Changing Soil Water Content: Main Trigger of the Multi-Phase Mobilization and Transformation of Petroleum Pollution Components—Insights from the Batch Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Pollutants and Media
2.2. Experimental Devices and Processes
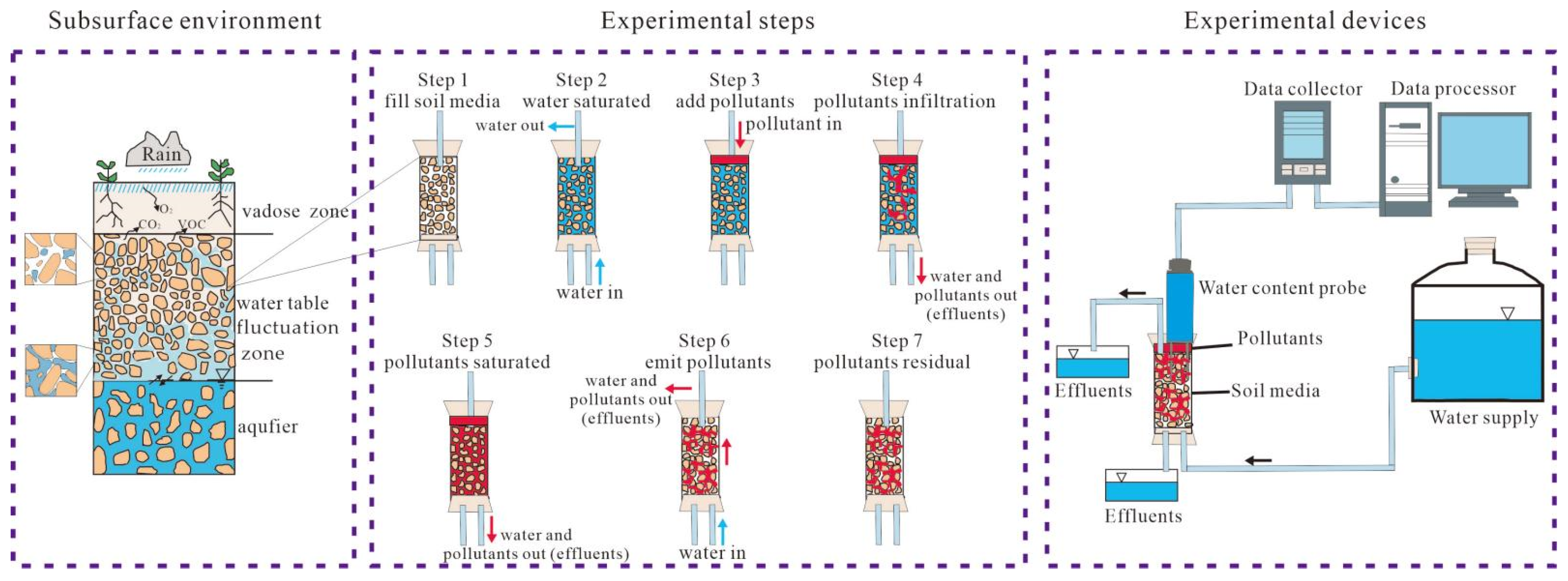
2.2.1. Experimental Aims
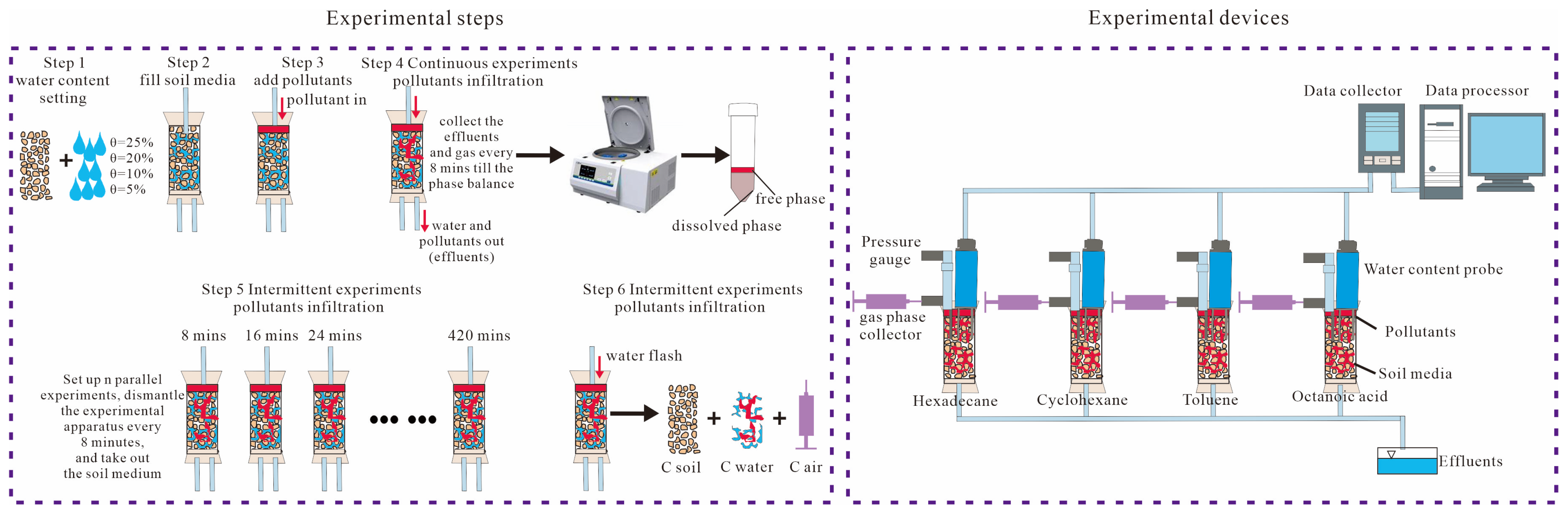
2.2.2. Experimental Devices and Steps
2.3. Sample Analysis
2.4. Saturation of Multi-Phase Pollutants
3. Results and Discussions
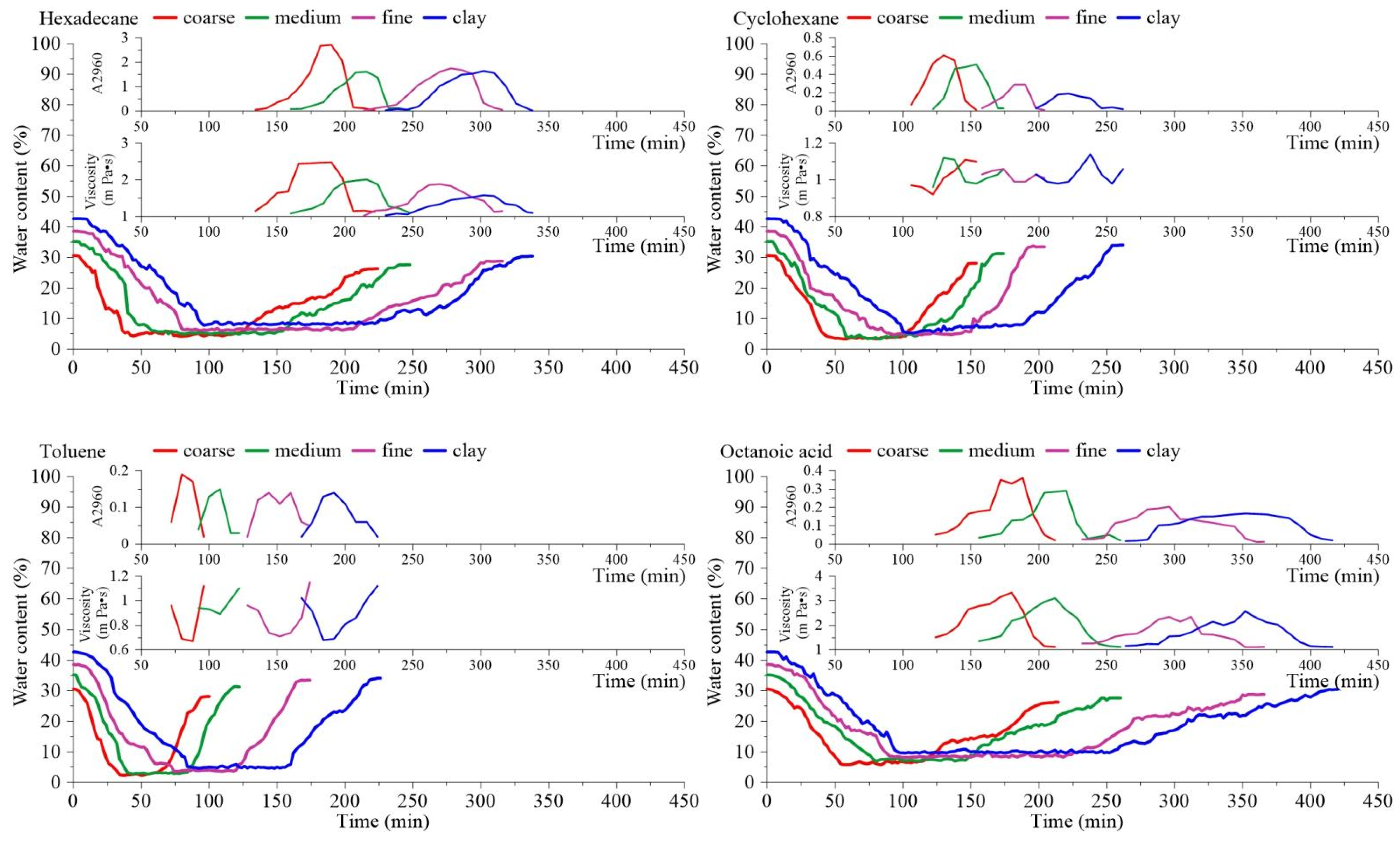
3.1. Analysis of Mobilization Morphology Characteristics of Pollution Components
3.1.1. Component Mobilization Morphology Formation Process
3.1.2. Hysteresis Effect Analysis
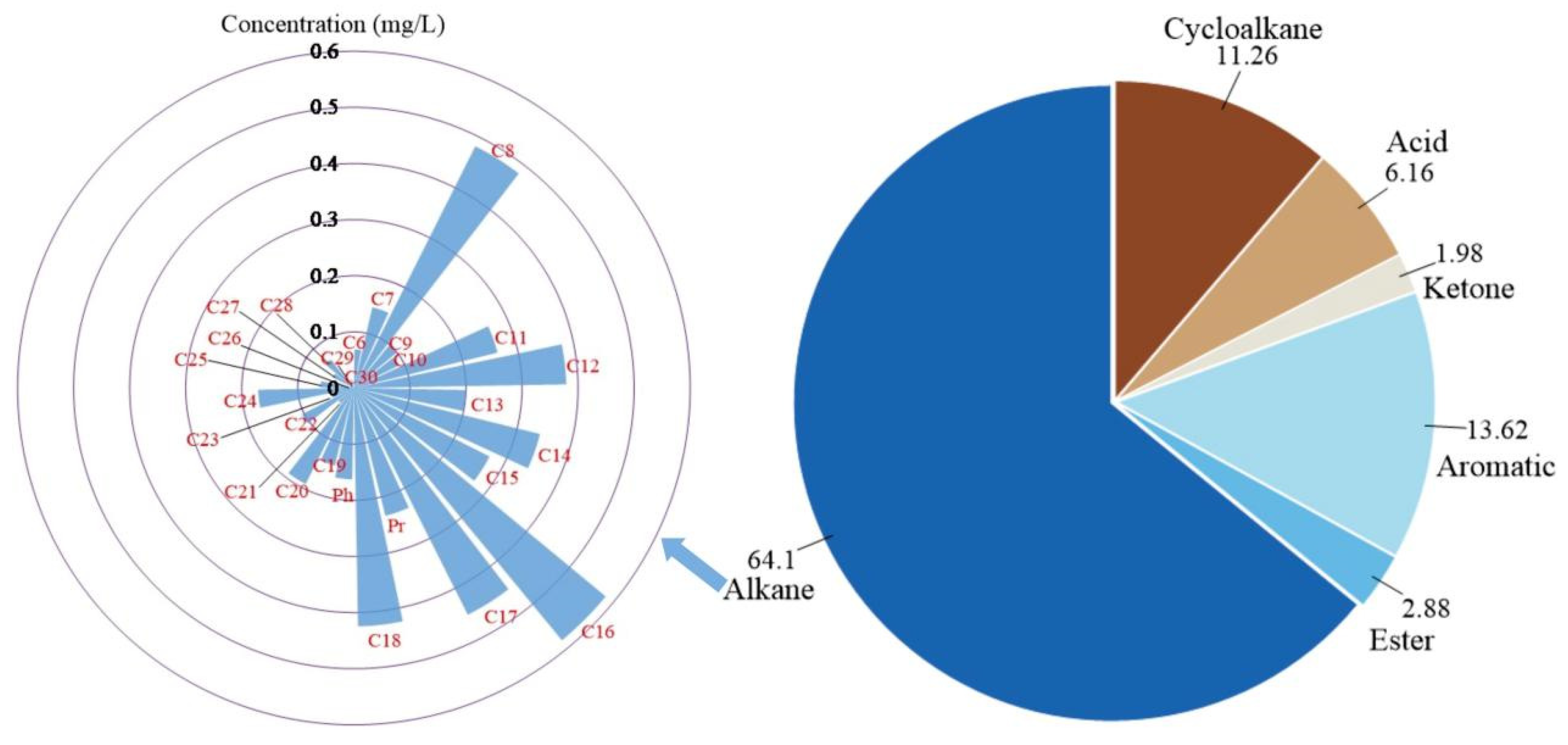
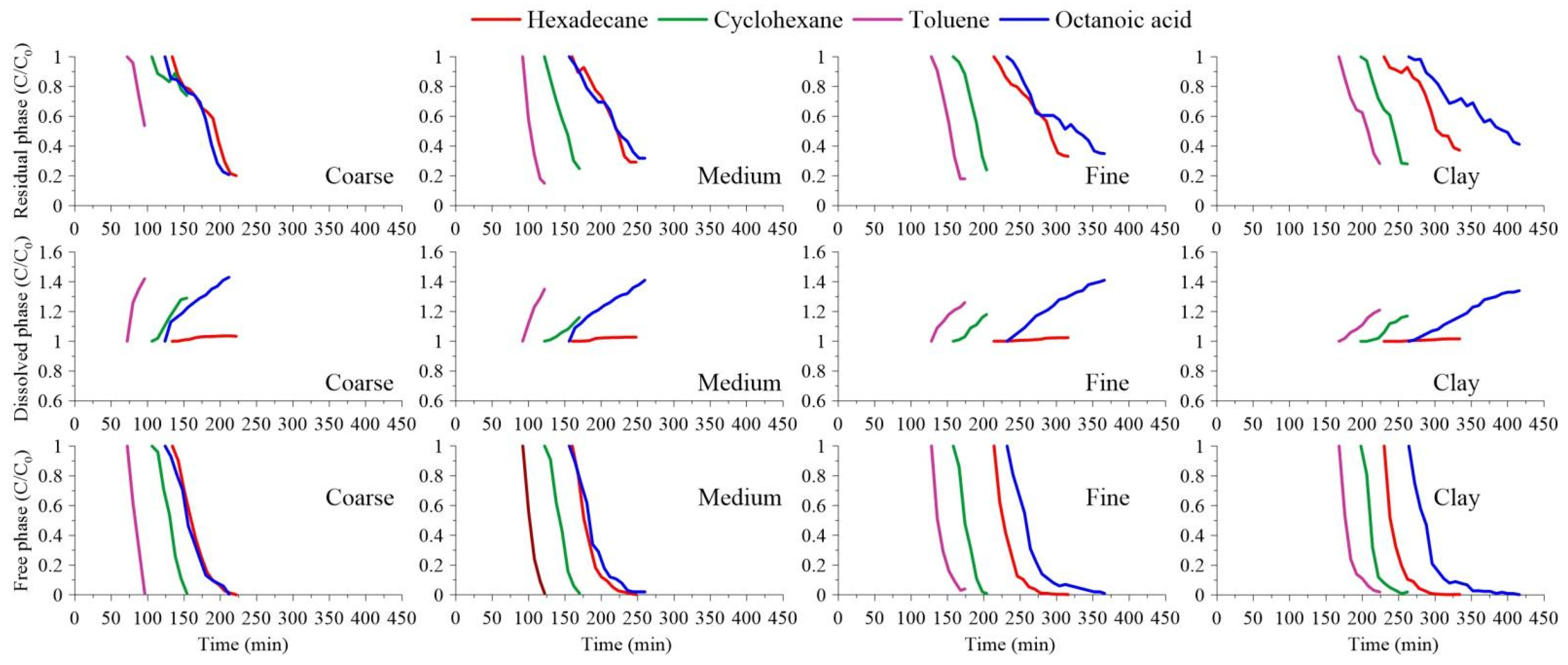
3.2. Mobilization Characteristics of Each Component
3.3. Different Transformation Processes
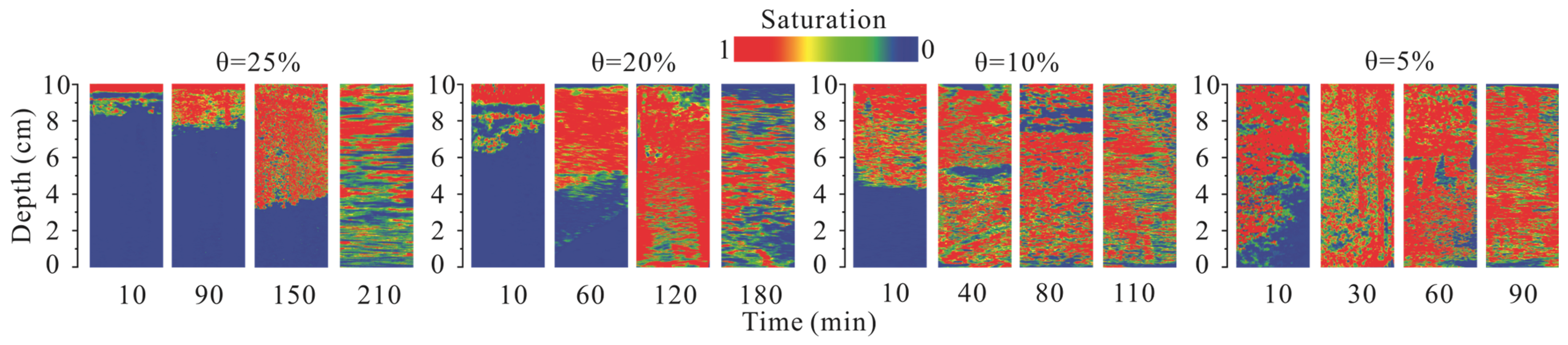
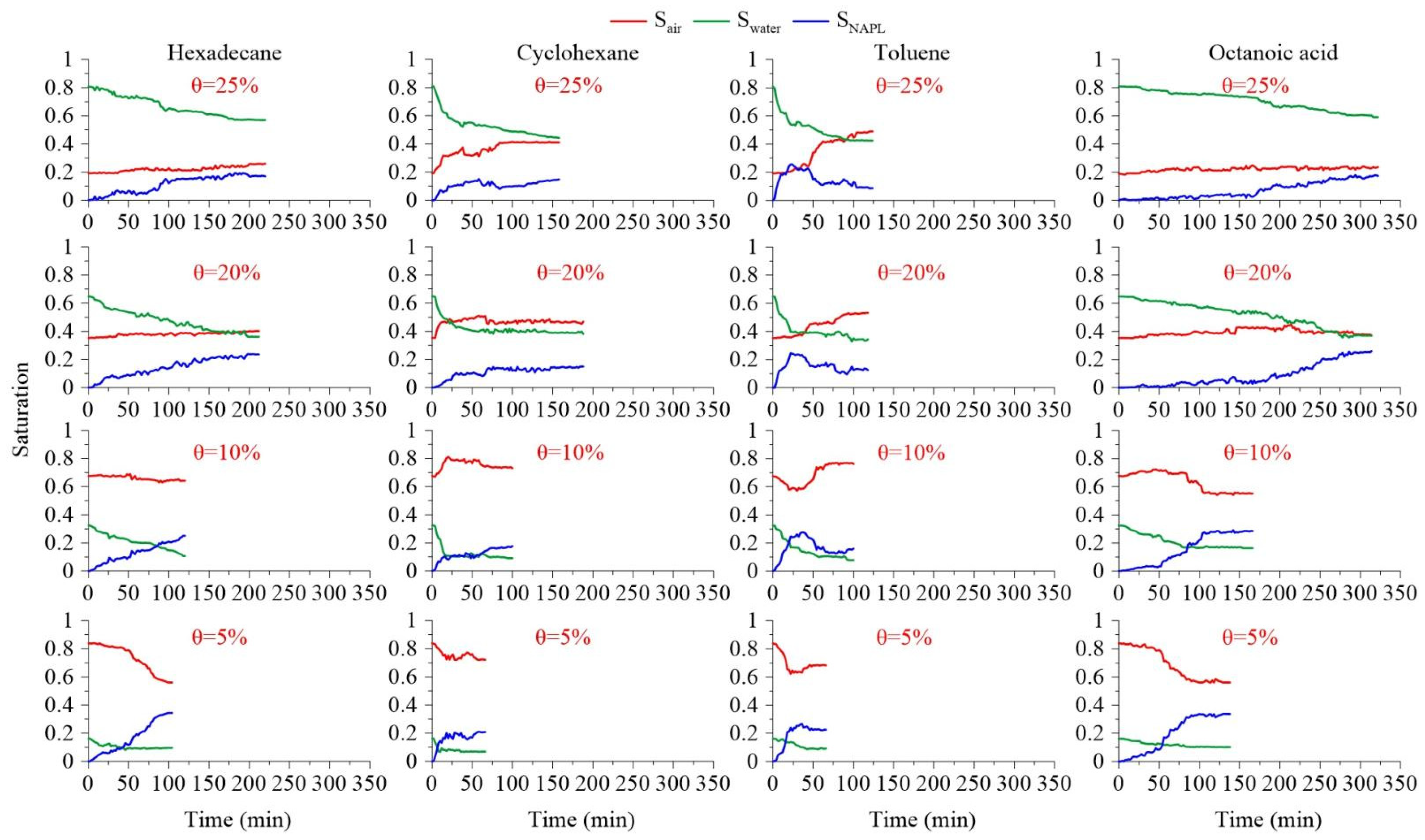
3.4. Effect of Soil Moisture Content on the Mobilization and Distribution of Pollution Components
3.4.1. Mobilization Characteristic Analysis
3.4.2. Differences in Component Mobilization under Different Moisture
Content Conditions
3.4.3. Phase Transformation Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Han, X.; Wang, G.; Mao, H.; Chen, X.; Zhou, L.; Huang, D.; Zhang, F.; Yan, X. Spatial distribution and driving factors of groundwater chemistry and pollution in an oil production region in the Northwest China. Sci. Total Environ. 2023, 875, 162635. [Google Scholar] [CrossRef] [PubMed]
- Sattar, S.; Hussain, R.; Shah, S.M.; Bibi, S.; Ahmad, S.R.; Shahzad, A.; Zamir, A.; Rauf, Z.; Noshad, A.; Ahmad, L. Composition, impacts, and removal of liquid petroleum waste through bioremediation as an alternative clean-up technology: A review. Heliyon 2022, 8, e11101. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wen, Z.; Cheng, Z.; Jakada, H. Contamination and natural attenuation characteristics of petroleum hydrocarbons in a fractured karst aquifer, North China. Environ. Sci. Pollut. R. 2020, 27, 22780–22794. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Quinn, M.; Al-Haddad, A.; Al-Khalid, A.; Al-Qallaf, H.; Rashed, T.; Bhandary, H.; Al-Salman, B.; Bushehri, A.; Boota, A. Pollution of fresh groundwater from damaged oil wells, North Kuwait. Environ. Earth Sci. 2017, 76, 145. