Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Overview of High Calcium and Magnesium Ionized External Drainage Water of Karamay Petrochemical Company
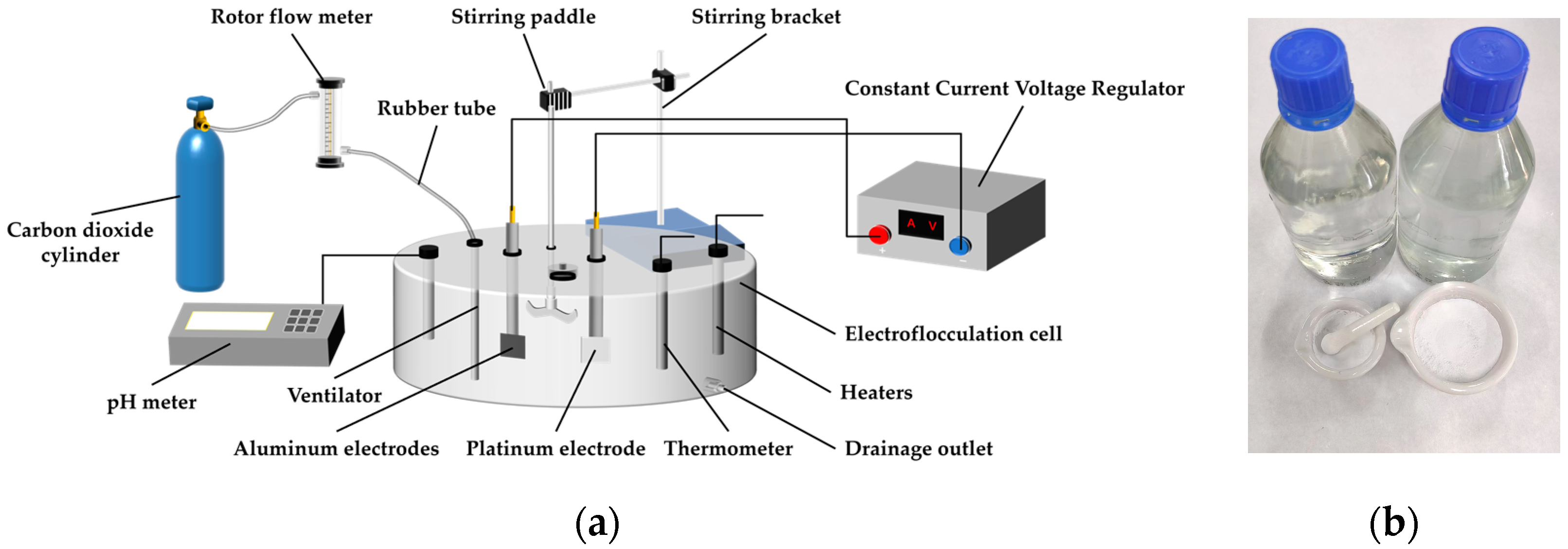
2.3. Experimental Setup
2.4. Process Description and Involved Reactions
2.5. Experimental Design
2.6. Sample Analysis and Calculation Methods
2.7. Solid Precipitate Characteristics
3. Results
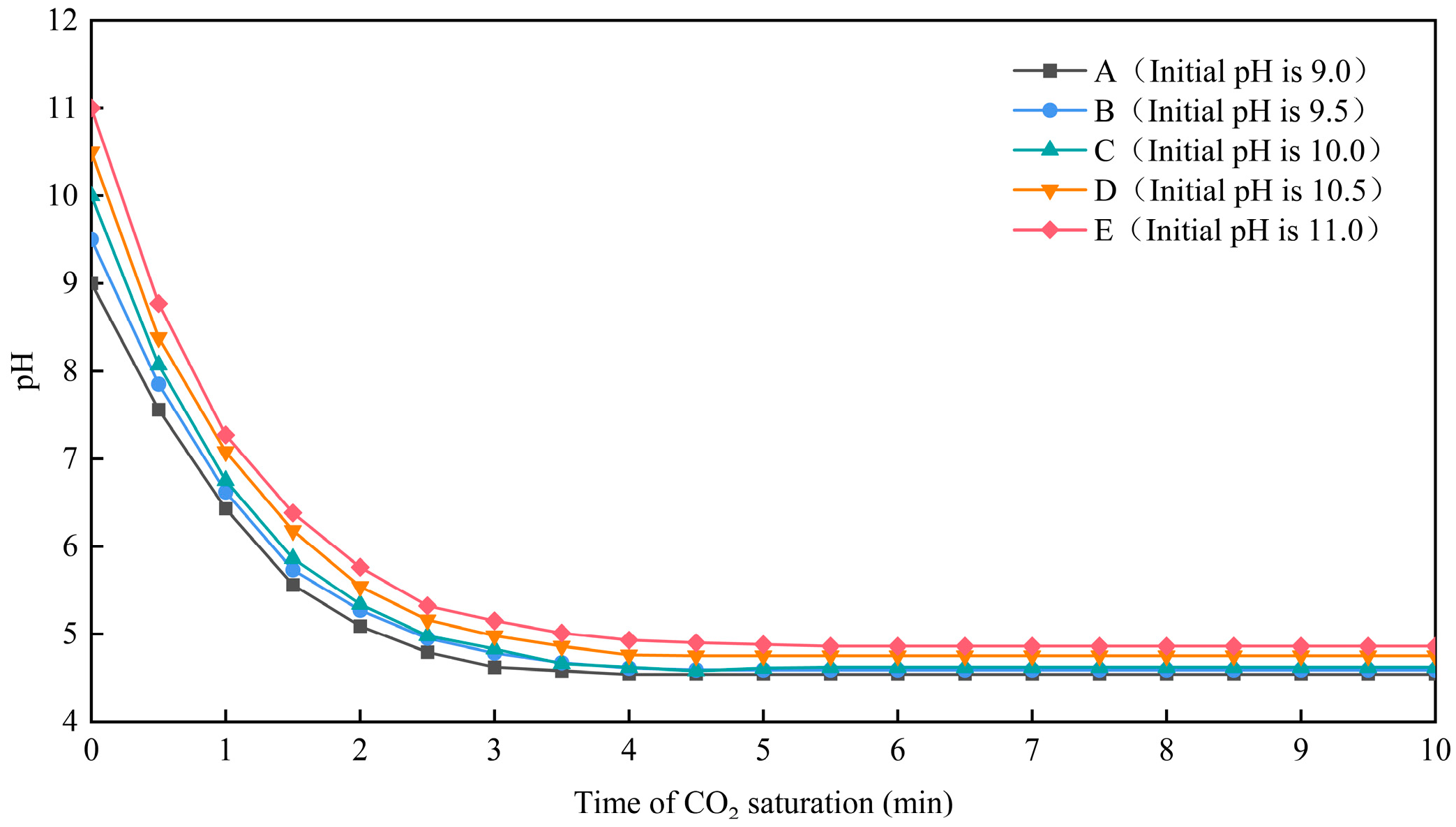
3.1. An Investigation of the Saturation Time of CO2 Aeration in a Sodium Hydroxide Base Solution
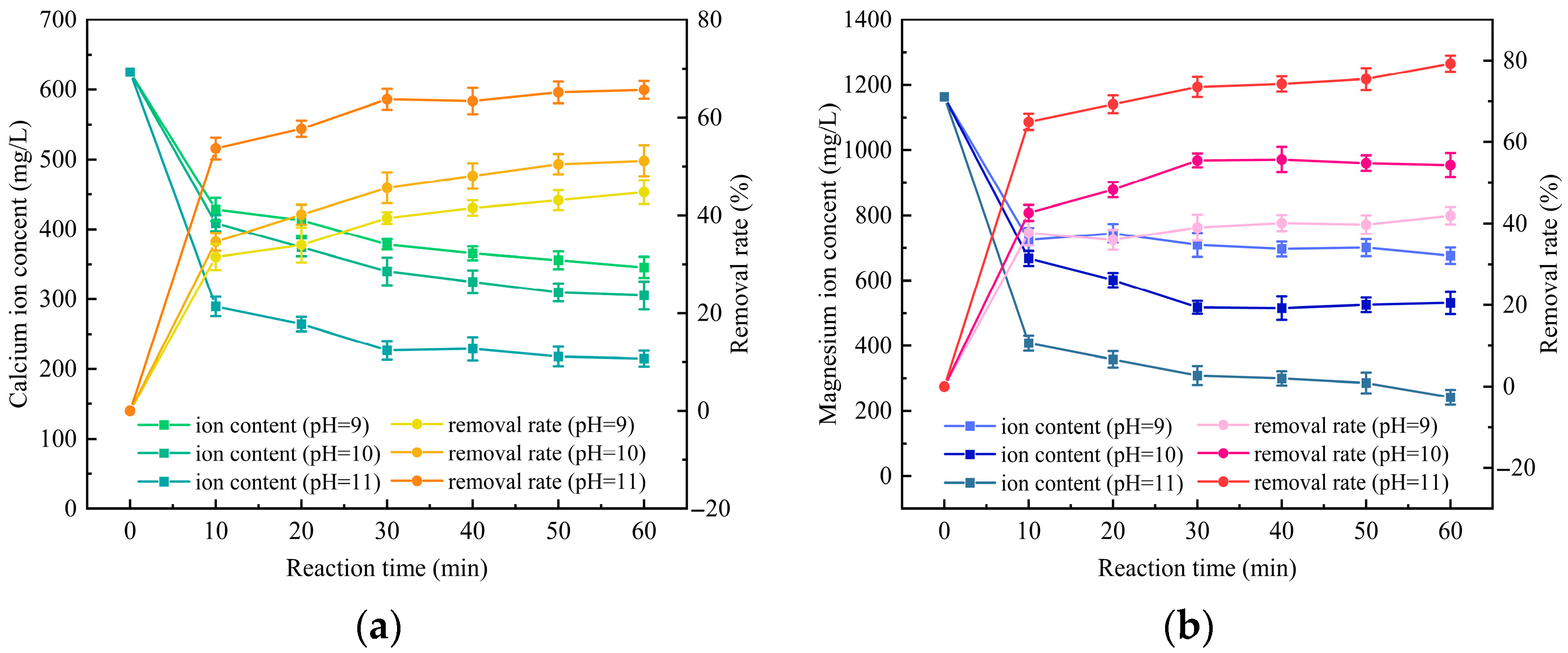
3.2. Investigation of the Removal Effect of pH Value on Calcium and Magnesium Ions in External Drainage Water
3.3. Investigation of the Removal Effect of Sodium Silicate on Calcium and Magnesium Ions in the External Drainage Water of Petrochemical Plants
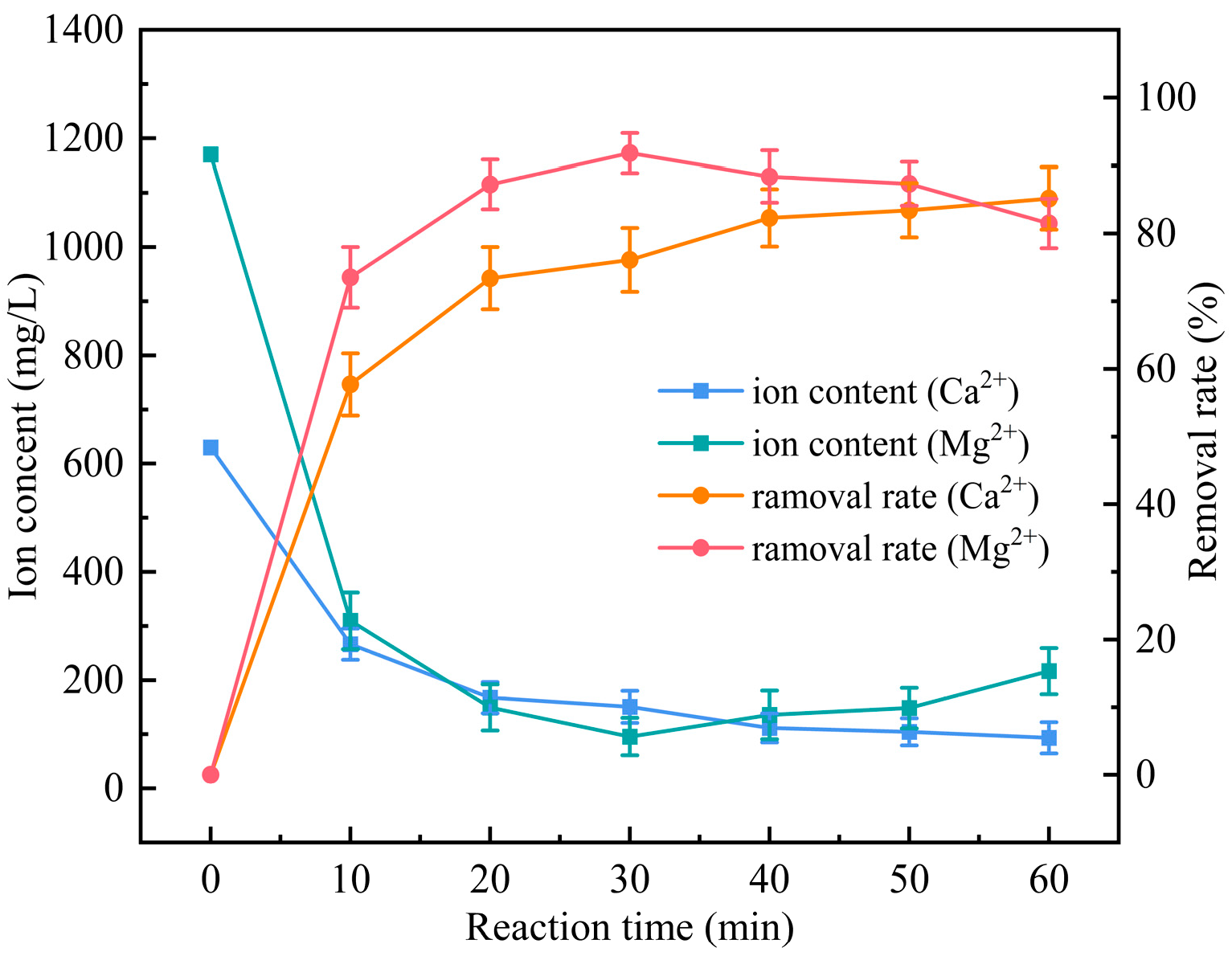
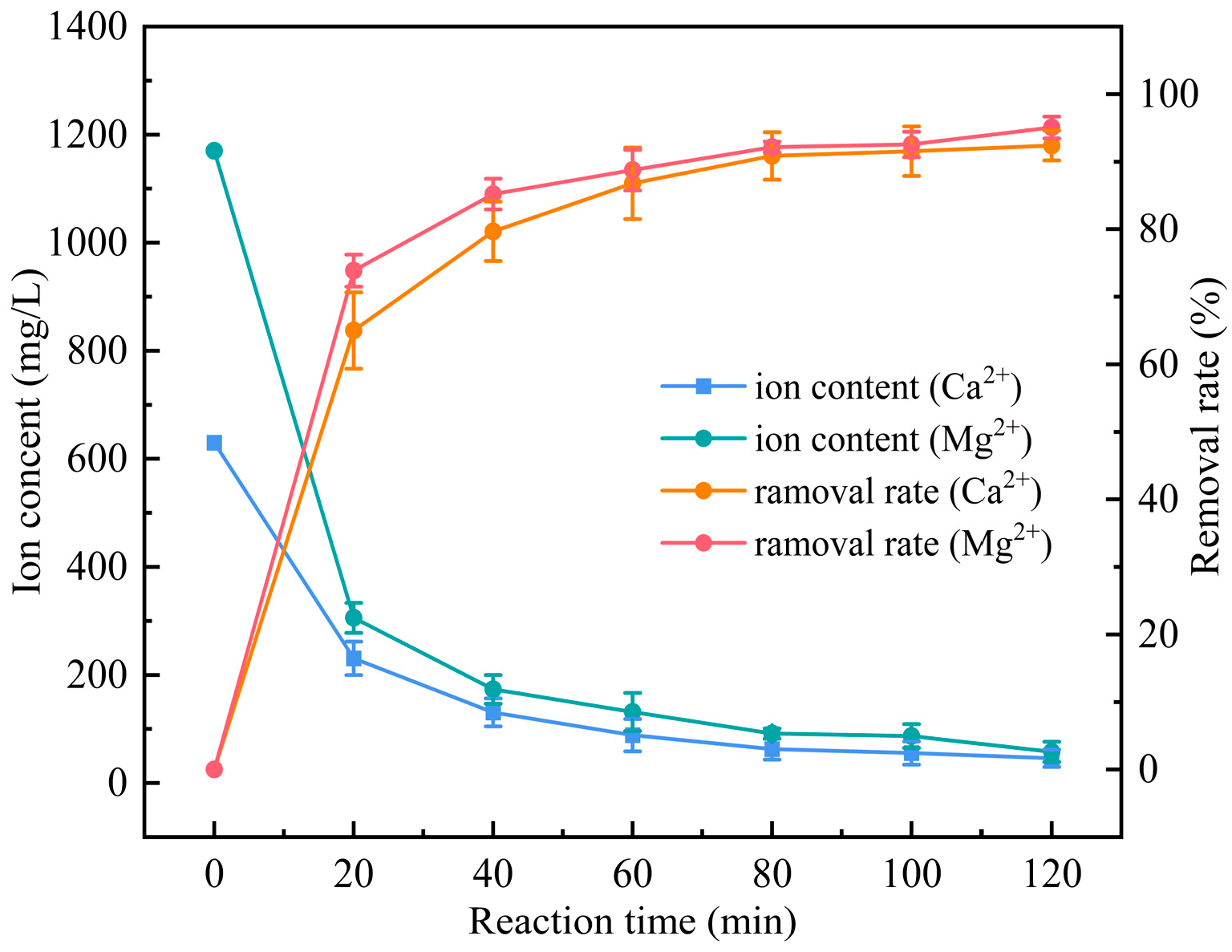
3.4. Investigation of the Removal Effect of Calcium and Magnesium Ions from the External Drainage Water of a Petrochemical Plant by Introducing Electrocoagulation Operation
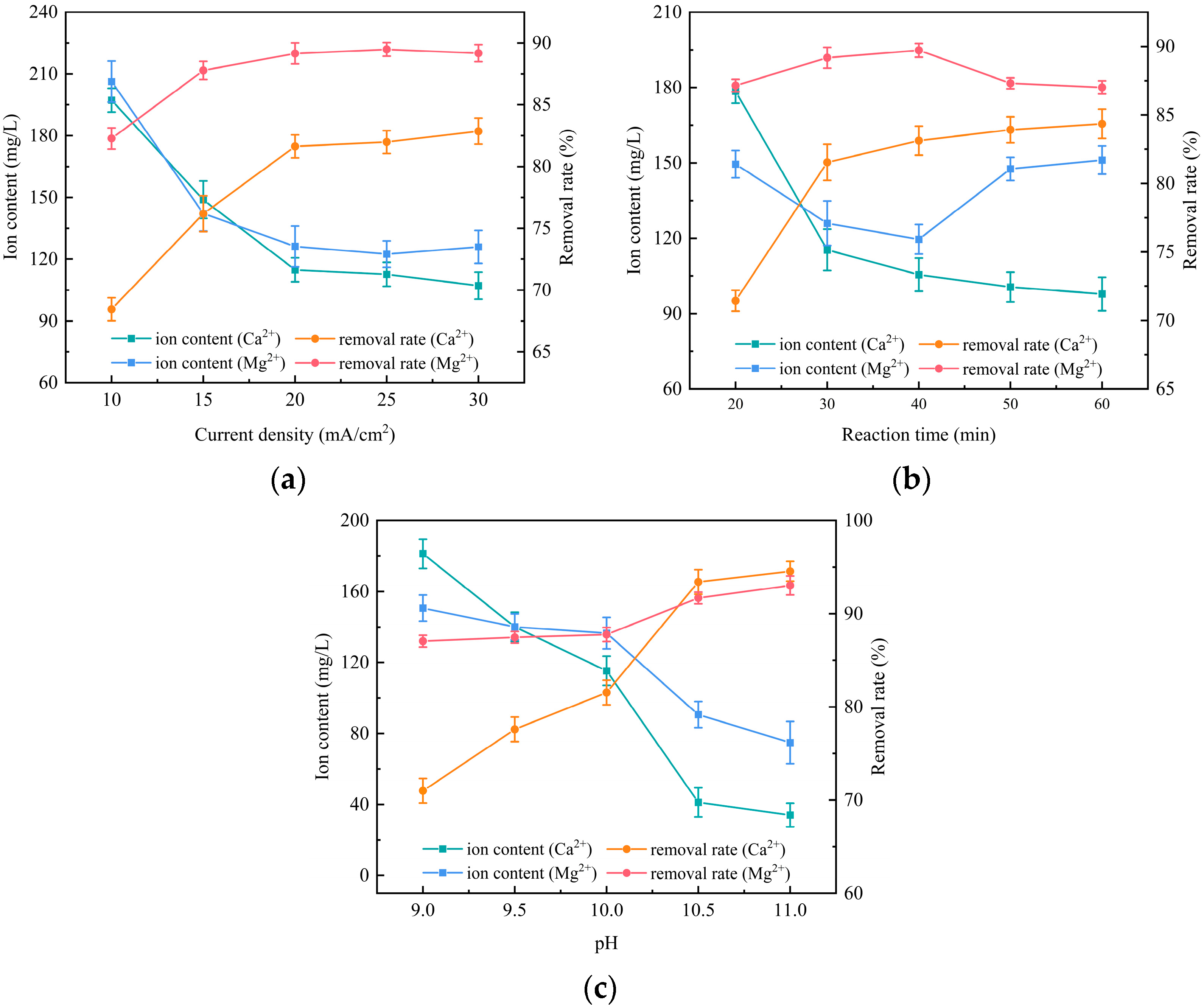
3.5. A One-Factor Experimental Investigation
3.6. Response Surface Optimization Experiments
3.6.1. Box–Behnken Design Results
3.6.2. Regression Equation Building and Model ANOVA
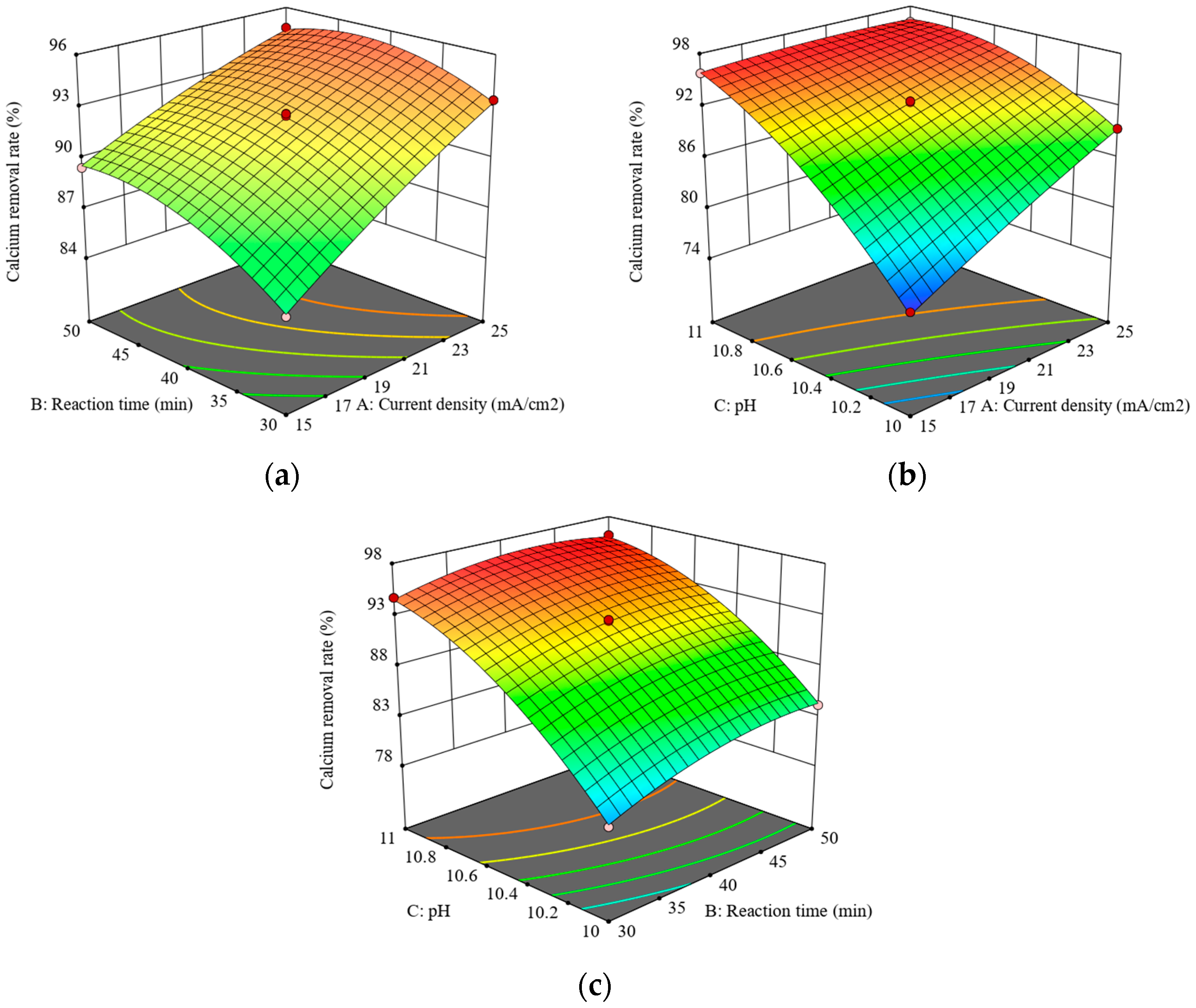
3.6.3. Response Surface Methodology (RSM)
3.6.4. Response Surface Optimization
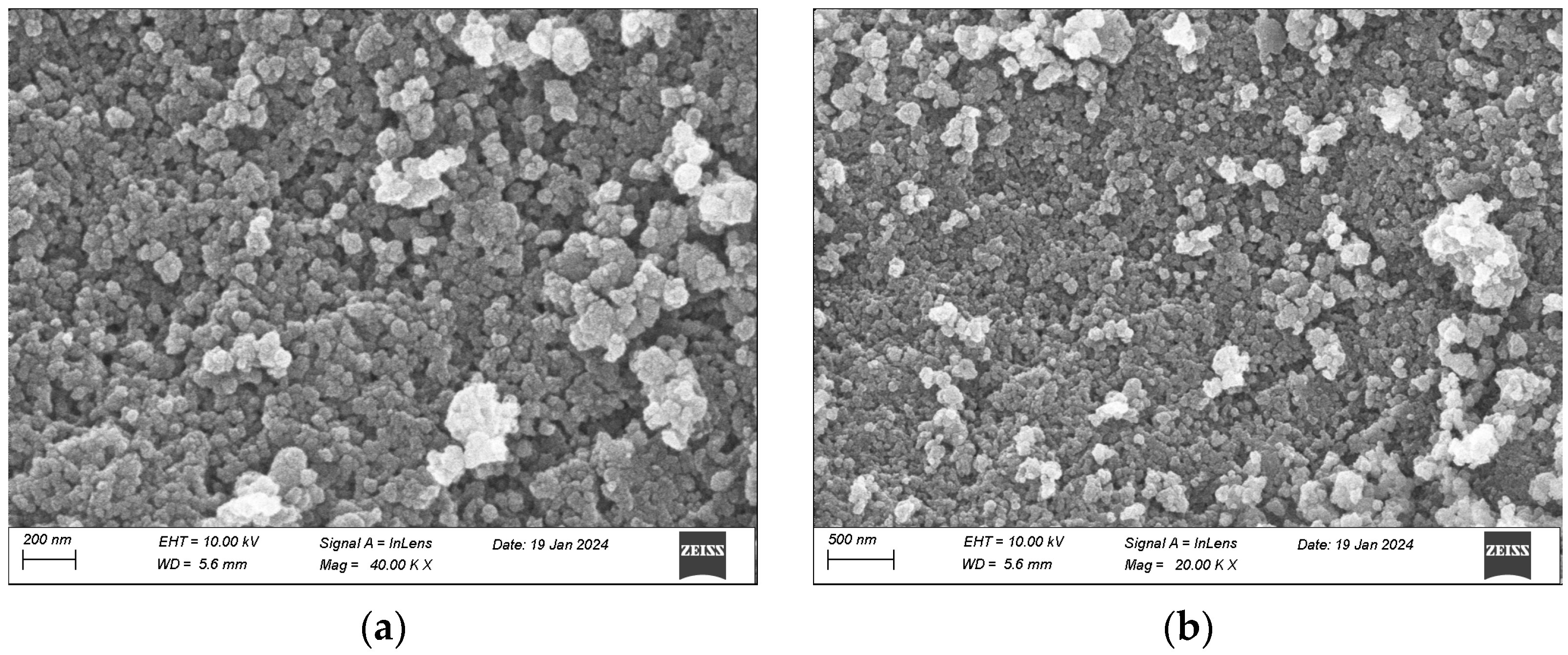
3.7. Solid Characteristics at Optimum Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Appendix A
| Name | CO32− mg/L | HCO3− mg/L | Cl− mg/L | SO42− mg/L | Ca2+ mg/L | Na+/K+ mg/L | Mineralization mg/L |
|---|---|---|---|---|---|---|---|
| Date | 91.98 | 758.37 | 5528.13 | 2658.15 | 2658.15 | 2658.15 | 14,672.45 |
| Level | ||||||
|---|---|---|---|---|---|---|
| Factors | Tag | Symbol | Units | −1 | 0 | +1 |
| Current density | CD | A | mA/cm2 | 15 | 20 | 25 |
| Reaction time | RT | B | min | 30 | 40 | 50 |
| pH | pH | C | - | 10 | 10.5 | 11 |
