Process Energy and Material Consumption Determined by Reaction Sequence: From AAO to OHO
Abstract
1. Introduction
2. Materials and Methods
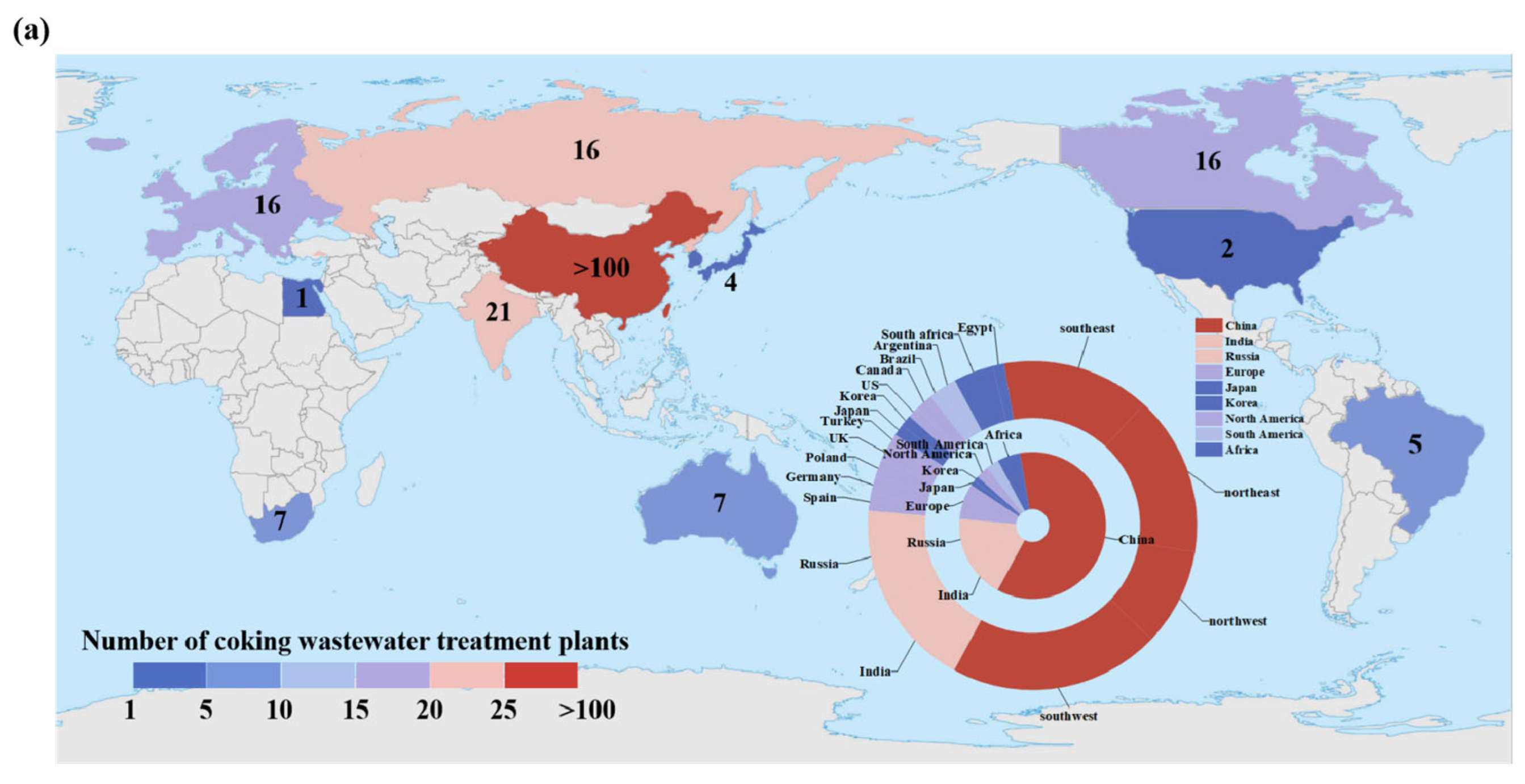
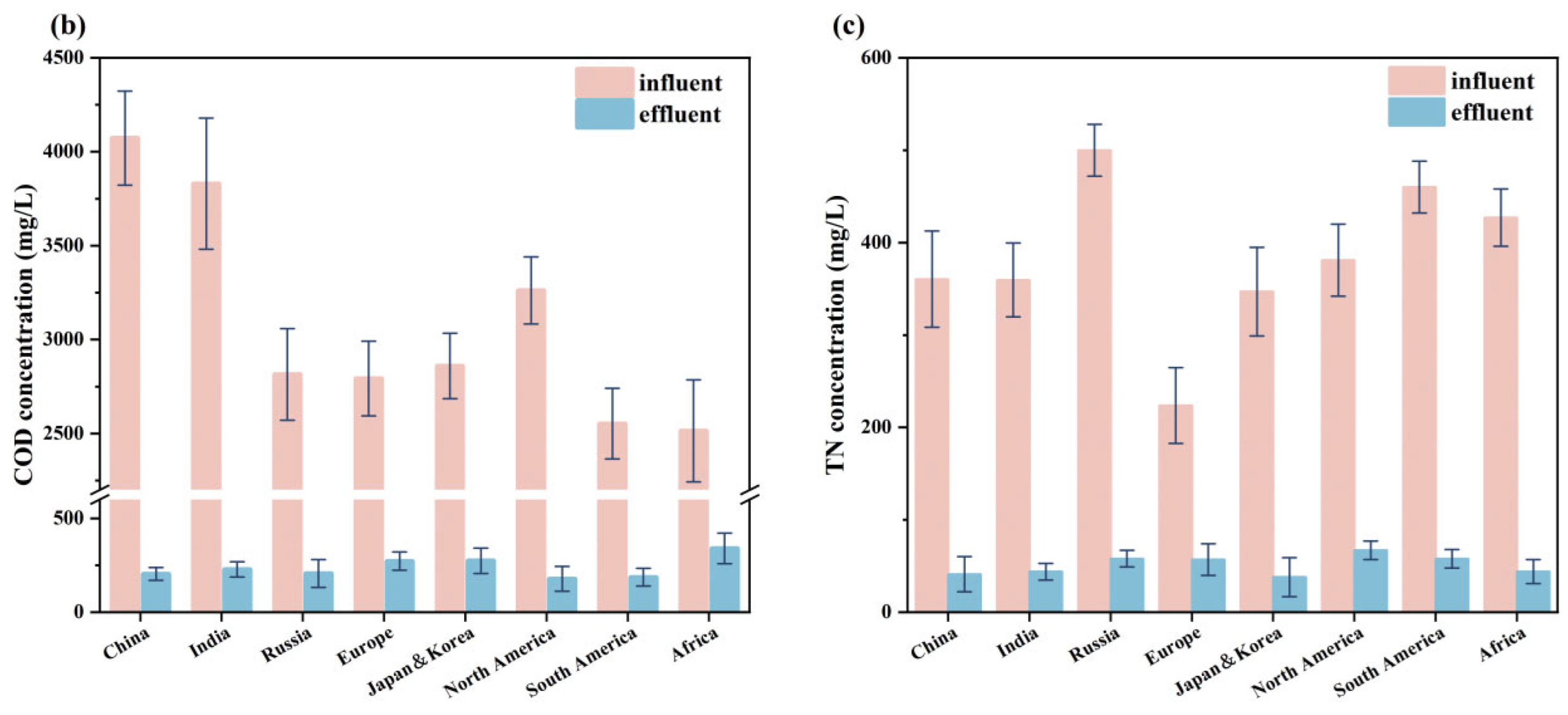
2.1. Data Collection and Analysis
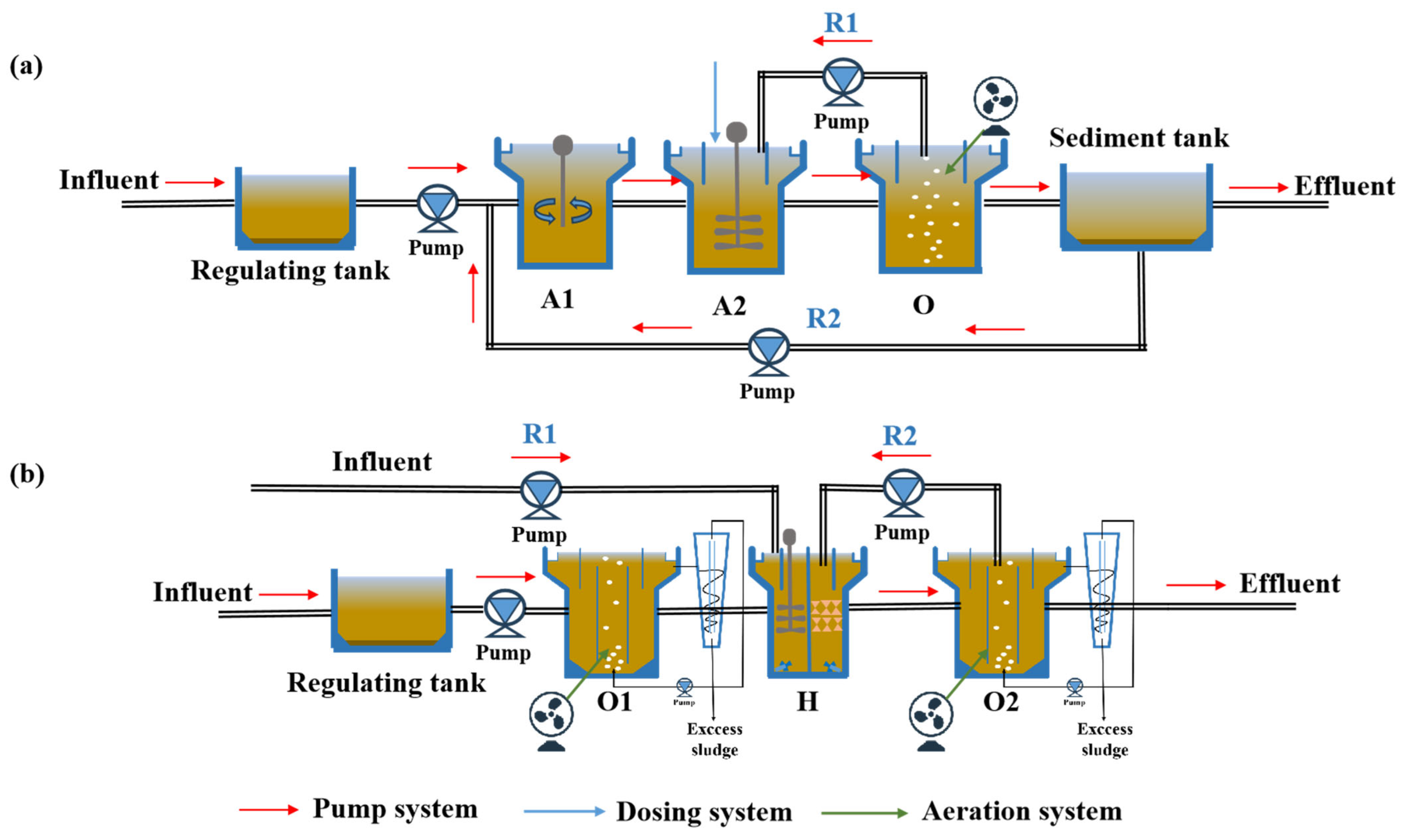
2.2. The Description of AAO and OHO
2.3. Analytic Method
2.4. Establishment of Energy Consumption Calculation Model
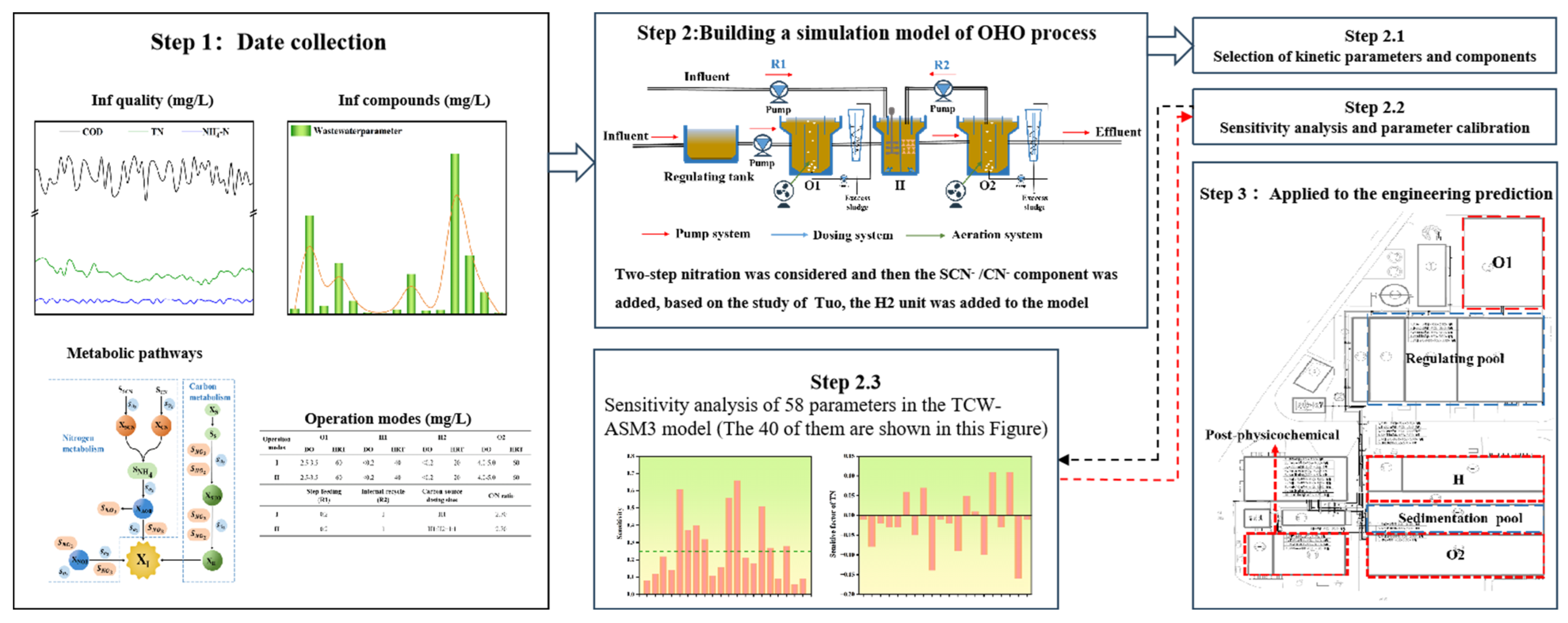
2.5. TCW-ASM3 Model Preparation
3. Results
3.1. Analysis of the Effect of Process Combinations on Pollutant Removal
| Parameter | Min | Max | Tolerance Limit | Partial Reference | |
|---|---|---|---|---|---|
| 1 | pH | 6.4 | 10.3 | 5.5~6.0 | Liu et al., 2020 [29], Smol et al., 2018 [30], Na et al., 2017 [31], S. Chakraborty et al., 2006 [32], Stefanos Giannakis et al., 2021 [33], Sohail et al., 2023 [34] |
| 2 | T, °C | 34 | 52 | / | Zhao et al., 2009 [35], Smol et al., 2018 [30], Chai et al., 2018 [36] |
| 3 | COD | 880 | 14,900 | 280 | Liu et al., 2020 [29], Zhao et al., 2009 [35], Zhao et al., 2009 [37], Smol et al., 2018 [30], Na et al., 2017 [31], E. Marañón et al., 2008 [38], Chakraborty et al., 2006 [32] |
| 4 | BOD | 303 | 4600 | 87 | Smol et al., 2018 [30], E. Marañón et al., 2008 [38], E. Raper et al., 2019 [39] |
| 5 | DO | / | / | 3.5~4.5 | Yang et al., 2018 [40], Vázquez et al., 2006 [41] |
| 6 | TOC | 770 | 4400 | / | Smol et al., 2018 [30], Stefanos Giannakis et al., 2021 [33], Stefanos Giannakis et al., 2021 [33] |
| 7 | 90 | 708 | 57 | Liu et al., 2020 [29], Zhao et al., 2009 [35], Zhao et al., 2009 [37], Smol et al., 2018 [30], Na et al., 2017 [31], E. Marañón etal., 2008 [38], Chakraborty et al., 2006 [32], Saswati Chakraborty et al., 2002 [42] | |
| 8 | 0.1 | 0.3 | / | Liu et al., 2020 [29], Smol et al., 2018 [30], E. Raper et al., 2019 [39] | |
| 9 | 1.8 | 3.3 | 1.1 | Liu et al., 2020 [29], Zhao et al., 2009 [37], Smol et al., 2018 [30] | |
| 10 | N | 58 | 108 | 37 | Yang et al., 2018 [40], Vázquez et al., 2006 [41] |
| 11 | TN | 370 | 1820 | 144 | Liu et al., 2020 [29], Zhao et al., 2009 [37], Smol et al., 2018 [30], E. Raper et al., 2019 [39] |
