1. Introduction
Water scarcity is considered as an imbalance condition that occurs when demands for freshwater become higher than its availability [
1]. Several factors can influence water scarcity, in particular, population growth and urbanization, individual consumption, pollution, and climate change [
2]. In the Middle East and North Africa as well as in numerous countries in South America and Southeast Asia, water scarcity is already a real concern [
3].
During the last seven decades, water desalination has become a key solution and an important source of freshwater production [
4], in particular, in the Middle East, considered as the leader of water desalination, which produces 50% of the total desalinated water in the world for only 2.9% of the world population [
3]. The two most common desalination technologies used to desalinize seawater, brackish water, and contaminated water are reverse osmosis and thermal-based desalination [
5]. However, these two techniques are expensive, energy consuming, and not environmentally friendly [
3,
5]. For these reasons, the use of solar energy in water desalination is considered as a promising alternative [
5].
The systems used for water desalination using solar energy are referred to as solar stills and are grouped into passive and active types [
6]. Solar stills are simple and cheap devices that require only simple maintenance [
7]. In addition, they can be developed in different ways, and a variety of types have been described in the literature [
7]. An emerging desalination technology using algae has recently been receiving more and more attention [
8].
In the context of water desalination, the term biodesalination is defined “as utilizing living organisms or biological elements directly or indirectly, mimicking their structures and mechanisms, borrowing concepts or inspiration from their desalination mechanisms for the production of sustainable fresh water” [
9]. According to this definition, not only the direct use of organisms is considered as a biodesalination process but also the inspiration from them or their mimetics. In fact, the use of halophilic organisms in desalination has been attracting the attention of researchers for several decades, in particular, the use of halophytes in soil desalination, also called phytodesalination [
10].
Sesuvium portulacastrum L. is a euhalophyte or obligate halophyte that requires salt for optimal growth. It is known for its high salt tolerance [
11] and its ability to accumulate enormous sodium and potassium quantities within its shoots [
12]. This halophyte showed a high phytodesalination capacity by removing high sodium quantities from soil [
13,
14]. In addition,
S. portulacastrum tolerates high temperatures. The aim of the present investigation is therefore to check whether this euhalophyte can be used in brackish water phytodesalination by growing it into a solar-still-like phytodesalinator.
2. Materials and Methods
2.1. Description of the Phytodesalinator
The device used in the present work received the Saudi patent number 10822 B1 in 2022. It was designed to function like a phytodesalinator with a base dimension of 100 × 100 cm (
Figure 1). The upper part of the device consisted of three straight sides and one side sloping with the slope of the longitude angle of the experiment site. The phytodesalinator was almost a closed system; there were only two small holes of 1 cm diameter in the back side. The phytodesalinator was positioned facing south to ensure the maximum sun light exposure every day throughout the experiment period. The base of the device had a slope of 2% towards its front to allow the collection of desalinated water into a tank. Plants were set into a floating foam board placed on the top of small rectangular containers filled with 6 L of reject brine each. With such a device, plants were grown in a closed system during the experiment.
2.2. Plant Material
S. portulacastrum cuttings were taken from mother plants grown in the garden of Qassim University. After rooting, obtained seedlings were hydroponically grown for 4 weeks on a fourth-strength Hoagland’s nutrient solution [
15]. Thereafter, plants were transferred into plastic pots (30 cm × 24 cm × 12 cm) containing 6 L of reject brine each.
2.3. Reject Brine Characterization
The reject brine used in this investigation was obtained from a local desalination plant. It was added with macro- and micronutrients according to fourth-strength Hoagland’s nutrient solution regardless of their initial concentrations. Two phytodesalination cycles that lasted 21 and 18 days, respectively, were performed. At the beginning of each cycle, reject brine samples were taken for EC determination and mineral analysis (
Table 1).
2.4. Phytodesalination Experiment
The experiment was conducted on Qassim University Campus (26°35′07″ N, 43°76′75″ W) during the period of 15 January–22 February 2022 under sunlight conditions and did not need any kind of added energy (average direct normal solar radiation and average air temperature during the experiment period are given as
supplementary data). Two different plant densities with 3 replicates each were used:
Low density (LD): 82 plants/container, which corresponds to 1140 plants/m2;
High density (HD): 209 plants/container, which corresponds to 2900 plants/m2.