| Coded Value | Calcium Removal Rate (%) | Magnesium Removal Rate (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | Experimental Value | Projected Value | Experimental Value | Projected Value | |
| 1 | 0 | 0 | 0 | 92.6431 | 92.4100 | 94.4085 | 94.4000 |
| 2 | −1 | 1 | 0 | 89.4525 | 89.5906 | 91.5005 | 91.5103 |
| 3 | 1 | 0 | −1 | 89.4525 | 89.3814 | 91.5005 | 91.7063 |
| 4 | 0 | 0 | 0 | 92.2212 | 92.4100 | 94.5057 | 94.4000 |
| 5 | 0 | 0 | 0 | 92.6167 | 92.4100 | 94.1110 | 94.4000 |
| 6 | 1 | 1 | 0 | 94.7262 | 94.5922 | 96.3573 | 96.1173 |
| 7 | 1 | 0 | 1 | 96.0447 | 96.3614 | 98.1787 | 98.2063 |
| 8 | 0 | 0 | 0 | 92.0893 | 92.4100 | 94.5360 | 94.4000 |
| 9 | −1 | 0 | −1 | 77.5865 | 77.2614 | 84.5187 | 84.4863 |
| 10 | 0 | −1 | −1 | 80.2234 | 80.4208 | 88.1614 | 87.9673 |
| 11 | 0 | 1 | −1 | 84.1787 | 84.3792 | 87.8578 | 87.8913 |
| 12 | −1 | −1 | 0 | 85.4971 | 85.6322 | 90.5898 | 90.8273 |
| 13 | 0 | 1 | 1 | 96.0447 | 95.8408 | 98.7858 | 98.9773 |
| 14 | 1 | −1 | 0 | 93.4078 | 93.2706 | 94.5360 | 94.2203 |
| 15 | −1 | 0 | 1 | 95.7810 | 95.8414 | 97.6323 | 97.4263 |
| 16 | 0 | −1 | 1 | 94.7262 | 94.5192 | 96.3573 | 96.3213 |
| 17 | 0 | 0 | 0 | 92.4849 | 92.4100 | 94.4450 | 94.4000 |
| Source | Square Sum | DF | Mean Square | F-Value | p-Value | Note |
|---|---|---|---|---|---|---|
| Model | 490.88 | 9 | 54.54 | 550.87 | <0.0001 | Significant |
| A-Current density | 80.10 | 1 | 80.10 | 808.99 | <0.0001 | |
| B-Reaction time | 13.91 | 1 | 13.91 | 140.45 | <0.0001 | |
| C-pH | 327.11 | 1 | 327.11 | 3303.73 | <0.0001 | |
| AB | 1.74 | 1 | 1.74 | 17.56 | 0.0041 | |
| AC | 33.65 | 1 | 33.65 | 339.89 | <0.0001 | |
| BC | 1.74 | 1 | 1.74 | 17.56 | 0.0041 | |
| A2 | 0.54 | 1 | 0.54 | 5.47 | 0.052 | |
| B2 | 6.92 | 1 | 6.92 | 69.84 | <0.0001 | |
| C2 | 22.98 | 1 | 22.98 | 232.11 | <0.0001 | |
| Error | 0.69 | 7 | 0.10 | |||
| Lack-of-Fit | 0.45 | 3 | 0.15 | 2.50 | 0.1987 | Not significant |
| Pure Error | 0.24 | 4 | 0.06 | |||
| Total | 491.58 | 16 |
| Source | Square Sum | DF | Mean Square | F-Value | p-Value | Note |
|---|---|---|---|---|---|---|
| Model | 244.82 | 9 | 27.20 | 485.05 | <0.0001 | Significant |
