1. Introduction
Increasing urban populations, expanding urban areas, and increasing building density and impervious soil cover play key roles in the deterioration of water resources, affecting both their quantity and quality in urbanised water basins [
1]. At the same time, global climate change is altering the spatial and temporal distributions of precipitation, leading to an increase in the frequency and intensity of precipitation [
2]. The interaction of impermeable areas with extreme and intense precipitation leads to the formation of local floods and an increase in stormwater runoff [
3,
4].
Stormwater runoff is the flow of water that results from rainfall or snowmelt and flows over a surface without penetrating the ground. In rural areas, the volume of stormwater runoff is less than 20% of the average annual rainfall, but in urban areas, this figure reaches 60–70% [
5].
Urbanisation of cities leads to changes in the characteristics of urban transport due to an increase in traffic and the spread of congestion. Urban transport is a source of a large number of organic pollutants due to exhaust emissions, tire and brake wear, and the spillage and leakage of fuels and lubricants that contaminate stormwater as a result of petroleum hydrocarbons (PHs) from diesel fuel, petrol, and used engine oil [
6]. Such water flows from roads, petrol stations, service stations, etc. The concentration of petroleum hydrocarbons in rainwater usually ranges from 0.2 to 277 mg/dm
3 [
7]. It should be emphasised that impervious surfaces themselves can also be sources of PHs, as coal tar pavement is believed to be a significant source of surfactants in the environment [
8].
The most common organic pollutants (OPs) are diesel fuel (DF) and used engine oil (UEO), which is the main source of energy worldwide. It is a complex chemical mixture of intermediate distillates of crude oil that has been separated by fractional distillation. The carbon number of diesel fuel ranges from 11 to 25, and the distillation range is from 180 to 380 °C. Diesel fuel contains between 2000 and 4000 hydrocarbons that cannot be completely separated by gas chromatography [
9]. Diesel fuel contains approximately 64% aliphatic hydrocarbons, 1–2% olefinic hydrocarbons, and 35% aromatic hydrocarbons and consists of four main structural classes: n-alkanes or n-paraffins (linear saturated hydrocarbons); isoalkanes or isoparaffins (branched saturated hydrocarbons); cycloalkanes or naphthenes (saturated cyclic alkanes); and polycyclic aromatic hydrocarbons [
10]. UEO contains polyaromatic hydrocarbons derived from petroleum, such as phenol, toluene, benzene and other compounds, as well as the metals V, Pb, Al, Ni, and Fe [
11]. Thus, UEO is considered one of the most dangerous environmental pollutants [
12].
Due to the harmful effects of OPs on humans and the environment, in particular the combination of genotoxicity, bioaccumulation, and their resistance to degradation, stormwater treatment is becoming increasingly important in the field of urban stormwater management [
13]. Solving this problem requires modern research and development on innovative and effective stormwater quality management technologies that can prevent further transport and spread of OPs into the environment, where they can bioaccumulate/biodegrade.
The choice of a stormwater treatment method and its application requires taking into account the fact that many OPs (benzene, toluene, ethylbenzene, xylene, and light aromatic hydrocarbons) are volatile and weakly hydrophilic, and are difficult to detect in stormwater in the particulate phase [
14]. Some types of OPs (PHs, phthalates, and polycyclic aromatic hydrocarbons (PAHs)) are hydrophobic, which allows them to be removed from the aqueous phase by simple methods, such as particle sedimentation [
15]. There are also hydrophilic–hydrophobic compounds, such as diesel hydrocarbons, alkylphenols (APs) and their ethoxylates (APEOs), diesel fuel with APs and APEOs, phthalates, and used engine oil components, which can form nano- and micro-dispersed emulsions in rainwater [
16]. Therefore, when choosing a stormwater treatment method, it is also advisable to take into account that such waters contain colloids, truly dissolved components, and emulsions of OPs.
Over the past decades, urban stormwater management paradigms have gained popularity, focusing on controlling stormwater directly near the source of its generation rather than on rapid drainage [
17]. One of the practices often used in these cases is rain gardens, which are implemented in residential areas for local stormwater management and contribute to slowing the rate of peak runoff, reducing its volume, recharging groundwater through infiltration, and removing pollutants from water before it reaches local streams.
The study of the quality of urban stormwater runoff, which is an important aspect of managing the quality of surface and groundwater sources, began in the mid-20th century, and the authors of [
18] were among the first to draw attention to this problem. The main problem was the presence of elevated concentrations of toxic pollutants in such waters. It was found that, in addition to organic contaminants, these waters contain total suspended solids (TSSs) [
19], metals (Cd, Cr, Cu, Ni, Pb, and Zn) [
20], pathogenic microorganisms [
21], and nutrients (N and P) [
22]. In addition, modern scientific studies often confirm the presence of pesticides [
23], microplastics [
24], and others.
One of the most effective ways to solve this problem is to organise rain gardens of various types and designs. Various scientific studies have shown that the efficiency of removing total phosphorus (TP) by rain gardens is 90% [
25], total nitrogen (TN) is 60–97% [
26], suspended solids (TSSs) is 53–60% [
27], zinc (Zn) is 80–95%, copper (Cu) is 72–95%, lead (Pb) is 95%, cadmium (Cd) is 81–99% [
28], mercury is 37%, methylmercury is 49% [
29], microplastics is 70–99% [
30], pesticides is 99.7% [
31], faecal coliform counts is 69% [
32], and E. coli is 71–83% [
33].
The authors of [
34] experimentally determined a reduction of more than 96% of oil and grease in laboratory-scale rain gardens and almost 100% in full-scale systems. Hong et al. [
35] observed an approximately 90% reduction in petroleum hydrocarbons in laboratory columns. DiBlasi et al. [
36] investigated the removal of PAHs by a rain garden design and reported an average reduction in the total load of 87%. The efficiency of removal of PHs and other organic pollutants in urban stormwater runoff by rain gardens was about 80–95% [
37]. In real-world conditions, rain gardens demonstrated high efficiency in the treatment of PHs and PAHs in stormwater, with a concentration reduction of 60–90% and an average annual reduction of 87%.
Recent studies have highlighted the effectiveness of supplementing traditional rain garden soil media with sorption materials to improve pollutant removal and hydrological performance. The authors of [
30] showed that petroleum hydrocarbons and PAHs were effectively removed by bioretention systems using sphagnum peat, biochar, and ash from municipal solid waste incineration with compost as sorption materials. In recent years, the use of biochar as a soil amendment has attracted much attention around the world. In [
38], the authors investigated the biochar production processes, the impacts of pyrolysis parameters on its properties, and the subsequent physicochemical impact of biochar on soil and water characteristics, noting the effective effects. Organic pollutants are removed by several different processes, the main ones being sedimentation on the surface of filters due to the sorption of larger particles, evaporation from the surface, sorption in solid materials, and biodegradation and/or phytoremediation by plants. In [
39], the sorption response of the two most common metals derived from PHs, hexavalent chromium (Cr
+6) and mercury (Hg
+2), was studied using two semi-arid soils from Saudi Arabia. Based on the empirical models (Langmuir and Freundlich), the nature of sorption (single-layer or heterogeneous) and its features were clarified. In addition, kinetic models were used to verify the type and nature of the sorption that occurs (pseudo-first or second order).
In [
40], laboratory experimental studies were conducted on the behaviour of PHs in three pilot rain gardens and the impact of vegetation on their removal. As a result of the experiment, the authors concluded that soil adsorption was the most effective mechanism for PH removal (>50%), although plant uptake was also significant. Weerasundara and Vithanage [
41] noted that when using a combination of phytoremediation and bioremediation systems, the rate of PH removal could be increased by 18–115%. Some studies confirm that microorganisms (
Pseudomonas,
Arthrobacter,
Rhodococcus,
Acinetobacter,
Flavobacterium,
Corynebacterium,
Xanthomonas,
Alcaligenes,
Nocardia,
Brevibacterium,
Candida,
Mycobacterium,
Beijerinkia,
Bacillus,
Enterobacteriaceae,
Klebsiella, and
Micrococcus) in rain gardens play important roles in the removal of oil pollutants [
42].
Since the process of PH removal is mainly based on adsorption by soil media, it can be assumed that these pollutants are not completely degraded within the system, which can lead to their accumulation over time. When it comes to the accumulation of PHs in the soil environment, the problem is complicated by the fact that these compounds can migrate by diffusion and capillary forces through saturated or unsaturated flow [
43]. Prolonged exposure to PHs can lead to a decrease in the affinity of the soil surface for water molecules, which disrupts its water-holding properties. Possible mechanisms that contribute to changes in soil moisture retention include the formation of a surface film at the «solid–air» interface and direct contamination of soil macro- and microchannels by PHs [
44]. Thus, it can be assumed that over time, the accumulation of PHs in the layers of the rain garden structure can directly affect the hydraulic characteristics of the entire system, with a violation of its functionality and efficiency.
The choice of plants for rain gardens is an important component, especially in solving the problem of petroleum hydrocarbon removal. Adaptation to new environmental conditions, high resistance to pollutants, and the ability to selectively adsorb pollutants are the main criteria for selecting vegetation for such systems. In addition, plants should have characteristics such as ease of maintenance, availability of planting materials, a fast growth rate, and a developed root system [
41].
There are a small number of studies on the possible consequences of plant resistance to PHs for the effective functioning of rain gardens. On the other hand, the phytotoxicity of petroleum hydrocarbons, in particular diesel fuel, has been widely studied by the authors of [
45]. Barrutia et al. [
46] studied the impact of DF on the physiological state of plants (
Trifolium repens and
Lolium perenne) and the characteristics of the soil microbial community in the context of the rhizoremediation of contaminated soils. For this purpose, a DF spill on soil was simulated in pot conditions. The authors assessed the germination of new sprouts, plant growth, and the condition of the root system. According to the results of the experiment,
L. perenne plants showed high resistance to diesel contamination of the soil; shoot and root growth, as well as physiological productivity, were almost unaffected. On the contrary, the fibrous root system of
L. perenne showed a greater potential for the rhizoremediation of diesel contaminated soil than
T. repens. It was concluded that plant tolerance is the main characteristic for the restoration of the physicochemical properties of soil contaminated with petroleum hydrocarbons. Another study [
47] indicates that plants of the
Fabaceae and
Poaceae species are draining and can contribute to the phytoremediation of soils contaminated with oil products. The use of herbs in this process is associated with their well-developed fibrous root system, which creates a high rhizosphere effect that is extremely important for the decomposition of organic contaminants.
