Iron Contamination in Groundwater: Risk Assessment and Remediation Techniques in Egypt’s New Valley
Abstract
1. Introduction
2. Methodology
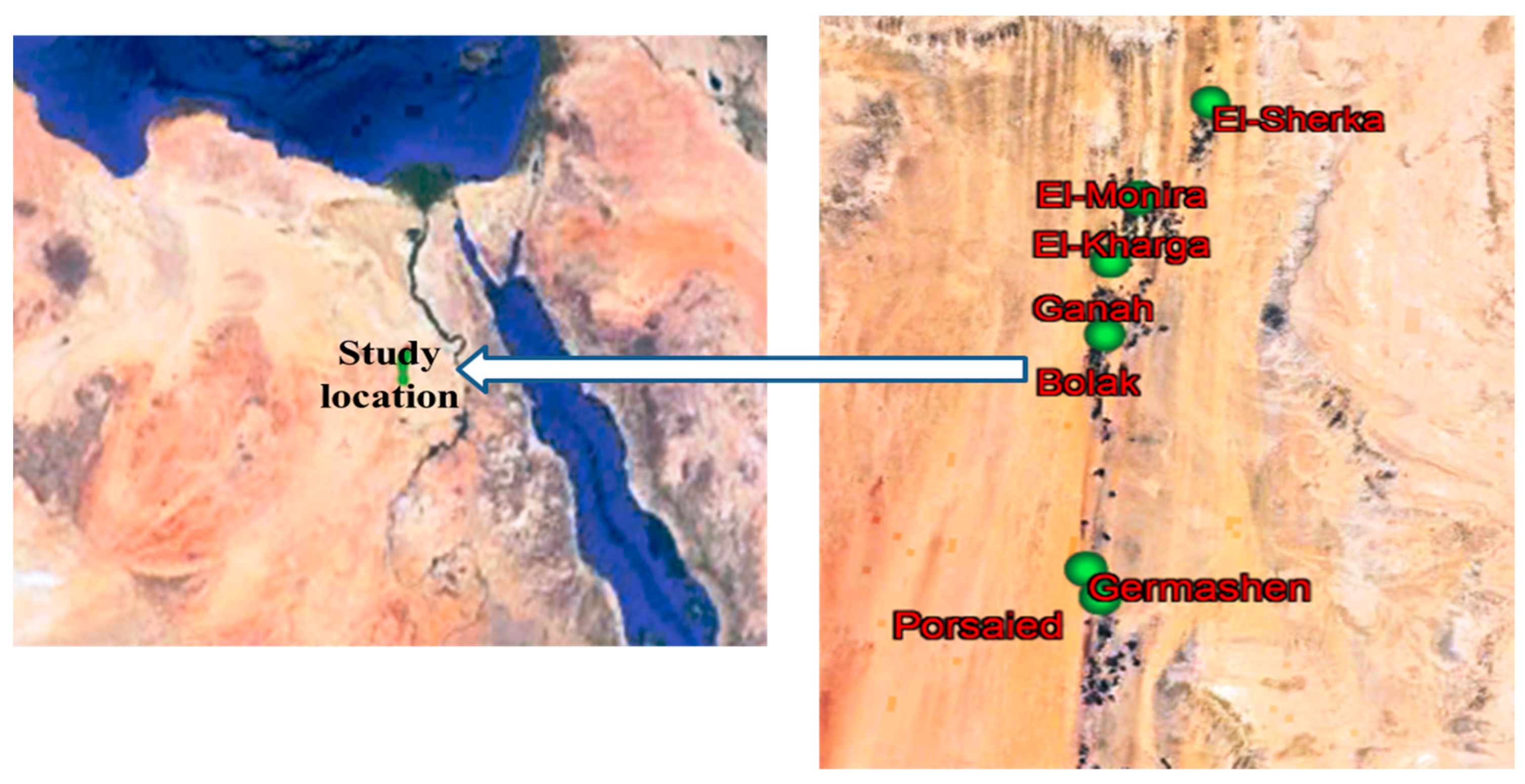
2.1. Study Location
2.2. Water Analyses
2.3. Risk Assessment
2.4. Efficiency of Different Sorbent Materials for Fe Removal from Aqueous Solution
2.4.1. Preparation of Fe Solutions
2.4.2. Batch Adsorption Studies
2.4.3. Adsorption Isotherms
2.4.4. Adsorption Kinetics Models
2.5. Regeneration
2.6. Data Analysis
3. Results and Discussion
3.1. Chemical Analyses of Water
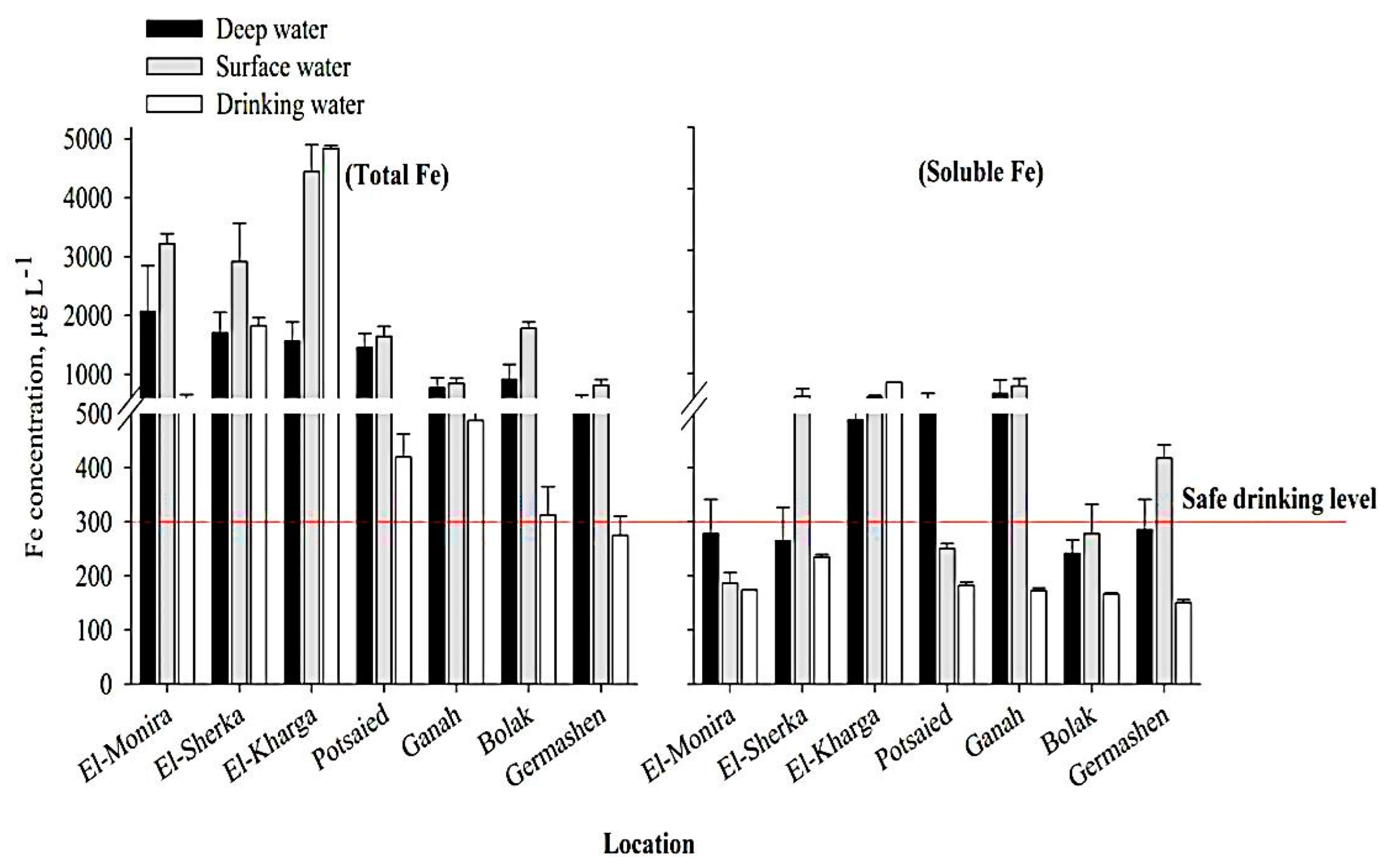
3.2. Total and Soluble Fe Contents
3.3. Risk Assessment Study
3.4. Characterization of Calcium Carbonate Soilds and Biochar
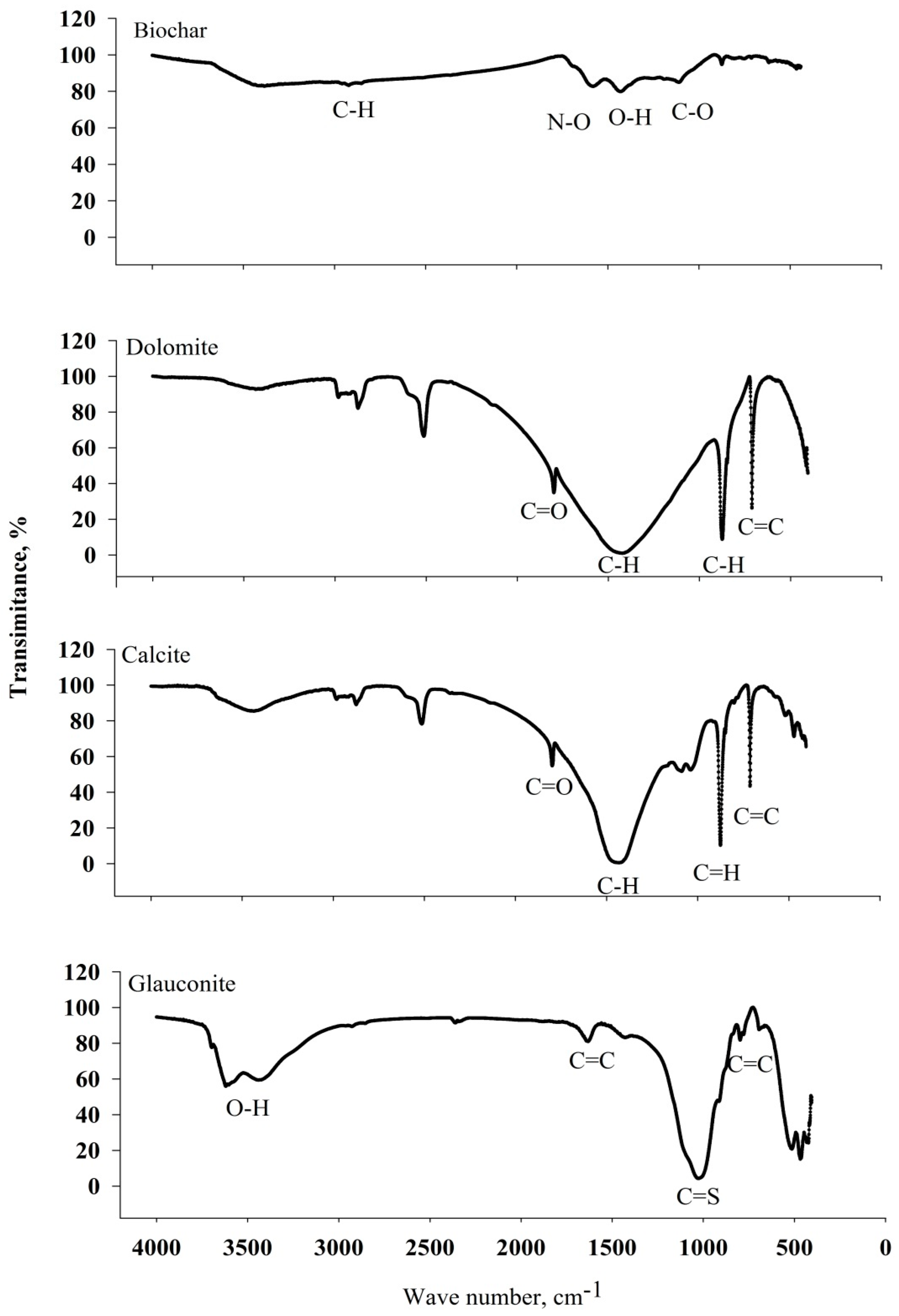
3.4.1. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
3.4.2. X-ray Diffraction Analysis (XRD) and Scanning Electron Microscopy (SEM) Analyses of Biochar Material
3.5. Factors Affecting Sorption of Fe by the Investigated Materials
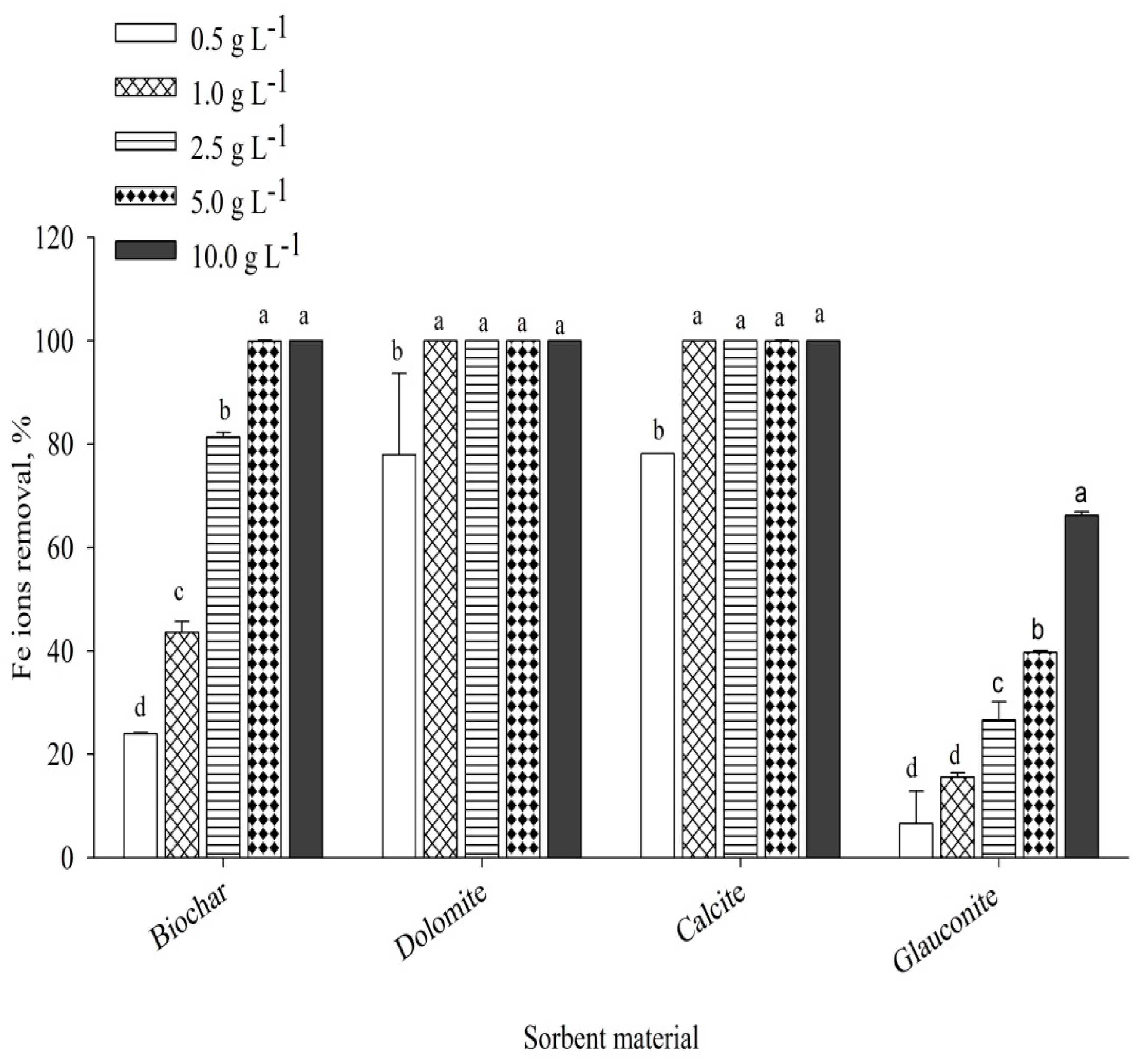
3.5.1. Sorbent Dosage
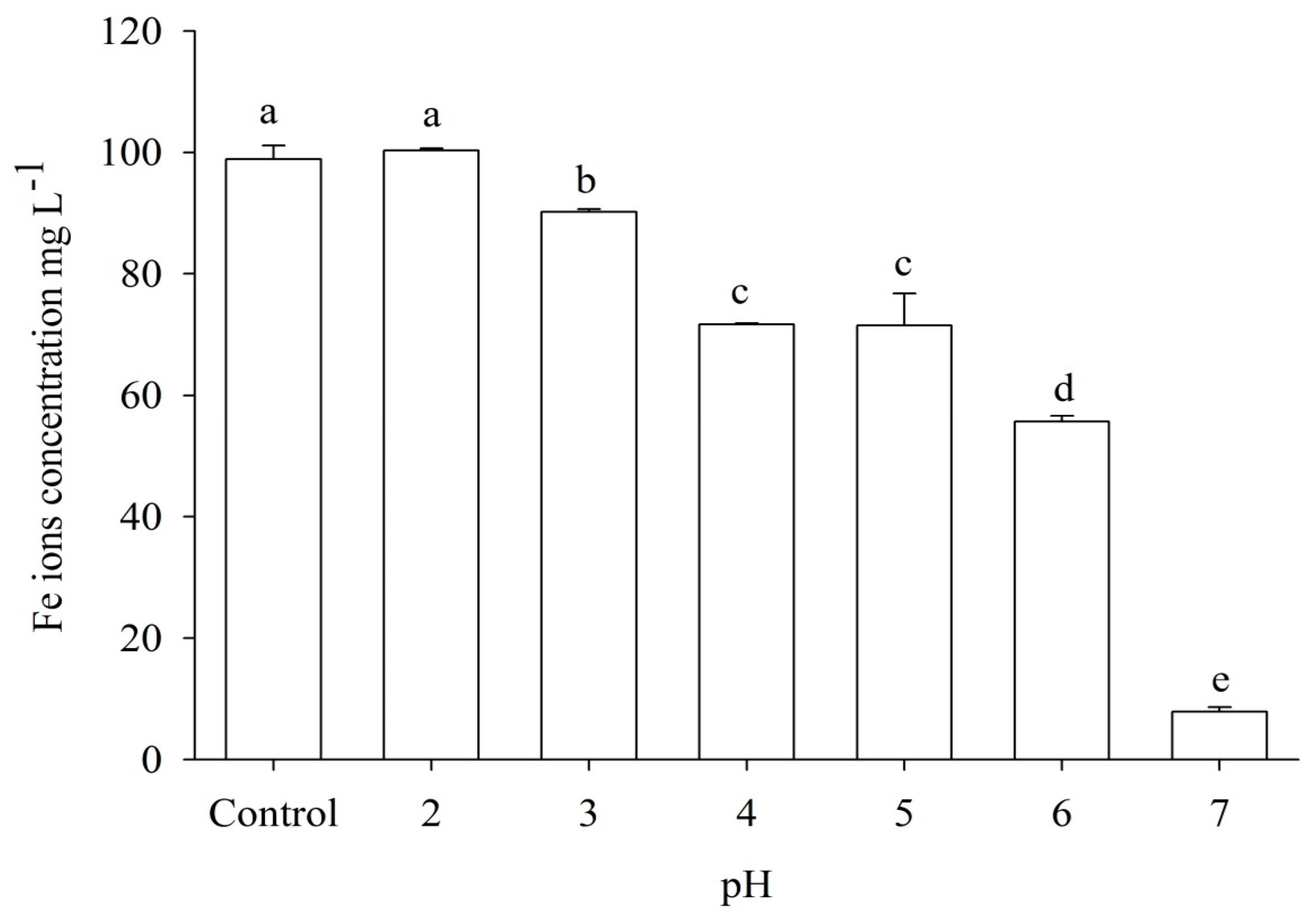
3.5.2. Effect of pH
3.6. Efficiency of Sorbent Materials for Fe Removal
3.6.1. Adsorption Isotherms
3.6.2. Kinetics of Fe Sorption
3.6.3. Regeneration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Barbieri, M.; Ricolfi, L.; Vitale, S.; Muteto, P.V.; Nigro, A.; Sappa, G. Assessment of groundwater quality in the buffer zone of Limpopo National Park, Gaza Province, Southern Mozambique. Environ. Sci. Pollut. Res. 2019, 26, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Baggio, G.; Qadir, M.; Smakhtin, V. Freshwater availability status across countries for human and ecosystem needs. Sci. Total Environ. 2021, 792, 148230. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, J.; Prell, C.; Bryan, B.A. Changes in supply and demand mediate the effects of land-use change on freshwater ecosystem services flows. Sci. Total Environ. 2021, 763, 143012. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.; Achari, V.S. Groundwater for drinking and industrial purposes: A study of water stability and human health risk assessment from black sand mineral rich coastal region of Kerala, India. J. Environ. Manag. 2024, 351, 119783. [Google Scholar] [CrossRef] [PubMed]
