Start-Up and Bacterial Enrichment of an Anammox Reactor with Polyurethane Porous Material: Performance and Microbial Community
Abstract
1. Introduction
2. Materials and Methods
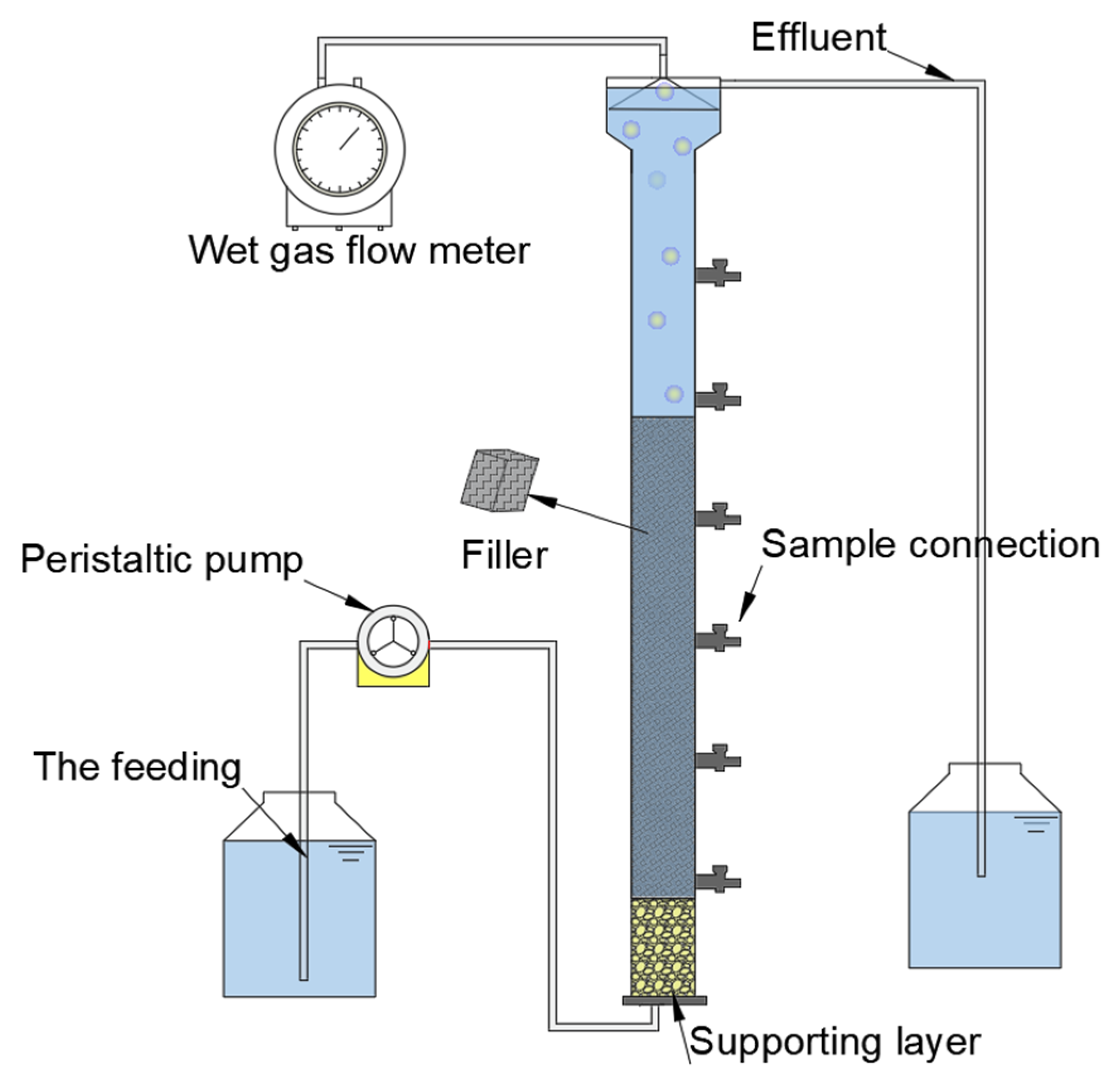
2.1. Experimental Setup
2.2. Influent and Sludge
2.3. Analytical Methods
2.3.1. Chemical Methods
2.3.2. Microbiological Analytical Methods
3. Results and Discussion
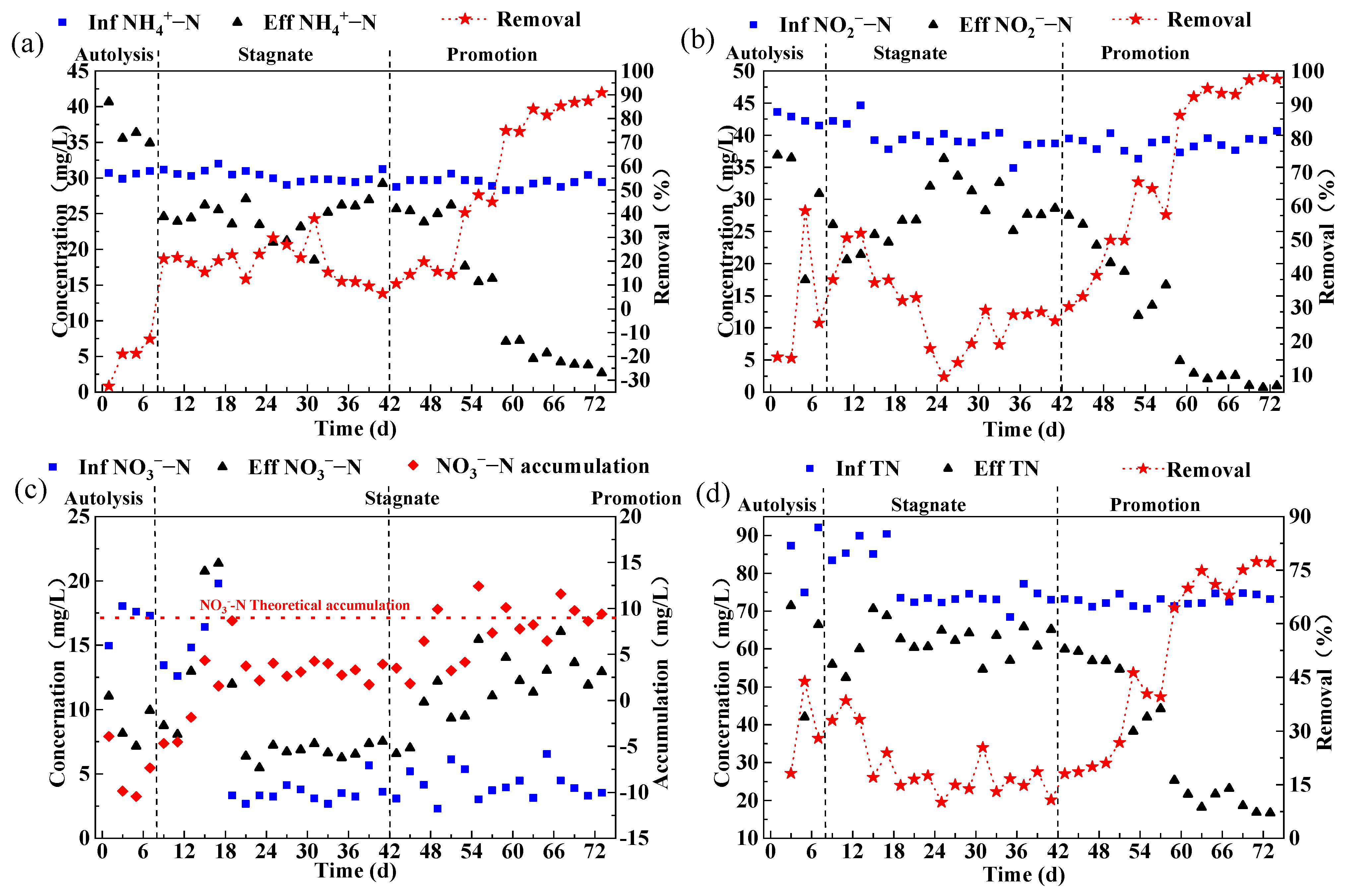
3.1. Start-Up of the Anammox Reactor
3.2. Enrichment of Anammox Bacteria
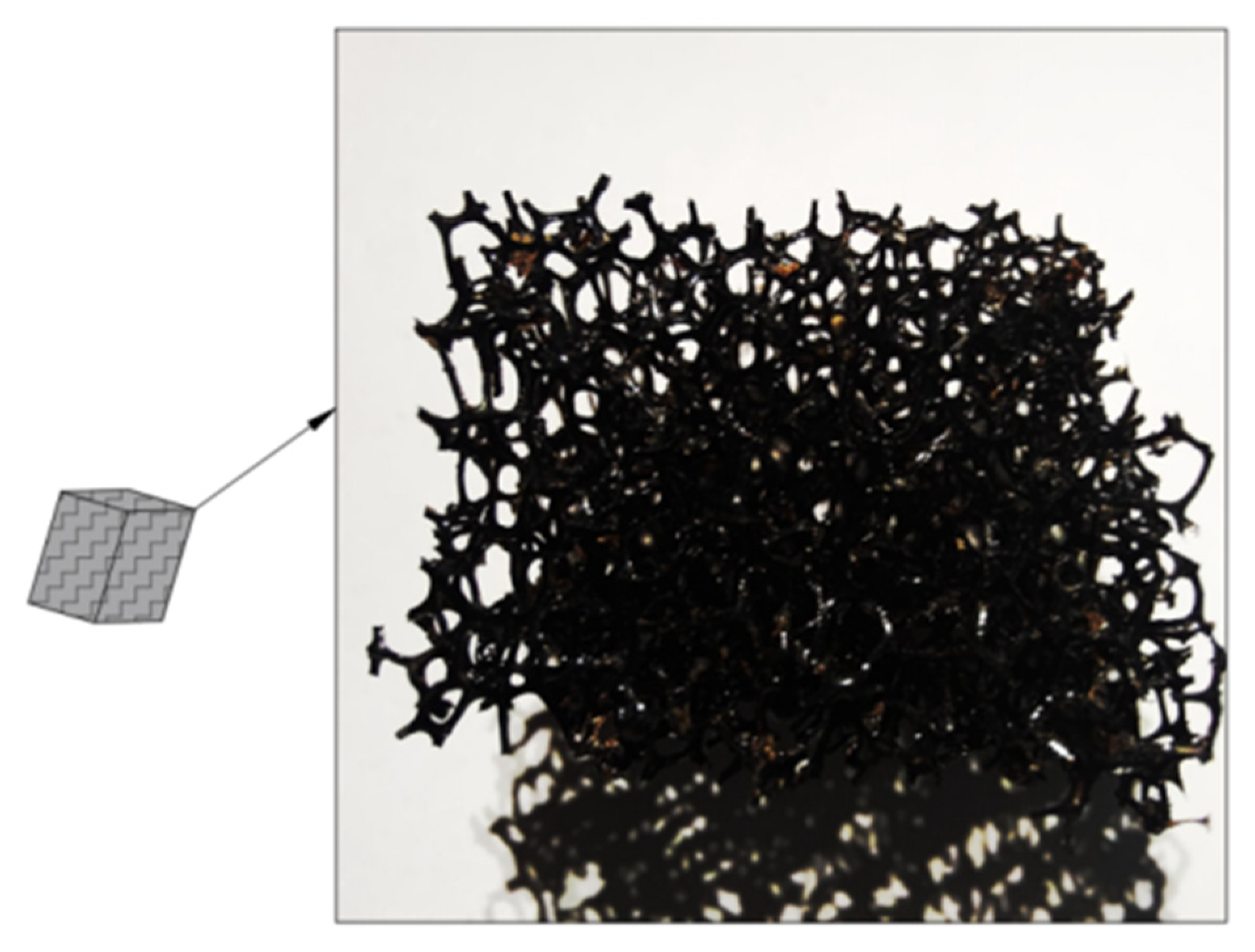
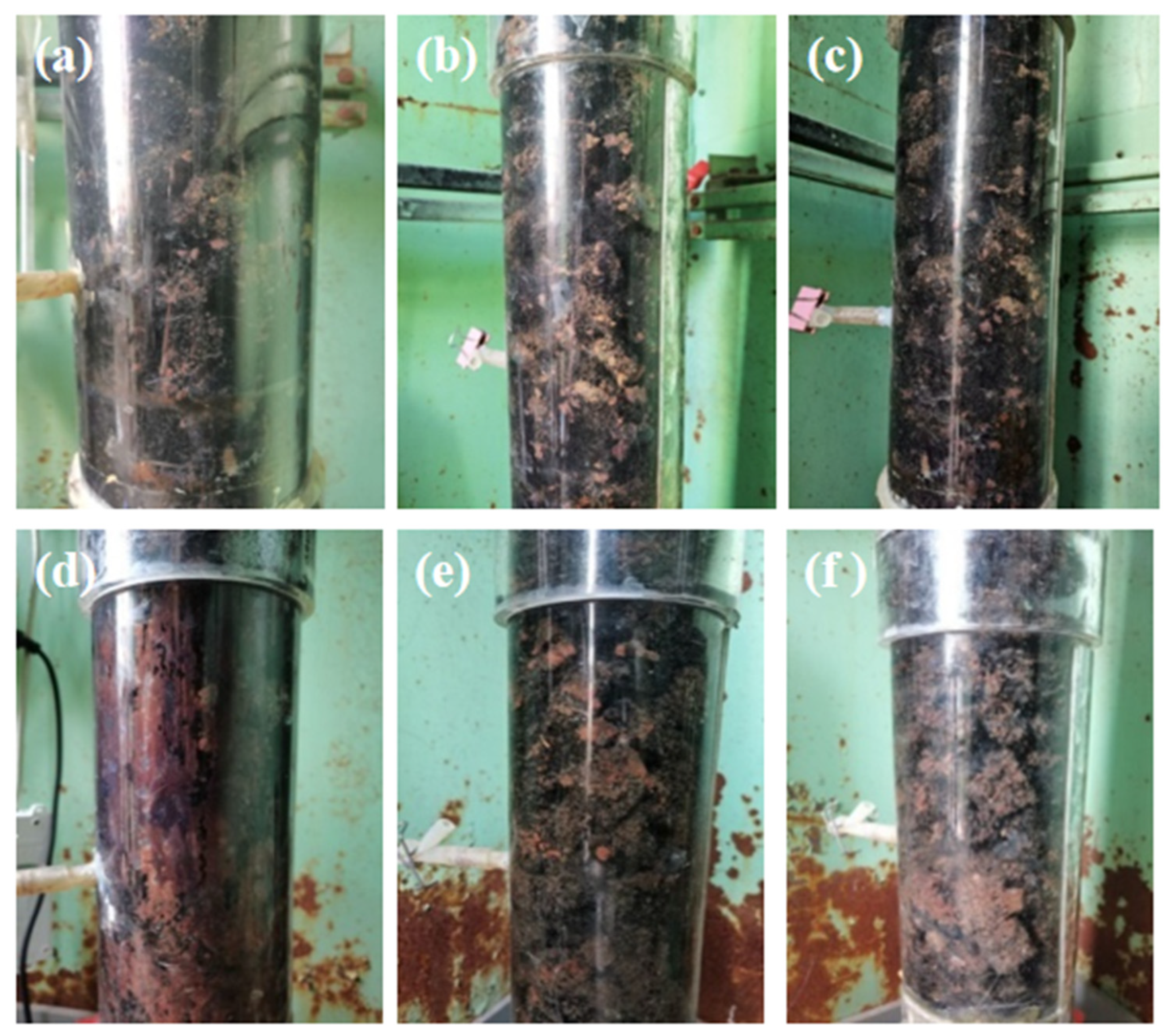
3.2.1. Changes in the Appearance of the Biofilm during Enrichment
3.2.2. Nitrogen Removal Effect of the Reactor
3.3. Microbial-Community Analysis after Enrichment
3.3.1. Analysis of Microbial Diversity and Richness
3.3.2. Classification-Level Analysis of Micropyle
3.3.3. Classification-Level Analysis of Microbe Genera
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Cui, N.; Xuan, K.; Xu, D.; Wang, D.; Li, C.; Li, Z.; Wang, Y. Start-up performance and process kinetics of a UASB-Anammox reactor at low substrate concentration. J. Environ. Chem. Eng. 2021, 9, 106726. [Google Scholar] [CrossRef]
- Ren, Z.-Q.; Wang, H.; Zhang, L.-G.; Du, X.-N.; Huang, B.-C.; Jin, R.-C. A review of anammox-based nitrogen removal technology: From microbial diversity to engineering applications. Bioresour. Technol. 2022, 363, 127896. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ran, X.; Li, J.; Wang, H.; Xue, G.; Wang, Y. Novel insights into carbon nanomaterials enhancing anammox for nitrogen removal: Effects and mechanisms. Sci. Total Environ. 2023, 905, 167146. [Google Scholar] [CrossRef] [PubMed]
- van der Star, W.R.L.; Abma, W.R.; Blommers, D.; Mulder, J.-W.; Tokutomi, T.; Strous, M.; Picioreanu, C.; van Loosdrecht, M.C.M. Startup of reactors for anoxic ammonium oxidation: Experiences from the first full-scale anammox reactor in Rotterdam. Water Res. 2007, 41, 4149–4163. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, J.; Feng, Y.; Li, H.; Wang, Z.; Chen, H.; Suo, N.; Yu, Y.; Shoubin, Z. Nitrogen removal performance and rapid start-up of anammox process in an electrolytic sequencing batch reactor (ESBR). Chemosphere 2022, 308, 136293. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Huang, X.-X.; Lei, C.-X.; Zhu, W.-J.; Chen, Y.-X.; Wu, W.-X. Improving Anammox start-up with bamboo charcoal. Chemosphere 2012, 89, 1224–1229. [Google Scholar] [CrossRef]
- Lu, G.; Ma, Y.; Zang, L.; Sun, Y.; Yu, F.; Xue, R. Effects of granular activated carbon and Fe-modified granular activated carbon on anammox process start-up††Electronic supplementary information (ESI) available. RSC Adv. 2021, 11, 10625–10634. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Huang, X.-W.; Li, X.-Y. Using anammox biofilms for rapid start-up of partial nitritation-anammox in integrated fixed-film activated sludge for autotrophic nitrogen removal. Sci. Total Environ. 2021, 791, 148314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, L.; Zhang, F.; Jiang, H.; Ren, S.; Wang, W.; Peng, Y. Enhanced nitrogen removal from nitrate-rich mature leachate via partial denitrification (PD)-anammox under real-time control. Bioresour. Technol. 2019, 289, 121615. [Google Scholar] [CrossRef]
- Fernández, I.; Vázquez-Padín, J.R.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Biofilm and granular systems to improve Anammox biomass retention. Biochem. Eng. J. 2008, 42, 308–313. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Wu, Y.; Lin, S. Enhancement of nitrogen removal and acceleration of anammox start-up with novel gravel contact carriers. Biochem. Eng. J. 2023, 200, 109084. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef]
- Adams, M.; Xie, J.; Chang, Y.; Kabore, A.W.J.; Chen, C. Start-up of Anammox systems with different biochar amendment: Process characteristics and microbial community. Sci. Total Environ. 2021, 790, 148242. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; De Costa, Y.G.; Tong, Z.; Cheng, J.J.; Zhou, L.; Zhuang, W.-Q.; Yu, K. The co-existence of anammox genera in an expanded granular sludge bed reactor with biomass carriers for nitrogen removal. Appl. Microbiol. Biotechnol. 