Assessment of Groundwater Quality through Hydrochemistry Using Principal Components Analysis (PCA) and Water Quality Index (WQI) in Kızılırmak Delta, Turkey
Abstract
1. Introduction
2. Materials and Methods
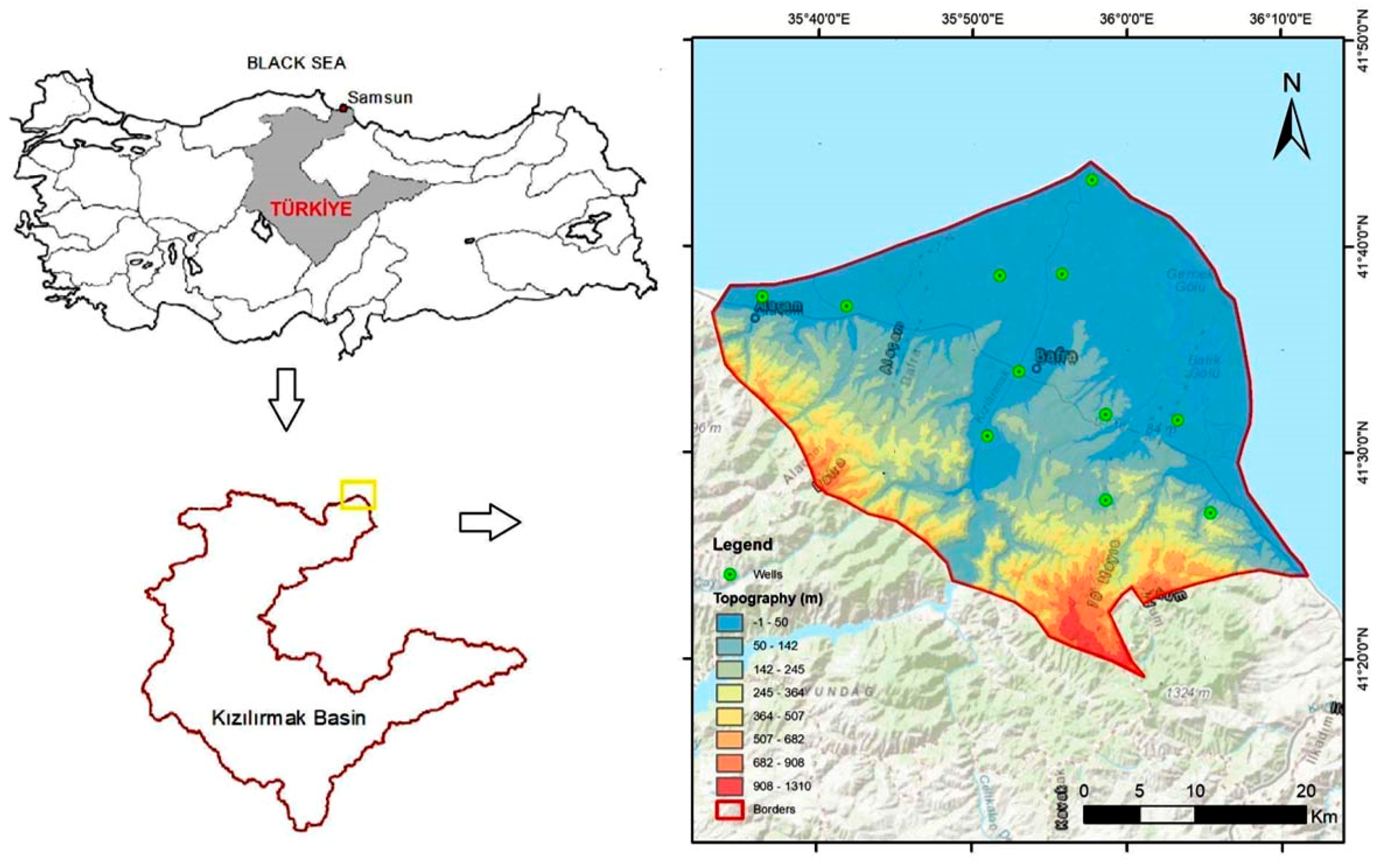
2.1. Study Area
2.2. Geology and Hydrogeology of the Study Area
2.3. Data Acquisition
2.3.1. Determination of the Water Quality Index for Groundwater
2.3.2. Canadian Council of Ministers of the Environment Water Quality Index (CCME WQI)
2.4. Principal Component Analysis (PCA)
- First, the covariance/correlation matrix is calculated (here, the most appropriate data set to perform PCA is the one with the highest correlation between the individual indicators).
- Determine the number of principal components to be considered according to the percentage of variance they explain.
2.5. Hierarchical Cluster Analysis (CA)
3. Results and Discussion
3.1. Hydrogeochemical Characteristics
| Parameters | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stations | X (m) | Y(m) | pH | EC | Na+ (mg/L) | K+ (mg/L) | Ca2+ (mg/L) | Mg2+ (mg/L) | HCO3− (mg/L) | Cl− (mg/L) | SO42− (mg/L) | Hardness | NO3− (mg/L) |
| Well 1 | 41°31′51″ | 35°58′37″ | 7.12 | 1015 | 142.768 | 3.128 | 136.42 | 386.771 | 546.739 | 24.106 | 37.463 | 37.700 | 3.782 |
| Well 2 | 41°27′41″ | 35°58′37″ | 8.5 | 1005 | 14.484 | 5.474 | 162.925 | 318.865 | 399.071 | 3.191 | 9.606 | 51.400 | 2.790 |
| Well 3 | 41°30′48″ | 35°50′59″ | 7.7 | 1162 | 50.118 | 11.339 | 122.044 | 308.531 | 233.097 | 6.027 | 13.929 | 40.900 | 2.604 |
| Well 4 | 41°33′55″ | 35°53′0″ | 7.6 | 1260 | 135.181 | 4.692 | 111.422 | 534.394 | 549.18 | 134.71 | 67.722 | 45.910 | 16.120 |
| Well 5 | 41°37′31″ | 35°56′23″ | 7.4 | 672 | 19.771 | 2.346 | 129.859 | 128.432 | 369.781 | 24.106 | 39.865 | 36.710 | <0.01 |
| Well 6 | 41°38′34″ | 35°51′46″ | 7.5 | 1546 | 207.599 | 5.083 | 151.903 | 615.586 | 375.273 | 243.896 | 340.533 | 58.730 | 26.600 |
| Well 7 | 41°42′53″ | 35°55′59″ | 7.5 | 2401 | 348.299 | 25.024 | 108.817 | 1778.851 | 685.865 | 383.215 | 475.497 | 87.410 | 31.000 |
| Well 8 | 41°38′39″ | 35°55′47″ | 7.3 | 1950 | 229.67 | 7.429 | 205.811 | 863.592 | 487.55 | 311.96 | 400.09 | 80.600 | 50.900 |
| Well 9 | 41°31′33″ | 35°3′16″ | 7 | 6112 | 1334.799 | 9.384 | 692.382 | 5183.026 | 732.85 | 638.1 | 1474.521 | 348.280 | 52.950 |
| Well 10 | 41°37′5″ | 35°41′51″ | 7.4 | 652 | 17.243 | 1.955 | 118.036 | 100.383 | 371.002 | 28.006 | 37.944 | 32.890 | 17.110 |
| Well 11 | 41°38′34″ | 35°51′46″ | 7.28 | 1606 | 187.598 | 5.083 | 130.26 | 1198.695 | 745.054 | 216.6 | 113.351 | 73.100 | 27.160 |
| Average | 7.5 | 1761 | 244.321 | 7.358 | 188.171 | 1037.921 | 499.587 | 183.083 | 273.684 | 81.239 | 23.102 | ||
| WHO standard [55,60]. | 7.5 | 500 | 200 | 10 | 75 | 50 | 500 | 250 | 250 | 100 | 50 | ||
| Parameters | Min | Max | Mean | Median | SD |
|---|---|---|---|---|---|
| pH | 7 | 8.5 | 7.5 | 7.4 | 0.39 |
| EC (μS/cm) | 652 | 6112 | 1761 | 1403 | 1472 |
| Na+ (mg/L) | 14.484 | 1334.799 | 244.321 | 165.100 | 359.281 |
| K+ (mg/L) | 1.955 | 25.024 | 7.358 | 5.242 | 21.799 |
| Ca2+ (mg/L) | 108.817 | 692.382 | 188.171 | 130.030 | 171.762 |
| Mg2+ (mg/L) | 100.383 | 5183.026 | 1037.921 | 574.900 | 1394.753 |
| HCO3− (mg/L) | 233.097 | 745.054 | 499.587 | 488.700 | 160.746 |
| Cl− (mg/L) | 3.191 | 638.100 | 183.083 | 81.350 | 199.711 |
| SO42− (mg/L) | 9.606 | 1474.521 | 273.684 | 53.750 | 420.049 |