The volume of obtained desalinated water was measured twice a week, and its EC was measured. At the end of each phytodesalination cycle, the remaining volume of reject brine and its EC were determined. No mineral analysis was performed for the remaining reject brine since, at the end of each desalination cycle, crystals appeared in the bottom of the containers and on the plant roots. In addition, at the beginning of the experiment as well as at the end of each phytodesalination cycle, plants were washed several times with distilled water to remove the salt crystals and root-adsorbed salts. Then, samples were taken for mineral analysis.
2.5. Plant Mineral Analysis
Shoot and root samples were oven-dried at 70 °C until they were a constant weight then ground to fine powder. After that, aliquots of about 20 mg ground plant material were digested with 0.5% HNO3. Potassium (K+) and sodium (Na+) concentrations were determined using a flame photometer apparatus (PFP7, Jenway LTD, Felsted, UK). Calcium and magnesium were analyzed by an atomic absorption flame emission spectrophotometer (AA-6200, Shimadzu, Kyoto, Japan).
2.6. Calculation
The following quantities were calculated using the measured data.
Cumulative volumes of desalinated water were calculated by adding the desalinated water volumes of all collects to each other. The daily productivity was calculated by dividing the volume of desalinated water obtained in each collect by the time of the collect (3 or 4 days), then scaled to a desalinating surface (reject brine surface) area of 1 m2. Each cycle started with 6 L brine water per container. At the end of the cycle, the remaining brine water volume was determined, then subtracted from the initial 6 L, giving the volume of desalinated water per container. The volume of desalinated water per plant during each phytodesalination cycle was obtained by dividing the volume of desalinated water in a given container by the number of plants in that container.
2.7. Statistical Analysis
When appropriate, data were subjected to One-Way ANOVA using IBM SPSS Statistics 25, and means were compared according to Duncan’s multiple-range test at p ≤ 0.05.
3. Results and Discussion
3.1. The First Time a Phytodesalinator Has Been Presented
As far as we know, this is the first time a phytodesalinator has been described in detail with an evaluation of its productivity. Indeed, the only work that discussed the use of similar plants (mangroves) was a study of the desalination of seawater carried out in a greenhouse [
16]. However, the system described by this author needed a heat exchanger, a condenser funnel, and a fan in addition to light sources. A thermal still with
Spirulina algae was also used to improve seawater evaporation and condensation as compared to a thermal still without algae [
17]. The present phytodesalinator beneficiated from the capacity of halophytes to desalinate brackish water through transpiration as in [
16] and used a euhalophyte instead of algae as in [
17]. The used euhalophyte does not need an added source of carbon as needed by algae. Moreover, the halophyte has the potential to be used for prolonged periods of time if new brackish water is added to replace the transpired water amounts.
3.2. Cumulative Desalinated Water and Daily Productivity
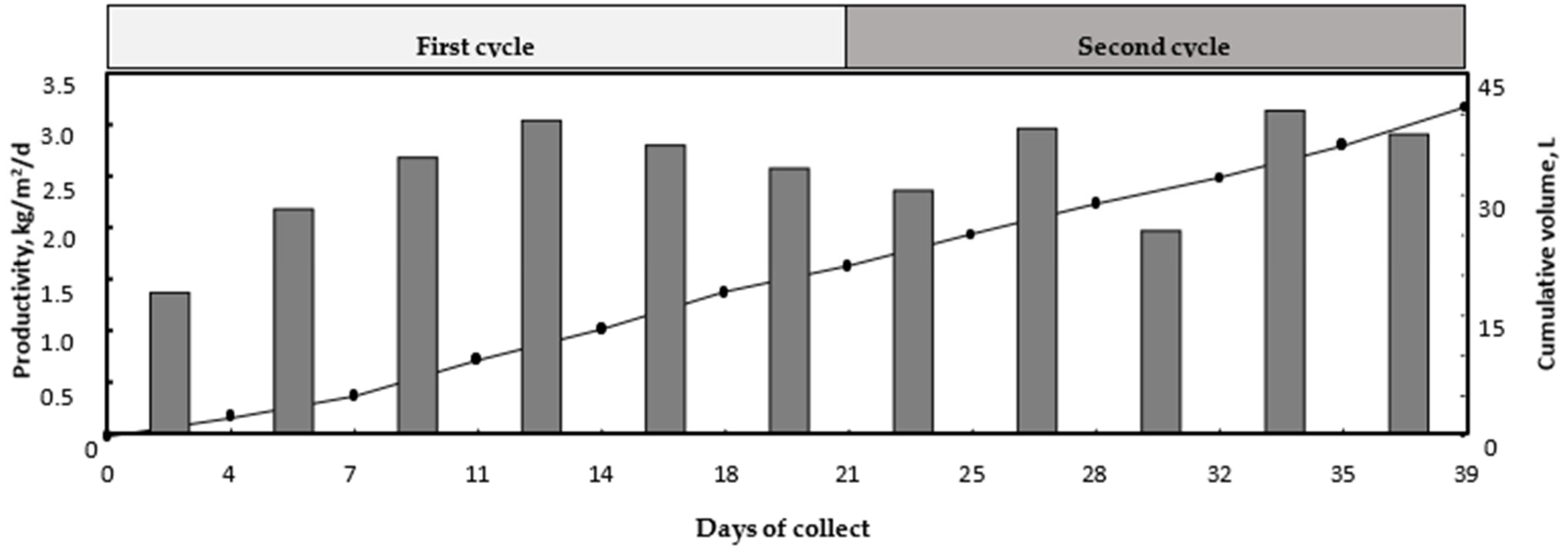