The main objectives of the work are as follows:
Determine the effectiveness of rain garden designs in removing petroleum hydrocarbons, such as diesel fuel and used engine oil, from rainwater;
Study the temporal and spatial characteristics of the distribution and accumulation of petroleum hydrocarbons in experimental rain gardens;
Study the impact of petroleum hydrocarbons on plants of the species Physocarpus opulifolia Diabolo;
Provide recommendations for the implementation and maintenance of rain gardens in real environmental conditions, in particular in areas with high levels of oil pollution, such as petrol stations, car parks, and car washes.
The object of the study is experimental filter columns that simulate rain gardens in the laboratory. The subject of the study is to determine the effectiveness of rain gardens in removing PHs from rainwater. The scientific novelty of the work is to establish the peculiarities of the temporal and spatial characteristics of the distribution and accumulation of PHs in rain gardens. The practical significance of the research is to study the impact of PHs on plants of the Physocarpus opulifolia Diabolo species, as well as to develop recommendations for the implementation and maintenance of rain gardens in real environmental conditions.
2. Materials and Methods
2.1. Experimental Filter Columns (Cylinders)
In this work, a set of three experiments in one was carried out based on the objectives. The experiment was carried out in the laboratory of environmental parameters control of the Department of Environmental Protection Technologies and Labour Protection of the Kyiv National University of Construction and Architecture (Ukraine) for 6 months (22 weeks).
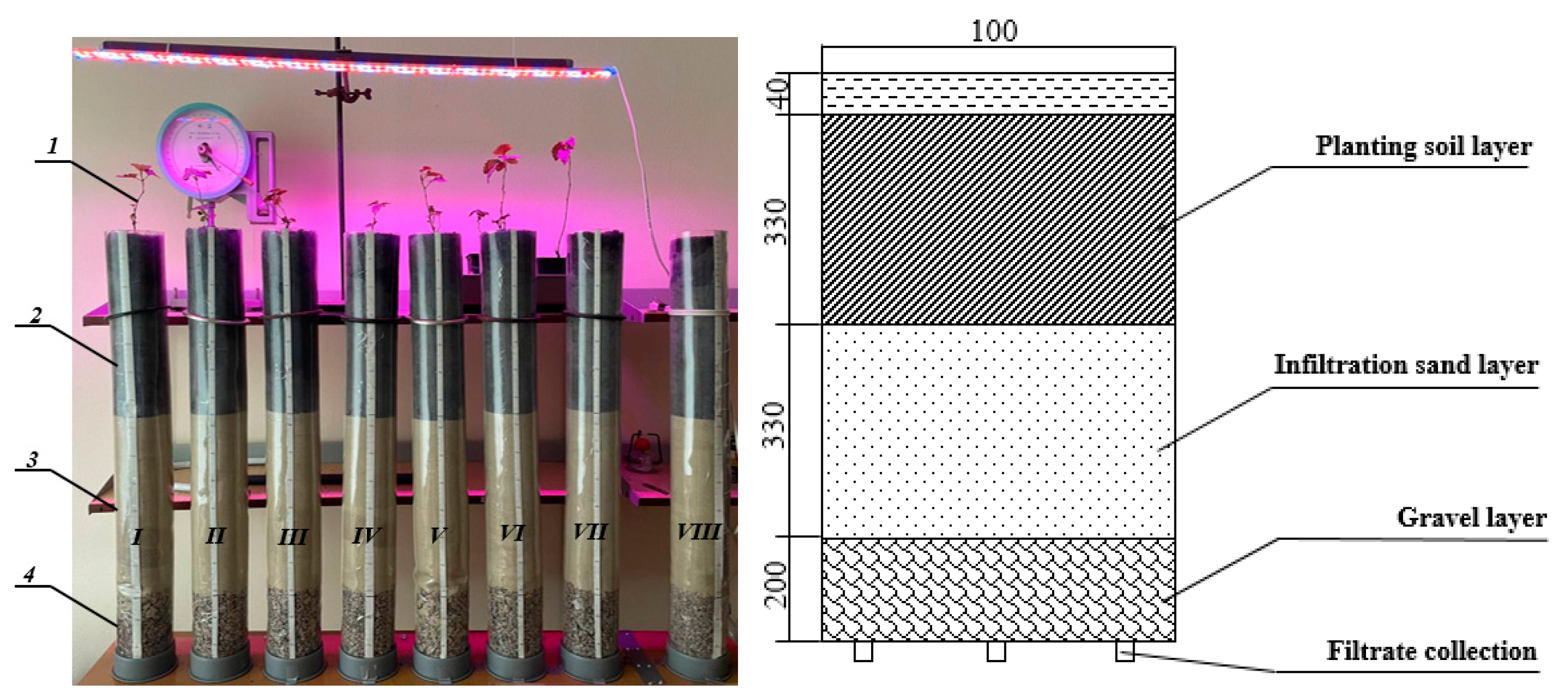
For the experimental studies, cylindrical columns (
Figure 1) were used, made of polyvinyl chloride materials, 100 mm in diameter, 900 mm in height and 2 mm in wall thickness, to create an economical and compact installation with dimensions that minimised the wall (edge) effect and dispersion in the columns. The wall effect is the phenomenon of increased hydraulic flow at the interface between the column walls and its holding surface [
48]. To reduce this effect, the inner surfaces of the columns were lightly ground to slow the fluid flow, and the distribution of solutions took place in the upper layer in the centre of the column. In addition, large-diameter columns increase the risk of dispersion, which can cause erosion of the filter media due to the formation of various channels for liquid flow, which are then eroded vertically [
49]. A total of eight experimental filter columns (cylinders) were used, which were studied depending on the type of PHs that penetrated the layers. A scale was installed on each column to measure the level of liquid seepage. Special holes were made in the bottom of each column to collect the filtrate.
In the vertical section of the experimental filtration columns, the layers were laid as follows (starting from the top): 330 mm of soil, 330 mm of sand, and 200 mm of gravel. The total volume of soil and sand in each column was approximately 2.6 dm
3, and the volume of gravel was 1.57 dm
3. The total height of the three different media layers in the column was 860 mm, with 40 mm of depth left for the water level. Since the main functions of the mulch layer in rain garden designs are to suppress weed growth, maintain the necessary moisture level for plants, and reduce the risk of waterlogging of the soil environment, it was decided to neglect this layer in the laboratory. In addition, according to the conclusions of a report [
50], the degree of PH removal in rain gardens with and without mulch did not differ significantly in terms of efficiency.
2.2. Characteristics of Experimental Samples
Soils. The soil for planting was collected from the surface layer at a depth of 50–100 mm from an uncontaminated area in one of the districts of Kyiv in September 2023. To avoid the presence of contaminants, the soil sampling site was chosen to be away from active road traffic or industrial facilities. The collected soil sample was rinsed with distilled water, air-dried, and sieved through a 2 × 2 mm sieve to ensure a homogeneous material and remove particles with a nominal size > 2 mm and long plant fibres. The texture of the soil was sandy loam, which is typical of Ukrainian soils. Natural river sand was used for the intermediate infiltration layer of the experimental columns [
51]. The drainage (gravel) layer is the lower part of a typical rain garden structure, which consists of fine, medium, or coarse gravel. This layer is more porous and is responsible for retaining and transporting treated water to the drainage system or surrounding soil, and prevents leaching of the engineered soil [
52]. For the drainage layer of the experimental columns, gravel with sizes of the main fractions of 5–7 mm and the accompanying fractions of 1–3 mm was selected.
The geotechnical analysis of soils was performed by the following methods. The hydraulic conductivity (filtration coefficient)
, mm/h, was determined at a given pressure on the soil and a variable pressure gradient when water was passed from top to bottom after pre-saturating the soil sample with water [
53]. The moisture content of the soil and sand was determined by drying in an oven at 105 °C for 3 h [
54], after which the samples were left in the oven for 30 min before weighing. The moisture content was calculated as the ratio between the mass, kg, of water in the sample (
) and the mass of the dry sample (
). The mass of water in the sample was determined as the difference between the mass of the wet and dry samples. The water-holding capacity of the materials was determined using the method validated by Nelson [
55] with a funnel and filter paper after two hours of gravity drainage from a saturated sample. The particle size analysis of soil and sand was performed using the sieving method [
56]. The particle size of the soil was less than 0.5 mm, while the grain size of the sand pores ranged from 0.06 mm to 0.5 mm and had an average size of 0.3 m. The physical and chemical properties of the soil filter materials are shown in
Table 1.
The soil was compacted in the columns by the methodology [
57]. The bulk density of the soil in the columns was 1.48 kg/dm
3, which is considered the optimal value for plant growth [
57]. The value of hydraulic conductivity for all experimental columns at the beginning of the experiment was 5.1 × 10
−5 m/s or 185 mm/h, which corresponds to the recommended range of 50 to 200 mm/h specified in the recommendations for a temperate climate [
58].
Plants. Resistance to petroleum hydrocarbons is a prerequisite for rain garden plants. This study focuses on plants with a well-developed fibrous root system, in particular on the widespread species
Physocarpus opulifolia Diabolo in Ukraine. It is a deciduous spreading shrub with branches that form a dense, hemispherical crown up to 3 m high and wide. This species is frost-resistant and light-tolerant, as it can grow in light and shade, undemanding to soil conditions, and resistant to urban environments. Plants of the
Physocarpus opulifolia Diabolo species are known for their effectiveness in the phytoremediation of air and removal of various organic substances and heavy metals [
59]. However, there are no data in the scientific literature on the impact of this plant species on the soil environment and water.
Model petroleum hydrocarbons. Rain gardens, whose main function is to clean PHs from stormwater, are installed along motorways, petrol stations, and parking areas where motor vehicles are the main source of diesel fuel, petrol, and used engine oil. The fractional composition of petroleum products is divided into light hydrocarbons (petrol and DF) and heavy hydrocarbons (fuel oil and UEO). To achieve the research objectives, representatives of each of these classes were selected as model oils. Due to the content of lighter and more volatile components, petrol has a higher degree of evaporation compared to DF, which makes it less suitable for long-term studies. Therefore, DF was chosen as the model fuel. Since UEO contains almost all types of hydrocarbons, this oil product was chosen as another model PH. The diesel fuel used in this study was purchased at a petrol station in Kyiv. The density of the diesel fuel was 0.83 g/cm3, and its viscosity at 20 °C was 5.8 mm2/s. The used engine oil was obtained from a car service station in Kyiv. Before starting the experiment, the degree of extraction of diesel components and UEO into water was checked. The degree of extraction was determined by intensively mixing 3 cm3 of distilled water with 0.15 cm3 of DF or UEO, followed by water-phase chromatography. Shaking was performed for 2 h.