| Hardness (mg/L) | 32.890 | 348.280 | 81.239 | 55.065 | 86.441 |
| NO3− (mg/L) | <0.01 | 52.950 | 23.102 | 16.616 | 18.845 |
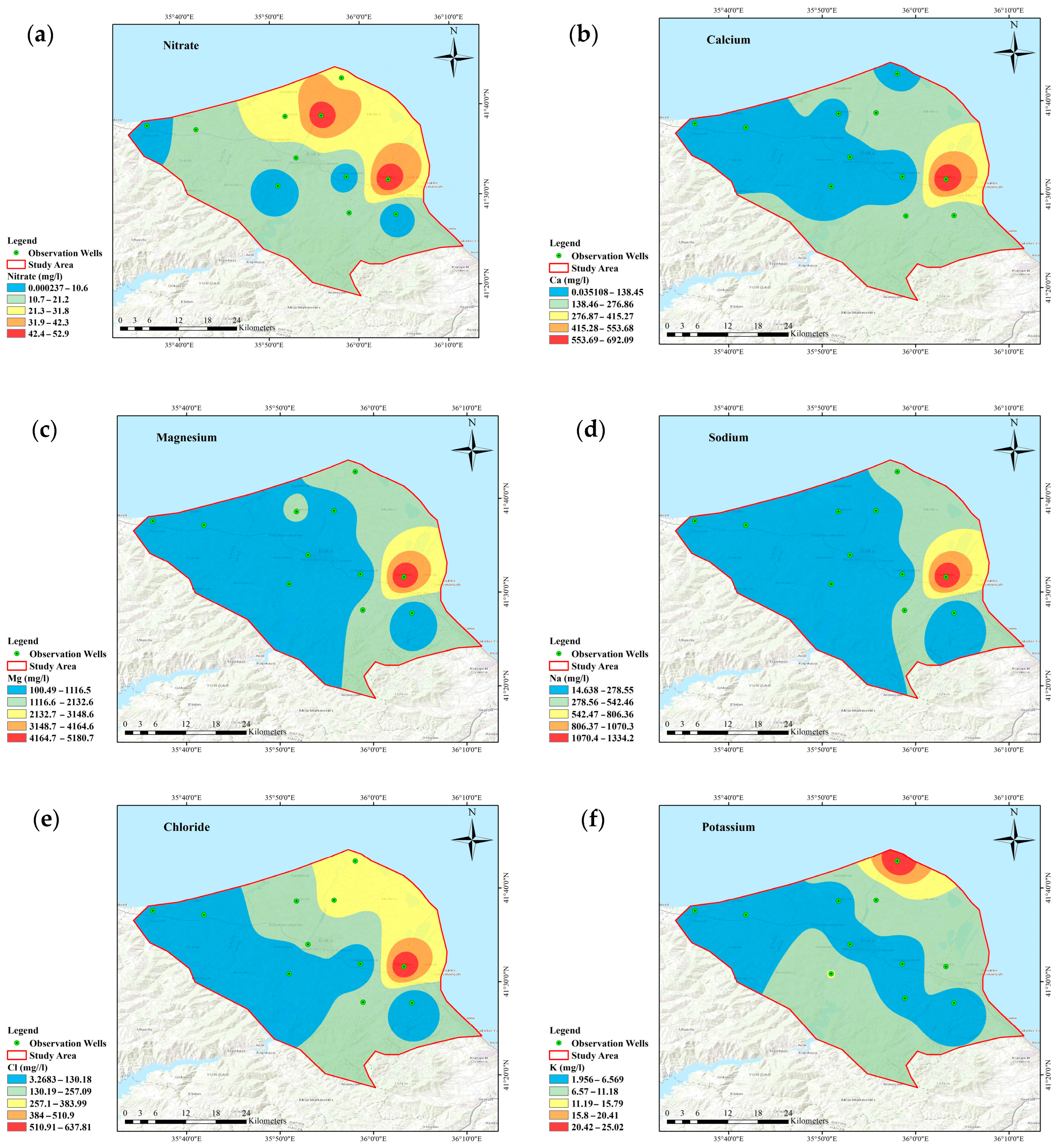
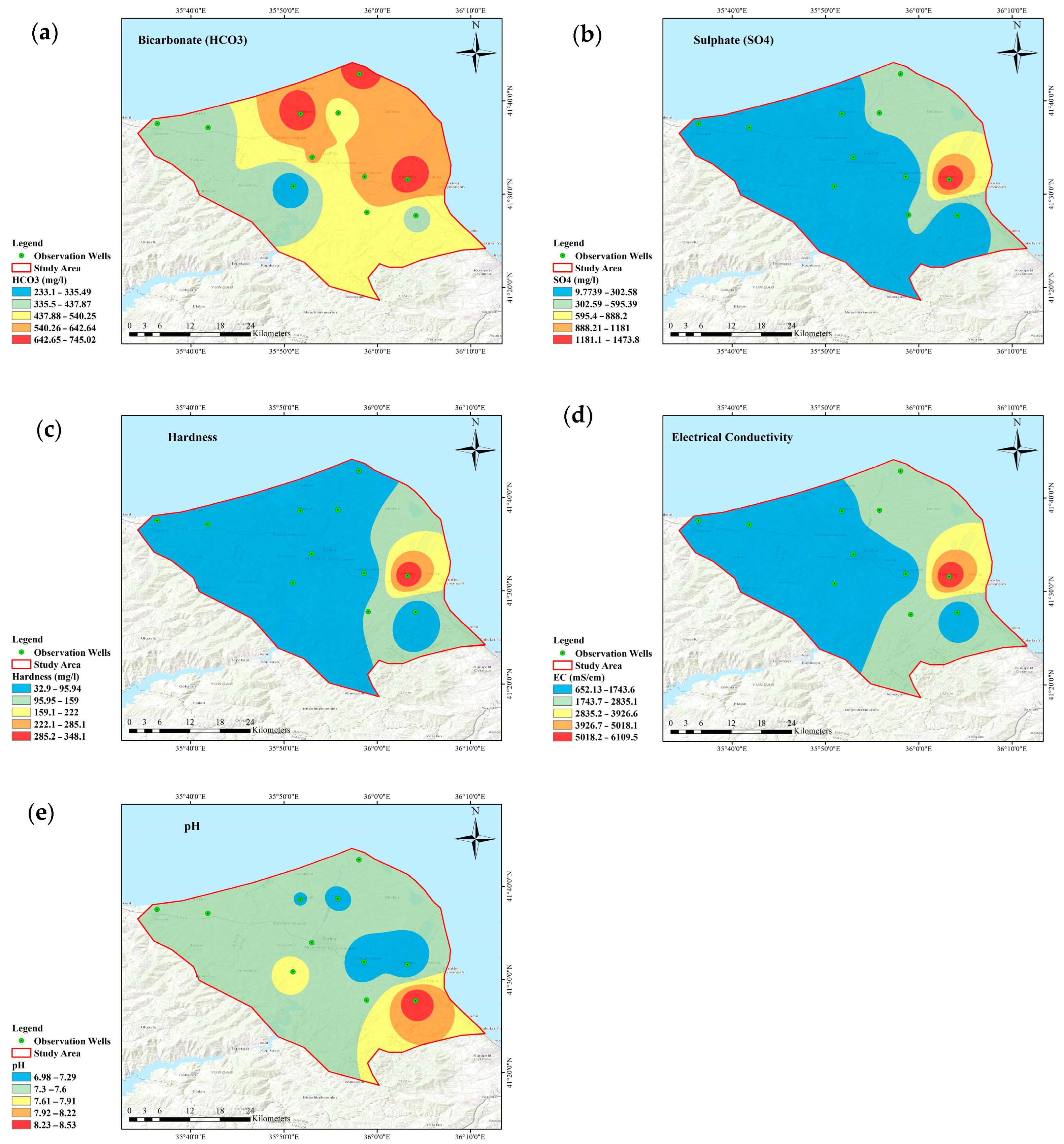
3.2. Groundwater Quality Mapping/Spatial Distribution Pattern
3.3. GIS-Based Groundwater Quality Index
3.3.1. GIS-Based Groundwater Quality Index by Brown et al. [33]
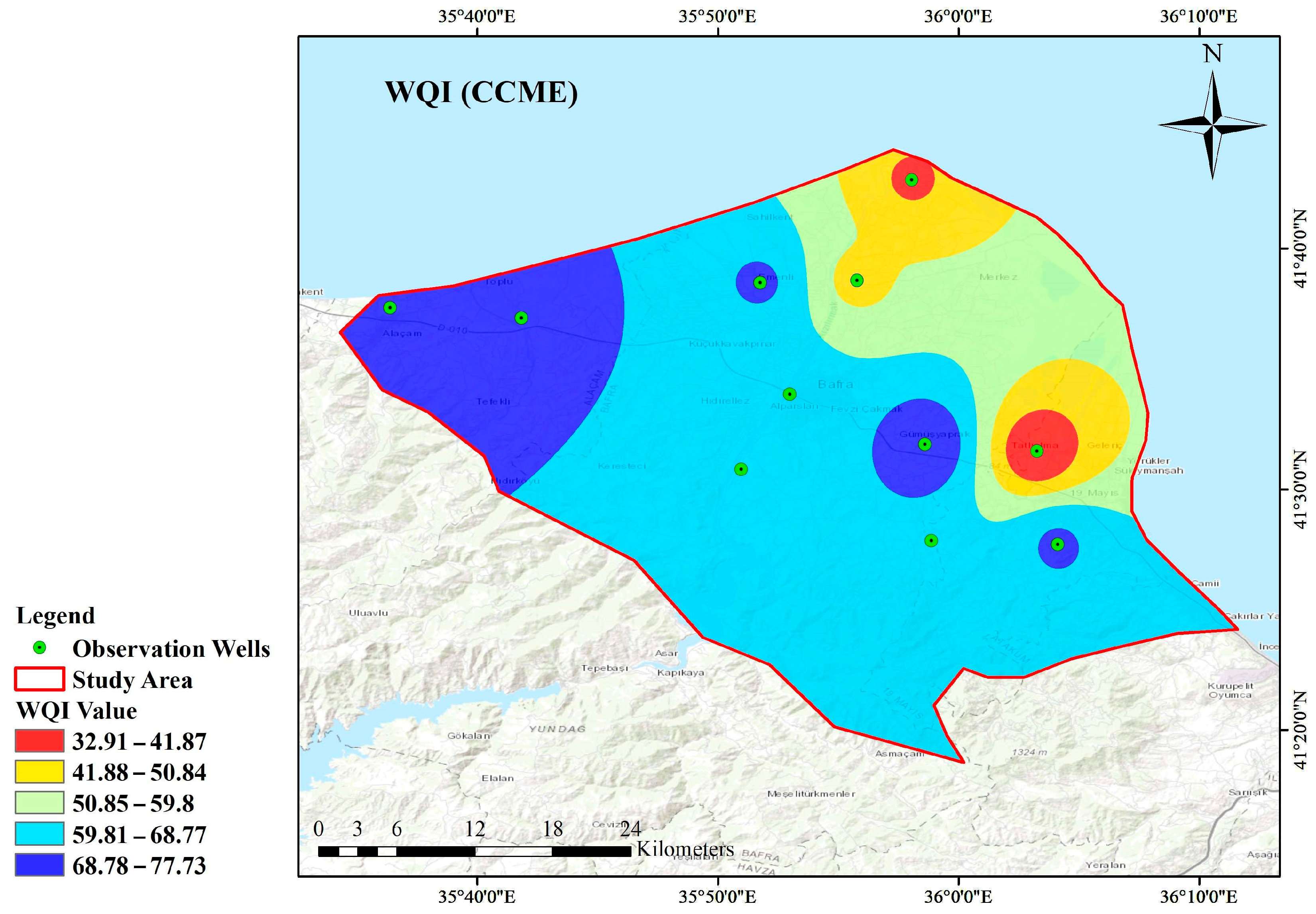
3.3.2. CCME WQI
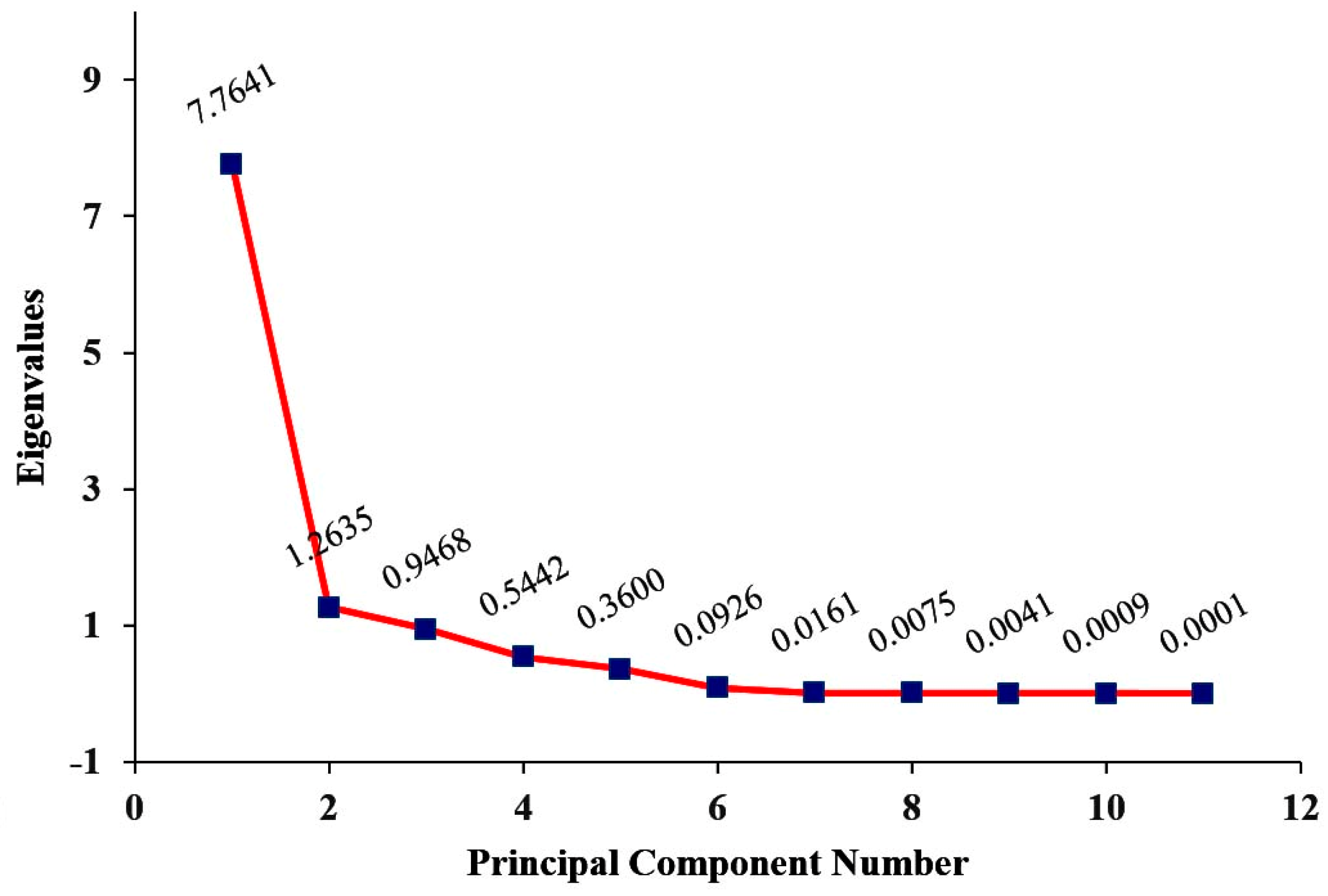
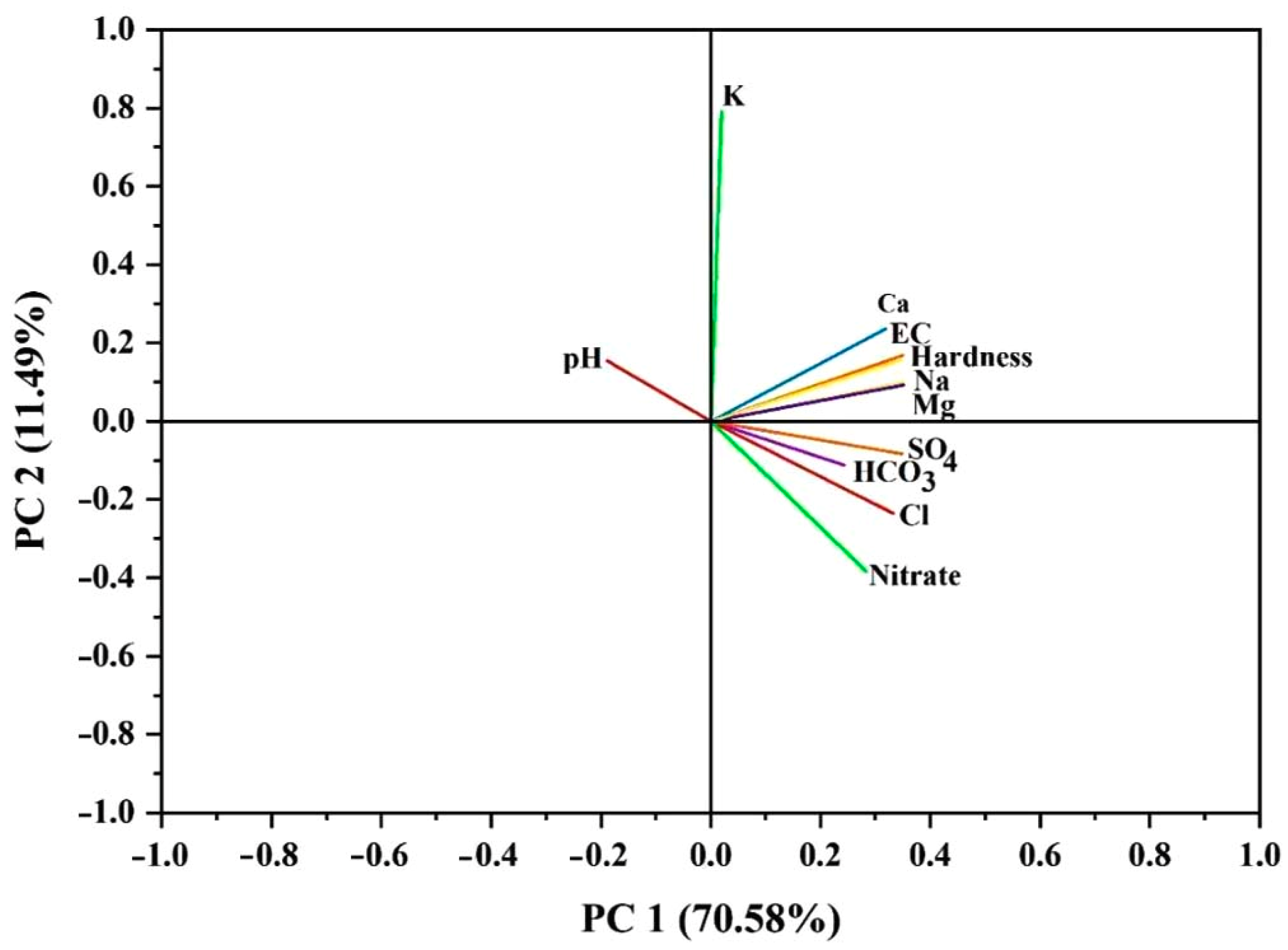
3.4. Principle Component Analysis (PCA)
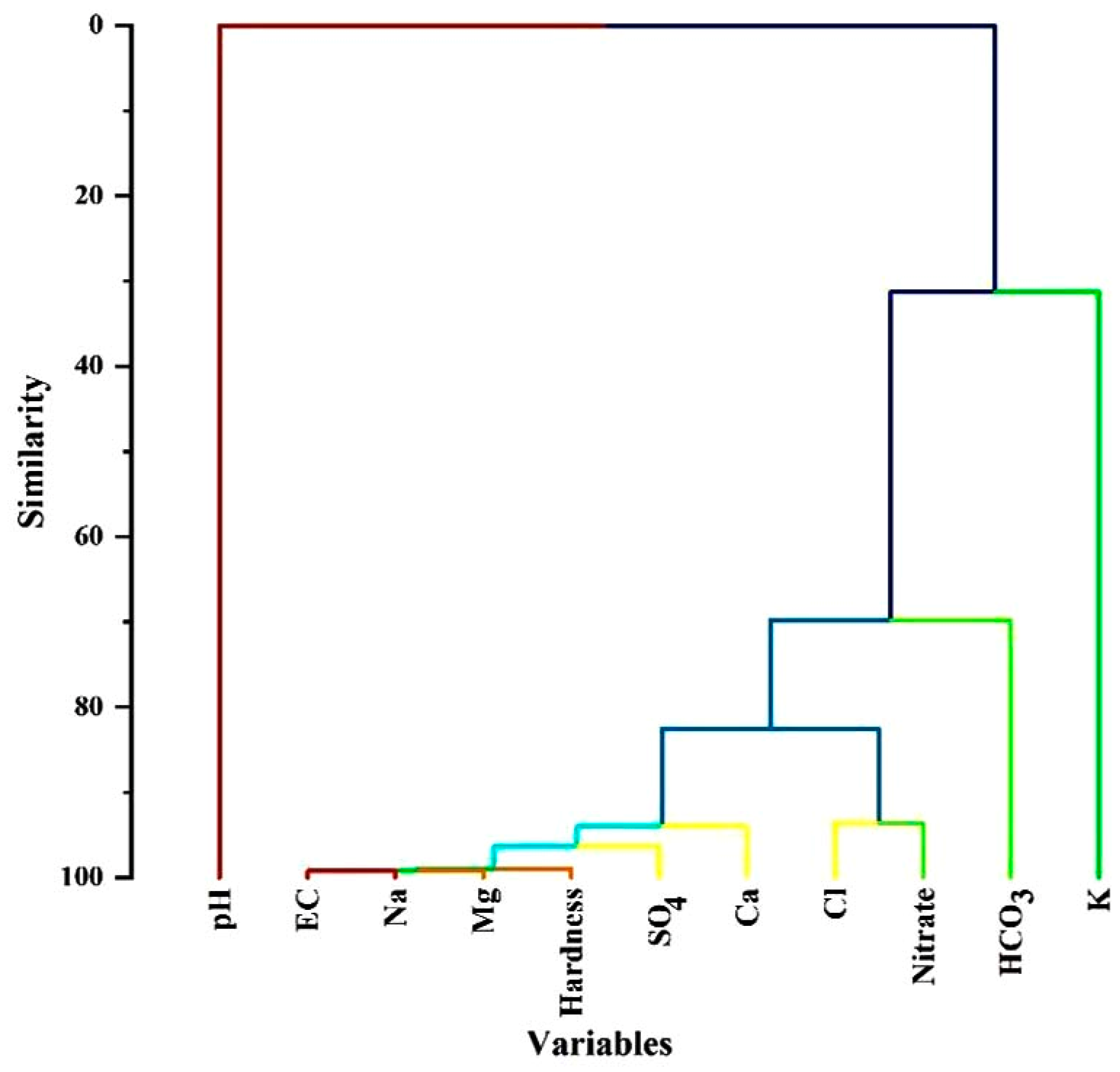
3.5. Hierarchical Cluster Analysis (HCA)
3.6. Correlation Coefficient Matrix Analysis
4. Conclusions
- ▪
- The WQI value was high due to the high values of Ca2+, Mg2+, and SO42− in some wells, indicating that the groundwater has the potential for salinization.
- ▪
- A low nitrate concentration was observed in this region. The reason is a combination of factors, including suitable agricultural activities, natural processes, land management practices, and geological conditions. In addition, since this region has international protection status, an important wetland and ecosystem health are also being protected.
- ▪
- Approximately 90% of wells had hardness levels within the desirable 100 mg/L limit. The low hardness may be because the hardness level decreases when mixed with groundwater and surface water or other sources with lower hardness. Another reason is that groundwater with a short residence time may need more contact with minerals in the aquifer to accumulate significant hardness.
- ▪
- According to the WQI values, most wells’ water is unsuitable for drinking and use. On the other hand, the CCME WQI method indicated that most sites have fair or lower than fair water quality or poor or marginal water quality, which is also unsuitable for drinking purposes.
- ▪
- Based on the spatial distribution of the water quality, it is estimated that the region’s western part is inadequate for drinking water and irrigation in the Kızılırmak Delta.
- ▪
- The correlation coefficient determined the relationships between the groundwater parameters, and the highest correlation was between Mg2+ and CaCO3.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siebert, S.; Burke, J.; Faures, J.-M.; Frenken, K.; Hoogeveen, J.; Döll, P.; Portmann, F.T. Groundwater use for irrigation-a global inventory. Hydrol. Earth Syst. Sci. 2010, 14, 1863–1880. [Google Scholar] [CrossRef]
- Eid, M.H.; Elbagory, M.; Tamma, A.A.; Gad, M.; Elsayed, S.; Hussein, H.; Moghanm, F.S.; Omara, A.E.-D.; Kovács, A.; Péter, S. Evaluation of groundwater quality for irrigation in deep aquifers using multiple graphical and indexing approaches supported with machine learning models and GIS techniques, Souf Valley, Algeria. Water 2023, 15, 182. [Google Scholar] [CrossRef]
- Taşan, S. Estimation of groundwater quality using an integration of water quality index, artificial intelligence methods and GIS: Case study, Central Mediterranean Region of Turkey. Appl. Water Sci. 2023, 13, 15. [Google Scholar] [CrossRef]
- Husna, A.; Akmalia, R.; Rohmat, F.I.W.; Rohmat, F.I.W.; Rohmat, D.; Wijayasari, W.; Alvando, P.V.; Wijaya, A. Groundwater Sustainability Assessment against the Population Growth Modelling in Bima City, Indonesia. Water 2023, 15, 4262. [Google Scholar] [CrossRef]