The cumulative quantities of desalinated water increased linearly with time during the experiment (
Figure 2). The obtained cumulative quantities were 21.3 and 41.1 kg at the end of the first and the second phytodesalination cycles, respectively.
The phytodesalinator daily productivity (daily average quantities of desalinated water per area unit) increased from 1.33 kg/m
2/d at the beginning of the experiment to 2.96 kg/m
2/d on days 11–14 of the first cycle, then decreased to 2.5 kg/m
2/d at the end of the first cycle (
Figure 2). The second cycle showed also the same trend for productivity. It started with 2.29 kg/m
2/d and finished with 2.82 kg/m
2/d, the highest productivity (3.05 kg/m
2/d) being recorded on days 32–35 of the experiment (days 11–14 of the second cycle).
During 39 d, the phytodesalinator used in this experiment produced 41.1 kg desalinated water, which corresponds to an average productivity of 2.44 kg/m
2/d. To our knowledge, there is no similar solar still using a halophyte that has been evaluated for productivity. A floating solar still simulating water uptake by plant roots was recently presented [
5]. These authors compared the productivity of their “bionic floating solar still” (about 1.5 kg/m
2/d) to those of four other different floating solar stills and found it the highest. The daily productivity of the modified basin solar still was water depth dependent; it increased from 1200 mL/m
2/d at 5 cm water depth to 1800 mL/m
2/d at 2 cm water depth [
18]. A circular, finned, single-basin, dual-slope solar still showed a daily productivity of 1491.7 mL/m
2/d at 10 mm water depth [
6].
3.3. Factors That Can Affect Daily Productivity
3.3.1. Plant Species
The choice of plant species that can be used in a phytodesalinator for phytodesalination of brackish water should be a euhalophyte. In addition, it should be an “includer”; it should not have salt glands such as
Aeluropus littoralis (Willd) Parl [
19] or glandular trichomes like
Atriplex halimus L. [
20]. Indeed, the existence of salt crystals due to such salt-secreting structures on the leaf surface may salinize the distillate. In addition, the chosen plant species should tolerate high temperatures and transpire at a high rate as it will be subjected to both high salinity and temperature. In the present study, the heat-tolerant euhalophyte
S. portulacastrum was efficient in desalinating reject brine through its high transpiration rate.
3.3.2. Phytodesalination Duration
During each phytodesalination cycle, the daily productivity of the phytodesalinator started with a relatively low value, increased to an optimum, then decreased (
Figure 2). The relatively low productivities at the beginning of the first cycle may be explained by two factors: (i) plant acclimation and (ii) low relative humidity inside the phytodesalinator. The productivity with which the second cycle started was 73% higher than that of the first cycle despite the higher reject brine salinity. This means that plants were already acclimated, and the opening of the phytodesalinator was responsible for the decrease in daily productivity because of the decline in relative humidity inside the phytodesalinator. The optimum daily productivity obtained in each phytodesalination cycle was followed by a decline, indicating that conditions, in particular, reject brine salinity, became increasingly unfavorable for the halophyte.