| A-Current density | 32.11 | 1 | 32.11 | 572.57 | <0.0001 | |
| B-Reaction time | 3.33 | 1 | 3.33 | 59.35 | <0.0001 | |
| C-pH | 189.30 | 1 | 189.30 | 3375.45 | <0.0001 | |
| AB | 0.37 | 1 | 0.37 | 6.57 | 0.0374 | |
| AC | 10.35 | 1 | 10.35 | 184.61 | <0.0001 | |
| BC | 1.87 | 1 | 1.87 | 33.27 | 0.0007 | |
| A2 | 1.19 | 1 | 1.19 | 21.26 | 0.0025 | |
| B2 | 2.06 | 1 | 2.06 | 36.69 | 0.0005 | |
| C2 | 3.50 | 1 | 3.50 | 62.39 | <0.0001 | |
| Error | 0.39 | 7 | 0.06 | |||
| Lack-of-Fit | 0.28 | 3 | 0.09 | 3.21 | 0.1449 | Not significant |
| Pure Error | 0.12 | 4 | 0.03 | |||
| Total | 245.22 | 16 |
References
- Chen, X.; Liu, L.; Yang, Q.; Xu, H.; Shen, G.; Chen, Q. Optimizing Biochar Application Rates to Improve Soil Properties and Crop Growth in Saline-Alkali Soil. Sustainability 2024, 16, 2523. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Wang, H.; Liu, X. Research Progress in the Treatment of High-Salinity Wastewater. J. Phys. Conf. Ser. 2024, 2706, 012042. [Google Scholar] [CrossRef]
- Jadoon, S.; Wang, J.; Mahmood, Q.; Li, X.-D.; Zeb, B.S.; Naseem, I.; Hayat, M.T.; Nawazish, S.; Ditta, A. Association of Nephrolithiasis with Drinking Water Quality and Diet in Pakistan. Environ. Eng. Manag. J. 2020, 19, 1289–1297. [Google Scholar]
- Na, H.; Yuan, Y.; Du, T.; Zhang, T.; Zhao, X.; Sun, J.; Qiu, Z.; Zhang, L. Multi-Process Production Occurs in the Iron and Steel Industry, Supporting ‘Dual Carbon’ Target: An in-Depth Study of CO2 Emissions from Different Processes. J. Environ. Sci. 2024, 140, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Tamersit, S.; Bouhidel, K.-E. Treatment of Tannery Unhairing Wastewater Using Carbon Dioxide and Zinc Cations for Greenhouse Gas Capture, Pollution Removal and Water Recycling. J. Water Process Eng. 2020, 34, 101120. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, S.; Americo-Pinheiro, J.H.P.; Vinthange, M.; Sher, F. Editorial: Emerging Approaches for Sustainable Management for Wastewater. Front. Environ. Sci. 2023, 10, 1122659. [Google Scholar] [CrossRef]