- Singh, A. Groundwater modelling for the assessment of water management alternatives. J. Hydrol. 2013, 481, 220–229. [Google Scholar] [CrossRef]
- Rohmat, F.I.; Labadie, J.W.; Gates, T.K. Deep learning for compute-efficient modeling of BMP impacts on stream-aquifer exchange and water law compliance in an irrigated river basin. Environ. Model. Softw. 2019, 122, 104529. [Google Scholar] [CrossRef]
- Barbieri, M.; Barberio, M.D.; Banzato, F.; Billi, A.; Boschetti, T.; Franchini, S.; Gori, F.; Petitta, M. Climate change and its effect on groundwater quality. Environ. Geochem. Health 2023, 45, 1133–1144. [Google Scholar] [CrossRef]
- Foster, S.; Hirata, R.; Eichholz, M.; Alam, M.-F. Urban self-supply from groundwater—An analysis of management aspects and policy needs. Water 2022, 14, 575. [Google Scholar] [CrossRef]
- Ram, A.; Tiwari, S.; Pandey, H.; Chaurasia, A.K.; Singh, S.; Singh, Y. Groundwater quality assessment using water quality index (WQI) under GIS framework. Appl. Water Sci. 2021, 11, 46. [Google Scholar] [CrossRef]
- Goodsell, T.; Carling, G.; Aanderud, Z.; Nelson, S.; Fernandez, D.; Tingey, D. Thermal groundwater contributions of arsenic and other trace elements to the middle Provo River, Utah, USA. Environ. Earth Sci. 2017, 76, 268. [Google Scholar] [CrossRef]
- Schreiber, M.; Gotkowitz, M.; Simo, J.; Freiberg, P. Mechanisms of arsenic release to ground water from naturally occurring sources, eastern Wisconsin. In Arsenic in Ground Water: Geochemistry and Occurrence; Springer: Boston, MA, USA, 2003; pp. 259–280. [Google Scholar]
- Selck, B.J.; Carling, G.T.; Kirby, S.M.; Hansen, N.C.; Bickmore, B.R.; Tingey, D.G.; Rey, K.; Wallace, J.; Jordan, J.L. Investigating anthropogenic and geogenic sources of groundwater contamination in a semi-arid alluvial basin, Goshen Valley, UT, USA. Water Air Soil Pollut. 2018, 229, 186. [Google Scholar] [CrossRef]
- Kushawaha, J.; Aithani, D. Geogenic pollutants in groundwater and their removal techniques. In Groundwater Geochemistry: Pollution and Remediation Methods; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; pp. 1–21. [Google Scholar]
- Yuan, L.; Wang, K.; Zhao, Q.; Yang, L.; Wang, G.; Jiang, M.; Li, L. An overview of in situ remediation for groundwater co-contaminated with heavy metals and petroleum hydrocarbons. J. Environ. Manag. 2024, 349, 119342. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; Kumar, A.; Shashtri, S.; Kumar, A.; Kumar, P.; Mallick, J. Multivariate statistical analysis and geochemical modeling for geochemical assessment of groundwater of Delhi, India. J. Geochem. Explor. 2017, 175, 59–71. [Google Scholar] [CrossRef]
- Karunanidhi, D.; Subramani, T.; Roy, P.D.; Li, H. Impact of groundwater contamination on human health. Environ. Geochem. Health 2021, 43, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lu, Y.; Wang, C.; Zhang, M.; Yuan, J.; Zhang, A.; Song, S.; Baninla, Y.; Khan, K.; Wang, Y. Hydrogeochemistry and quality of surface water and groundwater in the drinking water source area of an urbanizing region. Ecotoxicol. Environ. Saf. 2019, 186, 109628. [Google Scholar] [CrossRef] [PubMed]
- Mallick, J.; Singh, C.K.; AlMesfer, M.K.; Kumar, A.; Khan, R.A.; Islam, S.; Rahman, A. Hydro-geochemical assessment of groundwater quality in Aseer Region, Saudi Arabia. Water 2018, 10, 1847. [Google Scholar] [CrossRef]