| 12 | Total cyanides | 16 | 416 | 5 | Smol et al., 2018 [30], E. Marañón etal., 2008 [38], Chakraborty et al., 2006 [32], Saswati Chakraborty et al., 2002 [42], Cameron et al., 2007 [43] |
| 13 | Thiocyanate | 30 | 780 | / | E. Marañón etal., 2008 [38], Chakraborty et al., 2006 [32], 2006, Saswati Chakraborty et al., 2002 [42], E. Raper et al., 2019 [39], Cameron et al., 2007 [43] |
| 14 | Oil and grease | 5.3 | 80 | 10 | Smol et al., 2018 [30], E. Marañón etal., 2008 [38] |
| 15 | Sulfide | 28.8 | 1400 | 45.2 | Saswati Chakraborty et al., 2002 [42], Rodríguez-Iglesias et al., 2022 [44] |
| 16 | SS | 143 | 360 | 113 | Smol et al., 2018 [30], E. Marañón etal., 2008 [38], Chakraborty et al., 2006 [32], E. Raper et al., 2019 [39], Cameron et al., 2007 [43] |
| 17 | TDS | / | 1521.35 | / | Stefanos Giannakis et al., 2021 [33] |
| 18 | Chlorides | / | 2730 | / | Liu et al., 2020 [29], Zhao et al., 2009 [35], Chai et al., 2018 [36], Rodríguez-Iglesias et al., 2022 [44] |
| 19 | B/C | 0.08 | 0.89 | 0.03 | Stefanos Giannakis et al., 2021 [33], Yang et al., 2018 [40] |
| 20 | ORP, mV | −330 | −78 | 50 | Sohail et al., 2023 [34] |
| 21 | Conductivity, µs/cm | 3457 | 12,875 | 1245 | Smol et al., 2018 [30], 2018, E. Raper et al., 2019 [39] |
| 22 | Alkalinity | 220 | 650 | 180 | Zhao et al., 2009 [35], Smol et al., 2018 [30] |
| 23 | Turbidity | 71.2 | 691 | / | Zhao et al., 2009 [35], Zhao et al., 2009 [37], Smol et al., 2018 [30], Sohail et al., 2023 [34], |
| 24 | Hardness | / | 462.76 | 300 | Smol et al., 2018 [30], Rodríguez-Iglesias et al., 2022 [44] |
| 25 | Color | / | 2.5 × 104 | 500 | Zhao et al., 2009 [35], Smol et al., 2018 [30], Sohail et al., 2023 [34] |
3.2. Unit Reaction Analysis and Economic Cost Accounting
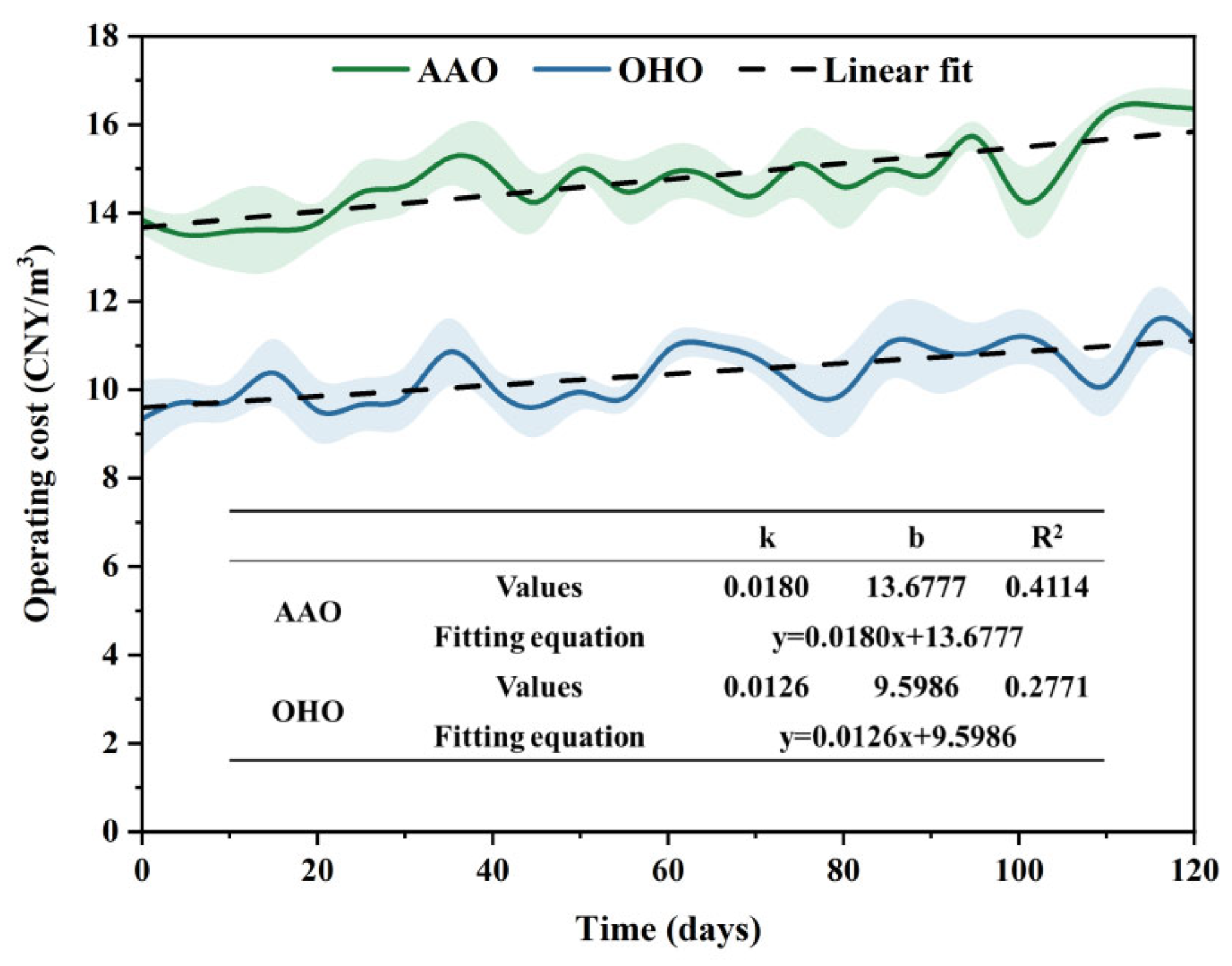
3.3. Actual Engineering Verification