- Ricolfi, L.; Barbieri, M.; Muteto, P.V.; Nigro, A.; Sappa, G.; Vitale, S. Potential toxic elements in groundwater and their health risk assessment in drinking water of Limpopo National Park, Gaza Province, Southern Mozambique. Environ. Geochem. Health 2020, 42, 2733–2745. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaki, C.; Badr, N.; Mohammedi, H.; Haidara, R.; Belarbi, H. Wastewater Reuse for Agriculture, Qualitative Aspects: A Case Study of Ain Temouchent, Algeria. In The Water, Climate, and Food Nexus: Linkages, Challenges and Emerging Solutions; Behnassi, M., Al-Shaikh, A.A., Gurib-Fakim, A., Barjees Baig, M., Bahir, M., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 355–377. [Google Scholar]
- Fouad, S.S.; Heggy, E.; Ramah, M.; Abotalib, A.Z.; Palmer, E.M.; Jomaa, S.; Weilacher, U. Egypt’s waterways conservation campaigns under growing intrinsic demand and Nile upstream damming. J. Hydrol. Reg. Stud. 2023, 50, 101537. [Google Scholar] [CrossRef]
- El-Shatla, H.; Ahmed, E.A.; Essa, R.; Mohamed, M.F. Economic efficiency and productivity of lifting and distribution methods of groundwater for wheat and tomato in New Valley governorate. Bull. Natl. Res. Cent. 2019, 43, 157. [Google Scholar] [CrossRef]
- Gabr, M.E. Land reclamation projects in the Egyptian Western Desert: Management of 1.5 million acres of groundwater irrigation. Water Int. 2023, 48, 240–258. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Abbas, M.H.H.; Kenawy, M.H.M.; Noureldeen, A.; Darwish, H.; Ewis, A.M.G.; Hamed, M.H. Evaluation of underground water quality for drinking and irrigation purposes in New Valley Governorate, Egypt. Environ. Technol. Innov. 2021, 22, 101486. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Hurrell, R.; Reddy, M.; Cook, J. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Molot, L.A.; Watson, S.B.; Creed, I.F.; Trick, C.G.; McCabe, S.K.; Verschoor, M.J.; Sorichetti, R.J.; Powe, C.; Venkiteswaran, J.J.; Schiff, S.L. A novel model for cyanobacteria bloom formation: The critical role of anoxia and ferrous iron. Freshw. Biol. 2014, 59, 1323–1340. [Google Scholar] [CrossRef]
- Aladejana, J.A.; Kalin, R.M.; Sentenac, P.; Hassan, I. Assessing the impact of climate change on groundwater quality of the shallow coastal aquifer of eastern dahomey basin, southwestern Nigeria. Water 2020, 12, 224. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Application of biological processes for the removal of arsenic from groundwaters. Water Res. 2004, 38, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Aoudj, S.; Khelifa, A.; Drouiche, N.; Hecini, M.; Hamitouche, H. Electrocoagulation process applied to wastewater containing dyes from textile industry. Chem. Eng. Process. Process Intensif. 