- Gautam, A.; Rai, S.; Rai, S.; Ram, K. Impact of anthropogenic and geological factors on groundwater hydrochemistry in the unconfined aquifers of Indo-Gangetic plain. Phys. Chem. Earth Parts A/B/C 2022, 126, 103109. [Google Scholar] [CrossRef]
- Ruiz-Pico, Á.; Pérez-Cuenca, Á.; Serrano-Agila, R.; Maza-Criollo, D.; Leiva-Piedra, J.; Salazar-Campos, J. Hydrochemical characterization of groundwater in the Loja Basin (Ecuador). Appl. Geochem. 2019, 104, 1–9. [Google Scholar] [CrossRef]
- Fırat-Ersoy, A.; Turan-Ayyıldız, N.; Arslan, H.; Kuleyin, A. Assessment of seawater intrusion in Kızılırmak delta coastal area (North Turkey) using hydrochemical and isotopic data. Environ. Earth Sci. 2021, 80, 400. [Google Scholar] [CrossRef]
- Karakuş, C.B. Evaluation of water quality of Kızılırmak River (Sivas/Turkey) using geo-statistical and multivariable statistical approaches. Environ. Dev. Sustain. 2020, 22, 4735–4769. [Google Scholar] [CrossRef]
- Samsunlu, A.; Akca, L.; Kinaci, C.; Findik, N.; Tanik, A. Significance of wetlands in water quality management-past and present situation of Kizilirmak Delta, Turkey. Water Sci. Technol. 2002, 46, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Demirci, I.; Gündoğdu, N.Y.; Candansayar, M.E.; Soupios, P.; Vafidis, A.; Arslan, H. Determination and evaluation of saltwater intrusion on bafra plain: Joint interpretation of geophysical, hydrogeological and hydrochemical data. Pure Appl. Geophys. 2020, 177, 5621–5640. [Google Scholar] [CrossRef]
- Mohebbi, M.R.; Saeedi, R.; Montazeri, A.; Vaghefi, K.A.; Labbafi, S.; Oktaie, S.; Abtahi, M.; Mohagheghian, A. Assessment of water quality in groundwater resources of Iran using a modified drinking water quality index (DWQI). Ecol. Indic. 2013, 30, 28–34. [Google Scholar] [CrossRef]
- Asadollah, S.B.H.S.; Sharafati, A.; Motta, D.; Yaseen, Z.M. River water quality index prediction and uncertainty analysis: A comparative study of machine learning models. J. Environ. Chem. Eng. 2021, 9, 104599. [Google Scholar] [CrossRef]
- Sánchez, E.; Colmenarejo, M.F.; Vicente, J.; Rubio, A.; García, M.G.; Travieso, L.; Borja, R. Use of the water quality index and dissolved oxygen deficit as simple indicators of watersheds pollution. Ecol. Indic. 2007, 7, 315–328. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Trivedi, M.K. Artificial neural network-based assessment of water quality index (WQI) of surface water in Gwalior-Chambal region. Int. J. Energy Environ. Eng. 2023, 14, 47–61. [Google Scholar] [CrossRef]
- Horton, R.K. An index number system for rating water quality. J. Water Pollut. Control. Fed. 1965, 37, 300–306. [Google Scholar]
- Lumb, A.; Sharma, T.; Bibeault, J.-F. A review of genesis and evolution of water quality index (WQI) and some future directions. Water Qual. Expo. Health 2011, 3, 11–24. [Google Scholar] [CrossRef]
- Davies, J.-M. Application and tests of the Canadian water quality index for assessing changes in water quality in lakes and rivers of central North America. Lake Reserv. Manag. 2006, 22, 308–320. [Google Scholar] [CrossRef]
- Wills, M.; Irvine, K.N. Application of the national sanitation foundation water quality index in Cazenovia Creek, NY, pilot watershed management project. Middle States Geogr. 1996, 1996, 95–104. [Google Scholar]
- Brown, R.M.; McClelland, N.I.; Deininger, R.A.; O’Connor, M.F. A water quality index—Crashing the psychological barrier. In Indicators of Environmental Quality: Proceedings of a Symposium Held during the AAAS Meeting, Philadelphia, Pennsylvania, 26–31 December 1971; Springer: Boston, MA, USA, 1972; pp. 173–182. [Google Scholar]