2019, 103, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, H. Nitrogen removal performance of anammox immobilized fillers in response to seasonal temperature variations and different operating modes: Substrate utilization and microbial community analysis. Sci. Total Environ. 2022, 829, 154574. [Google Scholar] [CrossRef]
- Verma, S.; Daverey, A.; Lin, J.-G. Successful start-up of anammox process from activated sludge and anaerobic sludge in a sequencing batch reactor using an unconventional strategy. Int. Biodeterior. Biodegrad. 2021, 156, 105132. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Sun, L.; Shen, J.; Wang, M. Achieving reliable partial nitrification and anammox process using polyvinyl alcohol gel beads to treat low-strength ammonia wastewater. Bioresour. Technol. 2021, 324, 124669. [Google Scholar] [CrossRef]
- Peng, Z.; Lei, Y.; Liu, Y.; Wan, X.; Yang, B.; Pan, X. Fast start-up and reactivation of anammox process using polyurethane sponge. Biochem. Eng. J. 2022, 177, 108249. [Google Scholar] [CrossRef]
- Wang, J.; Liang, J.; Ning, D.; Zhang, T.; Wang, M. A review of biomass immobilization in anammox and partial nitrification/anammox systems: Advances, issues, and future perspectives. Sci. Total Environ. 2022, 821, 152792. [Google Scholar] [CrossRef]
- Yang, S.; Peng, Y.; Zhang, S.; Han, X.; Li, J.; Zhang, L. Carrier type induces anammox biofilm structure and the nitrogen removal pathway: Demonstration in a full-scale partial nitritation/anammox process. Bioresour. Technol. 2021, 334, 125249. [Google Scholar] [CrossRef]
- Yang, S.; Peng, Y.; Zhang, Q.; Li, J.; Zhang, L. Biofilm phenotypes and internal community succession determines distinct growth of anammox bacteria in functional anammox biofilms. Bioresour. Technol. 2022, 349, 126893. [Google Scholar] [CrossRef]