2010, 49, 1176–1182. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Inyang, M.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.R.; Pullammanappallil, P.; Cao, X. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafez, A.A.; Li, J. Removal of Pb(II) from aqueous solution by using biochars derived from sugar cane bagasse and orange peel. J. Taiwan Inst. Chem. Eng. 2016, 61, 367–375. [Google Scholar] [CrossRef]
- Farid, I.M.; Siam, H.S.; Abbas, M.H.H.; Mohamed, I.; Mahmoud, S.A.; Tolba, M.; Abbas, H.H.; Yang, X.; Antoniadis, V.; Rinklebe, J.; et al. Co-composted biochar derived from rice straw and sugarcane bagasse improved soil properties, carbon balance, and zucchini growth in a sandy soil: A trial for enhancing the health of low fertile arid soils. Chemosphere 2022, 292, 133389. [Google Scholar] [CrossRef]
- Asaad, A.A.; El-Hawary, A.M.; Abbas, M.H.H.; Mohamed, I.; Abdelhafez, A.A.; Bassouny, M.A. Reclamation of wastewater in wetlands using reed plants and biochar. Sci. Rep. 2022, 12, 19516. [Google Scholar] [CrossRef]
- Kutergin, A.S.; Nedobukh, T.A.; Kutergina, I.N. Sorption treatment of surface waters from heavy metals by natural and granulated glauconite. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2020; Volume 2313. [Google Scholar] [CrossRef]
- Martemyanov, D.; Rudmin, M.; Zhuravkov, S.; Korotkova, E.; Godymchuk, A.; Haskelberg, M.; Martemyanova, I.; Chernova, A.; Tyabaev, A.; Artamonov, A.; et al. Application of ural glauconite for groundwater deironing and demanganation. J. Environ. Sci. Health Part A 2021, 56, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Nassar, M.Y.; El-Shahat, M.F.; Osman, A.; Sobeih, M.M.; Zaid, M.A. Adsorptive Removal of Manganese Ions from Polluted Aqueous Media by Glauconite Clay-Functionalized Chitosan Nanocomposites. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4050–4064. [Google Scholar] [CrossRef]
- Abdel-Ghafar, H.M.; Abdel-Aal, E.A.; El Anadouli, B.E. Iron removal from ground water using Egyptian cost-effective clay minerals. Appl. Chem. Eng. 2020, 3, 23–30. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Q.; Li, X.; Wu, L.; Liu, Y. Efficient heterogeneous precipitation and separation of iron in copper-containing solution using dolomite. Sep. Purif. Technol. 2020, 248, 117021. [Google Scholar] [CrossRef]
