A CFD Study on Optimization of Mass Transfer and Light Distribution in a Photocatalytic Reactor with Immobilized Photocatalyst on Spheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
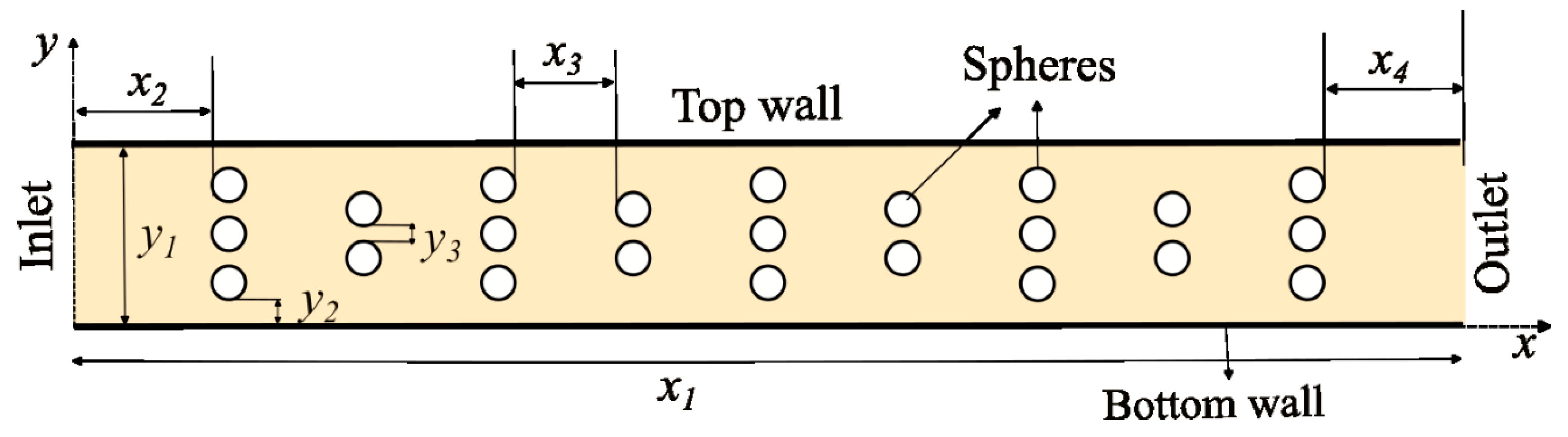
2.2. Computational Domain
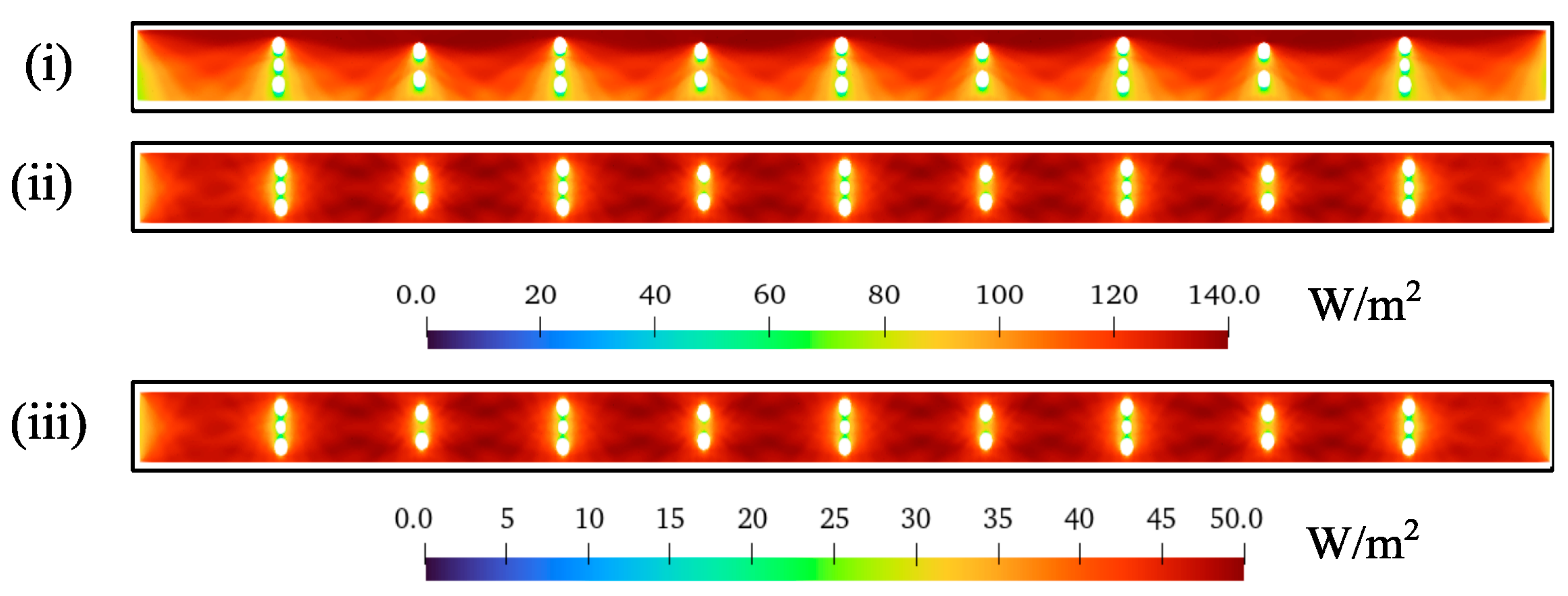
- (i)
- A channel with spheres and the horizontal distances and are halved.