- Jenkins, S.H. Standardized methods of water examination. Examination of water for pollution control, a reference handbook: Edited by M. J. Suess. Vol. 1 Sampling, data analysis and laboratory equipment, 360 pp. Vol. 2 Physical, chemical and radiological examination, 555 pp. Vol. 3 Biological, bacteriological and virological examination, 531 pp. published on behalf of the World Health Organization by Pergamon Press, Oxford, 1982. £175.00 or $350.00. Water Res. 1983, 17, 719–720. [Google Scholar] [CrossRef]
- Yan, Z.; Shen, L.; Jiao, L.; Tang, R. Effect of Fe (II) on nitrogen removal of anammox under organic matter inhibition. J. Water Process Eng. 2022, 46, 102632. [Google Scholar] [CrossRef]
- Ren, Z.; Li, N.; Yu, L.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. An Illumina MiSeq sequencing-based method using the mreB gene for high-throughput discrimination of Pseudomonas species in raw milk. LWT 2022, 163, 113573. [Google Scholar] [CrossRef]
- Qian, Y.; Ding, Y.; Ma, H.; Chi, Y.; Yuan, H.; Li, Y.-Y.; Tian, S.; Zhang, B. Startup and performance of a novel single-stage partial nitritation/anammox system for reject water treatment. Bioresour. Technol. 2021, 321, 124432. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Qi, Z.; Zhang, M.; Kang, R.; Liu, C.; Li, D. Quantitative effect of adding percentages of anammox granules on the start-up process and microbial community analysis. J. Environ. Manag. 2024, 349, 119361. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Li, J.; Zhang, Y.; Bian, W.; Wei, J.; Zhao, B.; Yang, J. Effects of carbon sources, COD/NO2−-N ratios and temperature on the nitrogen removal performance of the simultaneous partial nitrification, anammox and denitrification (SNAD) biofilm. Water Sci. Technol. 2017, 75, 1712–1721. [Google Scholar] [CrossRef]
- Li, H.; Zhou, S.; Ma, W.; Huang, G.; Xu, B. Fast start-up of ANAMMOX reactor: Operational strategy and some characteristics as indicators of reactor performance. Desalination 2012, 286, 436–441. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Yin, M.; Zheng, Y.; Wang, C.; Yan, L. Promoting performance of Anammox by iron loaded sludge biochar with hydrothermal carbonization (HTC-Fe-BC) addition. Process Saf. Environ. Prot. 2023, 170, 596–607. [Google Scholar] [CrossRef]