- Hamidi, M.D.; Kissane, S.; Bogush, A.A.; Karim, A.Q.; Sagintayev, J.; Towers, S.; Greenwell, H.C. Spatial estimation of groundwater quality, hydrogeochemical investigation, and health impacts of shallow groundwater in Kabul city, Afghanistan. Sustain. Water Resour. Manag. 2023, 9, 20. [Google Scholar] [CrossRef]
- Udeshani, W.; Dissanayake, H.; Gunatilake, S.; Chandrajith, R. Assessment of groundwater quality using water quality index (WQI): A case study of a hard rock terrain in Sri Lanka. Groundw. Sustain. Dev. 2020, 11, 100421. [Google Scholar] [CrossRef]
- Krishan, G.; Kumar, M.; Rao, M.S.; Garg, R.; Yadav, B.K.; Kansal, M.; Singh, S.; Bradley, A.; Muste, M.; Sharma, L. Integrated approach for the investigation of groundwater quality through hydrochemistry and water quality index (WQI). Urban Clim. 2023, 47, 101383. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Thirumalai, R.; Sundar, M.L.; Anusuya, M.; Kumar, P.M.; Hemalatha, E.; Prasad, M.M.; Munjal, N. Assessment of underground water quality and water quality index across the Noyyal River basin of Tirupur District in South India. Urban Clim. 2023, 49, 101436. [Google Scholar] [CrossRef]
- El Yousfi, Y.; Himi, M.; El Ouarghi, H.; Aqnouy, M.; Benyoussef, S.; Gueddari, H.; Ait Hmeid, H.; Alitane, A.; Chaibi, M.; Zahid, M. Assessment and Prediction of the Water Quality Index for the Groundwater of the Ghiss-Nekkor (Al Hoceima, Northeastern Morocco). Sustainability 2022, 15, 402. [Google Scholar] [CrossRef]
- Soujanya Kamble, B.; Saxena, P.R.; Kurakalva, R.M.; Shankar, K. Evaluation of seasonal and temporal variations of groundwater quality around Jawaharnagar municipal solid waste dumpsite of Hyderabad city, India. SN Appl. Sci. 2020, 2, 498. [Google Scholar] [CrossRef]
- Rabeiy, R.E. Assessment and modeling of groundwater quality using WQI and GIS in Upper Egypt area. Environ. Sci. Pollut. Res. 2018, 25, 30808–30817. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Noori, A.R. Groundwater quality assessment and modeling utilizing water quality index and GIS in Kabul Basin, Afghanistan. Environ. Monit. Assess. 2022, 194, 673. [Google Scholar] [CrossRef]
- Şener, E.; Şener, Ş.; Varol, S. Evaluation of irrigation water quality using GIS-based analytic hierarchy process (AHP) in Kızılırmak Delta (Turkey). Arab. J. Geosci. 2022, 15, 678. [Google Scholar] [CrossRef]
- SMM. Determination of the Kızılırmak Delta Water Footprint, Sub-Project Report; SMM: Samsun, Turkey, 2018; 594p. [Google Scholar]
- Chowdhury, R.M.; Muntasir, S.Y.; Hossain, M.M. Water quality index of water bodies along Faridpur-Barisal road in Bangladesh. Glob. J. Eng. Technol. 2012, 2, 1–8. [Google Scholar]
- Ponsadailakshmi, S.; Sankari, S.G.; Prasanna, S.M.; Madhurambal, G. Evaluation of water quality suitability for drinking using drinking water quality index in Nagapattinam district, Tamil Nadu in Southern India. Groundw. Sustain. Dev. 2018, 6, 43–49. [Google Scholar] [CrossRef]
- Tripathi, M.; Singal, S.K. Use of principal component analysis for parameter selection for development of a novel water quality index: A case study of river Ganga India. Ecol. Indic. 2019, 96, 430–436. [Google Scholar] [CrossRef]