- (ii)
- A channel with larger spheres i.e., middle sphere in three vertical sphere layer is of diameter and all others are of .
- (iii)
- Plain channel with no spheres, and hence, no photocatalyst is present.
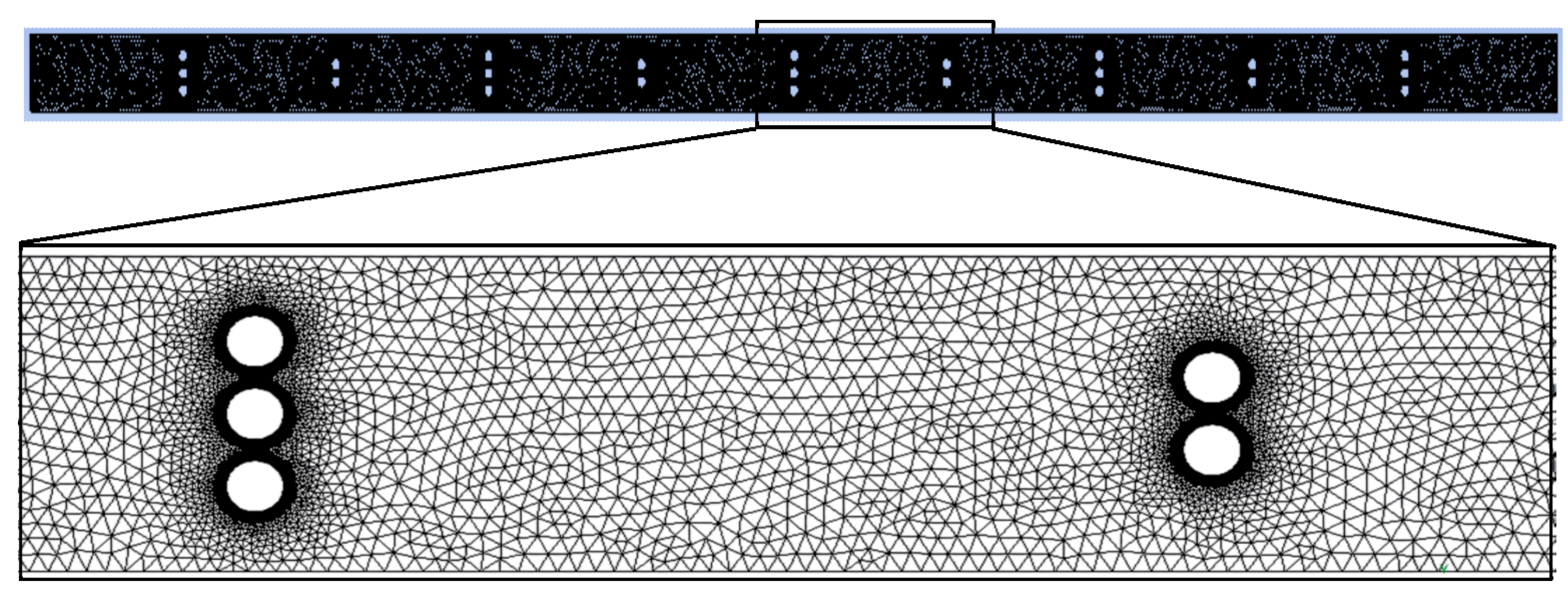
2.3. Meshing
2.4. Governing Equations
2.5. Boundary and Initial Conditions
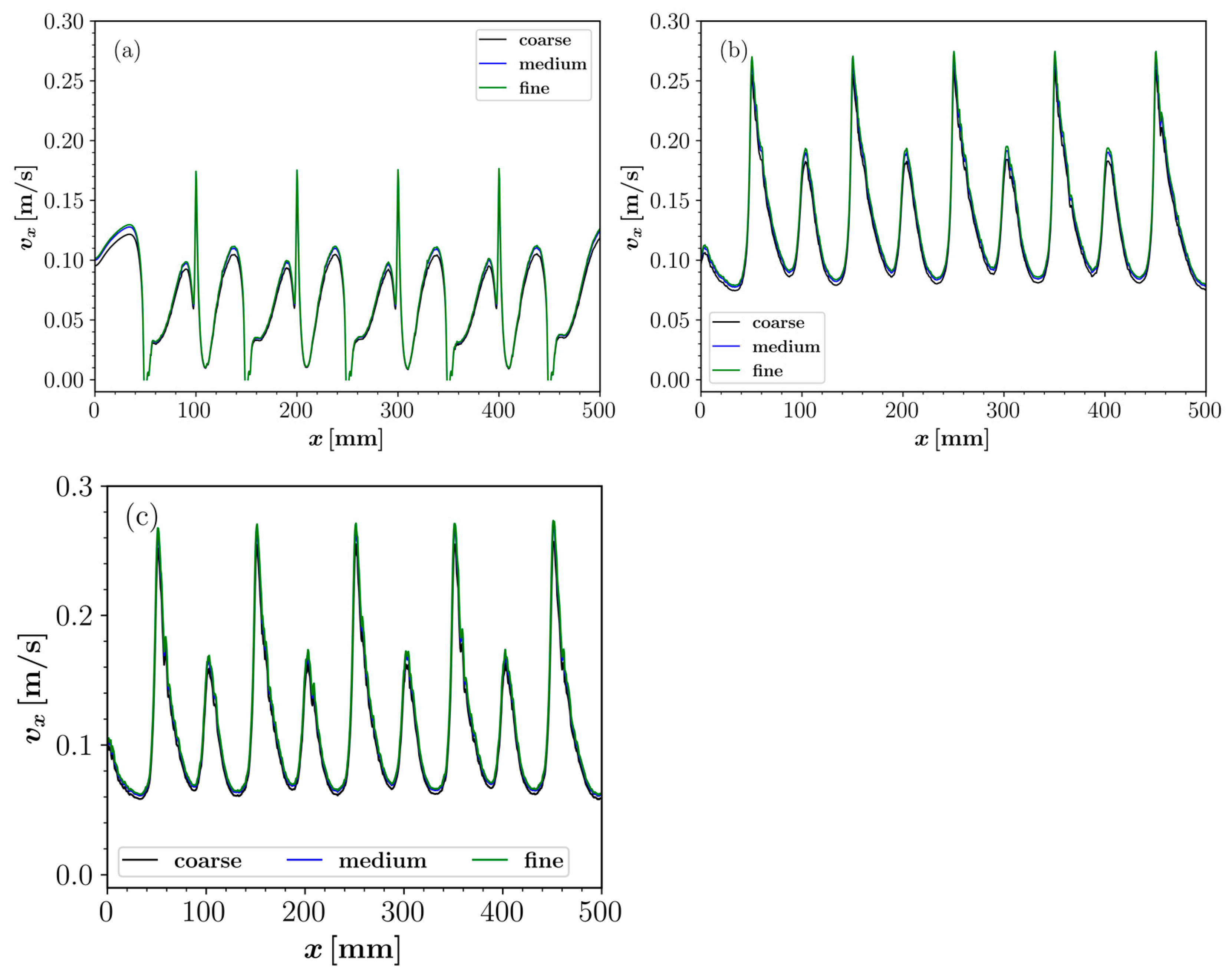
2.6. Mesh Convergence Study
3. Results
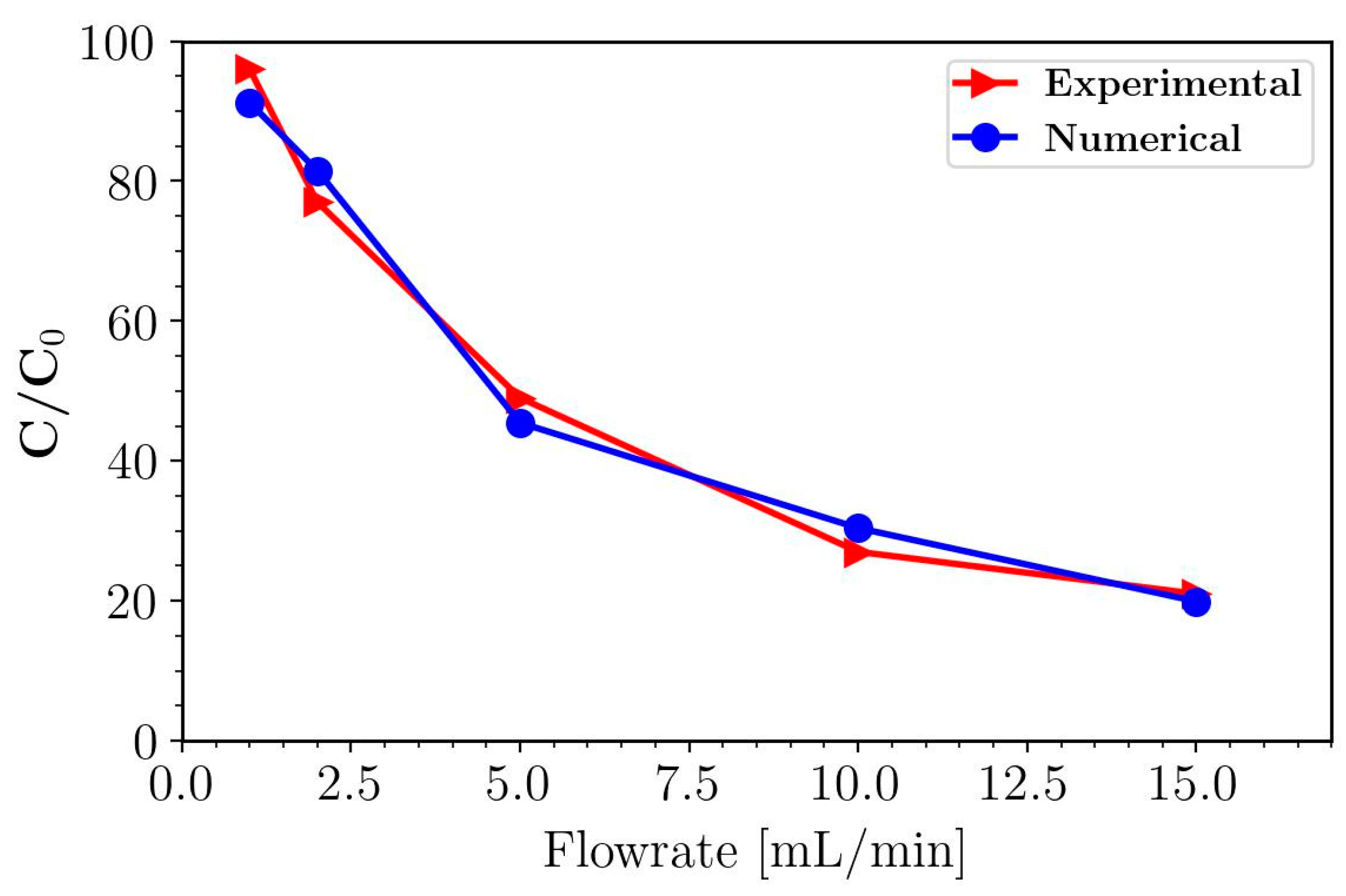
3.1. Validation with Experimental Data
3.2. Effect of Light Distribution
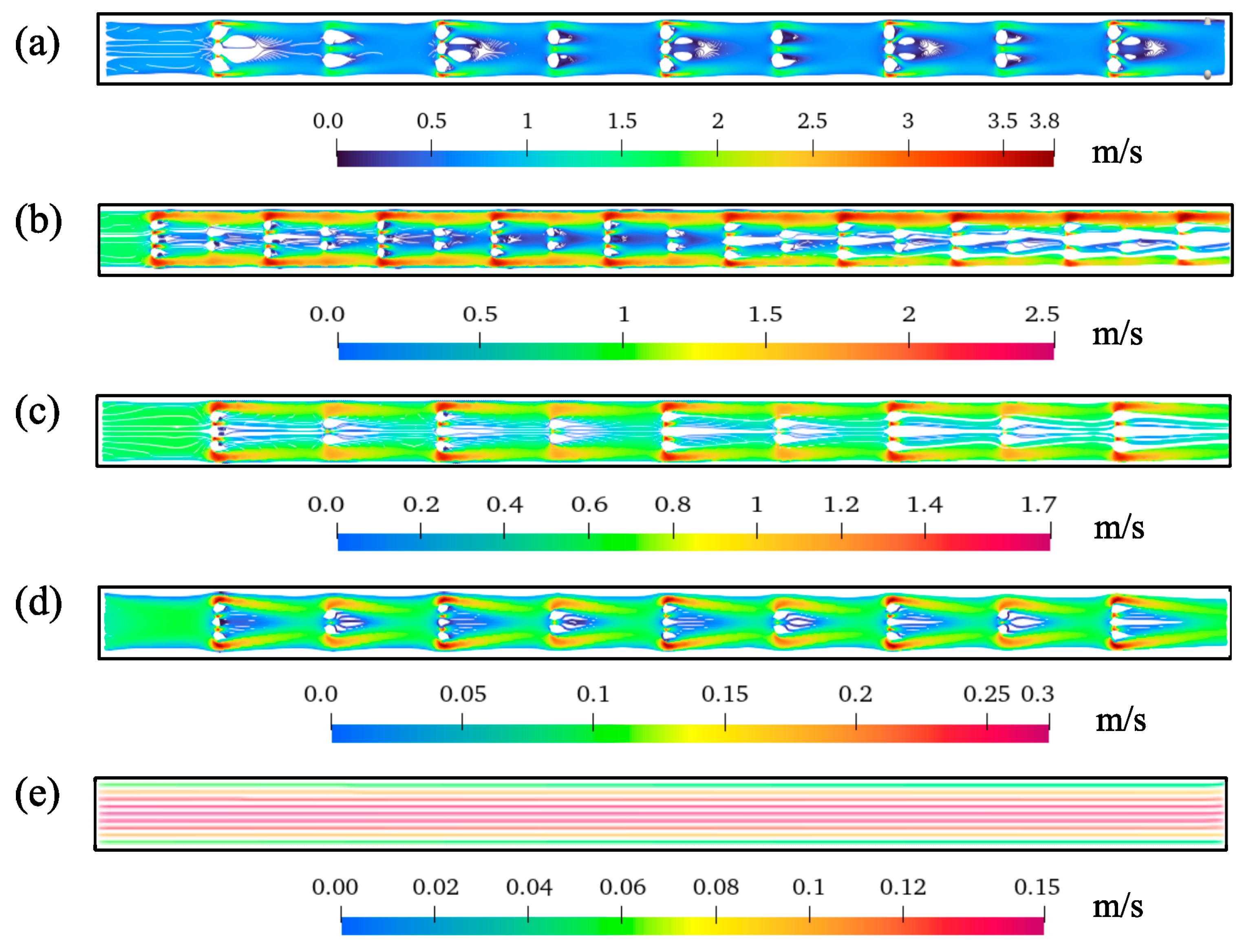
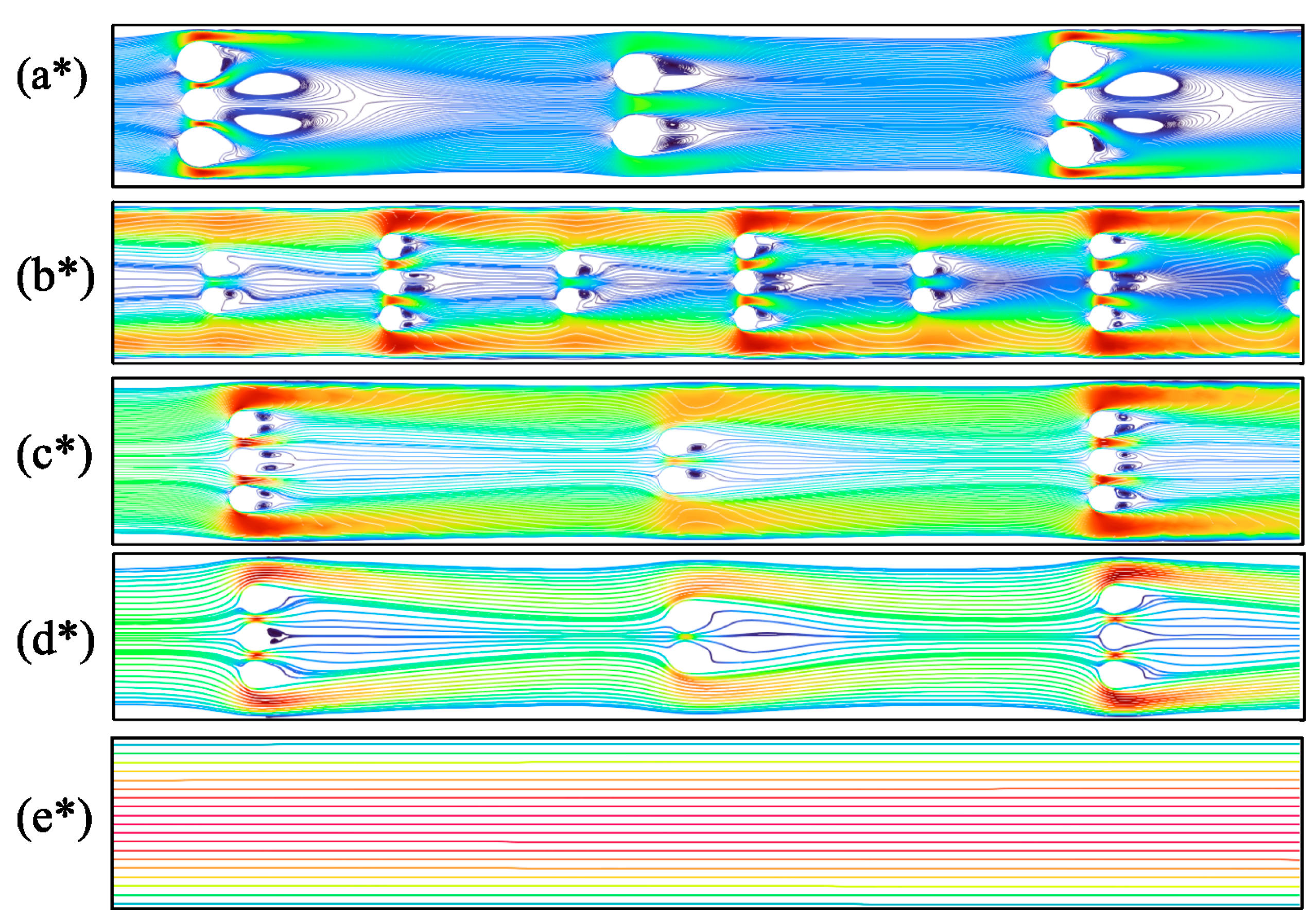
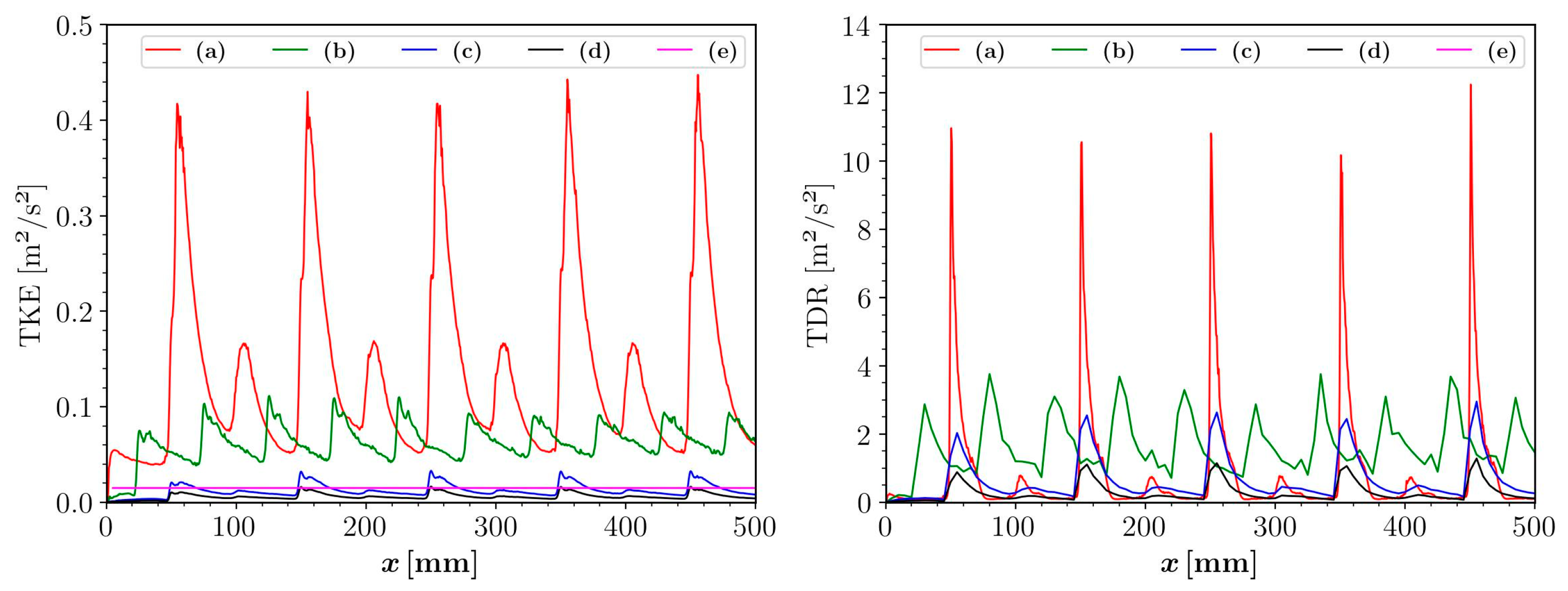
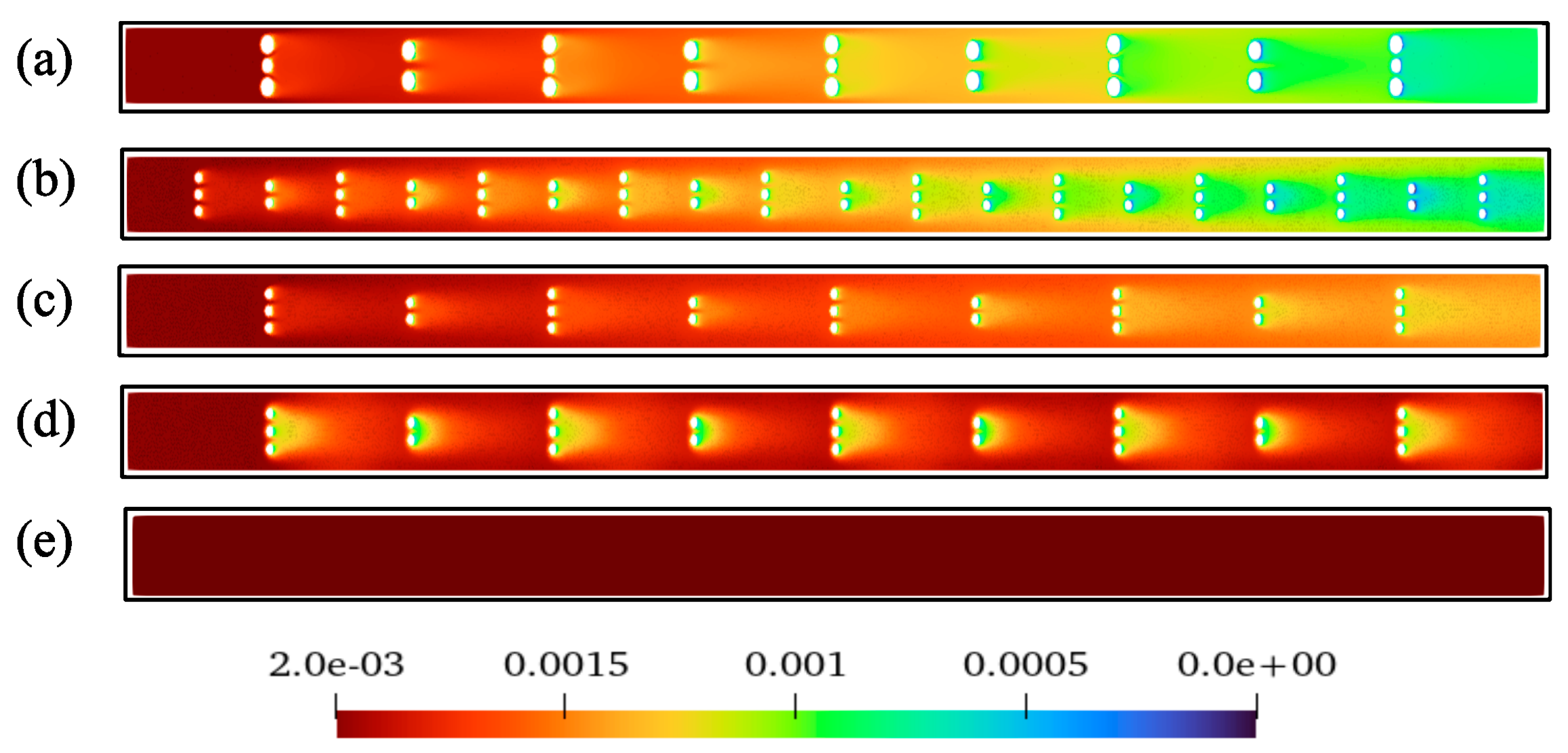
3.3. Effect on Flow Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent Developments in Photocatalytic Water Treatment Technology: A Review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H. Catalytic Ozonation for Water and Wastewater Treatment: Recent Advances and Perspective. Sci. Total Environ. 2020, 704, 135249. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A Review of the Existing and Emerging Technologies in the Combination of AOPs and Biological Processes in Industrial Textile Wastewater Treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of Antibiotic Wastewater by Coupled Photocatalytic and