Analysis of Influencing Factors on the Efficiency of Electrochemical Scaling Equipment
Abstract
1. Introduction
2. Electrochemical Descaling Simulation Experiment
2.1. Experimental Setup
- Solution conductivity is determined using a conductivity meter;
- Chloride ion concentration is measured using the Cl− concentration tester;
- Solution hardness (expressed as CaCO3) is detected according to GB/T 6909-2018 (methods of analysis for boiler water and cooling water) using the EDTA titration method [14].
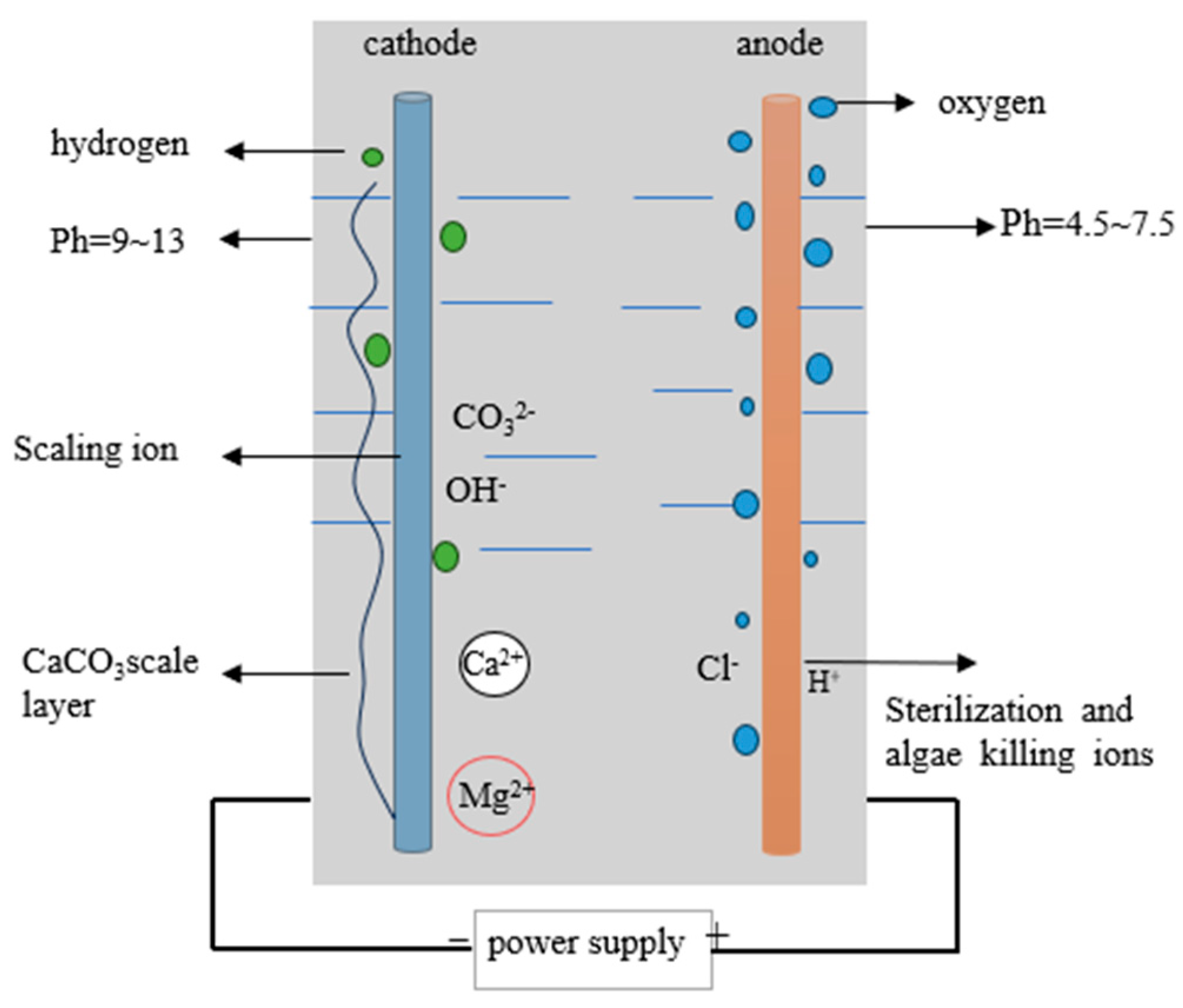
2.2. Experimental Principles
2.3. Experimental Procedures
- (1)
- Initial Setup:
- A predetermined amount of water is injected into the tank, recording the volume as 200 L.
- The water temperature is controlled at 30 °C.
- The initial measurement of conductivity is 756 μS/cm.
- The conductivity is increased to 1500 μS/cm by adding NaCl.
- The descaling machine is activated, recording a current of 10 A and a voltage of 24 V.