- Shah, K.H.; Fahad, M.; Ghazi, Z.A.; Ali, S.; Shahzad, A.; Din, S.U. Optimization, characterization and adsorption properties of natural calcite for toxic As(III) removal from aqueous solutions. Water SA 2022, 48, 295–303. [Google Scholar] [CrossRef]
- Liang-Tong, Z.; Li, Z.; Yuqing, Y.; Na, H.; Bate, B. Investigation of aqueous Fe(III) and Mn(II) removal using dolomite as a permeable reactive barrier material. Environ. Technol. 2023, 44, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Abdelhafez, A.A.; Li, J. Environmental Monitoring of Heavy Metal Status and Human Health Risk Assessment in the Agricultural Soils of the Jinxi River Area, China. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 952–971. [Google Scholar] [CrossRef]
- USEPA. Exposure Factors Handbook; Volume I General Factors; USEPA: Washington, DC, USA, 1997; EPA/600/P-96/002Fa. [Google Scholar]
- Abdelhafez, A.A.; Zhang, X.; Zhou, L.; Cai, M.; Cui, N.; Chen, G.; Zou, G.; Abbas, M.H.H.; Kenawy, M.H.M.; Ahmad, M.; et al. Eco-friendly production of biochar via conventional pyrolysis: Application of biochar and liquefied smoke for plant productivity and seed germination. Environ. Technol. Innov. 2021, 22, 101540. [Google Scholar] [CrossRef]
- ASTM E394-22; Standard Test Method for Iron in TRACE Quantities Using the 1,10-Phenanthroline Method. American Society for Testing and Materials. Available online: https://www.astm.org/e0394-22.html (accessed on 1 December 2023).
- Joo, J.-H.; Hassan, S.H.A.; Oh, S.-E. Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int. Biodeterior. Biodegrad. 2010, 64, 734–741. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption isotherms, kinetics, thermodynamics and desorption studies of 2,4,6-trichlorophenol on oil palm empty fruit bunch-based activated carbon. J. Hazard. Mater. 2008, 164, 473–478. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. The sorption of lead(II) ions on peat. Water Res. 1999, 33, 578–584. [Google Scholar] [CrossRef]
- Sawyer, C.N.; McCarty, P.L.; Parkin, G.F. (Eds.) Chemistry for Environmental Engineering and Science, 5th ed.; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Part 1, Rev. 1; FAO Irrigation and Drainage Paper 29; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994; Available online: https://www.fao.org/4/t0234e/t0234e00.htm (accessed on 1 December 2023).