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Athar, H.; Adetunji, C.O.; Aigbe, U.O.; Onyancha, R.B.; Abifarin, O. Environmental implications of petroleum spillages in the Niger Delta region of Nigeria: A review. J. Environ. Manag. 2021, 293, 112872. [Google Scholar] [CrossRef] [PubMed]
- Cagle, G.A.; Chen, H.; Fleeger, J.W.; Deis, D.R.; Lin, Q.; Hou, A. Spatial heterogeneity and oil pollution structured the soil microbial community in salt marshes in Barataria Bay, Louisiana, USA, eight years after the Deepwater Horizon oil spill. Ecol. Indic. 2024, 160, 111884. [Google Scholar] [CrossRef]
- Al-Mebayedh, H.; Niu, A.; Lin, C. Strategies for cost-effective remediation of widespread oil-contaminated soils in Kuwait, an environmental legacy of the first Gulf War. J. Environ. Manag. 2023, 344, 118601. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Wu, Y.; Fan, Q.; Li, P.; Liang, J.; Wang, Z.; Li, R.; Shi, L. Spatial distribution, composition, and source analysis of petroleum pollutants in soil from the Changqing Oilfield, Northwest China. Mar. Pollut. Bull. 2022, 185, 114338. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. An overview of total petroleum hydrocarbons. In Total Petroleum Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–27. [Google Scholar]
- Varjani, S.; Gnansounou, E.; Pandey, A. Comprehensive review on toxicity of persistent organic pollutants from petroleum refinery waste and their degradation by microorganisms. Chemosphere 2017, 188, 280–291. [Google Scholar] [CrossRef]
- Xu, C.; Qaria, M.A.; Xu, Q.; Zhu, D. The role of microorganisms in petroleum degradation: Current development and prospects. Sci. Total Environ. 2023, 865, 161112. [Google Scholar]
- Sheng, Y.; Zhang, X.; Zhai, X.; Zhang, F.; Li, G.; Zhang, D. A mobile, modular and rapidly-acting treatment system for optimizing and improving the removal of non-aqueous phase liquids (NAPLs) in groundwater. J. Hazard Mater. 2018, 360, 639–650. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Lai, C.; Pompilj, F.; Galli, G.; Tuccimei, P. Laboratory simulation of recent NAPL spills to investigate radon partition among NAPL vapours and soil air. Appl. Radiat. Isot. 2017, 120, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ossai, I.C.; Ahmed, A.; Hassan, A.; Hamid, F.S. Remediation of soil and water contaminated with petroleum hydrocarbon: A review. Environ. Technol. Innov. 2020, 17, 100526. [Google Scholar] [CrossRef]