- (2)
- Flow Rate Variation:
- The valve is closed and the values of the hardness, conductivity, and other parameters of the circulating water are measured and recorded during the operation of the scaling machine in still water at intervals of 6.
- The valve is opened, adjusting the flow rates to 0.35 m/s and 0.75 m/s, respectively.
- Changes in the above water quality parameters are monitored to investigate the effect of flow rate on fouling efficiency.
- (3)
- Voltage and Current Variation:
- A solution volume of 200 L is maintained, at a temperature of 30 °C, conductivity of 1500 μS/cm, and flow rate of 0.35 m/s.
- Experiments are conducted with different voltage and current settings for the descaling machine: 24 V/10 A, 20 V/7 A, and 16 V/5 A.
- Changes in water quality parameters are recorded to explore the impact of descaling machine voltage and current on fouling effects.
- (4)
- Temperature Variation:
- A solution flow rate of 0.35 m/s, descaling machine voltage of 24 V, and current of 10 A are maintained.
- The temperature control device is used to set the circulating water temperatures to 26 °C, 28 °C, 30 °C, 32 °C, 34 °C, and 36 °C.
- The influence of temperature on the descaling machine’s fouling efficiency is investigated by recording changes in water quality parameters.
3. Experimental Results and Analysis
3.1. Flow Rate
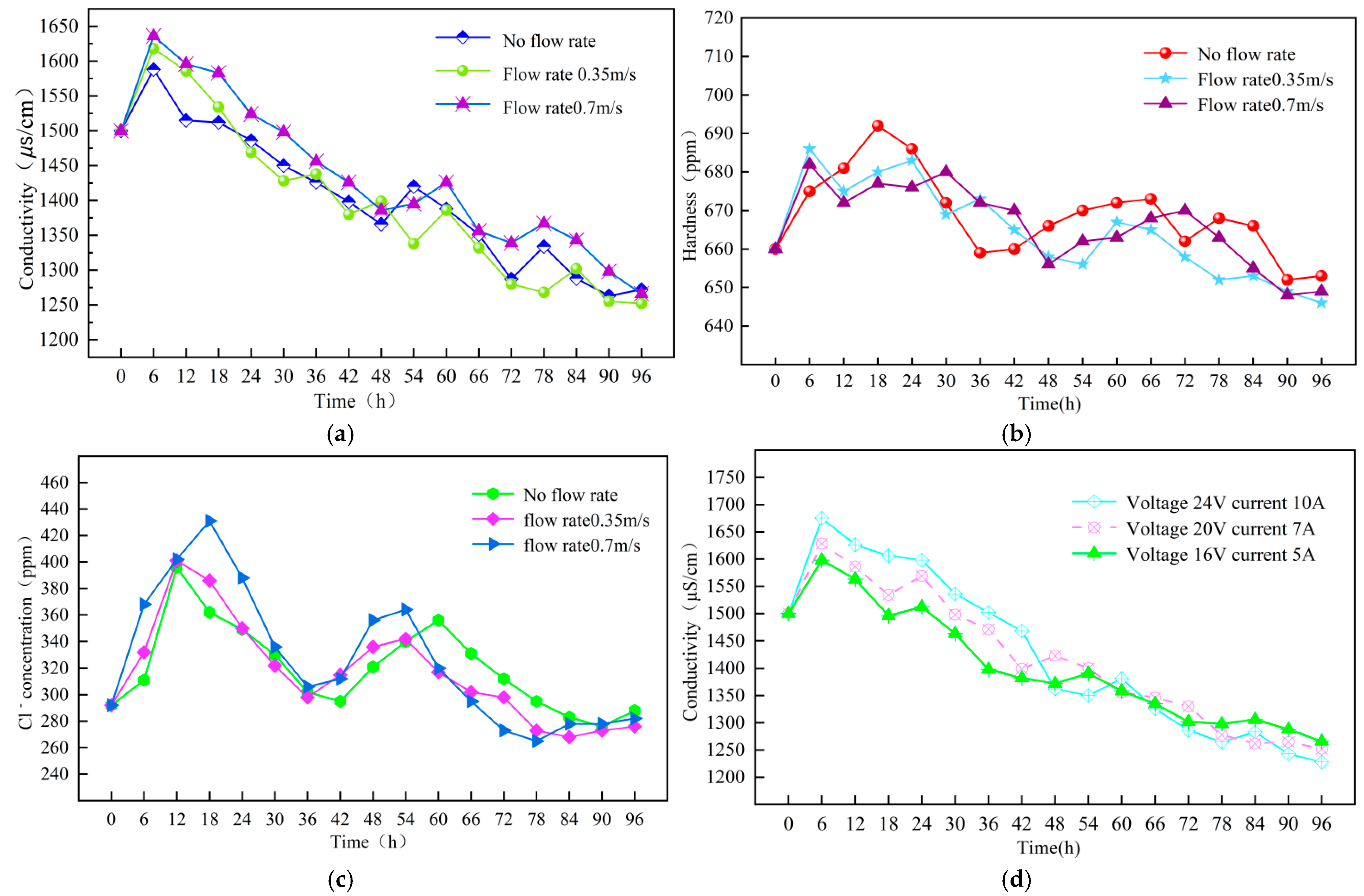
3.1.1. Impact of Flow Rate on Conductivity
3.1.2. Impact of Flow Rate on Hardness
3.1.3. Impact of Flow Rate on Cl− Concentration
3.2. Voltage and Current
3.2.1. Impact of Voltage and Current on Conductivity
3.2.2. Impact of Voltage and Current on Solution Hardness
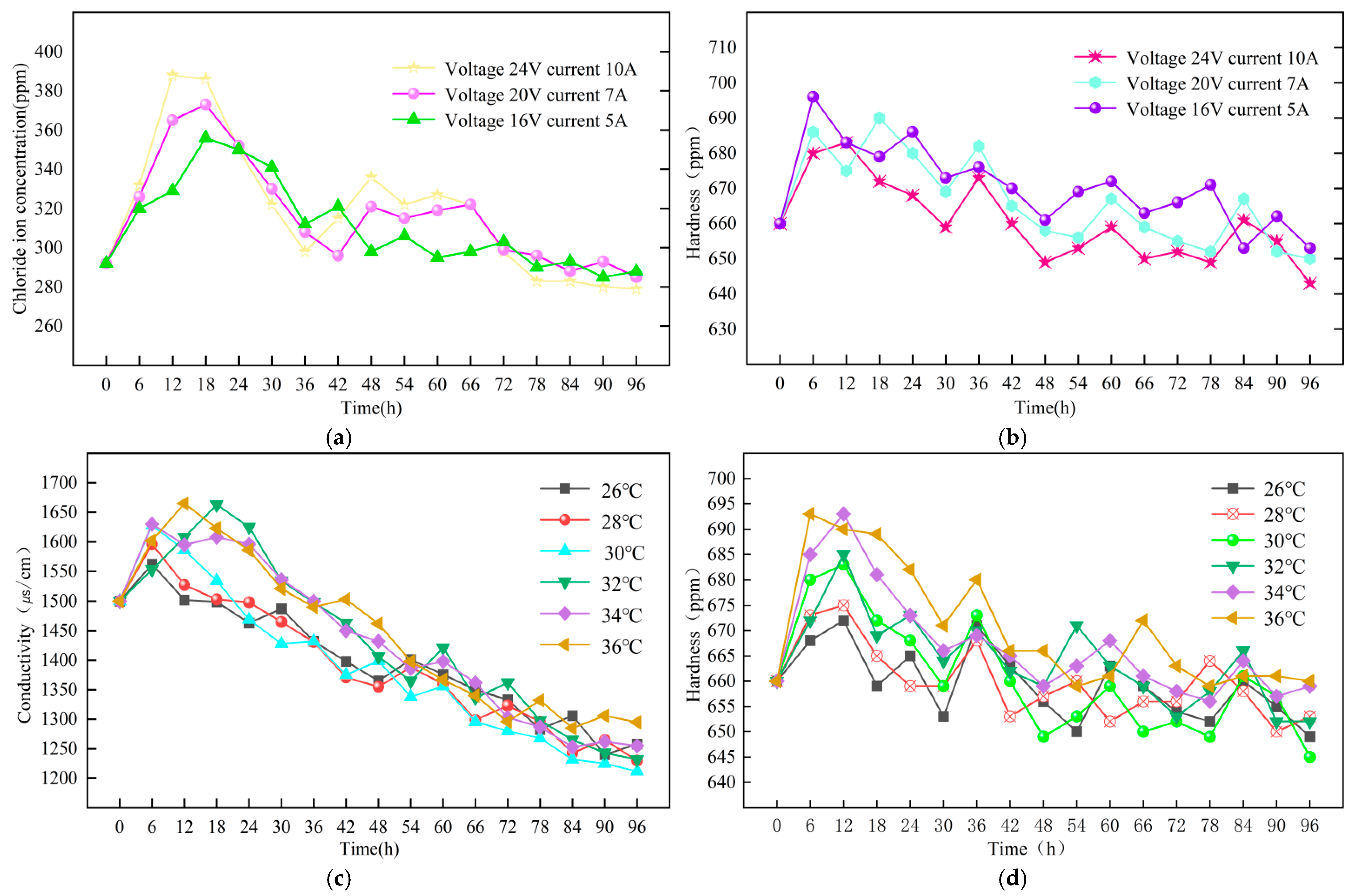
3.2.3. Impact of Voltage and Current on Solution Cl− Concentration
3.3. Temperature
4. Conclusions and Outlook
- (1)
- The descaling machine demonstrates effective scale inhibition. Through the electrolysis of water and the generation of a large number of OH− ions at the cathode, a high-alkalinity zone is formed, causing metal ions to rapidly precipitate from the water and adhere to the cathode surface, thereby reducing water conductivity and hardness and achieving scale inhibition. Additionally, the strongly oxidative substances generated at the anode, such as ozone (O3), hydrogen peroxide (H2O2), hydroxyl radicals (·OH), and the oxidation-stable OCl−, exhibit certain sterilization and algae-killing effects.