- Sassane, A.; Touati, M. Metallic groundwater contamination assessment using the heavy metal pollution index: A case study of the Guelma plain alluvial groundwater, Northeast of Algeria. Sustain. Water Resour. Manag. 2024, 10, 30. [Google Scholar] [CrossRef]
- Van Groeningen, N.; ThomasArrigo, L.K.; Byrne, J.M.; Kappler, A.; Christl, I.; Kretzschmar, R. Interactions of ferrous iron with clay mineral surfaces during sorption and subsequent oxidation. Environ. Sci. Process. Impacts 2020, 22, 1355–1367. [Google Scholar] [CrossRef]
- EPA. National Recommended Water Quality Criteria—Aquatic Life Criteria Table; EPA: Washington, DC, USA, 2016. [Google Scholar]
- Ravikumar, P.; Aneesul Mehmood, M.; Somashekar, R.K. Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl. Water Sci. 2013, 3, 247–261. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- USEPA. EPA’s Exposure Factors Handbook (EFH); EPA/600/R-09/052F; National Center for Environmental Assessment Office of Research and Development: Washington, DC, USA, 2011. [Google Scholar]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Miretzky, P.; Bisinoti, M.C.; Jardim, W.F. Sorption of mercury (II) in Amazon soils from column studies. Chemosphere 2005, 60, 1583–1589. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Murray, M.J.; Ahuja, M.; Smith, L.A. Anxiety, depression, and irritability in children with autism relative to other neuropsychiatric disorders and typical development. Res. Autism Spectr. Disord. 2011, 5, 474–485. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Masoumi, H.; Ghaemi, A. An insight into the potential of dolomite powder as a sorbent in the elimination of heavy metals: A review. Case Stud. Chem. Environ. Eng. 2023, 7, 100276. [Google Scholar] [CrossRef]
- Silva, R.; Cadorin, L.; Rubio, J. Sulphate ions removal from an aqueous solution: I. Co-precipitation with hydrolysed aluminum-bearing salts. Miner. Eng. 2010, 23, 1220–1226. [Google Scholar] [CrossRef]
- Encina, E.R.; Distaso, M.; Klupp Taylor, R.N.; Peukert, W. Synthesis of Goethite α-FeOOH Particles by Air Oxidation of Ferrous Hydroxide Fe(OH)2 Suspensions: Insight on the Formation Mechanism. Cryst. Growth Des. 2015, 15, 194–203. [Google Scholar] [CrossRef]
- Zhu, K.; Hopwood, M.J.; Groenenberg, J.E.; Engel, A.; Achterberg, E.P.; Gledhill, M. Influence of pH and Dissolved Organic Matter on Iron Speciation and Apparent Iron Solubility in the Peruvian Shelf and Slope Region. Environ. Sci. Technol. 2021, 55, 9372–9383. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.K.; Lui, J.H.; Zhou, C.H.; Keeling, J.; Glasmacher, U.A. Structure, genesis and resources efficiency of dolomite: New insights and remaining enigmas. Chem. Geol. 2021, 573, 120191. [Google Scholar] [CrossRef]
- Walker, G.M.; Connor, G.; Allen, S.J. Kinetics of iron (II) removal from aqueous solution using activated dolomite. Open Chem. Eng. J. 2007, 1, 23–29. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Li, H.; Lin, S. Advances in the study of heavy metal adsorption from water and soil by modified biochar. Water 2022, 14, 3894. [Google Scholar] [CrossRef]
- Colombo, C.; Palumbo, G.; He, J.-Z.; Pinton, R.; Cesco, S. Review on iron availability in soil: Interaction of Fe minerals, plants, and microbes. J. Soils Sediments 2014, 14, 538–548. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Kamarzamann, F.F.; Abdullah, M.M.A.B.; Abd Rahim, S.Z.; Abdul Kadir, A.; Jamil, N.H.; Wan Ibrahim, W.M.; Victor Sandu, A. Hydroxyapatite/Dolomite alkaline activated material reaction in the formation of low temperature sintered ceramic as adsorbent materials. Constr. Build. Mater. 2022, 349, 128603. [Google Scholar] [CrossRef]





| Parameter | Description | Unit | Adult | Children |
|---|---|---|---|---|
| C | Contaminant concentration in water | μg L−1 | -- | -- |
| IR | Ingestion rate | L day−1 | 2.2 | 1.8 |
| EF | Exposure frequency | L day−1 | 350 | |
| ED | Exposure duration | Years | 30.0 | 6.0 |
| BW | Body weight | Kg | 70.0 | 15 |
| AT | Average time | Days | 10,500 | 2100 |
| SA | Exposure skin area | cm2 | 28,000 | 6600 |
| ET | Exposure time | h day−1 | 0.6 | 1.0 |
| CF | Unit conservation factor | L/cm3 | 0.001 | |
| Kp, Dermal permeability coefficient | cm h−1 | 0.001 | ||