- Zargar, M.; Sarrafzadeh, M.H.; Taheri, B.; Keshavarz, A. Assessment of In Situ Bioremediation of Oil Contaminated Soil and Groundwater in a Petroleum Refinery: A Laboratory Soil Column Study. Petrol. Sci. Technol. 2014, 32, 1553–1561. [Google Scholar] [CrossRef]
- Liu, J.; Wei, K.; Xu, S.; Cui, J.; Ma, J.; Xiao, X.; Xi, B.; He, X. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, P.; Guo, H.; Wang, Y. Enhanced mass transfer of residual NAPL by convection in stagnant zone. J. Hydrol. 2023, 625, 130050. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Aminnaji, M.; Rezanezhad, F.; Ghazanfari, M.H.; Babaei, M. Dissolution and remobilization of NAPL in surfactant-enhanced aquifer remediation from microscopic scale simulations. Chemosphere 2022, 289, 133177. [Google Scholar] [CrossRef]
- Mobile, M.A.; Widdowson, M.A.; Stewart, L.; Nyman, J.; Deeb, R.A.; Kavanaugh, M.C.; Mercer, J.W.; Gallagher, D.L. In-situ determination of field-scale NAPL mass transfer coefficients: Performance, simulation and analysis. J. Contam. Hydrol. 2016, 187, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Sakhaei, Z.; Daryaee, R.; Moosavi, A.A.; Carrasco-Marin, F.; Betancur, S.; Bailón-García, E.; Pérez-Cadenas, A.F.; Riazi, M. Chemical-assisted biological methods for in situ remediation of petroleum hydrocarbon-contaminated soils. In Biotechnology of Emerging Microbes; Sarma, H., Joshi, S.J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 217–261. [Google Scholar]
- Guan, J.; Huang, J.; Sun, Y.; Li, C.; Wan, Y.; Guo, W.; Kang, R.; Pang, H.; Shi, Q.; McHugh, T.J. Understanding petroleum vapor fate and transport through high resolution analysis of two distinct vapor plumes. Sci. Total Environ. 2024, 912, 169464. [Google Scholar] [CrossRef]
- Fatehbasharzad, P.; Aliasghari, S.; Tabrizi, I.S.; Khan, J.A.; Boczkaj, G. Microbial fuel cell applications for removal of petroleum hydrocarbon pollutants: A review. Water Resour. Ind. 2022, 28, 100178. [Google Scholar] [CrossRef]