- (2)
- In the experiments examining the influence of different flow rates, temperatures, voltages, and currents on descaling efficiency, it is found that at a flow rate of 0.35 m/s, a temperature of 30 °C, and a descaling machine voltage of 24 V and current of 10 A, the conductivity, hardness, and Cl− concentration are the lowest. This indicates that under these conditions, the best descaling effect is achieved.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Liu, C.; Cao, Z.; Shan, M.; Bing, Y. Effect and mechanism of induced crystallization softening treatment on water quality in drinking water distribution system with high hardness water source. J. Environ. Chem. Eng. 2023, 11, 110474. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Y.; Xu, X.; Wang, J. Experimental Study on antiscaling characteristics of CaCO3 deposits on heat transfer surface under alternating magnetic field. Proc. CSEE 2022, 42, 7913–7922. [Google Scholar] [CrossRef]
- Wang, X.; Tang, R.; Chen, Z.; Yang, W. Influence of pulse current on the electrodeposition behaviors of CaCO3 scale in the industrial circulating water system. Desalination 2023, 554, 116495. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, Z.; Chang, H.; Fan, G.; Wang, Q.; Fu, X.; Qu, F.; Liang, H. Integrated membrane electrochemical Reactor-Membrane distillation process for enhanced landfill leachate treatment. Water Res. 2023, 230, 119559. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.U.; Moronshing, M.; Shestakova, M.; Al-Othman, A.; Sillanpää, M.; Zhan, Z.; Song, B.; Lei, Y. Electro-deionization technology for enhanced water treatment and desalination: A Review. Desalination 2023, 548, 116254. [Google Scholar] [CrossRef]
- Soleymani, F. Internal corrosion monitoring of pipelines using electrochemical techniques: A Comprehensive Review. Prot. Met. Phys. Chem. Surf. 2023, 59, 1045–1061. [Google Scholar] [CrossRef]
- He, C.; Xiao, N.; Li, J.; Liu, W. Research progress on electrochemical treatment technology for circulating cooling water. Ind. Water Treat. 2022, 42, 26–33. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, H.; Sun, Y.; Gong, Z.; Wang, Y.; Fu, Y.; Liu, K.; Sun, Z. Study on the removal effect of Ca2+ and Mg2+ in landfill leachate by alkali. Environ. Eng. 2022, 40, 55–61. [Google Scholar] [CrossRef]
- You, J.; Wang, Z.; Yu, J.; Zhang, C.; Sun, S.; Hu, S. Scaling trend prediction and influencing factors of formation water and injection water in huabei oilfield. Lithol. Reserv. 2017, 29, 162–168. [Google Scholar] [CrossRef]
- Xu, H.; Yuan, M.; Luo, Q. Pilot-Scale experimental study on electrochemical scale removal technology. Ind. Water Treat. 2019, 39, 37–41. [Google Scholar]
- Liu, Y.; Yu, Q.; Chen, Z.; Zhu, W.; Hu, Q.; Zheng, Z.; You, H.; Lv, Z.; Chen, B. Effect of current density on the electrochemical properties of Cobalt-Manganese Co-Doped lead dioxide anode materials. Mater. Rev. 2022, 36, 42–47. [Google Scholar] [CrossRef]
- Zhu, T.Z.; Yu, D.Z.; Zhang, M.Y.; Yao, G.Y.; Hu, X.G.; He, A.; Tao, L.; Teng, H.K. Enhancement of CO2 addition on electrochemical water softening in high hardness low alkalinity water. Ind. Water Treat. 2023, 43, 130–136. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Li, G.; Yu, H.; Dong, X.; Jiang, H. Research on the descaling characteristics of a new electrochemical water treatment device. Water Sci. Technol. 2023, 88, wst2023365. [Google Scholar] [CrossRef] [PubMed]
- GB/T 6909-2018; Methods of Analysis for Boiler Water and Cooling Water. State Administration for Market Regulation: Beijing, China, 2018.