3.3.3. Solar Radiation
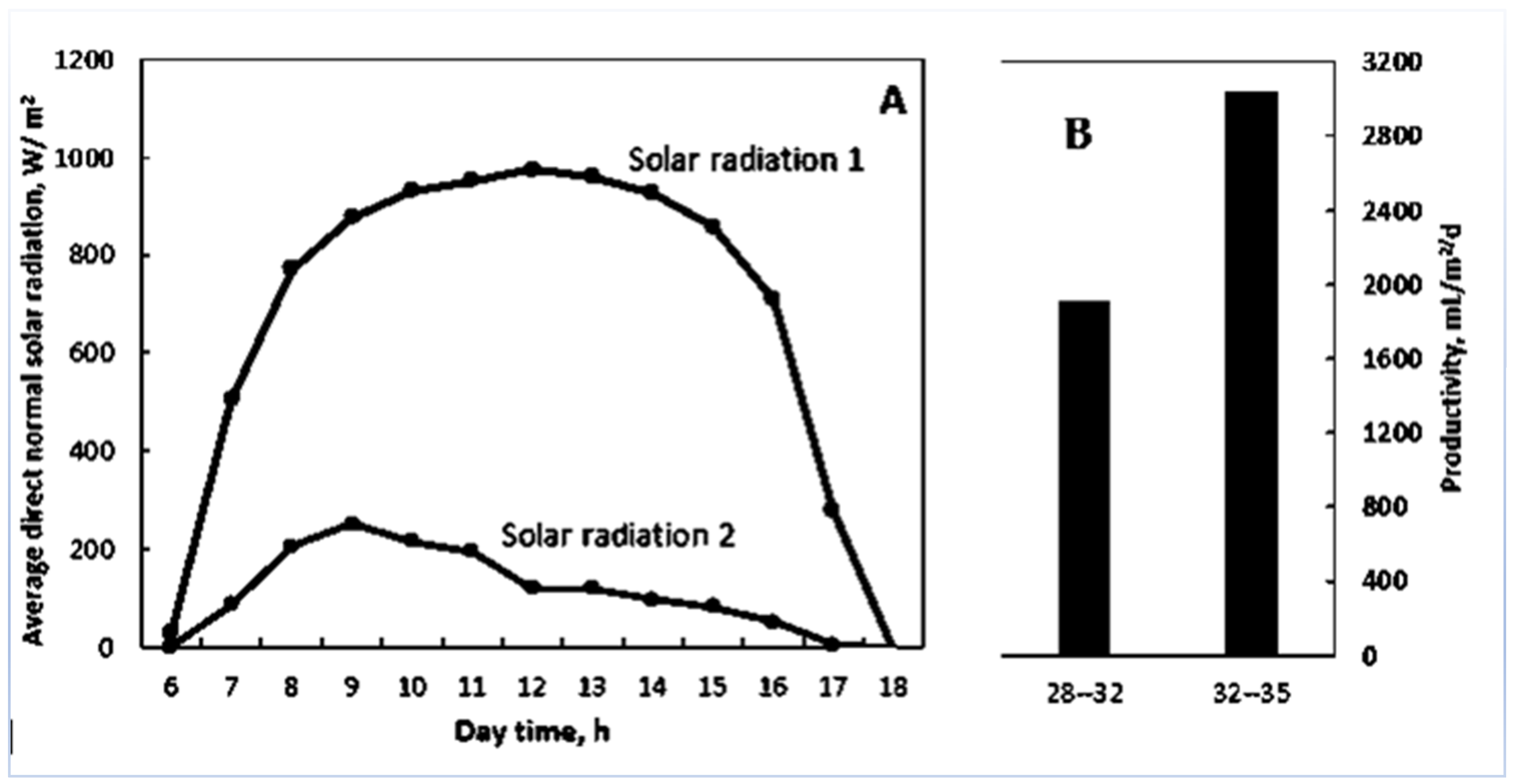
Figure 3A shows the average direct normal solar radiation (W/m
2) from 6:00 a.m. to 6:00 p.m. during the second cycle.