- Wu, L.; Li, Z.; Huang, S.; Shen, M.; Yan, Z.; Li, J.; Peng, Y. Low energy treatment of landfill leachate using simultaneous partial nitrification and partial denitrification with anaerobic ammonia oxidation. Environ. Int. 2019, 127, 452–461. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Zhang, C.; Song, Y.; Han, Y.; Hou, Y.; Zhang, D.; Li, C.; Wang, Y.; Guo, J. Responses of anammox and sulfur/pyrite autotrophic denitrification in one-stage system to high nitrogen load: Performance, metabolic and bacterial community. J. Environ. Manag. 2023, 332, 117427. [Google Scholar] [CrossRef]
- Karasuta, C.; Wang, X.; Zheng, X.; Chen, Y.; Chen, Z. Effect of hydraulic retention time on effluent pH in anammox bioreactors: Characteristics of effluent pH and pH as an indicator of reactor performance. J. Environ. Manag. 2021, 280, 111716. [Google Scholar] [CrossRef]
- Zhang, M.; Li, B.; Guan, Z.; Fan, Y.; He, L.; Wu, J. Rapid anammox startup in response to nitrogen loading variations: Reactor performance, microbial dynamics and mechanism exploration. J. Water Process Eng. 2024, 59, 104963. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Wang, W.; Li, J.; Li, B.; Huang, X. Characteristics of rapid-biofiltering anammox reactor (RBAR) for low nitrogen wastewater treatment. Bioresour. Technol. 2020, 318, 124066. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; He, S.; Han, M.; Wang, B.; Niu, Q.; Xu, Y.; Chen, Y.; Wang, H. Nitrogen removal performance and microbial community structure in the start-up and substrate inhibition stages of an anammox reactor. J. Biosci. Bioeng. 2018, 126, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Z.; Yang, Y.; Mei, X.; Wu, Z. Correlating microbial community structure and composition with aeration intensity in submerged membrane bioreactors by 454 high-throughput pyrosequencing. Water Res. 2013, 47, 859–869. [Google Scholar] [CrossRef]