Persulfate Oxidation System: A Critical Review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive Removal of Antibiotics from Water and Wastewater: Progress and Challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Matoh, L.; Žener, B.; Kovačić, M.; Kušić, H.; Arčon, I.; Levstek, M.; Lavrenčič Štangar, U. Photocatalytic Sol-Gel/P25 TiO2 Coatings for Water Treatment: Degradation of 7 Selected Pharmaceuticals. Ceram. Int. 2023, 49, 24395–24406. [Google Scholar] [CrossRef]
- Žener, B.; Matoh, L.; Rodič, P.; Škufca, D.; Heath, E.; Lavrenčič Štangar, U. Removal of 18 Bisphenols Co-Present in Aqueous Media by Effectively Immobilized Titania Photocatalyst. J. Environ. Chem. Eng. 2021, 9, 106814. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of Water and Wastewater by Advanced Oxidation Processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Akyol, A.; Bayramoglu, M. The Degradation of an Azo Dye in a Batch Slurry Photocatalytic Reactor. Chem. Eng. Process. Process Intensif. 2008, 47, 2150–2156. [Google Scholar] [CrossRef]
- Li Puma, G.; Yue, P.L. Modelling and Design of Thin-Film Slurry Photocatalytic Reactors for Water Purification. Chem. Eng. Sci. 2003, 58, 2269–2281. [Google Scholar] [CrossRef]