3.4. OHO Performance Optimization Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reid, E.; Igou, T.; Zhao, Y.; Crittenden, J.; Huang, C.; Westerhoff, P.; Rittmann, B.; Drewes, J.E.; Chen, Y. The Minus Approach Can Redefine the Standard of Practice of Drinking Water Treatment. Environ. Sci. Technol. 2023, 57, 7150–7161. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, A.; de Sario, S.; Attanasio, A.; Di Capua, F.; Gorgoglione, A.; Fratino, U.; Mascolo, M.C.; Pirozzi, F.; Trancone, G.; Spasiano, D. Phosphorus recovery as struvite and hydroxyapatite from the liquid fraction of municipal sewage sludge with limited magnesium addition. J. Environ. Qual. 2023, 52, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, P.; Yang, Z.; Judd, S.J. The status of potable water reuse implementation. Water Res. 2022, 214, 118198. [Google Scholar] [CrossRef] [PubMed]
- Trancone, G.; Spasiano, D.; Race, M.; Luongo, V.; Petrella, A.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. A combined system for asbestos-cement waste degradation by dark fermentation and resulting supernatant valorization in anaerobic digestion. Chemosphere 2022, 300, 134500. [Google Scholar] [CrossRef] [PubMed]
- Mažeikienė, A.; Grubliauskas, R. Biotechnological wastewater treatment in small-scale wastewater treatment plants. J. Clean. Prod. 2021, 279, 123750. [Google Scholar] [CrossRef]
- An, H.; Qian, Y.; Gu, X.; Tang, W.Z. Biological treatment of dye wastewaters using an anaerobic-oxic system. Chemosphere 1996, 33, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Li, Z.; Pan, J.; Fu, B.; Wei, J.; Chen, B.; Yang, X.; Ye, G.; Wei, C.; Luo, P.; et al. An Oxic–Hydrolytic–Oxic Process at the Nexus of Sludge Spatial Segmentation, Microbial Functionality, and Pollutants Removal in the Treatment of Coking Wastewater. ACS ES&T Water 2021, 1, 1252–1262. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Q.; Wu, Q.; Zhang, J.; Dzakpasu, M.; Wang, X.C. Nitrogen removal efficiency and mechanisms of an improved anaerobic-anoxic–oxic system for decentralized sewage treatment. Bioresour. Technol. 2024, 393, 129976. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, A.; Li, Z.; Ke, X.; Zhang, H.; Cheng, X.; Liang, Y.; Wei, G.; Qin, Z.; Guan, X.; et al. O/H/H/O Process for Total Nitrogen Removal: An Upgrade of the A/A/O Process for Coking Wastewater Treatment. Acs Est. Eng. 2023, 3, 1236–1247. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Chen, J.; Wang, X.; Li, D.; Hou, J.; He, X. Advanced nitrogen and phosphorus removal by combining endogenous denitrification and denitrifying dephosphatation in constructed wetlands. J. Environ. Manag. 2021, 294, 112967. [Google Scholar] [CrossRef]
- Ferro, T.N.; de Carvalho, K.Q.; de Lima, M.X.; Barana, A.C.; Kreutz, C.; Gauza, O.R.; Passig, F.H. Influence of HRT and carbon source on the enhancement of nutrient removal in an Anaerobic-Oxic-Anoxic (AOA) system. Environ. Technol. 2022, 43, 2478–2491. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Gong, B.; Wang, Y.; Lin, Z.; He, L.; Zhou, J.; He, Q. Metagenomic analysis reveals enhanced nutrients removal from low C/N municipal wastewater in a pilot-scale modified AAO system coupling electrolysis. Water Res. 2020, 173, 115530. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Fierro, V.; Sanhueza, J.; Arriagada, C.; Pereira, L.; Campos, V.; Gallardo, J.J.; Roeckel, M. The prediction of partial-nitrification-anammox performance in real industrial wastewater based on granular size. J. Environ. Manag. 2021, 286, 112255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Zhao, Y.; Cui, Y.; Zhang, Y.; Dai, H.; Li, D. Discrepant responses of polyvinyl chloride microplastics biofilms and activated sludge under sulfadiazine stress in an anaerobic/anoxic/oxic system. Chem. Eng. J. 2022, 446, 137055. [Google Scholar] [CrossRef]
- Li, K.; Wu, H.; Wei, J.; Qiu, G.; Wei, C.; Cheng, D.; Zhong, L. Simultaneous decarburization, nitrification and denitrification (SDCND) in coking wastewater treatment using an integrated fluidized-bed reactor. J. Environ. Manag. 2019, 252, 109661. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, W.; Li, Y.; Shao, Y.; Zhang, Z.; Zhang, M.; He, J.; Qiu, J.; Li, W.; Wang, J.; et al. Applying mass flow analysis and aeration optimization strategy to reduce energy consumption of a full-scale anaerobic/anoxic/oxic system. J. Water Process. Eng. 2023, 54, 104037. [Google Scholar] [CrossRef]