- Canadian Council of Ministers of the Environment. Canadian Environmental Quality Guidelines; Canadian Council of Ministers of the Environment: Winnipeg, MB, Canada, 2002; Volume 2. [Google Scholar]
- Jolliffe, I.T. Discarding variables in a principal component analysis. I: Artificial data. J. R. Stat. Soc. Ser. C Appl. Stat. 1972, 21, 160–173. [Google Scholar] [CrossRef]
- Mahapatra, S.; Sahu, M.; Patel, R.; Panda, B.N. Prediction of water quality using principal component analysis. Water Qual. Expo. Health 2012, 4, 93–104. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Discarding variables in a principal component analysis. II: Real data. J. R. Stat. Soc. Ser. C Appl. Stat. 1973, 22, 21–31. [Google Scholar] [CrossRef]
- Commission, J.R.C.-E. Handbook on Constructing Composite Indicators: Methodology and User Guide; OECD Publishing: Paris, France, 2008. [Google Scholar]
- Samtio, M.S.; Jahangir, T.M.; Mastoi, A.S.; Lanjwani, M.F.; Rajper, R.H.; Lashari, R.A.; Agheem, M.H.; Noonari, M.W. Impact of rock-water interaction on hydrogeochemical characteristics of groundwater: Using multivariate statistical, water quality index and irrigation indices of chachro sub-district, thar desert, sindh, Pakistan. Groundw. Sustain. Dev. 2023, 20, 100878. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.; Walsh, R. Soil water repellency: Its causes, characteristics and hydro-geomorphological significance. Earth-Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Gazette, R. Regulation on Surface Water Quality. Annex-5 (Amended: RG-10/8/2016-29797) Environmental QUALITY Standards for Some Parameters in Surface Water Bodies and Purposes of Use. (28483 Repeated), 30, 2012. Available online: https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=16806&MevzuatTur=7&MevzuatTertip=5 (accessed on 27 March 2024).
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 38, pp. 104–108. [Google Scholar]
- Jena, S.K. A review on potash recovery from different rock and mineral sources. Min. Metall. Explor. 2021, 38, 47–68. [Google Scholar] [CrossRef]
- Rajmohan, N.; Elango, L. Nutrient chemistry of groundwater in an intensively irrigated region of southern India. Environ. Geol. 2005, 47, 820–830. [Google Scholar] [CrossRef]
- Laghrib, F.; Bahaj, T.; El Kasmi, S.; Hilali, M.; Kacimi, I.; Nouayti, N.; Dakak, H.; Bouzekraoui, M.; El Fatni, O.; Hammani, O. Hydrogeochemical study of groundwater in arid and semi-arid regions of the Infracenomanian aquifers (Cretaceous Errachidia basin, Southeastern Morocco). Using hydrochemical modeling and multivariate statistical analysis. J. Afr. Earth Sci. 2024, 209, 105132. [Google Scholar] [CrossRef]
- Li, C.; Gao, X.; Li, S.; Bundschuh, J. A review of the distribution, sources, genesis, and environmental concerns of salinity in groundwater. Environ. Sci. Pollut. Res. 2020, 27, 41157–41174. [Google Scholar] [CrossRef] [PubMed]
- WHO. Use of Glycated Hemoglobin (HbA1c) in Diagnosis of Diabetes Mellitus: Abbreviated Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Haritash, A.; Kaushik, C.P.; Kaushik, A.; Kansal, A.; Yadav, A.K. Suitability assessment of groundwater for drinking, irrigation and industrial use in some North Indian villages. Environ. Monit. Assess. 2008, 145, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.; Bandyopadhyay, A.; Bhadra, A. Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India. Environ. Monit. Assess. 2010, 166, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Kumar, M. Sulphate contamination in groundwater and its remediation: An overview. Environ. Monit. Assess. 2020, 192, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hanor, J.S.; Wendeborn, F.C. Origin of sodium bicarbonate groundwaters, Southern Hills Aquifer System, USA by silicate hydrolysis. Appl. Geochem. 2023, 148, 105512. [Google Scholar] [CrossRef]