- Shan, A.Y.; Ghazi, T.I.M.; Rashid, S.A. Immobilisation of Titanium Dioxide onto Supporting Materials in Heterogeneous Photocatalysis: A Review. Appl. Catal. A Gen. 2010, 389, 1–8. [Google Scholar] [CrossRef]
- Protti, S.; Ravelli, D.; Fagnoni, M. Design Consideration of Continuous-Flow Photoreactors. In Photochemical Processes in Continuous-Flow Reactors; World Scientific: Amsterdam, The Netherlands, 2017; Volume 23, pp. 1–36. [Google Scholar]
- Cao, H.; Ye, C.; Yan, X.-F.; Liu, X.-N.; Wang, X.-K. Experimental Investigation of Turbulent Flows through a Boulder Array Placed on a Permeable Bed. Water Supply 2020, 20, 1281–1293. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Lyu, L.-M.; Lin, C.-Y.; Lee, G.-C.; Hsiao, K.-Y.; Lu, M.-Y. Improved Mass-Transfer Enhances Photo-Driven Dye Degradation and H2 Evolution over a Few-Layer WS2/ZnO Heterostructure. ACS Omega 2022, 7, 2217–2223. [Google Scholar] [CrossRef]
- Amiri, H.; Ayati, B.; Ganjidoust, H. Mass Transfer Phenomenon in Photocatalytic Cascade Disc Reactor: Effects of Artificial Roughness and Flow Rate. Chem. Eng. Process. Process Intensif. 2017, 116, 48–59. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laîné, J.-M. Oxidation of Organic Contaminants in a Rotating Disk Photocatalytic Reactor: Reaction Kinetics in the Liquid Phase and the Role of Mass Transfer Based on the Dimensionless Damköhler Number. Appl. Catal. B Environ. 2002, 38, 1–16. [Google Scholar] [CrossRef]