- Qi, S.; Luo, J.; O’Connor, D.; Wang, Y. A numerical model to optimize LNAPL remediation by multi-phase extraction. Sci. Total Environ. 2020, 718, 137309. [Google Scholar] [CrossRef]
- Mohamed, A.; El-Menshawy, N.; Saif, A.M. Remediation of saturated soil contaminated with petroleum products using air sparging with thermal enhancement. J. Environ. Manag. 2007, 83, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Petri, B.G.; Fučík, R.; Illangasekare, T.H.; Smits, K.M.; Christ, J.A.; Sakaki, T.; Sauck, C. Effect of NAPL source morphology on mass transfer in the vadose zone. Groundwater 2014, 53, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yang, M.; Tan, T.; Song, X. Vertical transportation diversity of petroleum pollutants under groundwater fluctuations and the instructions for remediation strategy. Sustainability 2023, 15, 6514. [Google Scholar] [CrossRef]
- Sookhak, L.K.; Rayner, J.L.; Davis, G.B. Towards optimizing LNAPL remediation. Water Resour. Res. 2019, 55, 923–936. [Google Scholar] [CrossRef]
- Podgorski, D.C.; Zito, P.; Kellerman, A.M.; Bekins, B.A.; Cozzarelli, I.M.; Smith, D.F.; Cao, X.; Schmidt-Rohr, K.; Wagner, S.; Stubbins, A. Hydrocarbons to carboxyl-rich alicyclic molecules: A continuum model to describe biodegradation of petroleum-derived dissolved organic matter in contaminated groundwater plumes. J. Hazard Mater. 2021, 402, 123998. [Google Scholar] [CrossRef]
- Ciampi, P.; Esposito, C.; Cassiani, G.; Deidda, G.P.; Rizzetto, P.; Papini, M.P. A field-scale remediation of residual light non-aqueous phase liquid (LNAPL): Chemical enhancers for pump and treat. Environ. Sci. Pollut. R. 2021, 28, 35286–35296. [Google Scholar] [CrossRef]
- Wei, K.; Ma, J.; Xi, B.; Yu, M.; Cui, J.; Chen, B.; Li, Y.; Gu, Q.; He, X. Recent progress on in-situ chemical oxidation for the remediation of petroleum contaminated soil and groundwater. J. Hazard Mater. 2022, 432, 128738. [Google Scholar] [CrossRef] [PubMed]
- Kechavarzi, C.; Soga, K.; Illangasekare, T.H. Two-dimensional laboratory simulation of LNAPL infiltration and redistribution in the vadose zone. J. Contam. Hydrol. 2005, 76, 211–233. [Google Scholar] [CrossRef]