During this cycle, there were 3 days (days 29, 30, and 31) of low solar radiation (solar radiation 2) contrary to the remaining days (solar radiation 1). These 3 days coincided with a decrease in productivity from 2.88 to 1.91 kg/m
2/d (
Figure 3B).
According to the present study, it is evident that solar radiation affected the phytodesalinator daily productivity through its effect on
S. portulacastrum transpiration. Upon soilless culture under greenhouse conditions, cucumber showed a 4-fold higher diurnal canopy transpiration rate at high solar radiation (up to 20 MJ/m
2/d) than at low solar radiation (up to 9 MJ/m
2/d) [
21]. Nevertheless, it was found that the same solar radiation levels had different transpiration rates in winter and summer, which may be partially due to differences in stomatal and aerodynamic conductance and in vapor pressure deficit [
22].
3.3.4. Plant Density
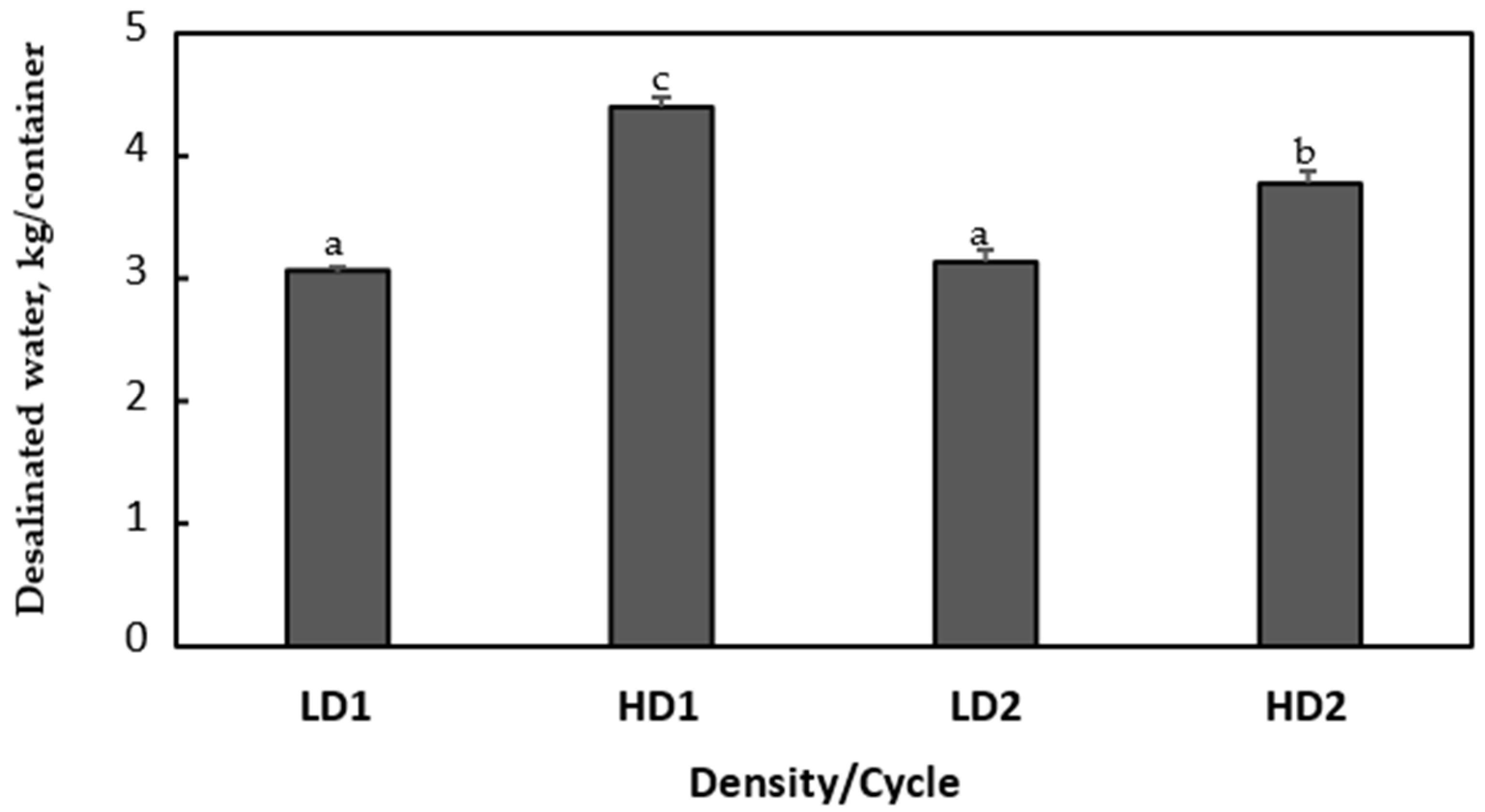
The comparison between the cumulative quantities of desalinated water per container obtained in each phytodesalination cycle with low (LD1 and LD2) and high (HD1 and HD2) plant densities showed a constant value for low plant density (3.1 kg/container) in both cycles (
Figure 4). As for high plant density, a high value was observed in the first cycle (4.4 kg/container) followed by a lower one (3.8 kg/container) in the second cycle.