- Amini, A.; Kim, Y.; Zhang, J.; Boyer, T.; Zhang, Q. Environmental and Economic Sustainability of Ion Exchange Drinking Water Treatment for Organics Removal. J. Clean. Prod. 2015, 104, 413–421. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of Salinity on Microorganisms in Activated Sludge Processes: A Review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Levchuk, I.; Rueda Marquez, J.J.; Sillanpaa, M. Removal of Natural Organic Matter (NOM) from Water by Ion Exchange—A Review. Chemosphere 2018, 192, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, N.; Judd, S. Chemical Cleaning of Potable Water Membranes: A Review. Sep. Purif. Technol. 2010, 71, 137–143. [Google Scholar] [CrossRef]
- Changmai, M.; Das, P.P.; Mondal, P.; Pasawan, M.; Sinha, A.; Biswas, P.; Sarkar, S.; Purkait, M.K. Hybrid Electrocoagulation-Microfiltration Technique for Treatment of Nanofiltration Rejected Steel Industry Effluent. Int. J. Environ. Anal. Chem. 2022, 102, 62–83. [Google Scholar] [CrossRef]
- Sharma, M.; Das, P.P.; Sood, T.; Chakraborty, A.; Purkait, M.K. Reduced Graphene Oxide Incorporated Polyvinylidene Fluoride/Cellulose Acetate Proton Exchange Membrane for Energy Extraction Using Microbial Fuel Cells. J. Electroanal. Chem. 2022, 907, 115890. [Google Scholar] [CrossRef]
- Joseph, T.M.; Al-Hazmi, H.E.; Śniatała, B.; Esmaeili, A.; Habibzadeh, S. Nanoparticles and Nanofiltration for Wastewater Treatment: From Polluted to Fresh Water. Environ. Res. 2023, 238, 117114. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-Q.; Zeng, L.-M.; Li, Q.; Liu, T.-Y.; Matsuyama, H.; Wang, X.-L. Selective Separation of Chloride and Sulfate by Nanofiltration for High Saline Wastewater Recycling. Sep. Purif. Technol. 2016, 166, 135–141. [Google Scholar] [CrossRef]
- Alonso-Vázquez, P.; Valle, C.; Sánchez-Arévalo, C.; Cuartas-Uribe, B.-E.; Vincent-Vela, M.-C.; Bes-Piá, A.; Álvarez-Blanco, S. Separation of Phenolic Compounds from Canned Mandarin Production Wastewater by Ultrafiltration and Nanofiltration. J. Water Process Eng. 2024, 59, 105041. [Google Scholar] [CrossRef]