- Luo, D.; Qian, J.; Fu, J.-X.; Liu, C.; Zhang, R.-X.; Huang, D.-N.; Zhang, L. Responses of anammox to long-term p-nitrophenol stress: From apparent and microscopic phenomena to mechanism simulation. Bioresour. Technol. 2022, 355, 127265. [Google Scholar] [CrossRef]
- Gamoń, F.; Banach-Wiśniewska, A.; Poprawa, I.; Cema, G.; Ziembińska-Buczyńska, A. Insight into the microbial and genetic response of anammox biomass to broad range concentrations of different antibiotics: Linking performance and mechanism. Chem. Eng. J. 2023, 451, 138546. [Google Scholar] [CrossRef]
- Huang, T.-H.; Tung, F.-T.; Chen, G.-F.; Chen, W.-H. Variations of N concentrations and microbial community in the start-up of anammox using anaerobic heterotrophic sludge: Influence of a long reaction-phase time and comparison of the efficiencies of attached-versus suspended-growth cultures. Chemosphere 2022, 287, 132151. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; An, N.; Shi, L.; Wei, Y.; Ma, B. Microbial community structure and nitrogen conversion rate of size-fractionated granules in partial denitrification and anammox reactor. J. Clean. Prod. 2023, 414, 137714. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Li, J.; Wei, P.; Luo, R.; Han, H. Fast start-up of ANAMMOX biofilm processes at low temperatures by economical quorum sensing regulation: The importance of endogenous N-acyl-homoserine lactones from enhanced inoculated sludge. Environ. Res. 2022, 214, 114097. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y.; Guo, Y.; Zhao, M.; Wang, S. Illumina MiSeq sequencing reveals the key microorganisms involved in partial nitritation followed by simultaneous sludge fermentation, denitrification and anammox process. Bioresour. Technol. 2016, 207, 118–125. [Google Scholar] [CrossRef]
- Xie, S.; Li, X.; Wang, C.; Kulandaivelu, J.; Jiang, G. Enhanced anaerobic digestion of primary sludge with additives: Performance and mechanisms. Bioresour. Technol. 2020, 316, 123970. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Jiang, B.; Feng, Y.; Zhu, T.; Tao, H.; Tang, X.; Liu, S. Genome-Centered Metagenomics Analysis Reveals the Symbiotic Organisms Possessing Ability to Cross-Feed with Anammox Bacteria in Anammox Consortia. Environ. Sci. Technol. 2018, 52, 11285–11296. [Google Scholar] [CrossRef]