- Chen, B.; Wei, G.; Zhang, T.; Wu, H.; Wu, C.; Chen, A.; Zhang, H.; Guan, X.; Ren, Y.; Feng, C.; et al. Flow drag force contributes high bio-treatment efficiency in a circulating fluidized bed reactor: Mechanism of selective separation of functional decayed sludge. Chem. Eng. J. 2023, 454, 140448. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, H.; Wu, C.; Qiu, G.; Feng, C.; Wei, C. Structure and function of microbial community involved in a novel full-scale prefix oxic coking wastewater treatment O/H/O system. Water Res. 2019, 164, 114963. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, J.; Zhao, L.; Liu, W.; Chen, L.; Cai, T.; Ji, X. Succession of microbial communities reveals the inevitability of anammox core in the development of anammox processes. Bioresour. Technol. 2023, 371, 128645. [Google Scholar] [CrossRef]
- Jeremias, J.S.D.; Lin, J.; Dalida, M.L.P.; Lu, M. Abatement technologies for copper containing industrial wastewater effluents—A review. J. Environ. Chem. Eng. 2023, 11, 109336. [Google Scholar] [CrossRef]
- Muscetta, M.; Bianco, F.; Trancone, G.; Race, M.; Siciliano, A.; Agostino, F.D.; Sprovieri, M.; Clarizia, L. Washing Bottom Sediment for The Removal of Arsenic from Contaminated Italian Coast. Processes 2023, 11, 902. [Google Scholar] [CrossRef]
- Garnier, J.; Laroche, L.; Pinault, S. Determining the domestic specific loads of two wastewater plants of the Paris conurbation (France) with contrasted treatments: A step for exploring the effects of the application of the European Directive. Water Res. 2006, 40, 3257–3266. [Google Scholar] [CrossRef]
- Li, Z.; Wei, C.; Chen, Y.; Chen, B.; Qiu, G.; Wan, J.; Wu, H.; Zhu, S.; Zhao, H. Achieving nitritation in an aerobic fluidized reactor for coking wastewater treatment: Operation stability, mechanisms and model analysis. Chem. Eng. J. 2021, 406, 126816. [Google Scholar] [CrossRef]