- Hualpa-Cutipa, E.; Acosta, R.A.S.; Sangay-Tucto, S.; Beingolea, X.G.M.; Gutierrez, G.T.; Zabarburú, I.N. Chapter 15—Recent Trends for Treatment of Environmental Contaminants in Wastewater: An Integrated Valorization of Industrial Wastewater. In Integrated Environmental Technologies for Wastewater Treatment and Sustainable Development; Kumar, V., Kumar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 337–368. ISBN 978-0-323-91180-1. [Google Scholar]
- Peng, H.; Guo, J. Removal of Chromium from Wastewater by Membrane Filtration, Chemical Precipitation, Ion Exchange, Adsorption Electrocoagulation, Electrochemical Reduction, Electrodialysis, Electrodeionization, Photocatalysis and Nanotechnology: A Review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Bharti, M.; Das, P.P.; Purkait, M.K. A Review on the Treatment of Water and Wastewater by Electrocoagulation Process: Advances and Emerging Applications. J. Environ. Chem. Eng. 2023, 11, 111558. [Google Scholar] [CrossRef]
- Zhi, S.; Zhang, S. Effect of Co-Existing Ions on Electrode Behavior in Electrocoagulation Process for Silica Removal. Desalin. Water Treat. 2015, 56, 3054–3066. [Google Scholar] [CrossRef]
- Mohammad, A.F.; Haris, S.; Mourad, A.A.-H.; Al-Marzouqi, A.H.; El-Naas, M.H.; van der Bruggen, B.; Al-Marzouqi, M.H. Evaluation of a Combined Approach for Sulfate and Ammonia Recovery from Treated Brine Using a Simultaneous Chemical Precipitation and Electrocoagulation Processes. Sustainability 2023, 15, 16534. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Amaral, M.C.S. Metallic Ions Recovery from Membrane Separation Processes Concentrate: A Special Look onto Ion Exchange Resins. Chem. Eng. J. 2021, 425, 131812. [Google Scholar] [CrossRef]
- Li, Y.-S.; Li, Q.-J.; Huang, B.; Gao, X.-F. On-Line Mini-Column Flow Injection Electrochemical Method for Researching on Resuscitation and Dehydration Performance of Deeply-Fouled Cation-Exchange Resins. Chem. Eng. J. 2018, 345, 517–525. [Google Scholar] [CrossRef]
- Zou, M.; Zhang, W.; Wu, R.; Jiang, H.; Cao, F.; Su, E. Removal of Ginkgotoxin from the Ginkgo biloba Seeds Powder by Adopting Membrane Separation Technology. J. Clean. Prod. 2021, 280, 124452. [Google Scholar] [CrossRef]