- Pareek, V.; Chong, S.; Tadé, M.; Adesina, A.A. Light Intensity Distribution in Heterogenous Photocatalytic Reactors. Asia-Pacific J. Chem. Eng. 2008, 3, 171–201. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Ang, M.; Pareek, V. CFD Simulation of a Pilot Scale Slurry Photocatalytic Reactor and Design of Multiple-Lamp Reactors. Chem. Eng. Sci. 2014, 111, 266–277. [Google Scholar] [CrossRef]
- Casado, C.; Marugán, J.; Timmers, R.; Muñoz, M.; Van Grieken, R. Comprehensive Multiphysics Modeling of Photocatalytic Processes by Computational Fluid Dynamics Based on Intrinsic Kinetic Parameters Determined in a Differential Photoreactor. Chem. Eng. J. 2017, 310, 368–380. [Google Scholar] [CrossRef]
- Duran, J.E.; Mohseni, M.; Taghipour, F. Computational Fluid Dynamics Modeling of Immobilized Photocatalytic Reactors for Water Treatment. AIChE J. 2011, 57, 1860–1872. [Google Scholar] [CrossRef]
- Versteeg, H.K.; Malalasekera, W. An Introduction to Computational Fluid Dynamics: The Finite Volume Method, 2nd ed.; Pearson Education Ltd.: Harlow, UK; New York, NY, USA, 2007; ISBN 978-0-13-127498-3. [Google Scholar]