| Fe oral and dermal reference dose | μg kg−1 day−1 | 700,000 | ||
| Village | Well | Depth, m | pH | EC dS m−1 | Turbidity, NTU |
|---|---|---|---|---|---|
| El-Monira | El-Monira 2 old (M1) | 706 | 7.07 ± 0.02 | 0.60 ± 0.02 | 16.4 ± 0.12 |
| El-Monira- country (M2) | 550 | 7.19 ± 0.03 | 0.69 ± 0.02 | 2.36 ± 0.31 | |
| El-Monira5 (M3) | 304 | 7.05 ± 0.04 | 0.67 ± 0.0 | 7.03 ± 1.80 | |
| El-Monira- Om ELkosour (M4) | 290 | 7.34 ± 0.02 | 0.46 ± 0.0 | 5.31 ± 0.10 | |
| El-Monira- 1/9 (M5) | 386 | 7.03 ± 0.0 | 0.66 ± 0.01 | 12.55 ± 0.92 | |
| El-Monira-surface 1 (MS1) | <100 | 6.81 ± 0.01 | 1.07 ± 0.0 | 29.55 ± 1.62 | |
| El-Monira- surface 2 (MS2) | <100 | 7.48 ± 0.11 | 0.55 ± 0.0 | 2.19 ± 0.16 | |
| El-Monira- drinking water (MD) | -- | 7.19 ± 0.02 | 0.96 ± 0.0 | 5.90 ± 0.21 | |
| El-Sherka | El-Sherka- 55 | 703 | 7.15 ± 0.01 | 0.54 ± 0.0 | 10.11 ± 0.42 |
| El-Sherka- 4 | 696 | 7.04 ± 0.002 | 0.51 ± 0.01 | 3.91 ± 0.11 | |
| El-Sherka- 8 | 643 | 7.80 ± 0.001 | 0.56 ± 0.04 | 1.28 ± 0.06 | |
| El-Sherka- 21/34 | 405 | 7.19 ± 0.00 | 0.72 ± 0.0 | 1.40 ± 0.08 | |
| El-Sherka- 5 | 332 | 7.08 ± 0.00 | 0.47 ± 0.0 | 7.17 ± 1.03 | |
| El-Sherka- 7 | 302 | 7.42 ± 0.05 | 0.723 ± 0.07 | 0.805 ± 0.06 | |
| El-Sherka- surface 1 | <100 | 7.13 ± 0.02 | 1.63 ± 0.0 | 21.60 ± 3.40 | |
| El-Sherka- surface 2 | <100 | 7.33 ± 0.00 | 0.68 ± 0.01 | 1.80 ± 0.57 | |
| El-Sherka- surface 3 | <100 | 7.22 ± 0.0 | 0.62 ± 0.0 | 1.32 ± 0.37 | |
| El-Sherka- drinking water | -- | 7.78 ± 0.02 | 0.72 ± 0.39 | 0.48 ± 0.08 | |
| El-Kharga | El-Kharga- 41 | 762 | 6.78 ± 0.06 | 0.36 ± 0.0 | 16.35 ± 3.61 |
| El-Kharga- 38 | 740 | 6.94 ± 0.06 | 0.35 ± 0.0 | 26.90 ± 0.71 | |
| El-Kharga- 12 | 509 | 6.95 ± 0.01 | 0.31 ± 0.02 | 34.25 ± 1.06 | |
| El-Kharga- 23 | 322 | 7.03 ± 0.01 | 0.44 ± 0.01 | 28.60 ± 0.71 | |
| El-Kharga- 24 | 283 | 6.90 ± 0.08 | 0.48 ± 0.01 | 39.05 ± 3.48 | |
| El-Kharga- 26 | 278 | 6.82 ± 0.02 | 0.60 ± 0.01 | 36.90 ± 0.28 | |
| El-Kharga- surface 1 | <100 | 6.99 ± 0.09 | 0.51 ± 0.0 | 24.90 ± 0.14 | |
| El-Kharga- surface 2 | <100 | 6.42 ± 0.03 | 1.76 ± 0.02 | 69.70 ± 1.98 | |
| El-Kharga- surface 3 | <100 | 6.64 ± 0.03 | 1.70 ± 0.01 | 80.15 ± 2.47 | |
| El-Kharga- drinking water | -- | 7.04 ± 0.09 | 0.82 ± 0.29 | 0.78 ± 0.07 | |
| Porsaied | Porsaied- Nassir new south | 573 | 6.75 ± 0.05 | 0.80 ± 0.0 | 34.70 ± 1.27 |
| Porsaied- Nassir new north | 512 | 6.76 ± 0.04 | 0.75 ± 0.02 | 44.852.19 | |
| Porsaied- Nassir tourist | 563 | 7.09 ± 0.04 | 0.48 ± 0.02 | 19.10 ± 0.14 | |
| Porsaied- new | 323 | 7.18 ± 0.01 | 0.32 ± 0.0 | 6.72 ± 0.0 | |
| Porsaied- surface | <100 | 6.78 ± 0.05 | 0.34 ± 0.0 | 16.45 ± 1.91 | |
| Porsaied- drinking water | -- | 7.265 ± 0.08 | 0.34 ± 0.0 | 0.89 ± 0.29 | |
| Ganah | Ganah- 5 | 800 | 6.82 ± 0.03 | 0.54 ± 0.0 | 42.80 ± 0.57 |
| Ganah- 2/4 | 804 | 6.85 ± 0.02 | 0.39 ± 0.0 | 26.35 ± 6.15 | |
| Ganah- 11/16 | 791 | 7.43 ± 0.03 | 0.38 ± 0.0 | 7.93 ± 0.30 | |
| Ganah- 20 country | 453 | 7.04 ± 0.15 | 0.61 ± 0.0 | 25.5 ± 14.42 | |
| Ganah- surface | <100 | 7.04 ± 0.04 | 0.35 ± 0.0 | 17.95 ± 1.20 | |
| Ganah- drinking water | -- | 7.32 ± 0.04 | 0.34 ± 0.0 | 0.77 ± 0.02 | |
| Bolak | Bolak- 3 new | 813 | 7.14 ± 0.03 | 0.47 ± 0.02 | 10.00 ± 0.57 |
| Bolak- 25 | 782 | 6.75 ± 0.04 | 0.91 ± 0.04 | 34.55 ± 2.76 | |
| Bolak- 20 | 633 | 6.65 ± 0.06 | 0.96 ± 0.0 | 18.53 ± 14.81 | |
| Bolak- 4/23 | 424 | 6.71 ± 0.01 | 0.94 ± 0.0 | 47.45 ± 2.90 | |
| Bolak- 6 | 346 | 6.87 ± 0.01 | 0.65 ± 0.0 | 27.6 ± 3.25 | |
| Bolk- palm relief | 150 | 6.76 ± 0.0 | 0.73 ± 0.03 | 31.4 ± 2.83 | |
| Bolak- surface 1 | <100 | 6.60 ± 0.30 | 1.15 ± 0.04 | 5.82 ± 3.27 | |
| Bolak- surface 2 | <100 | 6.87 ± 0.04 | 1.00 ± 0.01 | 12.1 ± 0.71 | |
| Bolak- drinking water | -- | 6.89 ± 0.01 | 1.05 ± 0.0 | 0.82 ± 0.04 | |
| Germashen | Germashen- 8 | 495 | 6.95 ± 0.02 | 0.91 ± 0.0 | 35.1 ± 0.71 |
| Germashen- 25 | 554 | 6.92 ± 0.03 | 0.84 ± 0.03 | 30.3 ± 2.69 | |
| Germashen- 1 | 495 | 7.09 ± 0.02 | 0.55 ± 0.02 | 15.95 ± 0.92 | |
| Germashen- 2G | 532 | 7.10 ± 0.04 | 0.60 ± 0.0 | 9.35 ± 3.05 | |
| Germashen- 16 | 573 | 6.91 ± 0.06 | 0.54 ± 0.01 | 23.3 ± 0.14 | |
| Germashen- surface | <100 | 6.80 ± 0.0 | 0.69 ± 0.0 | 21.15 ± 2.62 | |
| Germashen- drinking water | -- | 6.89 ± 0.12 | 0.64 ± 0.01 | 0.48 ± 0.02 | |
| WHO standard (drinking) | -- | 6.5–8.5 | <1.5 | -- | |