- He, Z.; Liang, F.; Meng, J.; Li, N. Effects of groundwater fluctuation on migration characteristics and representative elementary volume of entrapped LNAPL. J. Hydrol. 2022, 610, 127833. [Google Scholar] [CrossRef]
- Onaa, C.; Emmanuel Olaobaju, A.; Amro, M. Experimental and numerical assessment of Light Non-Aqueous Phase Liquid (LNAPL) subsurface migration behavior in the vicinity of groundwater table. Environ. Technol. Innov. 2021, 23, 101573. [Google Scholar] [CrossRef]
- Karthick, A.; Roy, B.; Chattopadhyay, P. A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J. Environ. Manag. 2019, 243, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Gao, M. Pore wettability for enhanced oil recovery, contaminant adsorption and oil/water separation: A review. Adv. Colloid. Interfac. 2021, 289, 102377. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Fu, B.; Huang, W. An evaluation model for in-situ bioremediation technology of petroleum hydrocarbon contaminated soil. Environ. Pollut. 2024, 344, 123299. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Tan, X.; Almatrafi, E.; Yang, Y.; Wang, W.; Luo, H.; Qin, F.; Zhou, C.; Zeng, G.; Zhang, C. Effects of biochar-based materials on the bioavailability of soil organic pollutants and their biological impacts. Sci. Total Environ. 2022, 826, 153956. [Google Scholar]
- Haruna, A.; Tanimu, G.; Ibrahim, I.; Garba, Z.N.; Yahaya, S.M.; Musa, S.G.; Merican, Z.M.A. Mitigating oil and gas pollutants for a sustainable environment—Critical review and prospects. J. Clean. Prod. 2023, 416, 137863. [Google Scholar] [CrossRef]
- Jiang, W.; Meng, L.; Liu, F.; Sheng, Y.; Chen, S.; Yang, J.; Mao, H.; Zhang, J.; Zhang, Z.; Ning, H. Distribution, source investigation, and risk assessment of topsoil heavy metals in areas with intensive anthropogenic activities using the positive matrix factorization (PMF) model coupled with self-organizing map (SOM). Environ. Geochem. Hlth. 2023, 45, 6353–6370. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Woo, H.; Kim, S.; Yun, S.; Chung, J.; Lee, S. Complex behavior of petroleum hydrocarbons in vadose zone: A holistic analysis using unsaturated soil columns. Chemosphere 2023, 326, 138417. [Google Scholar] [CrossRef]