- Ahmed, S.; Rasul, M.G.; Sattar, M.A.; Jahirul, M.I. Phenol Degradation of Waste and Stormwater on a Flat Plate Photocatalytic Reactor with TiO2 on Glass Slide: An Experimental and Modelling Investigation. J. Water Process Eng. 2022, 47, 102769. [Google Scholar] [CrossRef]
- Rasul, M.G.; Ahmed, S.; Sattar, M.A.; Jahirul, M.I. Modelling and Analysis of Hydrodynamics and Flow Phenomena of Fluid with Formic Acid as Pollutant in the Reactive Area of a Flat Plate Photocatalytic Reactor with Top and Bottom Turbulence Promote. Chem. Eng. J. 2023, 466, 142760. [Google Scholar] [CrossRef]
- Queffeulou, A.; Geron, L.; Archambeau, C.; Gall, H.L.; Marquaire, P.-M.; Zahraa, O. Kinetic Study of Acetaldehyde Photocatalytic Oxidation with a Thin Film of TiO2 Coated on Stainless Steel and CFD Modeling Approach. Ind. Eng. Chem. Res. 2010, 49, 6890–6897. [Google Scholar] [CrossRef]
- Duran, J.E.; Taghipour, F.; Mohseni, M. Irradiance Modeling in Annular Photoreactors Using the Finite-Volume Method. J. Photochem. Photobiol. A Chem. 2010, 215, 81–89. [Google Scholar] [CrossRef]
- Qi, N.; Zhang, H.; Jin, B.; Zhang, K. CFD Modelling of Hydrodynamics and Degradation Kinetics in an Annular Slurry Photocatalytic Reactor for Wastewater Treatment. Chem. Eng. J. 2011, 172, 84–95. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Experimental Analysis of a Photoreactor Packed with Pd-BiVO4-Coated Glass Beads. AIChE J. 2019, 65, 132–139. [Google Scholar] [CrossRef]