- Meng, H.; Liang, H.; Xu, T.; Bai, J.; Li, C. Crosslinked Electrospinning Membranes with Contamination Resistant Properties for Highly Efficient Oil-Water Separation. J. Polym. Res. 2021, 28, 347. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current Advances in Membrane Technologies for Saline Wastewater Treatment: A Comprehensive Review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Oh, M.; Lee, K.; Jeon, M.K.; Foster, R.I.; Lee, C.-H. Chemical Precipitation–Based Treatment of Acidic Wastewater Generated by Chemical Decontamination of Radioactive Concrete. J. Environ. Chem. Eng. 2023, 11, 110306. [Google Scholar] [CrossRef]
- Padmaja, K.; Cherukuri, J.; Reddy, M.A. A Comparative Study of the Efficiency of Chemical Coagulation and Electrocoagulation Methods in the Treatment of Pharmaceutical Effluent. J. Water Process Eng. 2020, 34, 101153. [Google Scholar] [CrossRef]
- Li, G.; Zheng, B.; Zhang, W.; Liu, Q.; Li, M.; Zhang, H. Phosphate Removal Efficiency and Life Cycle Assessment of Different Anode Materials in Electrocoagulation Treatment of Wastewater. Sustainability 2024, 16, 3836. [Google Scholar] [CrossRef]
- Ju, J.; Feng, Y.; Li, H.; Xu, C. Resource Utilization of Strongly Acidic Wastewater and Red Gypsum by a Harmless Self-Treatment Process. Process Saf. Environ. Prot. 2023, 172, 594–603. [Google Scholar] [CrossRef]
- Farmanbordar, S.; Kahforoushan, D.; Fatehifar, E. A New Method in the Removal of Ca and Mg Ions from Industrial Wastewater. Desalin. Water Treat. 2016, 57, 8904–8910. [Google Scholar] [CrossRef]
- Rinder, T.; Dietzel, M.; Leis, A. Calcium Carbonate Scaling under Alkaline Conditions—Case Studies and Hydrochemical Modelling. Appl. Geochem. 2013, 35, 132–141. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Ge, Y.; Su, M.; Zhong, Z. The Precipitation Mechanism of Calcium Carbonate in the Gas-Liquid-Solid Three Phase at Alkalescency Condition. Cryst. Res. Technol. 2017, 52, 1600229. [Google Scholar] [CrossRef]