2.3. Calculation of Model Solution Volumes
To simulate rain events, the volumes of model solutions were calculated by taking into account the typical drying and wetting regime in the city of Kyiv, which is characterised by a temperate climate. For this purpose, a rain event was simulated twice a week. The volume of water supplied to the experimental columns was calculated based on statistical data on the amount of precipitation for 2023, when 673 mm of precipitation fell [
60]. Most of the precipitation falls from April to October (400 mm), with a maximum of 85 mm in July and a minimum of 35 mm in March. In the period from November to March, respectively, just over 200 mm of precipitation falls. During the year, the average number of days with precipitation is about 160. Therefore, it can be assumed that it rains on average every 2.2 days, and the volume of water per rain event should be assumed to be 4.05 mm. The volume of rainwater supplied to the experimental columns was calculated by taking into account the runoff coefficient, typical rain events in the region, and the area of the experimental column, which was 0.00785 m
2.
It was assumed that the area of impermeable surfaces was 85% (the runoff coefficient was 0.85), and the area of the experimental column simulating the rain garden design was 7% of the catchment area (one column was designed for 0.112 m
2 of catchment) [
61].
The required volume of model rainwater (
) was calculated by the volumetric method [
62] using Equation (1):
where
is the catchment area of the runoff (m
2);
is the runoff coefficient; and
is the calculated rainfall depth (dm
3/m
2).
Based on these assumptions, it was calculated that each column should be irrigated with approximately 0.385 dm3 of rainwater per event (4.05 dm3/m2 × 0.112 m2 × 0.85 = 0.385 dm3). In this way, a typical wetting and drying pattern was reproduced in the laboratory according to the regional climate with a regular dosing regime. This approach allowed the columns to dry naturally between dosing events, which helped to avoid introducing artifacts in the time trends of hydraulic conductivity.
The volume of model petroleum hydrocarbons injected into the experimental columns was calculated from the typical volume of PHs that can be released during emergencies or gradually accumulate in the environment, for example, from car washing [
63]. We injected 5 cm
3 of diesel fuel and used engine oil twice a week into each experimental column with an area of 0.00785 m
2. This volume was chosen to simulate a moderate level of pollution, since the volume of each pollutant per 1 m
2 of area is 637.58 cm
3/m
2.
2.4. Reverse-Phase High-Performance Liquid Chromatography
Quantitative measurements of the concentrations of model PHs involve regular sampling of soil materials at different depths in the vertical direction of the experimental columns [
64]. In order not to violate the conditions of the processes taking place inside the experimental columns and to avoid affecting the functional development of
Physocarpus opulifolia Diabolo plants, the experiment did not involve interference with the system. Reverse-phase high-performance liquid chromatography (RP-HPLC) was used to analyse the original DF and UEO samples. Petroleum hydrocarbons are typically analysed using gas chromatography (GC)-based methods due to their non-polar and thermally stable nature. However, the analysis of samples containing aqueous extracts of contaminated soil or aqueous leachates is mainly carried out by the HPLC method. An advantage of HPLC is its compatibility with aqueous samples (or extracts), which minimises sample pretreatment steps. However, a disadvantage of HPLC is that analytes are only identified by their retention times. Identification should be performed when samples are complex and multiple peaks are detected, which can be achieved by ultraviolet (UV) detection that provides a match to specific UV spectra for the components of the PHs [
65]. Widely used HPLC detectors are based on the UV spectrum and the interaction of UV (absorption or fluorescence) with compounds. For this reason, PHs can be easily detected in soil or water samples by HPLC analysis, and the detection limit is higher than that of GC [
66]. The method under consideration was used by researchers to determine the concentration of benzo(a)pyrene in oil-contaminated soil [
67]. In this study, the individual and total concentrations of PHs present in contaminated soil samples were studied using HPLC with detection limits ranging from 0.2 to 12 ppm, with an average PH recovery of 85% to 105%. Samples of aqueous filtrates were extracted with dichloromethane, filtered and analysed by reverse-phase HPLC, as reported by the authors of [
68]. Therefore, the chosen HPLC method is suitable for the analysis of compounds with high molecular weights and boiling points and provides a high level of detail in the analysis of the PH components, which allows for a better assessment of their behaviours in the soil phase and water leaching.
The study was carried out on an Agilent 1100 system (Agilent Technologies, Inc., Santa Clara, CA, USA) with a four-channel pump and a diode array detector after pre-filtering through iPure filters with a nylon membrane with a pore diameter of 0.22 µm. A Zorbax SB C-18 4.6 × 250 mm chromatographic column with a 5 μm grain diameter was used to separate the substances in the samples. The sample volume was 5 mm3. The mobile phase consisted of a mixture of an aqueous solution of orthophosphoric acid (0.050 M) and acetonitrile. The elution was started with a proportion of acetonitrile of 5%, which was increased to 100% after 10 min. The flow rate was 0.6 cm3/min, and the total analysis time was 16 min. The column separation temperature was 20 °C. Detection was performed at a wavelength of 206 nm. Blank chromatograms were also recorded with 5 mm3 of pure hexane and water separately.
2.5. Experimental Procedure with Filter Columns
Taking into account the above, the following experimental procedure was adopted. Plants of the
Physocarpus opulifolia Diabolo species were planted on 12 October 2023 in experimental filter columns I to VI. Columns VII and VIII were left without vegetation. To support the physiological development of plants, red–blue artificial LED lighting was used, which was calculated according to the method described by the authors of [
69]. After planting, the plants were regularly watered with tap water without any additional nutrients to accelerate their adaptation period, which lasted 10 to 20 days after planting and was controlled by measuring the height of each sample and recording the appearance of new leaves.
The experiment to study the process of PH retention by filter columns was started on 13 November 2023 and completed on 13 April 2024 (22 weeks). Experimental columns I and II, in which plants of the Physocarpus opulifolia Diabolo species were planted, were watered with 5 cm3 of used engine oil, and after 30 min, with 385 cm3 of tap water, as provided for by the methodology. Columns III and IV with plants of the same species were watered with only 385 cm3 of tap water without adding model PHs to the system to have control variants for observing plant development. Experimental columns V and VI with plants of the Physocarpus opulifolia Diabolo species were watered with 5 cm3 of diesel fuel, and after 30 min, with 385 cm3 of tap water. Columns VII and VIII, which had identical soil layers to the other columns but no vegetation, were watered with 5 cm3 of UEO and DF, respectively, and after 30 min, with 385 cm3 of tap water. Watering of the experimental models was carried out according to the calculated volumes of pollutants and water with the same time interval—every 3 days. It was accompanied by recording the signs of development of the studied plant species. The purpose of the chosen methodology was to assess the efficiency of the filter layers of the experimental plants to remove significant loads of PHs in a short period.
A study by Zhang et al. [
70] showed that dry periods of at least 10 h are required for the biodegradation of pollutants to occur. Decomposition of petroleum hydrocarbon compounds with low degradation rates may take several days or weeks. Thus, in the experimental columns with uniform water flow, sorption processes prevail; so, the study of the biodegradation process was not the target of our work.
The quality of the leachate was monitored by taking samples at the outlet of the experimental columns at intervals of three months. The total volume of each sample taken for analysis was 100 cm3. The samples were collected in glass flasks and stored in a cooling bag until transported to the laboratory, where they were placed in refrigerators until further analysis. The content of PHs in the leachate samples was determined by the HPLC method at the Institute of High Molecular Weight Chemistry of the National Academy of Sciences of Ukraine. The first leachate samples were collected from columns I and II (with vegetation) and analysed on 13 January 2024 to determine the presence of UEO, from columns V and VI (with vegetation) to detect DF, and from columns VII and VIII (without vegetation) to determine the presence of both UEO and DF, respectively. Samples were not taken from columns III and IV (with vegetation), as these sites were not irrigated with model PHs and were used as controls to observe plant development.
To evaluate the efficiency of PH removal by the experimental columns imitating rain gardens, the concentrations of PH components in the leachate at the outlet of the columns was analysed and compared with the samples of the original model pollutants. To analyse the vertical accumulation of pollutants, samples of the topsoil and intermediate sand layer were taken after 6 months of operation of the experimental columns. The general samples of the sand and soil layers were obtained by quarting according to the following method: each layer of about 2.7 dm3 was scattered on a clean surface and formed a cone, which was divided into four equal parts. The first and third quarters were then removed and the remaining quarters were combined. This procedure was repeated until a 5 cm3 sample was obtained.
The resulting sand and soil samples were prepared by adding hexane at a ratio of 2 cm3 of hexane per 1 cm3 of sand/soil and then stirring the mixture for 2 h. The hexane phase was separated, while the residual aqueous phase was further extracted with 20 cm3 of hexane. Both hexane extracts were combined and then concentrated to approximately 1 cm3 by purging with nitrogen gas. The samples were then left to stand for one hour, after which a 1 cm3 aliquot was taken and passed through iPure filters with a 0.22 µm PTFE membrane. The filtered extract was weighed, transferred to a separatory funnel, and shaken for one hour. The filtered samples were analysed by HPLC using an Agilent 1100 system according to the same procedure as the original model samples. The total analysis time was 20 min. The analysis time was extended compared to the analysis of DF and UEO samples due to the presence of substances with longer retention times in the sand and soil extracts.
2.6. Study of Plant Resistance
This experiment, conducted with Physocarpus opulifolia Diabolo, was not aimed at studying the degradation of petroleum hydrocarbons in the process of bioremediation by plants and bacteria but allowed a study of the reaction of plants to pollutants and determination of the degree of their suitability for use in rain gardens.
The susceptibility of each plant sample to phytotoxicity was assessed by comparing the biomass of control and treated plants. A comparative susceptibility scale was used to assess the response of plants to the stress of PH-contaminated soil [
71]. Plants weighing less than 25% of the weight of control plants were considered highly sensitive. Plants weighing between 25% and 50% of the weight of the control plants were considered moderately sensitive. Plants weighing more than 50% of the weight of the control plants were considered resistant to stress caused by model petroleum hydrocarbon contamination.
The number of shoots and plant height (from the base of the shoot to the top) were measured weekly, starting from the first week after planting until the 22nd week of the experiment. At the end of the experiment, the plants were harvested to determine the dry and wet weights. Uprooted plants were washed with distilled water to remove any adhering deposits and separated into shoots (the part above the soil level) and roots. All samples were dried at 80 °C for 48 h in a forced-air oven. The dried shoots and roots were weighed separately to determine the masses of shoots and roots, and together to calculate the total biomass of the plant.
Growth analysis is a widely used analytical tool for characterising plant growth. One of the most important parameters commonly calculated is the relative growth rate (RGR), which is defined as the parameter r in Equation (2) [
72]:
where
and
are the plant dry weights at time
and
.
Relative growth rates (RGRs) of shoots and roots were calculated based on Equation (3):
where
is the dry weight of the shoot or root at the 4th week after planting;
is the dry weight of the shoot or root at 22 weeks after planting;
is the initial value of the time after planting and the end of the adaptation period of plants (4 weeks);
is the last value of the time after planting (22 weeks).