- Lin, G.-F.; Wu, M.-C. An RBF network with a two-step learning algorithm for developing a reservoir inflow forecasting model. J. Hydrol. 2011, 405, 439–450. [Google Scholar] [CrossRef]
- Singh, P.; Khan, I. Ground water quality assessment of Dhankawadi ward of Pune by using GIS. Int. J. Geomat. Geosci. 2011, 2, 688–703. [Google Scholar]
- Craswell, E. Fertilizers and nitrate pollution of surface and ground water: An increasingly pervasive global problem. SN Appl. Sci. 2021, 3, 518. [Google Scholar]
- Liu, G.; Wu, W.; Zhang, J. Regional differentiation of non-point source pollution of agriculture-derived nitrate nitrogen in groundwater in northern China. Agric. Ecosyst. Environ. 2005, 107, 211–220. [Google Scholar] [CrossRef]
- McCray, J.E.; Kirkland, S.L.; Siegrist, R.L.; Thyne, G.D. Model parameters for simulating fate and transport of on-site wastewater nutrients. Groundwater 2005, 43, 628–639. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The nitrogen cascade. Bioscience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Muniraj, K.; Thomas, M.; Ravichandran, N.; Bidorn, B. Identifying influencing groundwater parameter on human health associate with irrigation indices using the Automatic Linear Model (ALM) in a semi-arid region in India. Environ. Res. 2021, 202, 111778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, D.; Prabha, R.; Sharma, A.K. Role of cyanobacteria in nutrient cycle and use efficiency in the soil. Nutr. Use Effic. Basics Adv. 2015, 163–171. [Google Scholar]
- WHO. One Health; World Health Organization: Geneva, Switzerland, 2017; p. 736. [Google Scholar]
- Chaurasia, A.K.; Pandey, H.; Tiwari, S.; Pandey, P.; Ram, A. Groundwater vulnerability assessment using water quality index (WQI) under geographic information system (GIS) framework in parts of Uttar Pradesh, India. Sustain. Water Resour. Manag. 2021, 7, 40. [Google Scholar] [CrossRef]
- Changsheng, H.; Akram, W.; Rashid, A.; Ullah, Z.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M. Quality Assessment of Groundwater Based on Geochemical Modelling and Water Quality Index (WQI). Water 2022, 14, 3888. [Google Scholar] [CrossRef]
- Saqib, N.; Rai, P.K.; Kanga, S.; Kumar, D.; Đurin, B.; Singh, S.K. Assessment of Ground Water Quality of Lucknow City under GIS Framework Using Water Quality Index (WQI). Water 2023, 15, 3048. [Google Scholar] [CrossRef]
- Gaagai, A.; Aouissi, H.A.; Bencedira, S.; Hinge, G.; Athamena, A.; Heddam, S.; Gad, M.; Elsherbiny, O.; Elsayed, S.; Eid, M.H. Application of water quality indices, machine learning approaches, and GIS to identify groundwater quality for irrigation purposes: A case study of Sahara Aquifer, Doucen Plain, Algeria. Water 2023, 15, 289. [Google Scholar] [CrossRef]
- Howladar, M.F.; Chakma, E.; Koley, N.J.; Islam, S.; Al Numanbakth, M.A.; Ahmed, Z.; Chowdhury, T.R.; Akter, S. The water quality and pollution sources assessment of Surma river, Bangladesh using, hydrochemical, multivariate statistical and water quality index methods. Groundw. Sustain. Dev. 2021, 12, 100523. [Google Scholar] [CrossRef]