- Ge, C.-H.; Sun, N.; Kang, Q.; Ren, L.-F.; Ahmad, H.A.; Ni, S.-Q.; Wang, Z. Bacterial community evolutions driven by organic matter and powder activated carbon in simultaneous anammox and denitrification (SAD) process. Bioresour. Technol. 2018, 251, 13–21. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Cheng, J.; Zhang, S.; Li, X.; Peng, Y. Microbial community evolution in partial nitritation/anammox process: From sidestream to mainstream. Bioresour. Technol. 2018, 251, 327–333. [Google Scholar] [CrossRef]
- Kong, X.; Yu, S.; Fang, W.; Liu, J.; Li, H. Enhancing syntrophic associations among Clostridium butyricum, Syntrophomonas and two types of methanogen by zero valent iron in an anaerobic assay with a high organic loading. Bioresour. Technol. 2018, 257, 181–191. [Google Scholar] [CrossRef]
- Oyarzúa, P.; Bovio-Winkler, P.; Etchebehere, C.; Suárez-Ojeda, M.E. Microbial communities in an anammox reactor treating municipal wastewater at mainstream conditions: Practical implications of different molecular approaches. J. Environ. Chem. Eng. 2021, 9, 106622. [Google Scholar] [CrossRef]
- Thu, N.H.T.; Quy, L.V.; Anna, H.A.; Lund, N.J.; Halkjaer, N.P. High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems. FEMS Microbiol. Ecol. 2011, 76, 256–267. [Google Scholar] [CrossRef]
- Costa, M.C.; Carvalho, L.; Leal, C.D.; Dias, M.F.; Martins, K.L.; Garcia, G.B.; Mancuelo, I.D.; Hipólito, T.; Conell, E.F.; Okada, D.; et al. Impact of inocula and operating conditions on the microbial community structure of two anammox reactors. Environ. Technol. 2014, 35, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Antwi, P.; Li, J.; Boadi, P.O.; Meng, J.; Shi, E.; Chi, X.; Deng, K.; Ayivi, F. Dosing effect of zero valent iron (ZVI) on biomethanation and microbial community distribution as revealed by 16S rRNA high-throughput sequencing. Int. Biodeterior. Biodegrad. 2017, 123, 191–199. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, L.; Gong, Q.; Liu, X.; Li, X.; Zhang, Q.; Peng, Y. Rapid cultivation and enrichment of anammox bacteria solely using traditional activated sludge as inoculum and biocarrier in low-strength real sewage treatment. Bioresour. Technol. 2022, 358, 127354. [Google Scholar] [CrossRef]