The tolerance index (TI) was calculated as the ratio between the dry aboveground biomass of a plant in the PH-contaminated soil to the dry aboveground biomass of plants in the control soil [
73]:
where
is the biomass of the whole plant in the treated or contaminated soil;
is the biomass of the whole plant in the control or non-contaminated soil.
The tolerance of the studied plants is expressed on a scale where 1 and above mean full tolerance and from 0.5 to 1 means partial tolerance. These values will allow the development of recommendations for the use of this plant species in rain gardens in urban environments contaminated with PHs. If TI is in the range from 0 to 0.5, plants are considered not tolerant, and so they are not recommended for use in rain gardens.
3. Results and Discussion
3.1. Analysis of the Leachate from the Experimental Columns
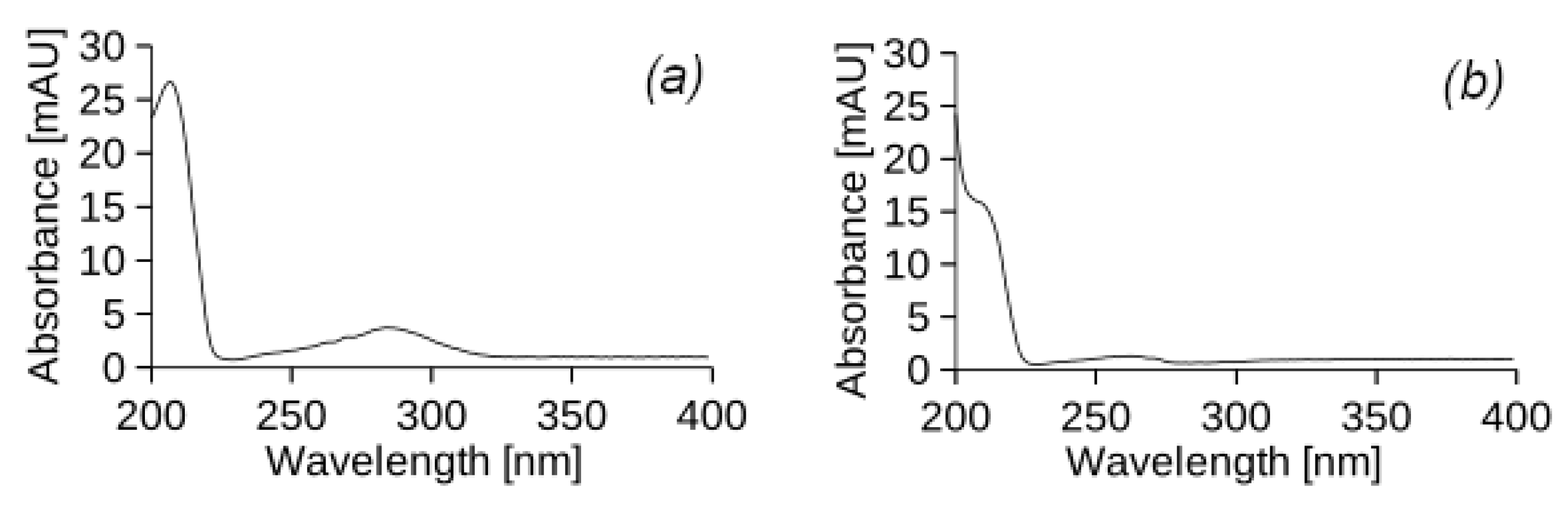
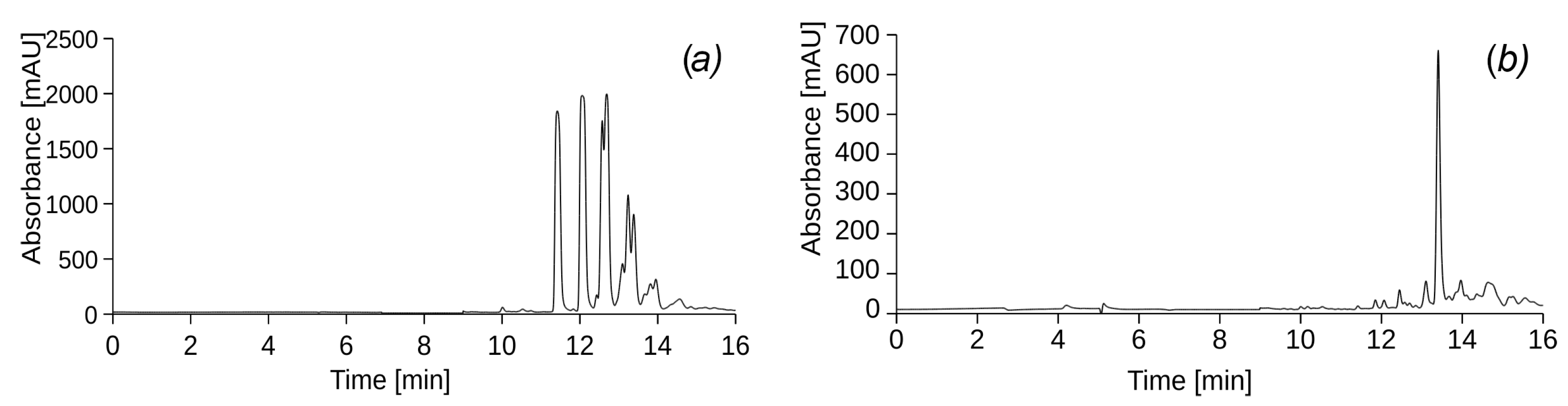
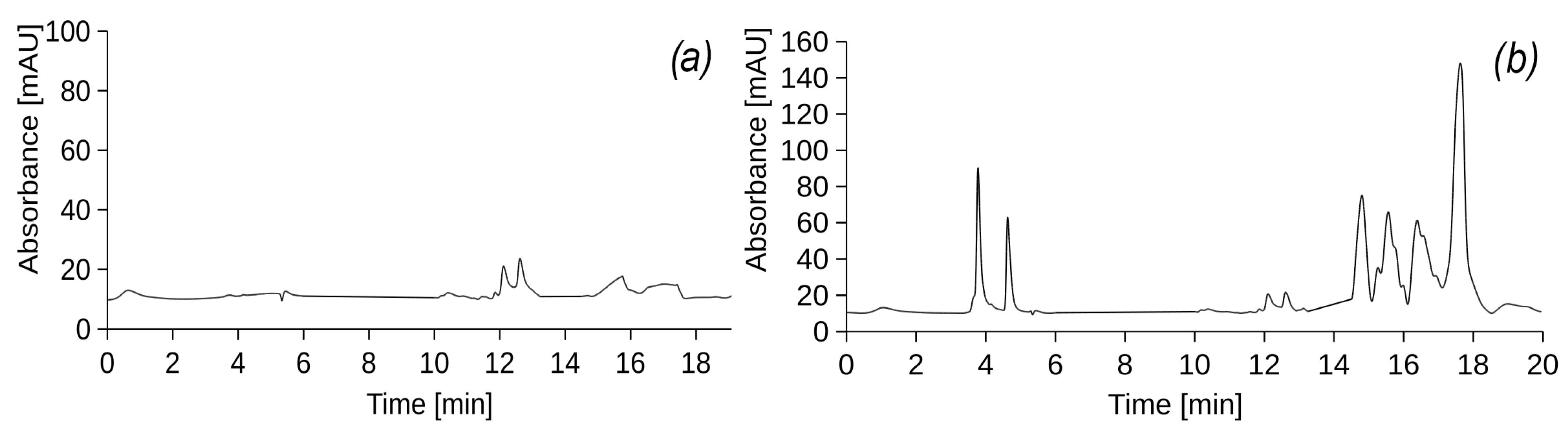
Figure 2 shows the chromatograms of samples of source diesel (
Figure 2a) and used engine oil (
Figure 2b) dissolved in hexane in a ratio of 1:40.
In addition,
Table 2 shows the mass percentages of substances in the initial DF and UEO and their retention times.
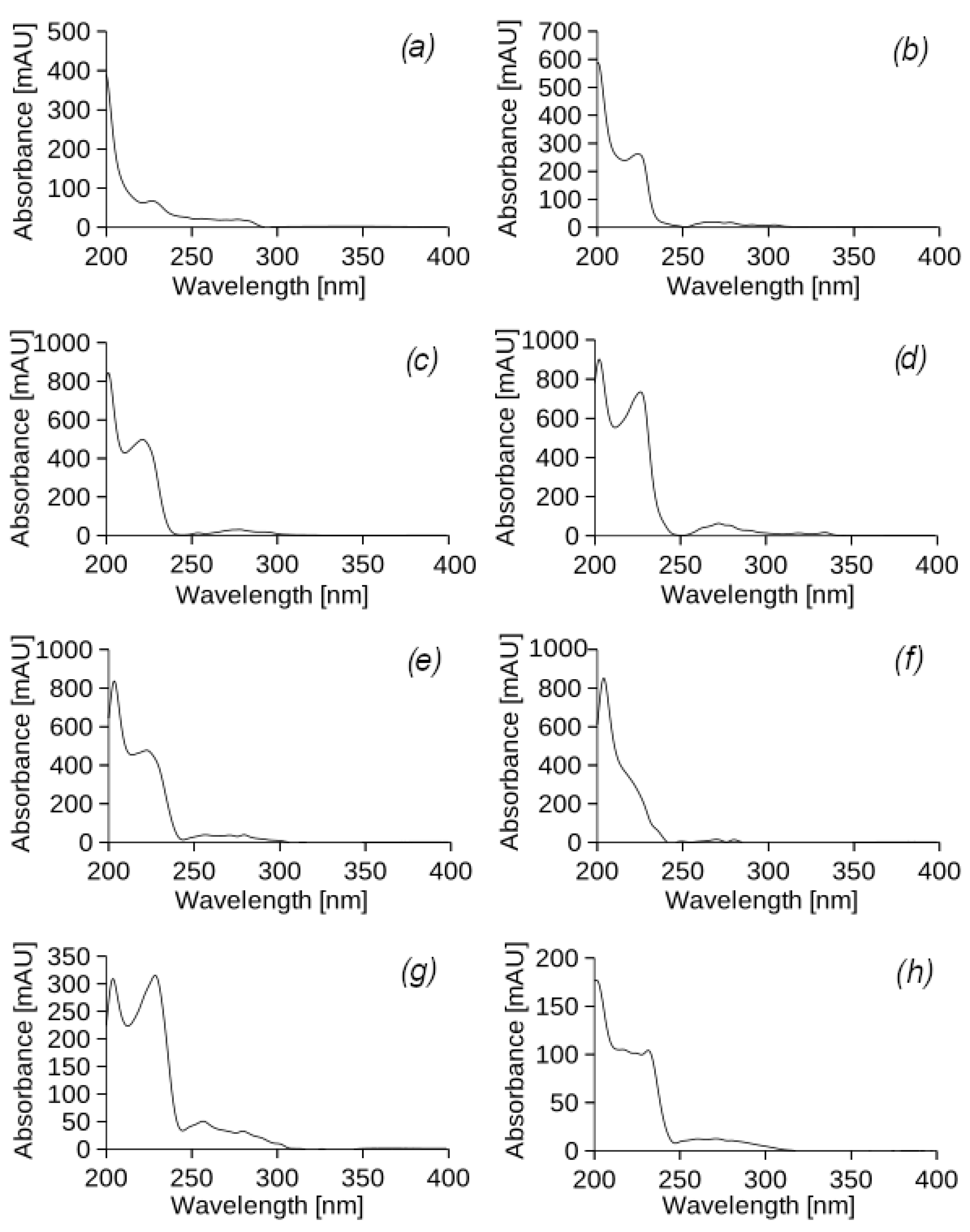
For each peak in the chromatogram of the original DF, the corresponding UV spectra were analysed to determine the nature of the constituents based on their UV absorption (
Figure A1). According to the spectral profiles, it was determined that the peaks of the chromatogram correspond to substances from the class of alkyl benzene and alkenes. Many of the PHs have very specific UV spectra. Despite the fact that most of the PHs are absorbed at 254 nm, this is not the only UV wavelength for all their components. The highest sensitivity and lowest detection limit can only be achieved by measuring at a specific UV wavelength. In this study, saturated PHs, which are the main component of DF and UEO, do not absorb UV radiation below 190 nm. This makes it difficult to detect them with the available detector. Therefore, the scan was carried out from 190 to 400 nm, which indicates a change in the absorption capacity of the DF at different UV wavelengths. By analysing the UV spectra, it is possible to detect the presence of diesel fuel components by correlating them with the peaks of alkyl benzene and alkenes on the chromatogram. Such spectral analysis helps to identify diesel components and determine their amounts, which is important for fuel quality control and detection of possible impurities or contaminants in leachate samples from the experimental columns.
