | 95.55 | 4.0 | 8.0 | 78.21 |
| RFR-Nelder–Mead | 95.55 | 4.0 | 8.0 | 78.11 |
| ETR-Nelder–Mead | 91.0 | 4.0 | 8.4 | 80.63 |
| 89.99 | 3.67 | 9.12 | 83.09 | |
| 88.978 | 3.94 | 9.43 | 84.08 | |
| 99.25 | 4.97 | 9.62 | 85.11 |
| Optimal Initial Concentration of Cr(VI) | Optimal pH | Optimal Adsorbent Dosage (g/L) | Optimal Cr(VI) Removal % | Cr(VI) Removal % through Experimentation | % Error |
|---|---|---|---|---|---|
| 99.25 | 4.97 | 9.62 | 85.11 | 79.75 | 6.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalabarige, L.R.; Krishna, D.; Potnuru, U.K.; Mishra, M.; Alharthi, S.S.; Koutavarapu, R. Tree-Based Machine Learning and Nelder–Mead Optimization for Optimized Cr(VI) Removal with Indian Gooseberry Seed Powder. Water 2024, 16, 2175. https://doi.org/10.3390/w16152175
Kalabarige LR, Krishna D, Potnuru UK, Mishra M, Alharthi SS, Koutavarapu R. Tree-Based Machine Learning and Nelder–Mead Optimization for Optimized Cr(VI) Removal with Indian Gooseberry Seed Powder. Water. 2024; 16(15):2175. https://doi.org/10.3390/w16152175
Chicago/Turabian StyleKalabarige, Lakshmana Rao, D. Krishna, Upendra Kumar Potnuru, Manohar Mishra, Salman S. Alharthi, and Ravindranadh Koutavarapu. 2024. "Tree-Based Machine Learning and Nelder–Mead Optimization for Optimized Cr(VI) Removal with Indian Gooseberry Seed Powder" Water 16, no. 15: 2175. https://doi.org/10.3390/w16152175
APA StyleKalabarige, L. R., Krishna, D., Potnuru, U. K., Mishra, M., Alharthi, S. S., & Koutavarapu, R. (2024). Tree-Based Machine Learning and Nelder–Mead Optimization for Optimized Cr(VI) Removal with Indian Gooseberry Seed Powder. Water, 16(15), 2175. https://doi.org/10.3390/w16152175