- Han, K.; Zuo, R.; Cao, X.; Xu, D.; Zhao, X.; Shi, J.; Xue, Z.; Xu, Y.; Wu, Z.; Wang, J. Spatial distribution characteristics and degradation mechanism of microorganisms in n-hexadecane contaminated vadose zone. Sci. Total Environ. 2024, 924, 171462. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Lu, H.; Xi, B.; Li, J.; Xiao, C.; Wang, Y.; Tang, J. Effect of water-level fluctuation on the removal of benzene from soil by SVE. Chemosphere 2021, 274, 129796. [Google Scholar] [CrossRef]
- Cavelan, A.; Faure, P.; Lorgeoux, C.; Colombano, S.; Deparis, J.; Davarzani, H.; Enjelvin, N.; Oltéan, C.; Tinet, A.J.; Domptail, F. An experimental multi-method approach to better characterize the LNAPL fate in soil under fluctuating groundwater levels. J. Contam. Hydrol. 2024, 262, 104319. [Google Scholar] [CrossRef] [PubMed]
- Koohbor, B.; Colombano, S.; Harrouet, T.; Deparis, J.; Lion, F.; Davarzani, H.; Ataie-Ashtiani, B. The effects of water table fluctuation on LNAPL deposit in highly permeable porous media: A coupled numerical and experimental study. J. Contam. Hydrol. 2023, 256, 104183. [Google Scholar] [CrossRef]
- Teramoto, E.H.; Chang, H.K. Field data and numerical simulation of btex concentration trends under water table fluctuations: Example of a jet fuel-contaminated site in Brazil. J. Contam. Hydrol. 2017, 198, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, J.; Feng, S.; Xiao, Z.; Chen, C. Effects of ecohydrological interfaces on migrations and transformations of pollutants: A critical review. Sci. Total Environ. 2022, 804, 150140. [Google Scholar] [CrossRef]
- Sakr, M.; El Agamawi, H.; Klammler, H.; Mohamed, M.M. A review on the use of permeable reactive barriers as an effective technique for groundwater remediation. Groundw. Sust. Dev. 2023, 21, 100914. [Google Scholar] [CrossRef]
- Gatsios, E.; García-Rincón, J.; Rayner, J.C.W.; McLaughlan, R.; Davis, G.B. LNAPL transmissivity as a remediation metric in complex sites under water table fluctuations. J. Environ. Manag. 2018, 215, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mineo, S. Groundwater and soil contamination by LNAPL: State of the art and future challenges. Sci. Total Environ. 2023, 874, 162394. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, G.; Arshadi, M.; Qin, T.; Goual, L. Micro-scale displacement of NAPL by surfactant and microemulsion in heterogeneous porous media. Adv. Water Resour. 2017, 105, 173–187. [Google Scholar] [CrossRef]