The UV-vis spectra of the peaks in the chromatogram of the original used engine oil (
Figure A1) indicate the presence of various unsaturated derivatives with both linear and cyclic structures. The available spectra do not allow for an accurate determination of the structure of specific components, but, as in the case of DF, they can serve for a general analysis of the composition of the original UEO. The detected peaks of the respective components can be used to track the presence of these components in the samples, which allows for quality control of the filtrate from the experimental columns and the detection of possible impurities or contaminants.
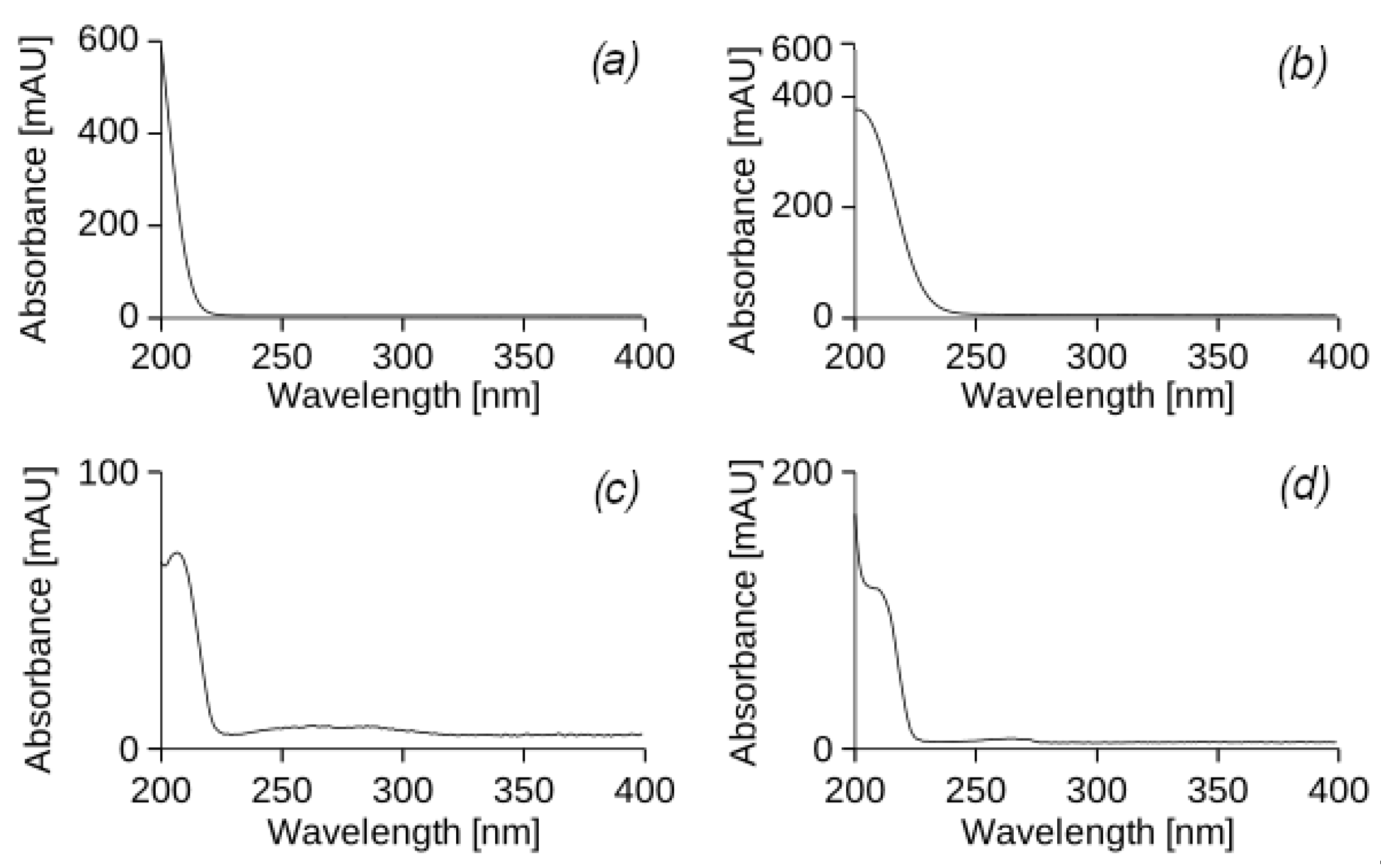
At the end of 18 irrigation cycles, 63 days (9 weeks) after the start of the experiment, samples of water that passed through the experimental columns contaminated with PHs were analysed. Sample 1 (
Figure 3a) and sample 2 (
Figure 3b) correspond to leachates from cylinders I and VII, which were irrigated with UEO and were with and without vegetation, respectively. Sample 3 (
Figure 3c) and sample 4 (
Figure 3d) correspond to leachates from cylinders V and VIII, which were watered with DF and were with and without vegetation, respectively. The results of the chromatographic analysis of leachates from cylinders II and VI, which contained vegetation and were irrigated with UEO and DF, showed a complete correspondence to the chromatograms of samples 1 and 3, respectively. Therefore, these results were not included in the report.
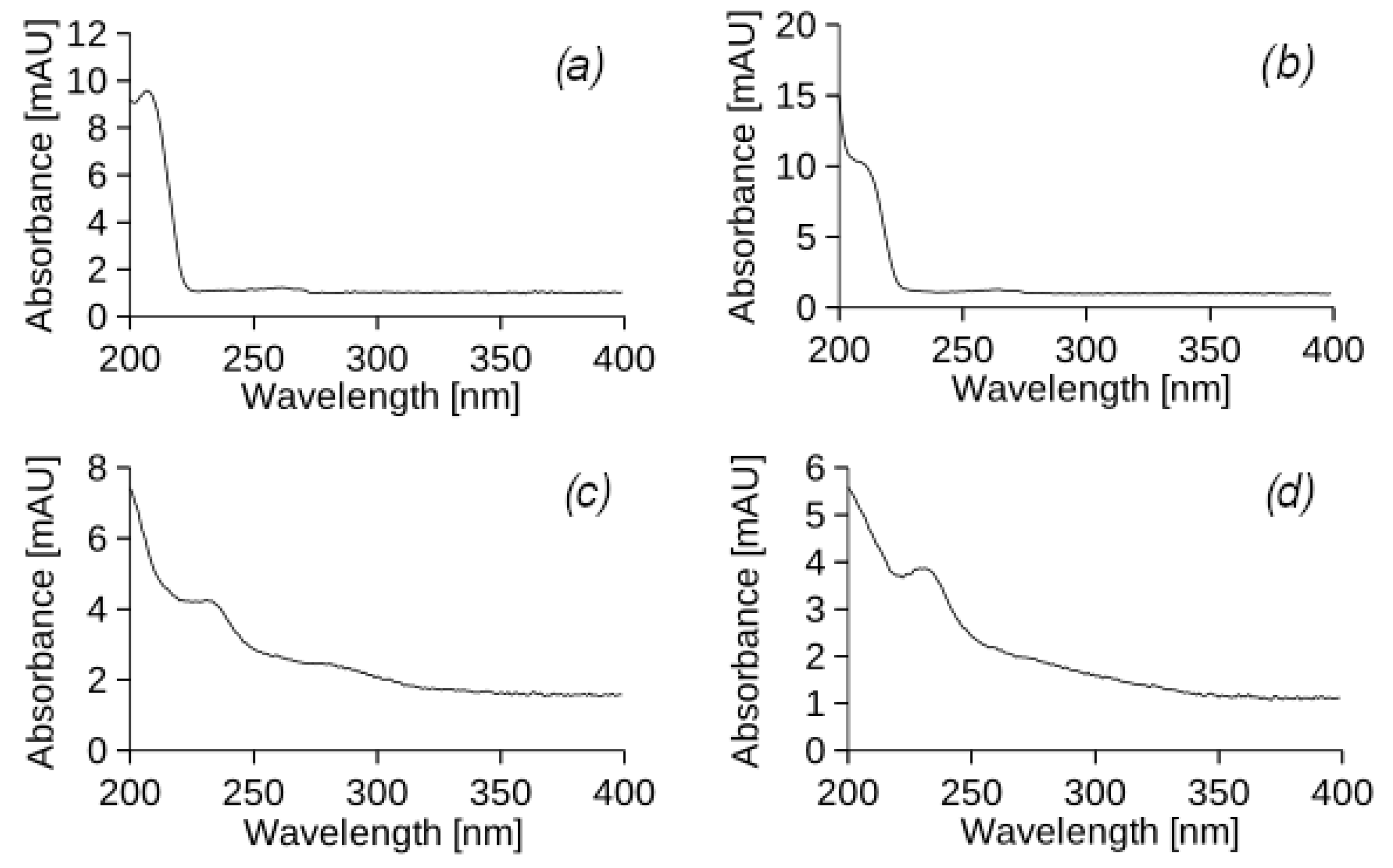
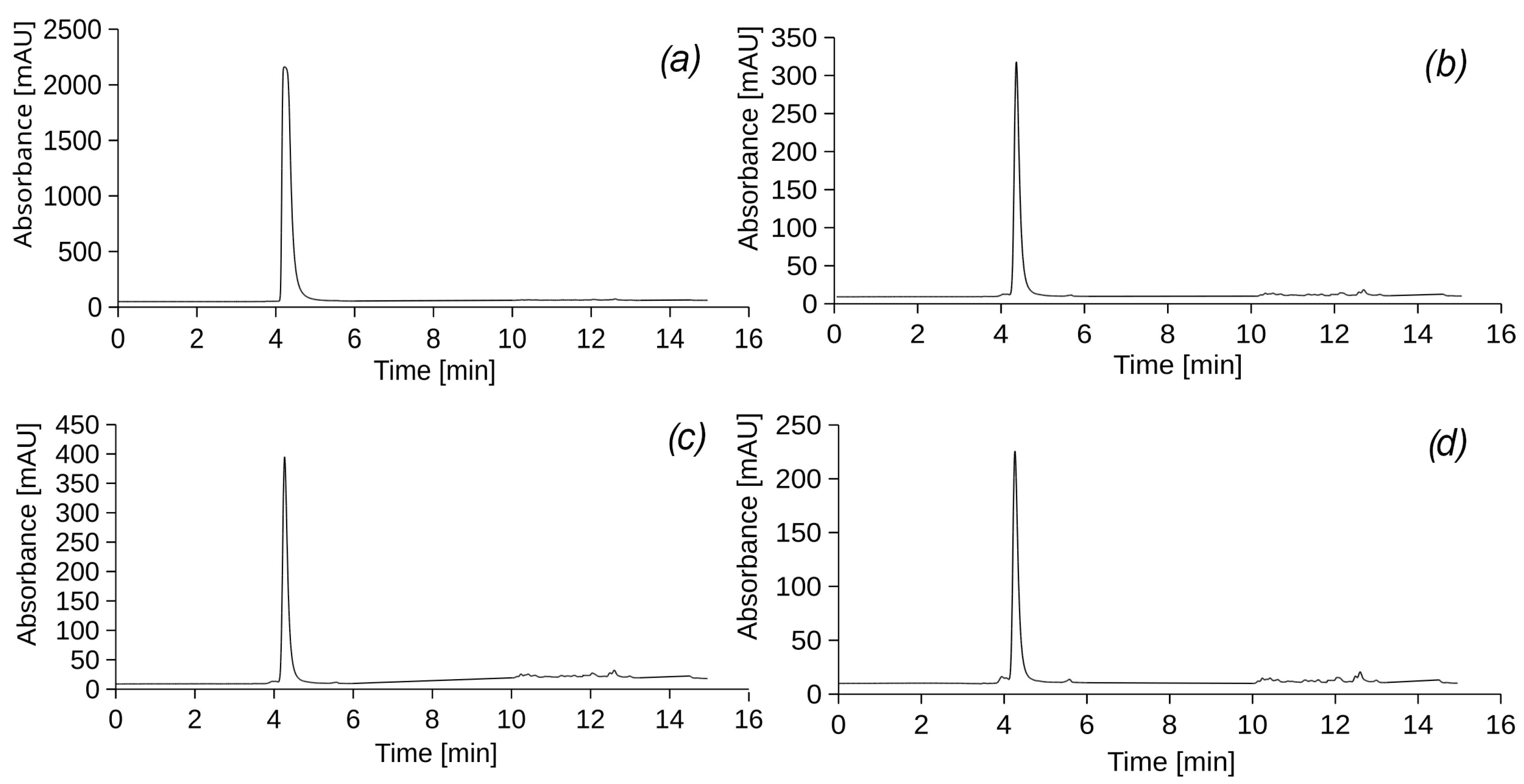
After 44 irrigation cycles, 154 days (22 weeks) from the start of the experiment, leachate 5 (
Figure 4a) from column I and leachate 6 (
Figure 4b) from column VI were analysed to analyse how the removal of model petroleum hydrocarbons changed over time.
The results of HPLC indicate the absence of UEO and DF components in the filtrates of all experimental columns. However, an intense peak with a retention time of 4.22 min was observed in each sample. The UV-vis spectrum (
Figure A2) of this peak does not correspond to any of the components of the model petroleum hydrocarbons. It can be assumed that the peak belongs to a substance released by the roots of the
Physocarpus opulifolia Diabolo plant into the soil, which requires further study of the bioremediation process. It should also be noted that at certain wavelengths (240 and 290 nm), PHs can affect other compounds absorbing UV light, such as fulvic and humic acids of the soil matrix, and these effects were not properly assessed. The soil used in this experiment was fertile sandy loam soil with a high organic matter content, and so humic compounds can be extracted together and detected in defined fractions on chromatograms.
By analysing the UV-vis spectrum of the peaks in the chromatogram of the leachate samples (
Figure A2), the retention time in the chromatographic column, and peak areas (
Table 3), it can be stated that this is a hydrophilic compound with a low molecular weight that contains a carbonyl or carboxyl group conjugated to a separate double bond.
According to the chromatographic analysis, the highest concentration of the identified hydrophilic compound was observed in sample 1 obtained from experimental column I (with vegetation), which was subjected to UEO irrigation. There was also a significant tendency for the concentrations of certain substances with a retention time of 12–13 min to increase in samples 4 and 5. It should be noted that these substances, although present in low concentrations (<10 mg/cm3), do not correspond to DF or UEO components in the UV spectrum and the retention times. The substances are the result of the transformation of petroleum hydrocarbons and are characterised by the presence of a long carbon chain, which determines their hydrophobicity.