- López, D.E.; Trembly, J.P. Desalination of Hypersaline Brines with Joule-Heating and Chemical Pre-Treatment: Conceptual Design and Economics. Desalination 2017, 415, 49–57. [Google Scholar] [CrossRef]
- Aupoil, J.; Champenois, J.-B.; d’Espinose de Lacaillerie, J.-B.; Poulesquen, A. Interplay between Silicate and Hydroxide Ions during Geopolymerization. Cem. Concr. Res. 2019, 115, 426–432. [Google Scholar] [CrossRef]
- Ng, J.F.; Ahmed, O.H.; Jalloh, M.B.; Omar, L.; Kwan, Y.M.; Musah, A.A.; Poong, K.H. Soil Nutrient Retention and pH Buffering Capacity Are Enhanced by Calciprill and Sodium Silicate. Agronomy 2022, 12, 219. [Google Scholar] [CrossRef]
- Acosta-Herrera, A.A.; Hernández-Montoya, V.; Tovar-Gómez, R.; Pérez-Cruz, M.A.; Montes-Morán, M.A.; Rangel-Vázquez, N.A.; Cervantes, F.J. Water Reclamation from Anodizing Wastewaters by Removing Reactive Silica with Adsorption and Precipitation Methods. J. Environ. Manag. 2023, 326, 116683. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, P.; Cai, Y.; Yu, X.; Yu, C.; Ran, Q. Carbonation Behavior of Calcium Silicate Hydrate (C-S-H): Its Potential for CO2 Capture. Chem. Eng. J. 2022, 431, 134243. [Google Scholar] [CrossRef]
- Ghods, B.; Rezaei, M.; Meshkani, F. Synthesis of Nanostructured Magnesium Silicate with High Surface Area and Mesoporous Structure. Ceram. Int. 2016, 42, 6883–6890. [Google Scholar] [CrossRef]
- Li, J.; Nong, Y.; Yin, S.; Chen, Z.; Su, T.; Yu, Q. Calcium and Magnesium Silicate Hydrates Formed in the Presence of Sodium Hydroxide: Insight from Experiments and DFT Simulation. Mater. Today Commun. 2022, 33, 104362. [Google Scholar] [CrossRef]
- Hsieh, I.-M.; Thakur, A.K.; Malmali, M. Comparative Analysis of Various Pretreatments to Mitigate Fouling and Scaling in Membrane Distillation. Desalination 2021, 509, 115046. [Google Scholar] [CrossRef]
- GB/T 19923-2005; The Reuse of Urban Recycling Water-Water Quality Standard for Industrial Uses. The General Administration of Quality supervision Inspection and Quarantine of the People’s Republic of China and The standardization Administration of the People’s Republic of China: Tianjin, China, 2005.