- Qiao, L.; Yuan, Y.; Mei, C.; Yin, W.; Zou, C.; Yin, Y.; Guo, Q.; Chen, T.; Ding, C. Reinforced nitrite supplement by cathode nitrate reduction with a bio-electrochemical system coupled anammox reactor. Environ. Res. 2022, 204, 112051. [Google Scholar] [CrossRef]
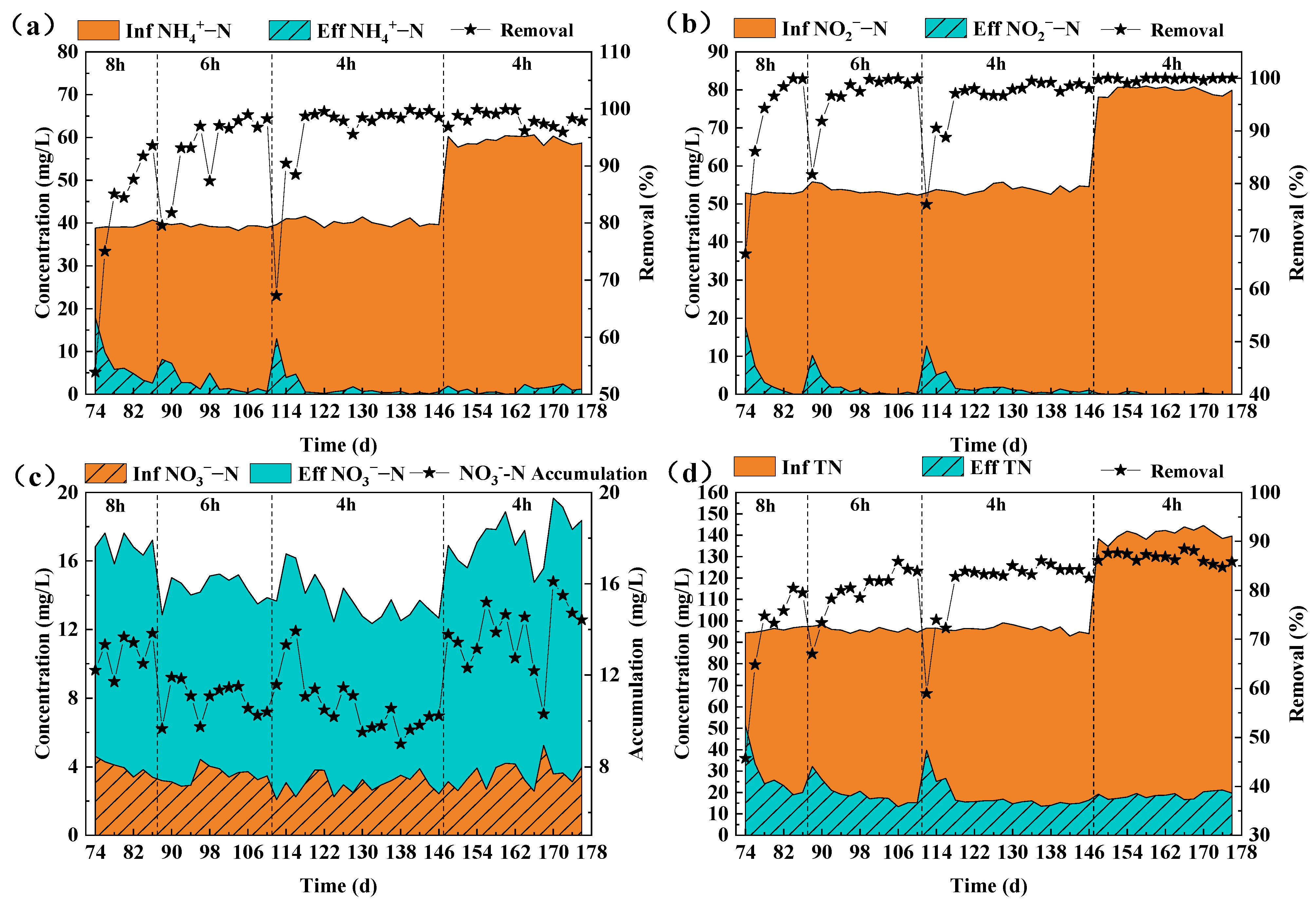
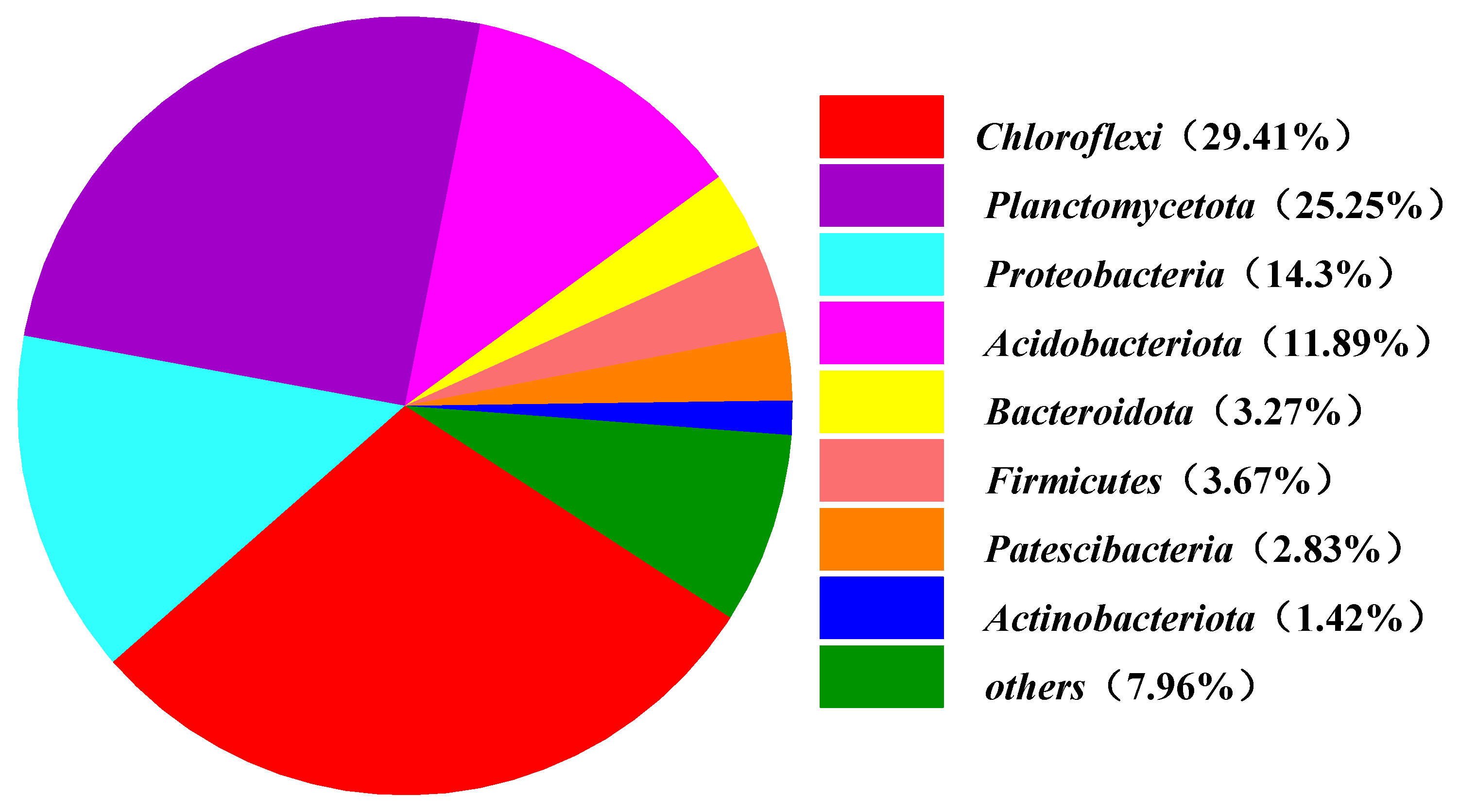
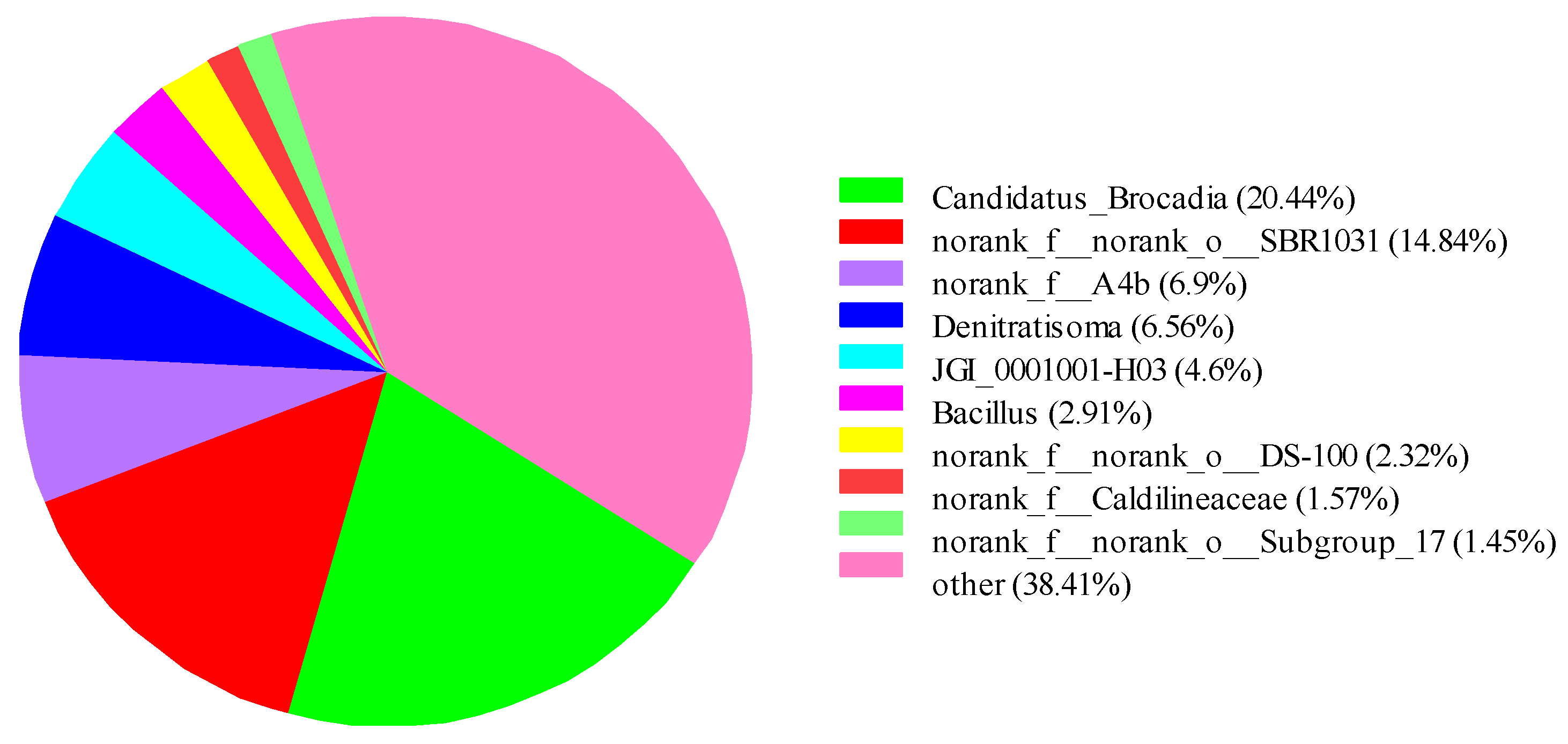
| Phase | Time/d | HRT/h | NH4+-N (mg/L) | NO2−-N (mg/L) |
|---|---|---|---|---|
| I | 74~86 | 8 | 40 ± 2 | 53 ± 2 |
| II | 87~110 | 6 | 40 ± 2 | 53 ± 2 |
| III | 111~146 | 4 | 40 ± 2 | 53 ± 2 |
| IV | 147~176 | 4 | 60 ± 2 | 80 ± 2 |
| Sample Number | Serial Number | Shannon | Simpson | Chao | Ace | Coverage Rate |
|---|---|---|---|---|---|---|
| 1 | 96154 | 4.37 | 0.052 | 912 | 917 | 0.9972 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Zhang, W.; Pei, Z.; Jiao, L. Start-Up and Bacterial Enrichment of an Anammox Reactor with Polyurethane Porous Material: Performance and Microbial Community. Water 2024, 16, 2116. https://doi.org/10.3390/w16152116
Yan Z, Zhang W, Pei Z, Jiao L. Start-Up and Bacterial Enrichment of an Anammox Reactor with Polyurethane Porous Material: Performance and Microbial Community. Water. 2024; 16(15):2116. https://doi.org/10.3390/w16152116
Chicago/Turabian StyleYan, Zichun, Weibin Zhang, Zhibin Pei, and Longzhen Jiao. 2024. "Start-Up and Bacterial Enrichment of an Anammox Reactor with Polyurethane Porous Material: Performance and Microbial Community" Water 16, no. 15: 2116. https://doi.org/10.3390/w16152116
APA StyleYan, Z., Zhang, W., Pei, Z., & Jiao, L. (2024). Start-Up and Bacterial Enrichment of an Anammox Reactor with Polyurethane Porous Material: Performance and Microbial Community. Water, 16(15), 2116. https://doi.org/10.3390/w16152116