Based on the results of chromatographic analysis of the filtrate from the experimental columns, which were subjected to regular irrigation with model petroleum hydrocarbons during all 62 cycles for the 154 days (22 weeks) of the experiment, no diesel fuel or used engine oil components were detected in the samples. These data allow us to conclude that with the given parameters of the experimental columns, properties of soil mixtures, dosages of pollutants and stormwater, the removal efficiency of both model PHs by the columns was 100%.
A similar laboratory study to evaluate the efficiency of PH removal by rain gardens under controlled laboratory conditions was conducted by the authors of [
16]. The experiment was carried out on two prototypes of modified rain garden structures with a soil substrate based on a specially developed mineral–organic mixture and a drainage channel. By analysing the results of the research, it was found that the pilot structures are characterised by a very high efficiency of PH removal from rainwater, close to 100%. Similar laboratory tests were conducted on two prototype rain gardens in Poland [
74]. The results showed that the bioretention structure is characterised by a high efficiency of removal of PHs from rainwater, and the efficiency of the reduction in these pollutants for both prototypes is close to 100%. The effectiveness of removing petroleum hydrocarbons by rain gardens was also tested in real conditions [
75]. Rain gardens in Vancouver (Canada) were tested on 18 soil environments of different compositions. Based on the results, all soil media showed a degree of PH removal that exceeded 99%, similar to the results of our laboratory study.
Despite the limited amount of available data on the performance of rain gardens in removing PHs from stormwater, existing studies and the results of our experiment show that these systems are highly effective at removing hydrophobic compounds. They can achieve a 90% to 100% reduction in the input load.
As noted above, sorption is the main kinetic process during stormwater infiltration, which depends on the duration of contact between the medium and the pollutant, i.e., the infiltration rate. Hydraulic conductivity (infiltration rate) and filter media properties are the main rain garden design configurations that need to be considered in the design. High infiltration can lead to increased hydraulic performance of the system, but at the same time, it can negatively affect pollutant removal efficiency.
In our experimental study, soil mixtures representing typical soils found in most of Ukraine (sandy loam and sandy loam soils) demonstrated a 100% efficiency in removing petroleum hydrocarbons. However, the relationship between the infiltration rate of other soil types (e.g., loams and clay soils) and their abilities to remove pollutants requires further investigation. This is necessary to obtain a more accurate understanding and improve the bioremediation process aimed at cleaning pollutants from the environment [
76].
3.2. Analysis of Soil Media
The chromatographic profiles of petroleum hydrocarbons in soil media were investigated in experimental columns II and V to study the ability of soil and sand to retain and/or remove PHs from rainwater in space and time, as well as the impact of long-term accumulation of pollutants.
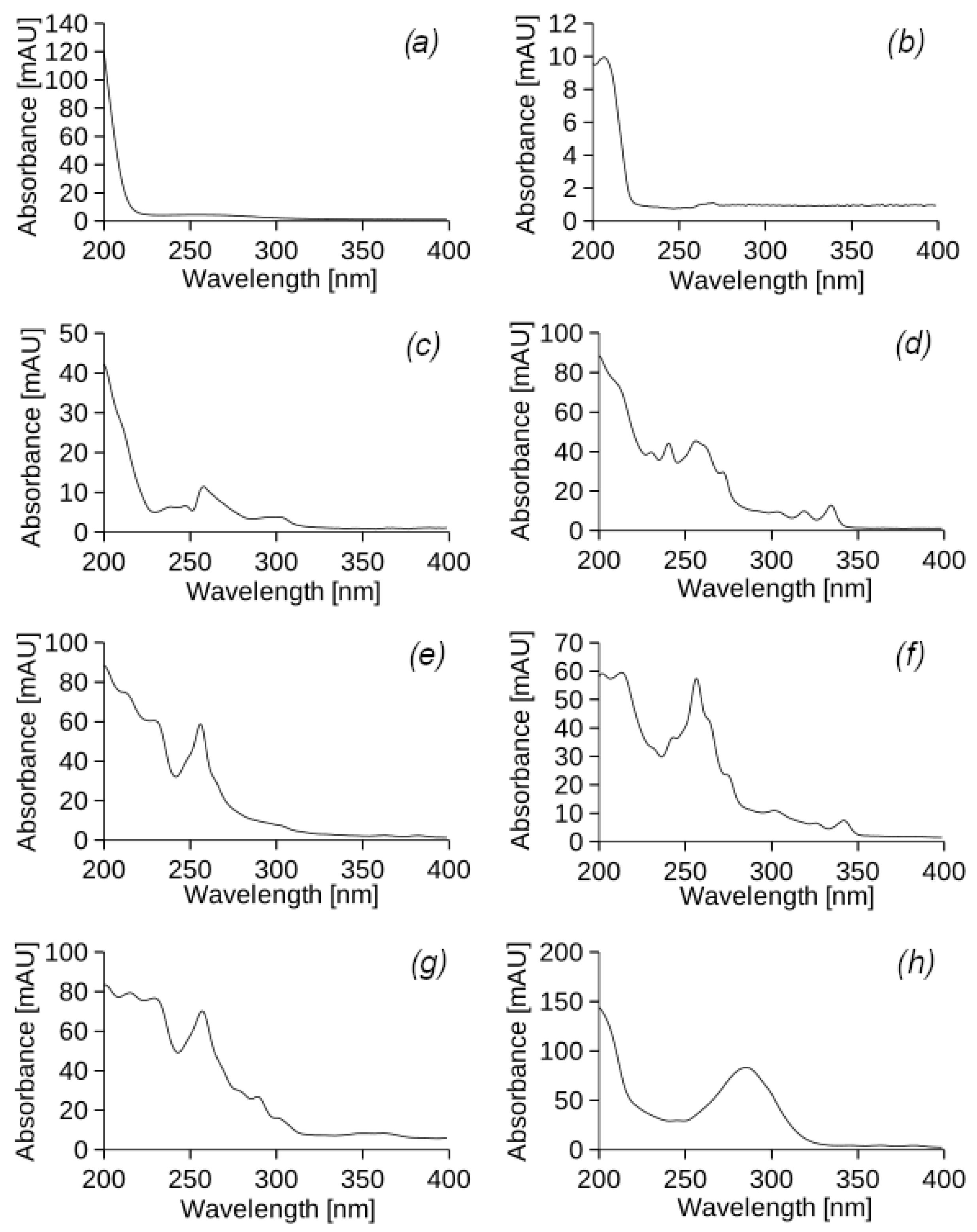
The chromatograms of samples of extracts from the sand (
Figure 5a) and soil layers (
Figure 5b) of experimental column II, which was exposed to UEO irrigation for 6 months (22 weeks) of the experiment, were obtained. For each peak on the chromatogram, the corresponding UV-vis spectra were obtained (
Figure A3 and
Figure A4).