- Sanjuán, I.; Benavente, D.; García-García, V.; Expósito, E.; Montiel, V. Paired electrolysis for simultaneous electrochemical water softening and production of weak acid solutions. Electrochem. Commun. 2019, 101, 88–92. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Zhao, G.; Tang, Y.; Li, J.; Li, F.; Zhuang, H.; Chen, J.; Lin, H.; Zhang, Y. Investigation on an electrochemical pilot equipment for water softening with an automatic descaling system: Parameter optimization and energy consumption analysis. J. Clean. Prod. 2020, 276, 123178. [Google Scholar] [CrossRef]
- Xu, H.; Xu, Z.; Guo, Y.; Guo, S.; Xu, X.; Gao, X.; Wang, L.; Yan, W. Research and application progress of electrochemical water quality stabilization technology for recirculating cooling water in China: A Short Review. J. Water Process Eng. 2020, 37, 101433. [Google Scholar] [CrossRef]
- Luan, J.X.; Wang, L.D.; Sun, W.; Li, X.H.; Zhu, T.Z.; Zhou, Y.Z.; Deng, H.T.; Chen, S.; He, S.H.; Liu, G.C. Multi-meshes coupled cathodes enhanced performance of electrochemical water softening system. Sep. Purif. Technol. 2019, 217, 128–136. [Google Scholar] [CrossRef]
- Jin, H.; Yu, Y.; Chen, X. Membrane-Based electrochemical precipitation for water softening. J. Membr. Sci. 2020, 597, 117639. [Google Scholar] [CrossRef]
- Mora, A.S.; McBeath, S.T.; Cid, C.A.; Hoffmann, M.R.; Graham, N.J.D. Diamond electrode facilitated electrosynthesis of water and wastewater treatment oxidants. Curr. Opin. Electrochem. 2022, 32, 100899. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, N.; Ren, J.; Wei, X.; Yu, G.; Li, J. Power dependence of reactive species generation in a water falling film dielectric barrier discharge system. Sep. Purif. Technol. 2023, 305, 122406. [Google Scholar] [CrossRef]