- Moukalled, F.; Mangani, L.; Darwish, M. The Finite Volume Method in Computational Fluid Dynamics: An Advanced Introduction with OpenFOAM and Matlab; Fluid mechanics and its applications; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany; New York, NY, USA; Dordrecht, The Netherlands; London, UK, 2016; ISBN 978-3-319-16874-6. [Google Scholar]
- Fluent, A. Ansys Fluent Theory Guide. Ansys Inc. USA 2011, 15317, 724–746. [Google Scholar]
- Rana, K.B.; Mavrič, B.; Zahoor, R.; Šarler, B. A Meshless Solution of the Compressible Viscous Flow in Axisymmetric Tubes with Varying Cross-Sections. Eng. Anal. Bound. Elem. 2022, 143, 340–352. [Google Scholar] [CrossRef]
- Maxworthy, T. Experiments on the Flow around a Sphere at High Reynolds Numbers. J. Appl. Mech. 1969, 36, 598–607. [Google Scholar] [CrossRef]
- Jung, E.C.; Lee, G.-H.; Shim, E.B.; Ha, H. Assessing the Impact of Turbulent Kinetic Energy Boundary Conditions on Turbulent Flow Simulations Using Computational Fluid Dynamics. Sci. Rep. 2023, 13, 14638. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, K.; Ray, A.K. Performance Enhancement of Photocatalytic Reactor Utilizing Flow Instability. Int. J. Chem. React. Eng. 2003, 1, 20121019. [Google Scholar] [CrossRef]