| FAO standard (irrigation) | -- | 6.5–8.5 | <3.0 | -- | |
| Village | Well | HCO3− | Cl− | SO4−− | Na+ | Ca++ | Mg++ | K+ |
|---|---|---|---|---|---|---|---|---|
| mmolc L−1 | ||||||||
| El-Monira | El-Monira- 2 | 0.70 ± 0.14 | 1.40 ± 0.28 | 6.48 ± 0.60 | 3.07 ± 0.03 | 2.83 ± 0.60 | 1.88 ± 0.39 | 0.82 ± 0.00 |
| El-Monira- 5 | 0.70 ± 0.14 | 1.60 ± 0.57 | 6.14 ± 0.55 | 3.20 ± 0.09 | 3.43 ± 0.11 | 1.00 ± 0.35 | 0.82 ± 0.00 | |
| Om eLksor | 1.30 ± 0.14 | 0.80 ± 0.0 | 2.74 ± 0.46 | 2.20 ± 0.03 | 1.10 ± 0.42 | 0.70 ± 0.14 | 0.84 ± 0.00 | |
| Monira Balad | 0.80 ± 0.0 | 1.40 ± 0.28 | 4.63 ± 1.29 | 3.35 ± 0.00 | 1.30 ± 0.14 | 1.40 ± 1.13 | 0.78 ± 0.02 | |
| Monira 1-9 | 0.50 ± 0.14 | 1.40 ± 0.28 | 3.88 ± 0.19 | 3.37 ± 0.03 | 1.40 ± 0.00 | 0.20 ± 0.00 | 0.81 ± 0.02 | |
| Monira surface- 1 | 0.80 ± 0.0 | 1.20 ± 0.0 | 8.25 ± 0.74 | 2.98 ± 0.03 | 4.33 ± 0.04 | 2.18 ± 0.67 | 0.77 ± 0.00 | |
| Monira surface- 2 | 0.90 ± 0.14 | 1.20 ± 0.57 | 3.84 ± 0.93 | 3.00 ± 0.06 | 1.98 ± 0.10 | 0.43 ± 0.32 | 0.54 ± 0.00 | |
| Monira drinking water | 0.80 ± 0.0 | 2.00 ± 0.57 | 4.65 ± 0.74 | 4.50 ± 0.03 | 1.63 ± 0.04 | 0.28 ± 0.11 | 1.05 ± 0.00 | |
| El-Sherka | El-Sherka- 5 | 0.70 ± 0.14 | 0.80 ± 0.0 | 5.75 ± 0.69 | 2.22 ± 0.00 | 3.03 ± 0.04 | 1.10 ± 0.71 | 0.91 ± 0.13 |
| El-Sherka- 55 | 0.90 ± 0.14 | 1.20 ± 0.0 | 5.65 ± 0.18 | 2.76 ± 0.03 | 3.20 ± 0.07 | 1.00 ± 0.28 | 0.79 ± 0.00 | |
| El-Sherka- 4 | 0.60 ± 0.0 | 1.00 ± 0.28 | 5.51 ± 0.04 | 2.57 ± 0.00 | 3.13 ± 0.04 | 0.60 ± 0.28 | 0.82 ± 0.00 | |
| El-Sherka- 7 | 1.20 ± 0.0 | 1.40 ± 0.28 | 5.21 ± 0.14 | 3.52 ± 0.00 | 3.15 ± 0.00 | 0.50 ± 0.42 | 0.64 ± 0.00 | |
| El-Sherka- 8 | 0.90 ± 0.14 | 1.20 ± 0.0 | 5.18 ± 0.18 | 2.44 ± 0.00 | 3.28 ± 0.04 | 0.80 ± 0.28 | 0.77 ± 0.00 | |
| El-Sherka- 21-34 | 1.00 ± 0.57 | 1.60 ± 0.0 | 5.62 ± 0.31 | 3.74 ± 0.06 | 3.30 ± 0.07 | 0.50 ± 0.14 | 0.68 ± 0.02 | |
| El-Sherka surface- G2 | 1.00 ± 0.28 | 3.60 ± 1.13 | 9.41 ± 1.38 | 5.92 ± 0.25 | 5.45 ± 0.21 | 1.90 ± 0.99 | 0.74 ± 0.00 | |
| El-Sherka surface- H2 | 0.80 ± 0.0 | 1.60 ± 0.0 | 5.67 ± 0.67 | 3.61 ± 0.00 | 3.23 ± 0.04 | 0.70 ± 0.71 | 0.54 ± 0.00 | |
| El-Sherka surface- I 2 | 2.10 ± 0.14 | 8.00 ± 0.0 | 26.65 ± 0.18 | 11.31 ± 0.0 | 14.43 ± 0.53 | 9.40 ± 0.57 | 1.61 ± 0.00 | |
| El-Sherka drinking water | 0.70 ± 0.14 | 1.60 ± 0.0 | 6.65 ± 0.19 | 3.55 ± 0.03 | 3.58 ± 0.04 | 1.00 ± 0.28 | 0.83 ± 0.02 | |
| El-Kharga | El-Kharga- 26 | 0.90 ± 0.14 | 1.00 ± 0.28 | 5.32 ± 1.36 | 1.09 ± 0.00 | 3.73 ± 0.04 | 1.35 ± 0.4 | 1.06 ± 0.02 |
| El-Kharga- 12 | 0.50 ± 0.14 | 0.80 ± 0.57 | 3.65 ± 0.42 | 1.13 ± 0.00 | 2.25 ± 0.00 | 0.80 ± 0.0 | 0.77 ± 0.00 | |
| El-Kharga- 24 | 0.80 ± 0.28 | 1.40 ± 0.28 | 4.22 ± 0.89 | 1.02 ± 0.03 | 3.28 ± 0.04 | 0.98 ± 0.08 | 1.15 ± 0.00 | |
| El-Kharga- 38 | 0.50 ± 0.14 | 1.60 ± 0.57 | 3.57 ± 0.73 | 1.26 ± 0.00 | 2.38 ± 0.04 | 1.23 ± 0.04 | 0.81 ± 0.02 | |
| El-Kharga- 41 | 0.30 ± 0.14 | 1.00 ± 0.28 | 3.57 ± 0.65 | 1.24 ± 0.03 | 2.33 ± 0.04 | 0.50 ± 0.14 | 0.81 ± 0.02 | |
| El-Kharga- 23 | 0.70 ± 0.42 | 1.80 ± 0.28 | 3.47 ± 0.34 | 0.94 ± 0.03 | 2.98 ± 0.04 | 1.00 ± 0.28 | 1.06 ± 0.01 | |
| El-Kharga surface- D2 | 0.90 ± 0.14 | 1.20 ± 0.57 | 5.03 ± 0.70 | 1.11 ± 0.03 | 3.43 ± 0.04 | 1.60 ± 0.28 | 1.00 ± 0.00 | |
| El-Kharga surface- E2 | 0.50 ± 0.14 | 2.40 ± 0.57 | 10.97 ± 0.72 | 5.98 ± 0.09 | 5.23 ± 0.03 | 2.10 ± 0.14 | 0.56 ± 0.00 | |
| El-Kharga surface- F2 | 0.70 ± 0.42 | 2.60 ± 0.28 | 11.01 ± 0.29 | 6.18 ± 0.06 | 5.10 ± 0.07 | 2.50 ± 0.42 | 0.54 ± 0.00 | |
| El-Kharga drinking water | 0.70 ± 0.14 | 1.40 ± 0.28 | 3.39 ± 0.39 | 1.07 ± 0.03 | 2.38 ± 0.11 | 1.10 ± 0.14 | 0.95 ± 0.04 | |
| Porsaied | Porsaied Nasir new sud | 0.50 ± 0.14 | 1.80 ± 0.28 | 6.69 ± 0.59 | 2.02 ± 0.03 | 4.50 ± 0.21 | 1.30 ± 0.65 | 1.16 ± 0.01 |
| Porsaied Nasir new south | 0.40 ± 0.0 | 1.60 ± 0.0 | 6.95 ± 1.26 | 1.96 ± 0.00 | 4.33 ± 0.04 | 1.50 ± 0.09 | 1.16 ± 0.02 | |
| Porsaied tourism | 0.90 ± 0.14 | 1.20 ± 0.0 | 5.24 ± 0.55 | 1.48 ± 0.00 | 3.23 ± 0.04 | 1.70 ± 0.07 | 0.93 ± 0.03 | |
| Porsaied new | 0.60 ± 0.0 | 2.60 ± 0.28 | 1.77 ± 0.48 | 1.22 ± 0.00 | 2.48 ± 0.03 | 0.50 ± 0.14 | 0.78 ± 0.02 | |
| Porsaied surface | 0.60 ± 0.0 | 9.80 ± 1.98 | 17.35 ± 2.68 | 7.07 ± 0.95 | 10.88 ± 1.52 | 6.60 ± 1.70 | 3.21 ± 0.49 | |