- Wei, T.; Ban, Z.; Ke, X.; Chen, A.; Guan, X.; Gan, H.; Pan, J.; Li, Z.; Wei, C.; Qiu, G.; et al. A combined process model for wastewater treatment based on hydraulic retention time and toxicity inhibition. Chemosphere 2023, 329, 138660. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Pan, J.; Ke, X.; Gan, H.; Chen, A.; Guan, X.; Ban, Z.; Li, Z.; Wei, G.; Wei, C.; et al. Evaluation of Carbon and Nitrogen Removal Performance of the Oxic-Hydrolytic and Denitrification-Oxic Process in Coking Wastewater Treatment. ACS ES&T Water 2023, 3, 236–245. [Google Scholar] [CrossRef]
- Guo, H.; Yao, H.; Huang, Q.; Li, T.; Show, D.; Ling, M.; Yan, Y.; Show, K.; Lee, D. Anaerobic–anoxic–oxic biological treatment of high-strength, highly recalcitrant polyphenylene sulfide wastewater. Bioresour. Technol. 2023, 371, 128640. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Guan, X.; Pang, Z.; Ke, X.; Qin, Z.; Chen, Y.; Wei, G.; Wu, H.; Qiu, G.; Hu, Y.; et al. Catalytic oxidation of biorefractory cyanide-containing coking wastewater by deconjugation effect of bimetal copper-loaded activated carbon. J. Environ. Chem. Eng. 2023, 11, 111283. [Google Scholar] [CrossRef]
- Wei, G.; Wei, T.; Li, Z.; Wei, C.; Kong, Q.; Guan, X.; Qiu, G.; Hu, Y.; Wei, C.; Zhu, S.; et al. BOD/COD ratio as a probing index in the O/H/O process for coking wastewater treatment. Chem. Eng. J. 2023, 466, 143257. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Z.; Peng, P.; Xie, H.; Li, X.; Xu, J.; Li, W. A pilot-scale three-dimensional electrochemical reactor combined with anaerobic-anoxic-oxic system for advanced treatment of coking wastewater. J. Environ. Manag. 2020, 258, 110021. [Google Scholar] [CrossRef]
- Smol, M.; Włóka, D.; Włodarczyk-Makuła, M. Influence of Integrated Membrane Treatment on the Phytotoxicity of Wastewater from the Coke Industry. Water Air Soil Pollut. 2018, 229, 154. [Google Scholar] [CrossRef]
- Na, C.; Zhang, Y.; Quan, X.; Chen, S.; Liu, W.; Zhang, Y. Evaluation of the detoxification efficiencies of coking wastewater treated by combined anaerobic-anoxic-oxic (A2O) and advanced oxidation process. J. Hazard Mater. 2017, 338, 186–193. [Google Scholar] [CrossRef]