- Duran, J.E.; Mohseni, M.; Taghipour, F. Modeling of Annular Reactors with Surface Reaction Using Computational Fluid Dynamics (CFD). Chem. Eng. Sci. 2010, 65, 1201–1211. [Google Scholar] [CrossRef]
- Matoh, L.; Žener, B.; Skalar, T.; Štangar, U.L. Synthesis of Nanostructured TiO2 Microparticles with High Surface Area. Catalysts 2021, 11, 1512. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, Q.; Rana, K.B.; Matoh, L. A CFD Study on Optimization of Mass Transfer and Light Distribution in a Photocatalytic Reactor with Immobilized Photocatalyst on Spheres. Water 2024, 16, 1828. https://doi.org/10.3390/w16131828
Jamil Q, Rana KB, Matoh L. A CFD Study on Optimization of Mass Transfer and Light Distribution in a Photocatalytic Reactor with Immobilized Photocatalyst on Spheres. Water. 2024; 16(13):1828. https://doi.org/10.3390/w16131828
Chicago/Turabian StyleJamil, Qasim, Khush Bakhat Rana, and Lev Matoh. 2024. "A CFD Study on Optimization of Mass Transfer and Light Distribution in a Photocatalytic Reactor with Immobilized Photocatalyst on Spheres" Water 16, no. 13: 1828. https://doi.org/10.3390/w16131828
APA StyleJamil, Q., Rana, K. B., & Matoh, L. (2024). A CFD Study on Optimization of Mass Transfer and Light Distribution in a Photocatalytic Reactor with Immobilized Photocatalyst on Spheres. Water, 16(13), 1828. https://doi.org/10.3390/w16131828