| Porsaied drinking water | 0.30 ± 0.13 | 0.80 ± 0.0 | 3.76 ± 0.28 | 1.24 ± 0.03 | 2.38 ± 0.04 | 0.30 ± 0.14 | 0.95 ± 0.0 | |
| Ganah | Ganah 5 | 0.80 ± 0.0 | 1.23 ± 0.02 | 4.99 ± 0.18 | 1.61 ± 0.0 | 3.38 ± 0.04 | 1.10 ± 0.14 | 0.93 ± 0.01 |
| Ganah 2-4 | 0.50 ± 0.14 | 1.25 ± 0.01 | 3.31 ± 0.13 | 1.57 ± 0.0 | 2.50 ± 0.00 | 0.20 ± 0.00 | 0.79 ± 0.00 | |
| Ganah 11-16 | 1.30 ± 0.14 | 1.51 ± 0.08 | 2.22 ± 0.25 | 1.39 ± 0.06 | 2.53 ± 0.03 | 0.30 ± 0.01 | 0.81 ± 0.02 | |
| Ganah 20 | 0.60 ± 0.0 | 1.30 ± 0.01 | 5.75 ± 1.06 | 1.26 ± 0.00 | 3.68 ± 0.04 | 1.70 ± 0.09 | 1.01 ± 0.02 | |
| Ganah-surface | 0.40 ± 0.0 | 1.24 ± 0.14 | 4.11 ± 1.42 | 1.28 ± 0.03 | 2.55 ± 0.00 | 1.10 ± 0.03 | 0.82 ± 0.04 | |
| Ganah drinking water | 0.60 ± 0.28 | 1.31 ± 0.02 | 3.11 ± 0.05 | 1.22 ± 0.00 | 2.45 ± 0.07 | 0.40 ± 0.02 | 0.95 ± 0.00 | |
| Bolak | Bolak 3 new | 0.50 ± 0.14 | 1.20 ± 0.0 | 4.94 ± 0.14 | 1.72 ± 0.03 | 3.13 ± 0.04 | 1.00 ± 0.28 | 0.79 ± 0.00 |
| Bolak dates | 0.70 ± 0.13 | 2.20 ± 0.28 | 5.57 ± 0.08 | 2.07 ± 0.03 | 4.48 ± 0.03 | 0.80 ± 0.21 | 1.13 ± 0.00 | |
| Bolak 25 | 0.50 ± 0.12 | 2.60 ± 0.25 | 8.56 ± 0.11 | 2.76 ± 0.02 | 5.00 ± 0.00 | 2.80 ± 0.00 | 1.10 ± 0.00 | |
| Bolak 4-32 | 0.80 ± 0.28 | 2.40 ± 0.0 | 8.23 ± 0.39 | 2.89 ± 0.09 | 5.03 ± 0.18 | 2.50 ± 0.42 | 1.01 ± 0.02 | |
| Bolak 6 | 0.20 ± 0.0 | 1.80 ± 0.23 | 7.01 ± 1.06 | 2.11 ± 0.01 | 4.03 ± 0.04 | 1.90 ± 0.71 | 0.97 ± 0.00 | |
| Bolak 20 | 0.50 ± 0.11 | 2.80 ± 0.57 | 7.60 ± 2.14 | 2.94 ± 0.01 | 5.05 ± 0.07 | 1.80 ± 0.17 | 1.11 ± 0.02 | |
| Bolak surface 1 | 0.70 ± 0.10 | 3.40 ± 0.20 | 8.35 ± 0.29 | 3.59 ± 0.09 | 5.38 ± 0.03 | 2.60 ± 0.00 | 0.88 ± 0.02 | |
| Bolak surface 2 | 0.70 ± 0.09 | 3.20 ± 0.52 | 7.47 ± 1.72 | 3.20 ± 0.02 | 5.03 ± 0.04 | 2.20 ± 0.14 | 0.95 ± 0.18 | |
| Bolak drinking water | 0.90 ± 0.11 | 3.40 ± 0.21 | 7.90 ± 0.68 | 2.89 ± 0.03 | 5.25 ± 0.14 | 2.70 ± 0.42 | 1.36 ± 0.00 | |
| Germashen | Germashen 8 | 0.40 ± 0.0 | 3.00 ± 0.26 | 9.90 ± 0.04 | 2.94 ± 0.08 | 4.98 ± 0.46 | 4.40 ± 0.28 | 0.98 ± 0.05 |
| Germashen 25 | 0.50 ± 0.14 | 2.80 ± 0.53 | 8.68 ± 0.58 | 2.65 ± 0.0 | 4.63 ± 0.04 | 3.90 ± 0.09 | 0.81 ± 0.02 | |
| Germashen 1 | 0.40 ± 0.0 | 1.60 ± 0.0 | 6.07 ± 0.25 | 1.98 ± 0.03 | 3.45 ± 0.0 | 1.80 ± 0.28 | 0.84 ± 0.0 | |
| Germashen 2 G | 0.70 ± 0.13 | 1.80 ± 0.28 | 5.71 ± 0.88 | 2.54 ± 0.02 | 3.50 ± 0.0 | 1.30 ± 0.42 | 0.87 ± 0.0 | |
| Germashen 16 | 0.60 ± 0.28 | 1.60 ± 0.48 | 5.71 ± 0.44 | 2.28 ± 0.01 | 3.30 ± 0.0 | 1.50 ± 0.14 | 0.83 ± 0.02 | |
| Germashen- Surface | 0.80 ± 0.25 | 2.20 ± 0.28 | 6.32 ± 0.07 | 2.33 ± 0.02 | 4.18 ± 0.04 | 2.00 ± 0.0 | 0.82 ± 0.0 | |
| Germashen drinking water | 0.60 ± 0.20 | 2.00 ± 0.0 | 6.05 ± 0.0 | 2.13 ± 0.0 | 3.75 ± 0.0 | 1.80 ± 0.28 | 0.97 ± 0.0 | |
| 6.56 | 7.05 | 0.38 | 8.70 | 3.7 | 2.057 | 0.31 | ||
| 2.0 | <3.0 | <4.0 | <3.0 | 20 | 5.02 | 2.0 |
| Sorbents | Pseudo-1st | Pseudo-2nd | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | R² | K1/K2 | qe (mg g−1) | Slope | Intercept | R² | K1/K2 | qe (mg g−1) | |
| Biochar | 0.0117 | 1.834 | 0.914 | −0.03 | 68.23 | 0.009 | 0.1274 | 0.990 | 0.00 | 106.38 |
| Dolomite | 0.0289 | 1.5153 | 0.841 | −0.07 | 32.76 | 0.01 | 0.016 | 0.999 | 0.006 | 101.01 |
| Calcite | 0.0215 | 0.729 | 0.584 | −0.05 | 5.36 | 0.010 | 0.0038 | 1.000 | 0.026 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abed, E.A.M.; Alaboudi, K.A.N.; Abbas, M.H.H.; Attia, T.M.S.; Abdelhafez, A.A. Iron Contamination in Groundwater: Risk Assessment and Remediation Techniques in Egypt’s New Valley. Water 2024, 16, 1834. https://doi.org/10.3390/w16131834
Abed EAM, Alaboudi KAN, Abbas MHH, Attia TMS, Abdelhafez AA. Iron Contamination in Groundwater: Risk Assessment and Remediation Techniques in Egypt’s New Valley. Water. 2024; 16(13):1834. https://doi.org/10.3390/w16131834
Chicago/Turabian StyleAbed, Ehdaa A. M., Khalid A. N. Alaboudi, Mohamed H. H. Abbas, Tamer M. S. Attia, and Ahmed A. Abdelhafez. 2024. "Iron Contamination in Groundwater: Risk Assessment and Remediation Techniques in Egypt’s New Valley" Water 16, no. 13: 1834. https://doi.org/10.3390/w16131834
APA StyleAbed, E. A. M., Alaboudi, K. A. N., Abbas, M. H. H., Attia, T. M. S., & Abdelhafez, A. A. (2024). Iron Contamination in Groundwater: Risk Assessment and Remediation Techniques in Egypt’s New Valley. Water, 16(13), 1834. https://doi.org/10.3390/w16131834