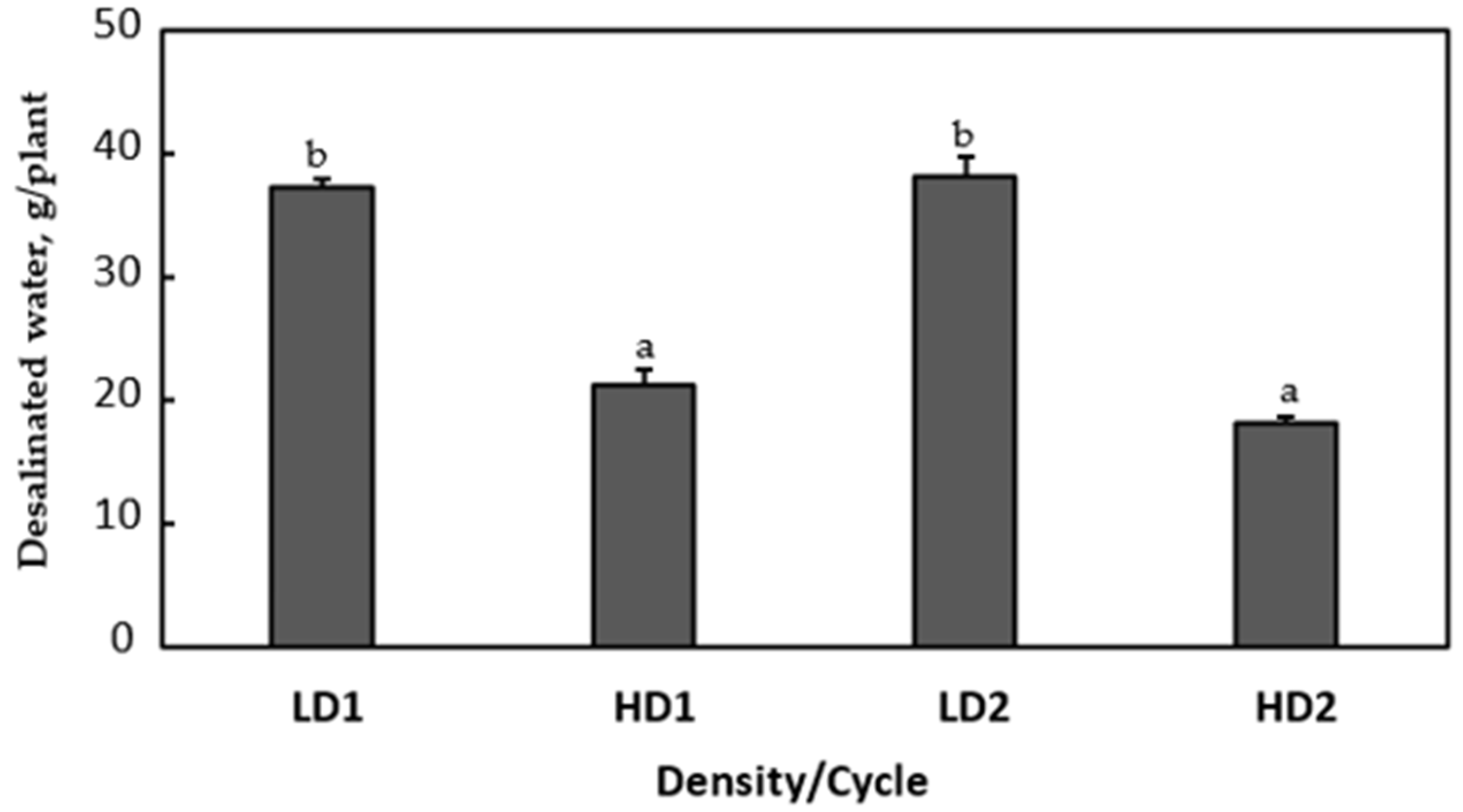
Considering the cumulative volumes of desalinated water per plant obtained in each phytodesalination cycle, it was obvious that, although the total volume of desalinated water was higher for HD plants, plants grown in low density were more able to desalinate brine water (37.3–38.2 g/plant) as compared to plants grown in high density (18.2–21.2 g/plant;
Figure 5).