- Yang, Z.; Verpoort, F.; Dong, C.; Chen, C.; Chen, S.; Kao, C. Remediation of petroleum-hydrocarbon contaminated groundwater using optimized in situ chemical oxidation system: Batch and column studies. Process Saf. Environ. 2020, 138, 18–26. [Google Scholar] [CrossRef]
- Sun, Y.; Gu, Y.; Zha, Q. A novel surface imprinted resin for the selective removal of metal-complexed dyes from aqueous solution in batch experiments: ACB GGN as a representative contaminant. Chemosphere 2021, 280, 130611. [Google Scholar] [CrossRef]
- Negarestani, M.; Reisi, S.; Sohrabi, M.; Shayesteh, H.; Farimaniraad, H.; Mollahosseini, A.; Hosseinzadeh, M.; Tavassoli, S. In-situ growth of Al/Ni layered double hydroxide onto polyaniline-wrapped sisal fibers for highly efficient removal of pharmaceutical Ketoprofen and Ibuprofen contaminants: Batch and fixed-bed column studies. J. Water Process Eng. 2024, 57, 104657. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, B.; Yang, Y.; Du, X.; Yang, M. Quantitative assessment of organic mass fluxes and natural attenuation processes in a petroleum-contaminated subsurface environment. Appl. Sci. 2023, 13, 12782. [Google Scholar] [CrossRef]
- Stafford, B.P.; Cápiro, N.L.; Alvarez, P.J.J.; Rixey, W.G. Pore Water Characteristics following a release of neat ethanol onto pre-existing NAPL. Ground Water Monit. R. 2009, 29, 93–104. [Google Scholar] [CrossRef]
- Rhee, S.; Lee, S.; Park, J. Effect of soil organic carbon on the quantification of jet-fuels in soil using partitioning tracer method. J. Hazard Mater. 2010, 184, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yue, G.; Ma, J. Transport and natural attenuation of benzene vapor from a point source in the vadose zone. Chemosphere 2023, 323, 138222. [Google Scholar] [CrossRef]
- Brost, E.J.; DeVaull, G.E. Non-aqueous phase liquid (NAPL) mobility limits in soil. API Soil Groundw. Res. Bull. 2000, 9, 1–9. [Google Scholar]

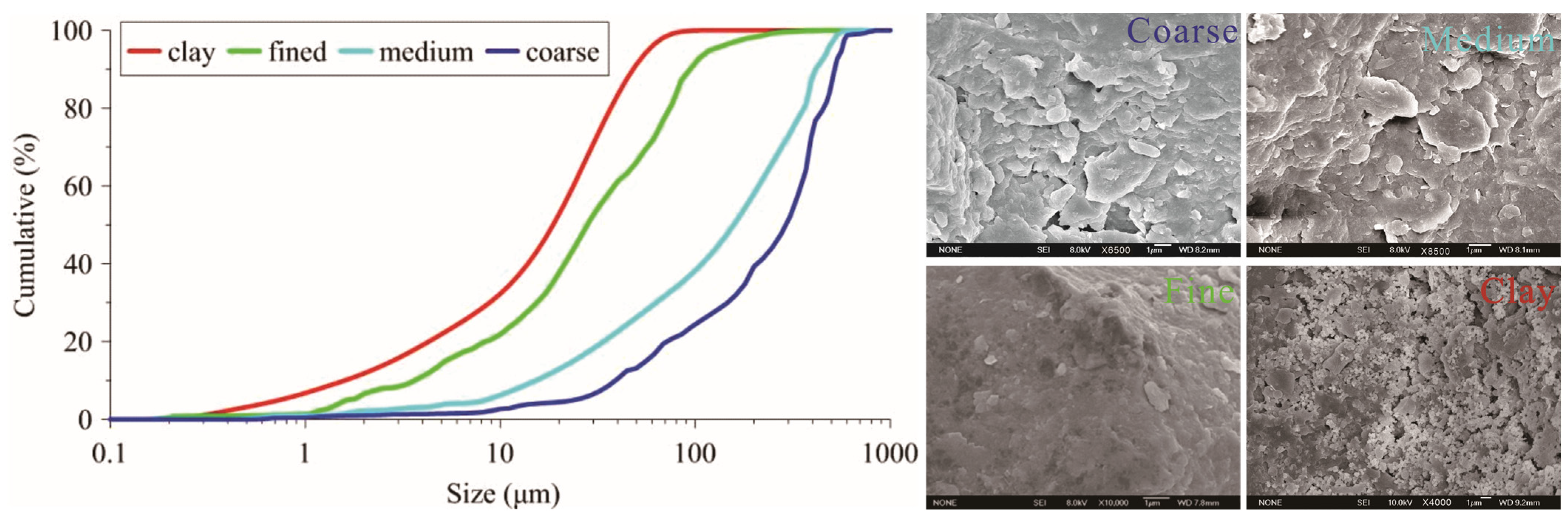


| Media Type | Coarse Sand | Medium Sand | Fine Sand | Clay |
|---|---|---|---|---|
| Packing density (g/cm3) | 1.83 | 1.72 | 1.63 | 1.52 |
| Porosity (%) | 30.9 | 35.2 | 38.6 | 42.7 |
| Specific surface area (m2/kg) | 58.8 | 126.4 | 231.8 | 449.6 |