- Chakraborty, S.; Veeramani, H. Effect of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate and ammonia in an anaerobic–anoxic–aerobic continuous system. Process. Biochem. 2006, 41, 96–105. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Sohail, N.F.; Zeshan; Iftikhar, R.; Saleem, S. Microalgal treatment of high-nutrient wastewater using twin layer cultivation system. J. Environ. Chem. Eng. 2023, 11, 109248. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Lee, D. Enhanced treatment of coke plant wastewater using an anaerobic–anoxic–oxic membrane bioreactor system. Sep. Purif. Technol. 2009, 66, 279–286. [Google Scholar] [CrossRef]
- Chai, Q.; Hu, A.; Qian, Y.; Ao, X.; Liu, W.; Yang, H.; Xie, Y.F. A comparison of genotoxicity change in reclaimed wastewater from different disinfection processes. Chemosphere 2018, 191, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Huang, X.; Lee, D.; Wang, X.; Shen, Y. Use of submerged anaerobic–anoxic–oxic membrane bioreactor to treat highly toxic coke wastewater with complete sludge retention. J. Membrane Sci. 2009, 330, 57–64. [Google Scholar] [CrossRef]
- Marañón, E.; Vázquez, I.; Rodríguez, J.; Castrillón, L.; Fernández, Y. Coke Wastewater Treatment by a Three-Step Activated Sludge System. Water Air Soil Pollut. 2008, 192, 155–164. [Google Scholar] [CrossRef]
- Raper, E.; Fisher, R.; Anderson, D.R.; Stephenson, T.; Soares, A. Nitrogen removal from coke making wastewater through a pre-denitrification activated sludge process. Sci. Total Environ. 2019, 666, 31–38. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Hua, M.; Zhang, Y.; Shi, X. Characterization of effluent organic matter from different coking wastewater treatment plants. Chemosphere 2018, 203, 68–75. [Google Scholar] [CrossRef]
- Vázquez, I.; Rodríguez, J.; Marañón, E.; Castrillón, L.; Fernández, Y. Simultaneous removal of phenol, ammonium and thiocyanate from coke wastewater by aerobic biodegradation. J. Hazard Mater. 2006, 137, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Veeramani, H. Anaerobic-anoxic-aerobic sequential degradation of synthetic wastewaters. Appl. Biochem. Biotech. 2002, 102, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Staib, C.; Lant, P. Thiocyanate degradation during activated sludge treatment of coke-ovens wastewater. Biochem. Eng. J. 2007, 34, 122–130. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, J.; Alcalá, L.; Megido, L.; Castrillón, L. Removal of fluoride from coke wastewater by aluminum doped chelating ion-exchange resins: A tertiary treatment. Environ. Sci. Pollut. R 2022, 29, 8705–8715. [Google Scholar] [CrossRef] [PubMed]
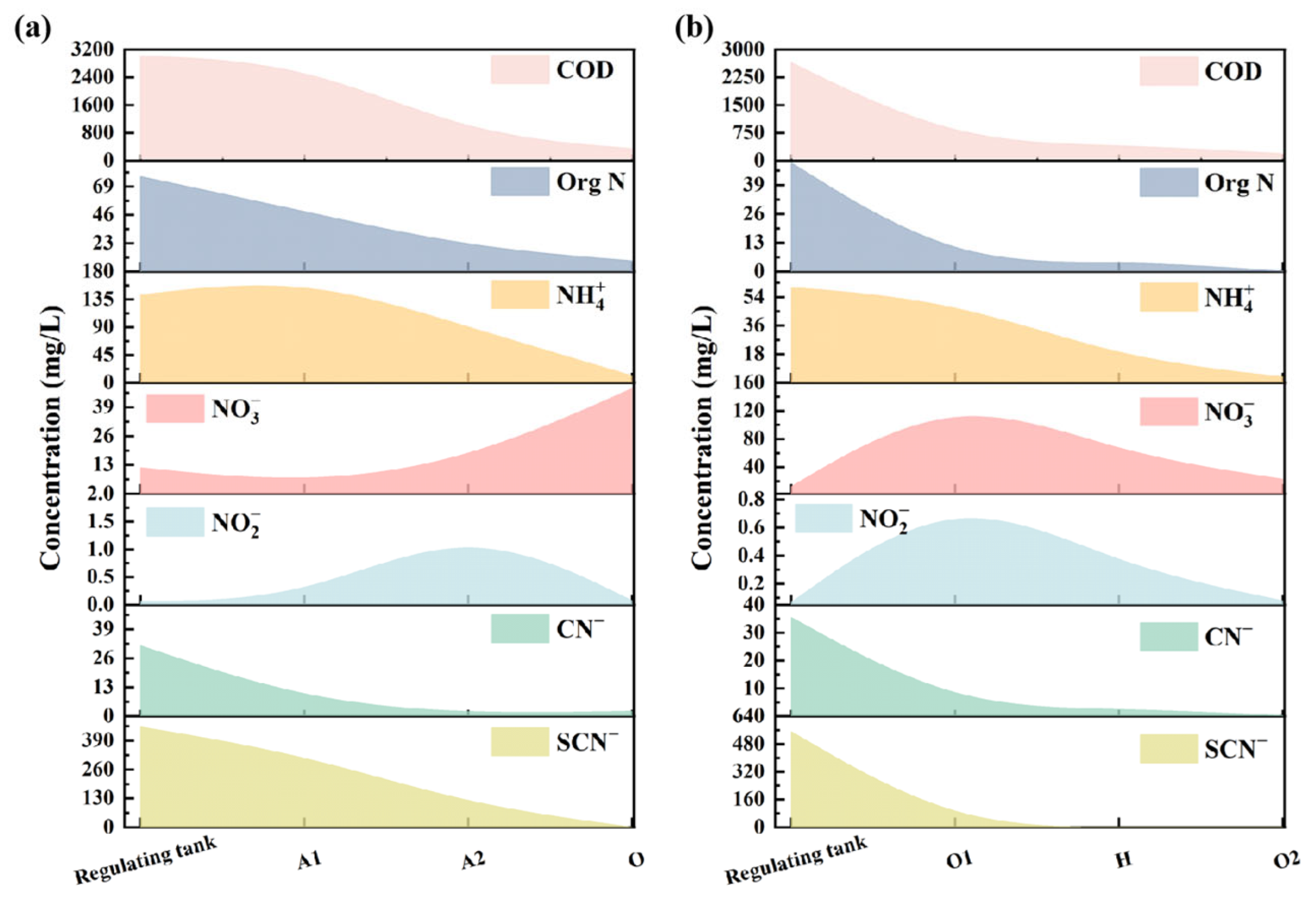


| Unit | Functions | Removal | Putative Biotransformation |
|---|---|---|---|
| O tank of AAO | Decarburization Nitrification Biotransformation of refractory organics | COD TN -N -N CN− SCN− | Organics + O2 → H2O + CO2 + H2O |
| O1 tank of OHO | Biodegradation of most biodegradable compounds Denitrogen Ammonification Partial nitrification | COD TN -N CN− SCN− | Organics + O2 → H2O + CO2 CN− + O2 + H2O → N2 + CO2 + OH− |
| O2 tank of OHO | Complete nitrification and mineralization Biotransformation of refractory organics | COD TN -N CN− SCN− | Organics + O2 → H2O + CO2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Ke, X.; Wei, T.; Chen, Y.; Qin, Z.; Chen, A.; Zhang, H.; Huang, H.; Yang, Y.; Qiu, G.; et al. Process Energy and Material Consumption Determined by Reaction Sequence: From AAO to OHO. Water 2024, 16, 1796. https://doi.org/10.3390/w16131796
He X, Ke X, Wei T, Chen Y, Qin Z, Chen A, Zhang H, Huang H, Yang Y, Qiu G, et al. Process Energy and Material Consumption Determined by Reaction Sequence: From AAO to OHO. Water. 2024; 16(13):1796. https://doi.org/10.3390/w16131796
Chicago/Turabian StyleHe, Xuguang, Xiong Ke, Tuo Wei, Yao Chen, Zhi Qin, Acong Chen, Heng Zhang, Hua Huang, Yudi Yang, Guanglei Qiu, and et al. 2024. "Process Energy and Material Consumption Determined by Reaction Sequence: From AAO to OHO" Water 16, no. 13: 1796. https://doi.org/10.3390/w16131796
APA StyleHe, X., Ke, X., Wei, T., Chen, Y., Qin, Z., Chen, A., Zhang, H., Huang, H., Yang, Y., Qiu, G., Wu, H., & Wei, C. (2024). Process Energy and Material Consumption Determined by Reaction Sequence: From AAO to OHO. Water, 16(13), 1796. https://doi.org/10.3390/w16131796