The UV-vis spectra show that the peaks in the chromatogram of the sand layer extracts (
Figure A3), obtained by scanning in the wavelength range from 200 to 400 nm, usually appear between 12 and 18 min. All the spectra have a large peak at 210–230 nm and a weak peak at 250–270 nm, which corresponds to the two maximum UV absorption levels for PHs.
According to the UV-vis spectra (
Figure A4), the peaks in the chromatogram of the soil extract obtained by scanning in the wavelength range from 200 to 400 nm are observed between 4 and 18 min. The spectra have maximum peaks at 210–220 nm and weak peaks at 300–320 nm, indicating that most of the extracted petroleum hydrocarbon compounds tend to interact with the mobile phase.
A comparison of the results of chromatographic analysis of the sand and soil layers of experimental column II showed that approximately 95% of the UEO substances remained in the soil layer and only 5% reached the sand layer. The UV-vis spectra of the peaks in the chromatograms of the sand and soil layers differ significantly from the UV-vis spectra of the peaks in the chromatogram of the original UEO. This indicates that the oil components underwent a series of chemical transformations after entering the soil. The increase in absorbance in the wavelength range of 250–300 nm is due to the formation of new conjugated systems, possibly aromatic. The chemical transformations of the components also led to an increase in their hydrophobicity, which was confirmed by higher retention times.
Figure 6 shows the chromatograms of samples of extracts from the sand (a) and soil layers (b) of experimental column V, which was watered with DF. The corresponding UV-vis spectrum was obtained for each peak on the chromatogram (
Figure A5 and
Figure A6).
The UV-vis spectra (
Figure A5) show that the peaks in the chromatogram of the sand layer extracts obtained by scanning in the wavelength range from 200 to 400 nm usually appear between 12 and 13 min. In total, two spectra were detected with maximum peaks at 200–210 nm and minimum peaks at 260–320 nm, which correspond to the two maximum UV absorption levels for the PHs.
According to the UV-vis spectra (
Figure A6), the peaks in the chromatogram of the soil extract obtained by scanning in the wavelength range from 200 to 400 nm are observed between 13 and 18 min. The spectra have maximum peaks at 210–240 nm and weak peaks at 270–300 nm.
The total peak areas in the chromatograms of the sand and soil samples in experimental columns II and V are shown in
Table 4. The results of chromatographic analysis showed that almost all DF components were absorbed by 95% in the upper soil layer. According to the UV-vis spectra, it can be concluded that the DF components underwent a chemical transformation in the soil, which led to the appearance of an absorption band with a maximum around 225 nm. This can be explained by the formation of conjugated double-bond systems, which caused changes in the UV-vis spectra. In addition, there was an increase in peak retention time, which may be due to the formation of larger molecules from the original DF components. Similar to the chromatographic analysis of the original DF, four components with more than 50% of the total were observed, but all of them differed in their UV spectra and retention times.
As can be seen from the results of the experimental study, the retention of most (95%) of the model petroleum hydrocarbons occurs in the surface layer of the soil medium for planted plants due to the sorption process. During the experimental study, the DF and UEO components introduced into the soil environment underwent a biochemical transformation. This was confirmed by changes in UV-visible spectra. Oxidation processes may be responsible for the formation of oxygen-containing functional groups, such as hydroxyl, carbonyl, epoxy, and carboxyl groups, as well as for the possible reduction of the carbon skeleton. However, the retention time of the new substances increased compared to the original components, indicating the absence of polar groups and an increase in molecular size. As a rule, the increase in the size of the molecules of the PHs leads to simultaneous increases in hydrophobicity, electrochemical stability, high sorption capacity, and stability in the soil.
The results obtained correlate with the conclusions drawn in [
77]. Three laboratory-scale columns were packed with soil. Activated carbon (0.5% by weight) was added to two columns. For 28 days, synthetically modelled stormwater saturated with petroleum hydrocarbons was passed through the column layers. The desorbed amounts of contaminants from the soils were determined by gas chromatography and mass spectrometry, which showed that the contaminants were strongly sorbed in the upper soil layer to a depth of about a centimetre.
3.3. Changes in Hydraulic Conductivity
The hydrological processes in a rain garden design include surface infiltration, percolation through the soil medium, and drainage through the gravel layer. Surface infiltration is the key process in these systems, as it determines the rate at which stormwater can reach the underlying layers for infiltration and drainage. The rate of surface infiltration is determined by the saturated hydraulic conductivity of the soil media, the height of the water column at the soil surface, and the moisture content of the soil. In practice, the saturated hydraulic conductivity is usually used to control the infiltration capacity of rain gardens. However, over time, the deposition of pollutants, which are transported within the system by water flows, can cause clogging and changes in saturated hydraulic conductivity. In long-term studies, the change in the saturated hydraulic conductivity of soil media is used as an indicator of clogging development.
The effect of fouling on the efficiency of PH removal by the experimental columns was not specifically investigated in this work. However, during the experimental study, which lasted 22 weeks, a change in hydraulic conductivity
(mm/h) was detected over time, especially for the experimental columns that were subjected to irrigation with used engine oil (
Table 5). This indicates a clogging of the experimental rain gardens, which manifested itself in the delayed infiltration of water inside the columns.
The value of hydraulic conductivity for all experimental columns at the beginning of the experiment was 185 mm/h. After 10 weeks of research (20 irrigation cycles), the
value decreased by 26 and 12% for columns I, II, V, and VI with vegetation and the corresponding type of PHs. For columns without vegetation, VII and VIII, the decrease in
was 33 and 15%, respectively. As can be seen from the results in
Table 5, the hydraulic conductivity decreased with time for all configurations of the experimental columns (with and without vegetation). For the systems with vegetation (I, II, V, and VI), the hydraulic conductivity was higher, although not significantly, but remained below the initial value of 185 mm/h. By the end of the experiment (22 weeks and 62 irrigation cycles), the k value had significantly decreased for columns I, II, and VII, which were irrigated with UEO, in contrast to columns V, VI, and VIII, which were irrigated with DF. Moreover, the percentage of reduction in
depending on the presence of plants did not differ significantly, since at this stage, the development of the outer part of the plant and its root system was already inhibited. This result is similar to the results of Hatt et al. [
78], who showed a decrease in the hydrological conductivity of the system after 40 weeks of operation.
The results obtained can be explained by the ability of UEO, unlike DF, to change the hydrophilic properties of the air-dispersed soil medium to hydrophobic ones. Used engine oil contains alkyl sulfonates, which can change the interfacial properties of the soil. When water penetrates the oil-filled soil pores, it does not wet the mineral particles and does not displace the oil, as is required by physical laws [
79]. Thus, water seepage through the UEO adsorbed on hydrophobic mineral surfaces is slower, which leads to a decrease in the hydraulic performance of the system as a whole.
The change in hydraulic conductivity of experimental columns III and IV, which were planted with vegetation and were not subjected to watering with any of the model PHs, but only with tap water, should be separately justified. This allows us to draw a conclusion and compare it with the results available in the literature regarding the change in the hydrological parameters of the system during its operation in the absence of the pollutant factor. As can be seen from the results presented in
Table 5, the percentage of reduction in k for columns III and IV after 10 and 22 weeks was 2.7 and 15.1%, respectively. That is, the modelled flow in the columns showed a slight decrease in k values at the end of the experiment, which correlates with the results obtained in studies [
80,
81]. Rain garden systems «clog up» over time and the hydraulic conductivity decreases by an average of 3.6 times during 72 weeks of testing due to the top layer. Other authors [
82] studied an infiltration basin built on sandy loam soils. They found that hydraulic conductivity increased from 20% to 40% (of its original value) after replacing the top 5 cm of soil. Removal of 15 cm of soil was required to restore the hydraulic conductivity of the system to its original value (68%). High concentrations of contaminants, especially of petroleum origin, in the filter material can pose a risk to humans or wildlife through acute or chronic toxicity [
80].
The results of some studies of hydrological parameters in the field differ significantly from laboratory experiments. From a hydrological point of view, rain garden systems function well in real conditions over time, based on the results of a study of 22 non-novel rain garden designs in Ontario that had been in operation for up to 10 years [
83]. The hydraulic conductivity of 20/22 systems was 25 mm/h, which is the recommended minimum according to local standards. Similar values have been observed in other studies [
84,
85]. For example, in North Carolina, 98% of the studied designs had medium to high permeability [
85]. Additionally, studies with rain gardens aged 10–22 years showed that hydraulic conductivity values in most systems exceeded local minimum recommendations [
84].