- El-Naas, M.H.; Al-Zuhair, S.; Al-Lobaney, A.; Makhlouf, S. Assessment of Electrocoagulation for the Treatment of Petroleum Refinery Wastewater. J. Environ. Manag. 2009, 91, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Liu, J.; Gao, J.; Ji, Z.; Guo, X.; Liu, J.; Yuan, J. Trash to Treasure: Seawater Pretreatment by CO2 Mineral Carbonation Using Brine Pretreatment Waste of Soda Ash Plant as Alkali Source. Desalination 2017, 407, 85–92. [Google Scholar] [CrossRef]
- Bang, J.-H.; Chae, S.-C.; Song, K.; Lee, S.-W. Optimizing Experimental Parameters in Sequential CO2 Mineralization Using Seawater Desalination Brine. Desalination 2021, 519, 115309. [Google Scholar] [CrossRef]
- Shokri, A.; Fard, M.S. A Critical Review in Electrocoagulation Technology Applied for Oil Removal in Industrial Wastewater. Chemosphere 2022, 288, 132355. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, M.; Yasri, N.; Roberts, E.P.L. Electrode Passivation, Faradaic Efficiency, and Performance Enhancement Strategies in Electrocoagulation-a Review. Water Res. 2020, 187, 116433. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Liu, S.; Zhang, X.; Zhang, F.; Yang, X.; Xu, M.; Hou, J. Reliability Treatment of Silicon in Oilfield Wastewater by Electrocoagulation. Water 2023, 15, 206. [Google Scholar] [CrossRef]
- Bahmanzadegan, F.; Ghaemi, A. Exploring the Effect of Zeolite’s Structural Parameters on the CO2 Capture Efficiency Using RSM and ANN Methodologies. Case Stud. Chem. Environ. Eng. 2024, 9, 100595. [Google Scholar] [CrossRef]
- Aghajanzadeh, I.; Ramezanianpour, A.M.; Amani, A.; Habibi, A. Mixture Optimization of Alkali Activated Slag Concrete Containing Recycled Concrete Aggregates and Silica Fume Using Response Surface Method. Constr. Build. Mater. 2024, 425, 135928. [Google Scholar] [CrossRef]
- Hagemann, S.E.; Gastaldini, A.L.G.; Cocco, M.; Jahn, S.L.; Terra, L.M. Synergic Effects of the Substitution of Portland Cement for Water Treatment Plant Sludge Ash and Ground Limestone: Technical and Economic Evaluation. J. Clean. Prod. 2019, 214, 916–926. [Google Scholar] [CrossRef]
- Bendjeffal, H.; Mamine, H.; Metidji, T.; Djebli, A.; Diaf, R.; Bouhedja, Y. A Box-Behnken Design-Based Chemometric Approach to Optimize the Removal of Phosphate Ions from Water Using Punica granatum Shells. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 632–644. [Google Scholar] [CrossRef]
- Srinivasa, A.S.; Swaminathan, K.; Yaragal, S.C. Microstructural and Optimization Studies on Novel One-Part Geopolymer Pastes by Box-Behnken Response Surface Design Method. Case Stud. Constr. Mater. 2023, 18, e01946. [Google Scholar] [CrossRef]
- Sibiya, N.P.; Amo-Duodu, G.; Kweinor Tetteh, E.; Rathilal, S. Response Surface Optimisation of a Magnetic Coagulation Process for Wastewater Treatment via Box-Behnken. Mater. Today Proc. 2022, 62, S122–S126. [Google Scholar] [CrossRef]
- Song, C.; Kitamura, Y.; Li, S. Optimization of a Novel Cryogenic CO2 Capture Process by Response Surface Methodology (RSM). J. Taiwan Inst. Chem. Eng. 2014, 45, 1666–1676. [Google Scholar] [CrossRef]
- Calvo, L.M.; Domingo, R. Influence of Process Operating Parameters on CO2 Emissions in Continuous Industrial Plants. J. Clean. Prod. 2015, 96, 253–262. [Google Scholar] [CrossRef]
- Nigri, E.M.; Santos, A.L.A.; Rocha, S.D.F. Removal of Organic Compounds, Calcium and Strontium from Petroleum Industry Effluent by Simultaneous Electrocoagulation and Adsorption. J. Water Process Eng. 2020, 37, 101442. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Liu, Q.; Liu, Y.; Li, Y.; Wang, W.; Feng, Z.; Song, W.; Jiang, B. Coupled Electrochemical Crystallization-Electrocoagulation-Flocculation Process for Efficient Removal of Hardness and Silica from Reverse Osmosis Concentrate. Desalination 2024, 580, 117549. [Google Scholar] [CrossRef]
- Feng, Q.; Guo, K.; Gao, Y.; Liu, B.; Yue, Q.; Shi, W.; Feng, C.; Zhou, J.; Wang, G.; Gao, B. Effect of Coagulation Treatment on Sludge Dewatering Performance: Application of Polysilicate and Their Mechanism. Sep. Purif. Technol. 2022, 301, 121954. [Google Scholar] [CrossRef]
- Den, W.; Wang, C.-J. Removal of Silica from Brackish Water by Electrocoagulation Pretreatment to Prevent Fouling of Reverse Osmosis Membranes. Sep. Purif. Technol. 2008, 59, 318–325. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, Y.-J.; Lee, Y.-H.; Guim, H.; Han, S.M. Behavior and Characteristics of Amorphous Calcium Carbonate and Calcite Using CaCO3 Film Synthesis. Mater. Des. 2016, 112, 367–373. [Google Scholar] [CrossRef]
- Blue, C.R.; Giuffre, A.; Mergelsberg, S.; Han, N.; De Yoreo, J.J.; Dove, P.M. Chemical and Physical Controls on the Transformation of Amorphous Calcium Carbonate into Crystalline CaCO3 Polymorphs. Geochim. Cosmochim. Acta 2017, 196, 179–196. [Google Scholar] [CrossRef]
- Huang, F.; Liang, Y.; He, Y. On the Pickering Emulsions Stabilized by Calcium Carbonate Particles with Various Morphologies. Colloids Surf. Physicochem. Eng. Asp. 2019, 580, 123722. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, B.; Li, G.; Guo, F.; Lu, S.; Peng, Y.; Hou, J. Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water. Water 2024, 16, 1715. https://doi.org/10.3390/w16121715
Chang B, Li G, Guo F, Lu S, Peng Y, Hou J. Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water. Water. 2024; 16(12):1715. https://doi.org/10.3390/w16121715
Chicago/Turabian StyleChang, Baoqi, Guangpu Li, Fuqiang Guo, Shuang Lu, Yuhao Peng, and Junwei Hou. 2024. "Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water" Water 16, no. 12: 1715. https://doi.org/10.3390/w16121715
APA StyleChang, B., Li, G., Guo, F., Lu, S., Peng, Y., & Hou, J. (2024). Research on Carbon Dioxide-Assisted Electrocoagulation Technology for Treatment of Divalent Cations in Water. Water, 16(12), 1715. https://doi.org/10.3390/w16121715