- Jiani, L.; Zhicheng, X.; Hao, X.; Dan, Q.; Zhengwei, L.; Wei, Y.; Yu, W. Pulsed electrochemical Oxidation of acid red and crystal violet by PbO2 anode. J. Environ. Chem. Eng. 2020, 8, 103773. [Google Scholar] [CrossRef]
- Kim, S.K.; Shin, D.-M.; Rhim, J.W. Designing a high-efficiency hypochlorite ion generation system by combining cation exchange membrane aided electrolysis with chlorine gas recovery stream. J. Membr. Sci. 2021, 630, 119318. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P. Amoxicillin electro-catalytic Oxidation using Ti/RuO2 Anode: Mechanism, Oxidation products, and degradation pathway. Electrochim. Acta 2019, 296, 856–866. [Google Scholar] [CrossRef]
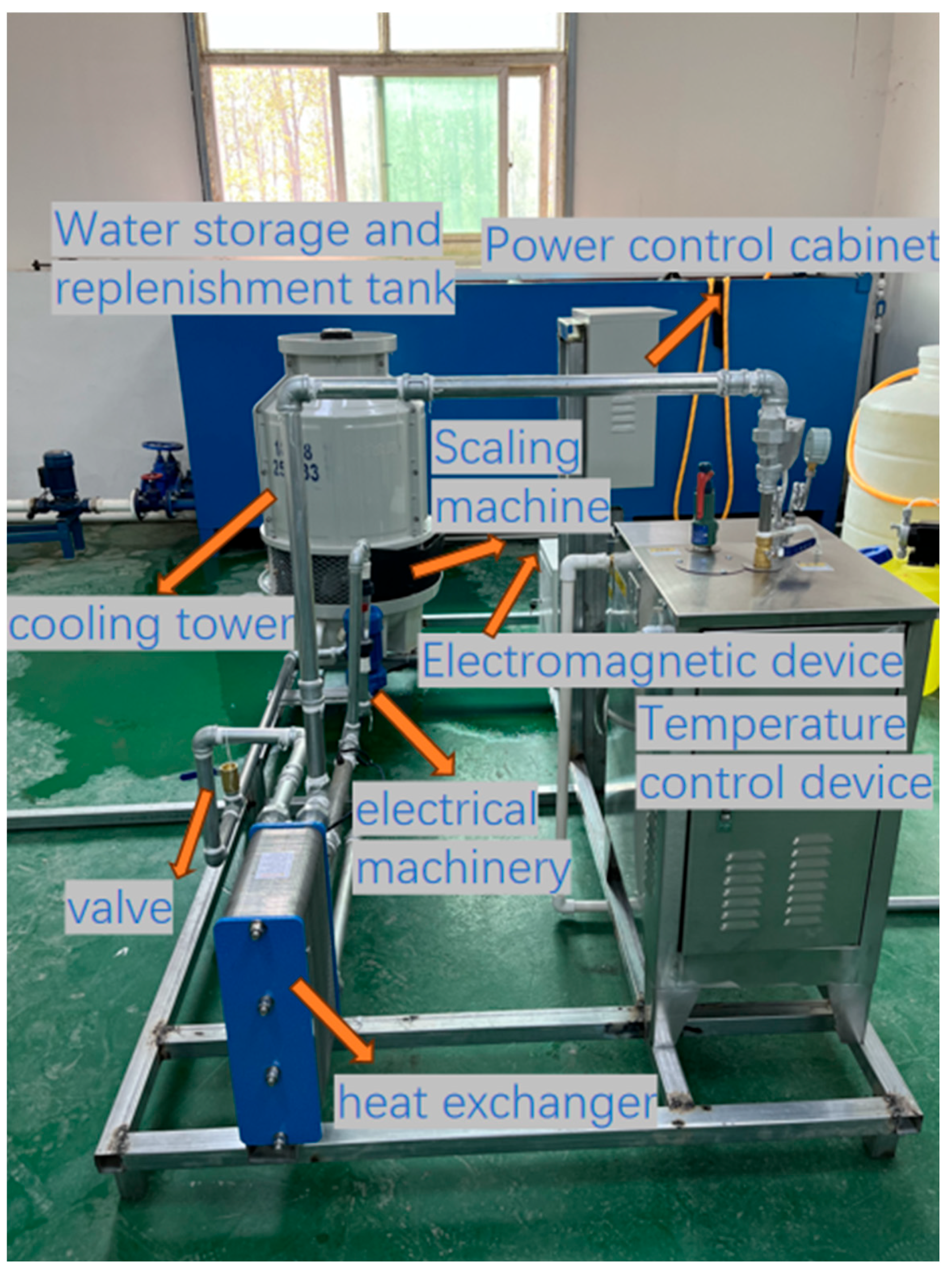
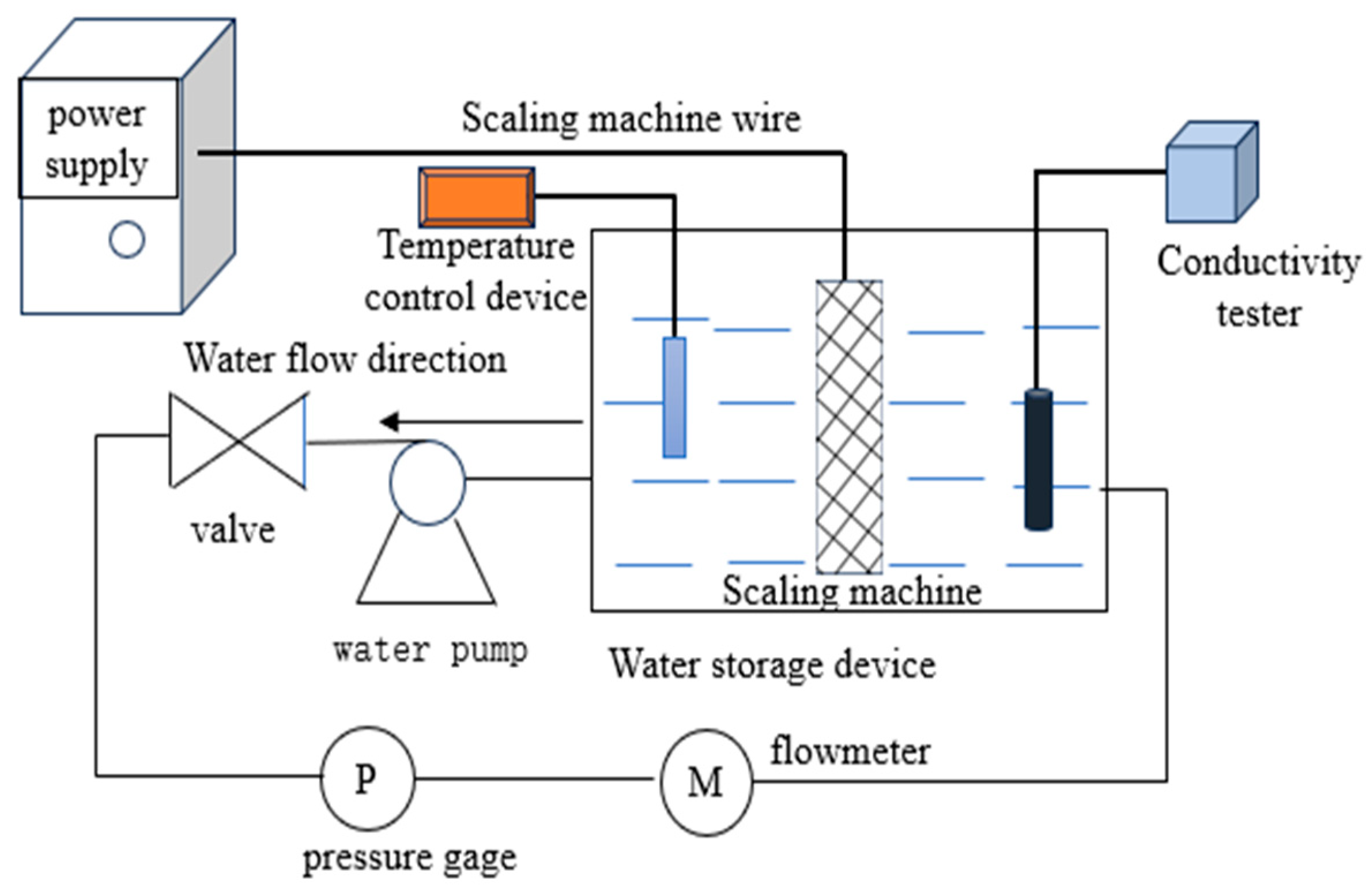
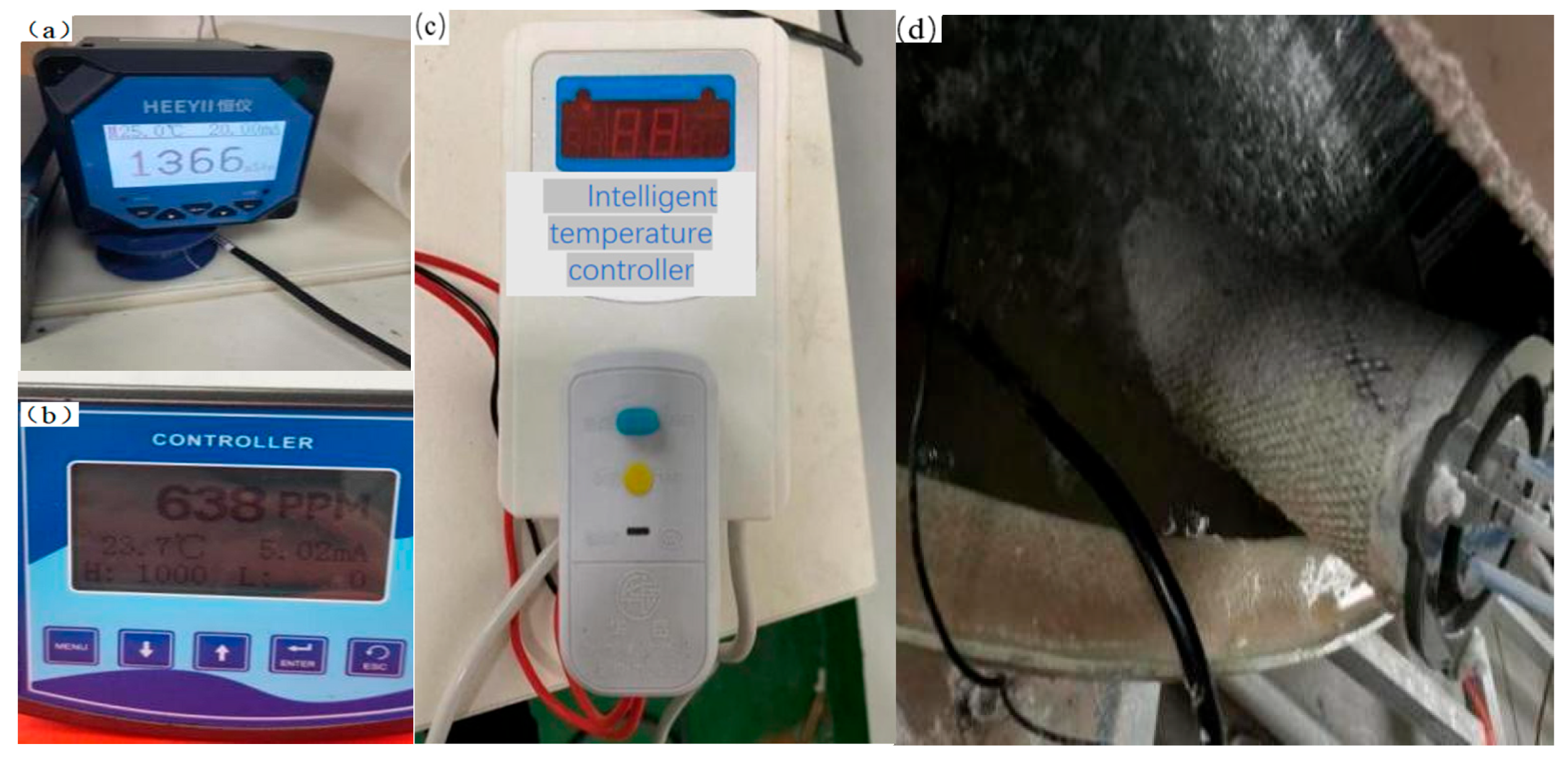

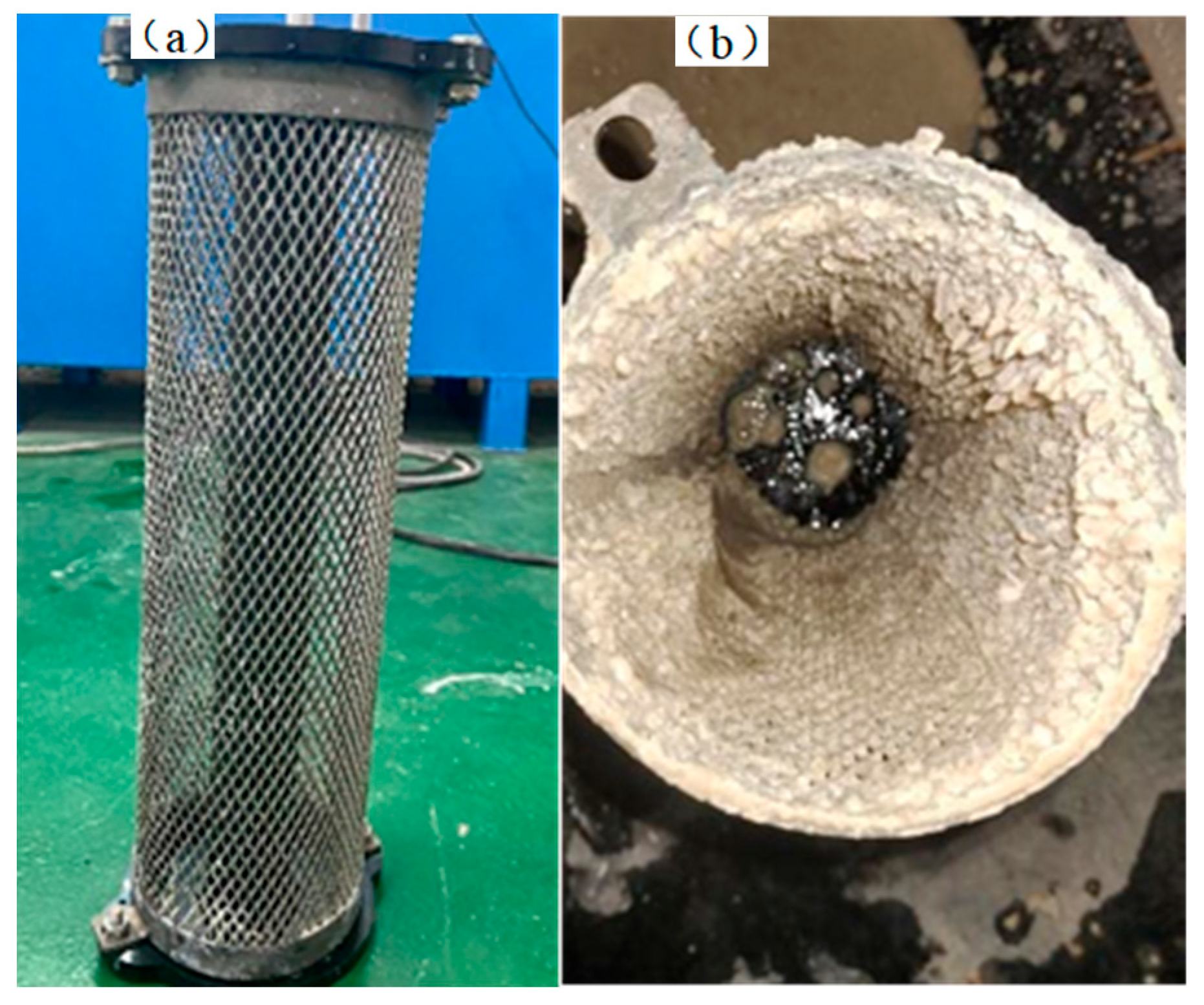
| Water Quality Parameters | pH | Conductivity (μs/cm) | Hardness (ppm) | Cl− Concentration (ppm) | Temperature (°C) |
|---|---|---|---|---|---|
| Raw water and supplementary water | 6.8 ± 0.2 | 1500 | 660 | 290 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wang, D.; Li, G.; Yu, H.; Dong, X.; Jiang, H. Analysis of Influencing Factors on the Efficiency of Electrochemical Scaling Equipment. Water 2024, 16, 2171. https://doi.org/10.3390/w16152171
Zhang S, Wang D, Li G, Yu H, Dong X, Jiang H. Analysis of Influencing Factors on the Efficiency of Electrochemical Scaling Equipment. Water. 2024; 16(15):2171. https://doi.org/10.3390/w16152171
Chicago/Turabian StyleZhang, Saiwei, Dongqiang Wang, Gangsheng Li, Hechun Yu, Xuewu Dong, and Haiqin Jiang. 2024. "Analysis of Influencing Factors on the Efficiency of Electrochemical Scaling Equipment" Water 16, no. 15: 2171. https://doi.org/10.3390/w16152171
APA StyleZhang, S., Wang, D., Li, G., Yu, H., Dong, X., & Jiang, H. (2024). Analysis of Influencing Factors on the Efficiency of Electrochemical Scaling Equipment. Water, 16(15), 2171. https://doi.org/10.3390/w16152171