A comparison of the results obtained in the field and laboratory shows uncertainty about whether hydraulic conductivity improves or deteriorates with time and age of rain garden structures if no contamination factors are present. Laboratory experiments are typically conducted over short time scales, such as weeks or months, and do not reproduce the same dry periods between wet periods that are typical of field systems. Modelling experiments do not allow sufficient time for slow biological, soil formation, or flow path formation processes to become apparent and possibly affect the ability of systems to maintain infiltration rates. Furthermore, hydraulic conductivity is not only a function of the permeability of the soil media in rain gardens but also of the density and viscosity of the water. Therefore, as the temperature decreases,
will decrease [
86]. The relationship between water permeability and hydraulic conductivity
, together with the tabulated values of water density and viscosity at different temperatures, suggest that the value of
measured at 22.5 °C will be reduced by 25% at 12 °C and by another 50% at 0 °C. Therefore,
values in the field can be much lower than those measured at room temperature.
3.4. Plant Resistance
All plant samples survived in the control (C) and DF- and UEO-contaminated soil throughout the study period (22 weeks). However, not all plant samples grew equally, demonstrating different sensitivities to a particular type of pollutant, which led to significant differences in growth rates (
Figure 7,
Table 6).
Table 6 shows the results of the reaction of the studied plant samples to stress from petroleum hydrocarbons in the soil compared to control plants. According to the conservative susceptibility scale [
71], which is used to assess the response of plants to oil pollution in the soil, the samples of
Physocarpus opulifolia Diabolo grown in columns V and VI were the most resistant, showing decreases in development by 2%, 6%, 12.5%, 18.8%, and 19.6% at 2, 5, 8, 11, and 22 weeks of the study, respectively. The reductions in the development of plants grown in columns I and II, which were watered with UEO, were 10%, 12.5%, 24.9%, 43.7%, and 56.2% at 2, 5, 8, 11, and 22 weeks of the study, respectively.
The first signs of plant phytotoxicity, such as leaf chlorosis and growth retardation, began to appear at week 5 after the start of irrigation with model petroleum hydrocarbons and increased during the experiment (
Figure 8).
The samples of Physocarpus opulifolia Diabolo were the least sensitive to DF soil contamination in experimental cylinders V and VI, while the shoot height and growth rate of Physocarpus opulifolia Diabolo were most affected by UEO contamination compared to the uncontaminated soil (C).
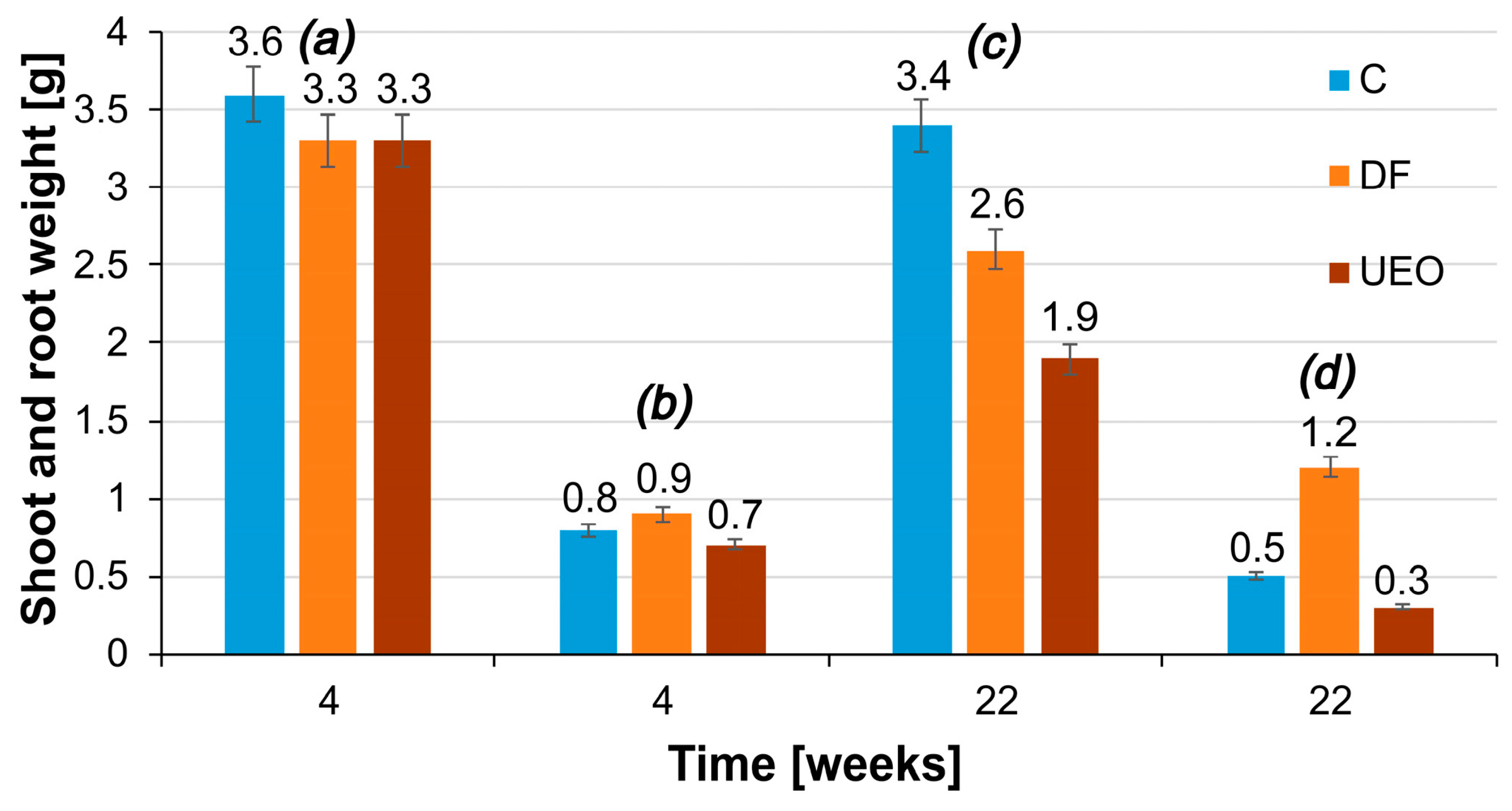
Figure 9 shows the results of measuring the masses of the shoots and roots of plant samples grown in soil contaminated with different petroleum hydrocarbons and control soil on the 4th and 22nd weeks of the experiment.
In the 4th week of the study, the decrease in the dry weight of shoots was the same for the samples grown in soil contaminated with DF and UEO. The dry weight of shoots was 8.4% lower compared to the control. In the 22nd week of the experiment, the weakest inhibitory effect on the dry weight of shoots was observed for samples grown in soil contaminated with UEO. The dry weight of shoots was 24% and 44% lower for the samples grown in the soil contaminated with DF and UEO, respectively, compared to the control.
A decrease in root weight was observed in the samples of Physocarpus opulifolia Diabolo grown in uncontaminated soil and soil contaminated with UEO. At weeks 4 and 22, the highest root weights were observed in samples grown in DF-contaminated soil, followed by plants grown in the control, uncontaminated soil. The highest sensitivity was shown by the samples grown in the soil irrigated with UEO.
The tolerance index (TI) showed that Physocarpus opulifolia Diabolo samples grown in soil contaminated with diesel fuel (DF) grew even better than those in the control group (TI > 1.03). This indicates the complete tolerance of this plant species to diesel fuel contamination. The samples grown in the soil contaminated with UEO were moderately tolerant to pollution, with a TI value of 0.75.
Thus, the plants of
Physocarpus opulifolia Diabolo that developed in the UEO-contaminated soil showed faster growth and development inhibition compared to those watered with DF. This was observed in the specimens that formed new shoots and leaves. In addition, the root system at the end of the experimental study was more developed in the samples exposed to DF compared to the plants in column II, whose root system was completely or partially necrotic. This can be explained by the fact that plants improve stormwater runoff quality through mechanisms such as phytodegradation, phytoextraction and rhizosphere interactions. Phytodegradation, also known as phytotransformation, promotes the breakdown of complex organic chemicals, including PHs, through metabolic processes in plants, resulting in the production of specific enzymes. This process reduces the toxicity of contaminants and converts them into simpler compounds that support plant growth [
37]. In addition, Bakina et al. [
87] found that the presence of PHs in the soil has a certain stimulating effect on chlorophyll biosynthesis, increasing its content in leaves and stimulating growth. It has been proven [
88] that microorganisms, due to their characteristics such as P solubilisation, ACC-deaminase activity and siderophore formation, can improve plant growth even under conditions of stress from pollutants or toxic environments. This requires further investigation in the case of the
Physocarpus opulifolia Diabolo species.
Different plant responses to DF and UEO pollution have been described previously in the literature, including those that contradict our results. For example, a more pronounced inhibition of plant growth and development was shown after diesel fuel pollution than UEO pollution [
89]. Differences in plant responses to petroleum hydrocarbon pollution are due to their species-specific characteristics, which have already been confirmed in our study with plants of the
Physocarpus opulifolia Diabolo species.
Decreases in the growth rate and biomass accumulation were observed for all plant specimens exposed to irrigation with petroleum hydrocarbons and was most pronounced for experimental sites I and II, which were irrigated with UEO. This is consistent with the findings of researchers [
47] who also emphasise that soil contamination with petroleum hydrocarbons impairs plant growth and development. The plant specimens planted in experimental columns III and IV, which were watered with tap water, also showed growth throttling at the end of the experimental period and had visual signs of developmental inhibition.
It is important to note that the plants under study are deciduous, and the experiment was conducted in the autumn when the plants were dormant. This fact is important because the death and fall of leaves in winter is a natural part of the plant life cycle. Thus, the detected signs of phytotoxicity may be partially related to the natural processes of leaf death in autumn, as well as to the fact that young shoots, which are usually less adapted to negative environmental influences, were tested during the experiment.
Abiotic stress caused by soil pollution affected the plants during the experimental period and the root system was exposed to high soil toxicity. Some authors [
46,
90] have previously reported on the resistance of plants to compounds of petroleum origin. In our study,
Physocarpus opulifolia Diabolo showed resistance to diesel fuel pollution, which was manifested in the appearance of young shoots at the beginning of the experiment and the development of the root system. Probably, the improved physiological state of
Physocarpus opulifolia Diabolo plants under the influence of diesel fuel compared to used engine oil can be explained by the more active activity of the rhizosphere microbial community. The latter reduces the negative impact of pollution on the rhizosphere and root system of plants. This hypothesis was confirmed in [
91], which compared the content of petroleum hydrocarbons in the two analysed plant parts (roots and leaves) and found that higher accumulation of PHs occurs through the roots. These results indicate that the uptake of petroleum hydrocarbons by plant roots is enhanced by the rhizodegradation mechanism. During rhizodegradation, organic pollutants are decomposed in the soil through the bioactivity of the plant rhizosphere or soil microorganisms such as bacteria or fungi.