With a simple estimation, it was found that LD exhibited throughout the two cycles an average productivity of 2.21 kg/m
2/d, while HD displayed an average productivity of 2.91 kg/m
2/d. The difference between the two values was not high considering the huge difference in densities, HD being 2.5-fold higher than LD. Consequently, the individual plant phytodesalination yield was much higher in LD compared to HD (
Figure 5). This can be partially explained by the faster increase in reject brine salinity in HD since the phytodesalination process was faster at the level of the whole container.
3.4. Reject Brine Salinity after the Two Cycles of Phytodesalination
At the beginning of the experiment and after the addition of nutrients to the reject brine, EC was about 16 dS/m in both LD and HD containers (
Table 2). After the first phytodesalination cycle, it increased by 61 and 123%, respectively. At the beginning of the second cycle, desalinated water quantities were replaced by equal quantities of reject brine, then nutrients were added. The second cycle started with EC values of about 20 dS/m for both LD and HD containers. These values increased by, respectively, 79 and 145% at the end of the experiment.
3.5. Plant Mineral Composition
Plant Na
+, K
+, Ca
++, and Mg
++ contents at the beginning of the experiment and at the end of each phytodesalination cycle are presented in
Table 3. It seems that this halophyte maintained a relatively constant mineral composition during the experiment, except in the case of its K
+ content, which increased from 30.2 mg/g DW at the beginning of the experiment to 51.6 mg/g DW at its end with no significant difference between the two phytodesalination cycles. Hence, plant tissues were not invaded by salt ions, which indicated its ability to control ion uptake and transport [
23].
3.6. Cost Analysis
The device cost/meter squared was USD 125. The expected device life is about 20 years. It was estimated also that one patch of fresh plants per 3 months costs USD 15. The plant nutrient solution was estimated to cost USD 10/month. Hence, the total annual cost could be about USD 0.45/kg of desalinated water. This unit is a prototype, and a lot of its parts were manually manufactured. If commercially produced, the cost/kg of water is expected to decrease.
3.7. Advantages of the Phytodesalinator
This device showed relatively high daily productivity. It needs only sunlight, which is a sustainable energy, but, alternatively, any other source of light can be used. It was conceived to be used during cold periods when basic solar stills cannot work. It is based on the process of plant transpiration and not on the process of evaporation. It is a closed system that does not need any kind of maintenance. It does not require a given water depth. After harvest, plants can be used for several purposes (ornamental, fodder, dune fixation, etc.). It is not an expensive device.
4. Conclusions
The presented phytodesalinator is designed to operate in regions with sunny winters and to replace basic solar stills since it does not require high temperatures for desalination. Unlike basic solar stills that need to heat water to initiate evaporation, this system maintains evaporation through a plant’s physiological process (transpiration) as long as the plant is exposed to light. However, the system’s performance is limited by the plant’s ability to tolerate heat, making it unsuitable for use under high solar radiation intensity. Despite several factors influencing its productivity, the phytodesalinator has notable advantages over basic solar stills. It can function under low-solar-radiation conditions and produces very little high-salinity water at the end of the desalination cycle compared to traditional solar stills, which require running water and produce twice the amount of saline water for each unit of desalinated water. The total annual cost is estimated to be about USD 0.45 per kilogram of desalinated water, with the cost per kilogram expected to decrease with larger units. Further research should explore the use of different types of euhalophytes and various improved designs of this prototype to reduce costs and increase productivity per unit area.