| Organic matter content (%) | 1.5 | 2.2 | 3.6 | 3.8 |
| Mean diameter (μm) | 653.55 | 281.21 | 129.32 | 45.53 |
| MW (g/mol) | FG | A (cm−1) | S (mg/L) | ρ (g/cm3) | BP (℃) | η (mPa·s) | H (cm3/cm3) | log Kow | Koc (cm3/g) | Dair (cm2/s) | Dwater (cm2/s) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexadecane | 226.4 | -(CH2)n- | 2960 | 26 | 0.773 | 287 | 3.34 | 1.6 × 102 | 8.24 | 8.47 × 106 | 0.037 | 4.2 × 10−6 |
| Cyclohexane | 84.2 |  | 1450 | 55 | 0.779 | 80.7 | 0.98 | 7.8 × 100 | 3.44 | 9.63 × 102 | 0.084 | 9.1 × 10−6 |
| Toluene | 92.1 |  | 748 | 515 | 0.866 | 110.6 | 0.59 | 2.7 × 10−1 | 2.69 | 2.34 × 102 | 0.087 | 8.6 × 10−6 |
| Octanoic acid | 144.2 | -COOH | 1244 | 680 | 0.911 | 239.7 | 5.83 | 5.0 × 102 | 3.05 | 1.10 × 104 | 0.023 | 3.6 × 10−6 |
| Residual Saturation (%) | Hexadecane | Cyclohexane | Toluene | Octanoic Acid |
|---|---|---|---|---|
| Coarse sand | 13.3 | 10.6 | 8.2 | 14.2 |
| Medium sand | 16.2 | 15.2 | 11.1 | 21.8 |
| Fine sand | 21.6 | 18.8 | 13.2 | 25.3 |
| Clay | 28.1 | 24.2 | 20.1 | 28.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Wang, B.; Xia, Y.; Qiu, Y.; Li, C.; Cao, Z. Changing Soil Water Content: Main Trigger of the Multi-Phase Mobilization and Transformation of Petroleum Pollution Components—Insights from the Batch Experiments. Water 2024, 16, 1775. https://doi.org/10.3390/w16131775
Yang M, Wang B, Xia Y, Qiu Y, Li C, Cao Z. Changing Soil Water Content: Main Trigger of the Multi-Phase Mobilization and Transformation of Petroleum Pollution Components—Insights from the Batch Experiments. Water. 2024; 16(13):1775. https://doi.org/10.3390/w16131775
Chicago/Turabian StyleYang, Mingxing, Bing Wang, Yubo Xia, Yan Qiu, Chunling Li, and Zhendong Cao. 2024. "Changing Soil Water Content: Main Trigger of the Multi-Phase Mobilization and Transformation of Petroleum Pollution Components—Insights from the Batch Experiments" Water 16, no. 13: 1775. https://doi.org/10.3390/w16131775
APA StyleYang, M., Wang, B., Xia, Y., Qiu, Y., Li, C., & Cao, Z. (2024). Changing Soil Water Content: Main Trigger of the Multi-Phase Mobilization and Transformation of Petroleum Pollution Components—Insights from the Batch Experiments. Water, 16(13), 1775. https://doi.org/10.3390/w16131775