- Frías, S.; Conde, J.E.; Rodríguez-Bencomo, J.J.; García-Montelongo, F.; Pérez-Trujillo, J.P. Classification of commercial wines from the Canary Islands (Spain) by chemometric techniques using metallic contents. Talanta 2003, 59, 335–344. [Google Scholar] [CrossRef]

| WQI Level | Water Quality Status | Grade |
|---|---|---|
| 0–25 | Excellent water quality | A |
| 26–50 | Good water quality | B |
| 51–75 | Poor water quality | C |
| 76–100 | Very poor water quality | D |
| >100 | Unsuitable for drinking | E |
| Water Quality | CCMEWQI Value | Description |
|---|---|---|
| Excellent | 95–100 | Water quality is protected with a virtual absence of threat or impairment, conditions very close to natural or pristine levels. |
| Very good | 89–94 | Water quality is protected with a slight threat or impairment, conditions close to natural or pristine levels. |
| Good | 80–88 | Water quality is protected with only a minor threat or impairment; conditions rarely depart from natural or desirable levels. |
| Fair | 65–79 | Water quality is usually protected but occasionally threatened or impaired; conditions sometimes depart from natural or desirable. |
| Poor (Marginal) | 45–64 | Water quality is frequently threatened or impaired; conditions often depart from natural or desirable levels. |
| Poor | 0–44 | Water quality is almost always threatened or impaired; conditions usually depart from natural or desirable levels. |
| Stations | GIS-Based WQI | Remarks |
|---|---|---|
| Well 1 | 69.14688 | Poor |
| Well 2 | 200.0549 | Unsuitable |
| Well 3 | 144.0467 | Unsuitable |
| Well 4 | 143.5819 | Unsuitable |
| Well 5 | 66.03841 | Poor |
| Well 6 | 157.0077 | Unsuitable |
| Well 7 | 367.2081 | Unsuitable |
| Well 8 | 184.5786 | Unsuitable |
| Well 9 | 772.5291 | Unsuitable |
| Well 10 | 63.94814 | Poor |
| Well 11 | 211.5382 | Unsuitable |
| Stations | CCME WQI | Remarks |
|---|---|---|
| Well 1 | 77.728 | Fair |
| Well 2 | 70.306 | Fair |
| Well 3 | 62.884 | Fair |
| Well 4 | 62.880 | Fair |
| Well 5 | 77.731 | Fair |
| Well 6 | 62.878 | Fair |
| Well 7 | 40.577 | Poor |
| Well 8 | 48.027 | Marginal |
| Well 9 | 32.887 | Poor |
| Well 10 | 77.728 | Fair |
| Well 11 | 70.278 | Fair |
| Variables | Component Matrix | |
|---|---|---|
| PC1 | PC2 | |
| pH | −0.18876 | 0.15442 |
| EC | 0.35053 | 0.16871 |
| Na+ | 0.35206 | 0.09999 |
| K+ | 0.02028 | 0.79085 |
| Ca2+ | 0.31866 | 0.23649 |
| Mg2+ | 0.35237 | 0.09261 |
| HCO3− | 0.24332 | −0.11207 |
| Cl− | 0.3326 | −0.23585 |
| SO42− | 0.34852 | −0.0826 |
| CaCO3 | 0.34591 | 0.15731 |
| NO3− | 0.28371 | −0.38469 |
| Eigenvalues | 7.764 | 1.26353 |
| Variability (%) | 70.50 | 11.49 |
| Cumulative (%) | 70.50 | 82.07 |
| pH | EC | Na+ | K+ | Ca2+ | Mg2+ | HCO3− | Cl− | SO42− | CaCO3 | NO3− | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | −0.436 | −0.488 | −0.054 | −0.296 | −0.443 | −0.468 | −0.470 | −0.448 | −0.411 | −0.469 |
| EC | 1 | 0.989 | 0.197 | 0.928 | 0.987 | 0.605 | 0.854 | 0.944 | 0.985 | 0.678 | |
| Na+ | 1 | 0.099 | 0.918 | 0.989 | 0.610 | 0.855 | 0.960 | 0.988 | 0.673 | ||
| K+ | 1 | 0.164 | 0.101 | 0.078 | −0.116 | −0.080 | 0.129 | −0.210 | |||
| Ca2+ | 1 | 0.902 | 0.383 | 0.711 | 0.873 | 0.962 | 0.577 | ||||
| Mg2+ | 1 | 0.669 | 0.871 | 0.950 | 0.982 | 0.679 | |||||
| HCO3− | 1 | 0.680 | 0.547 | 0.563 | 0.566 | ||||||
| Cl− | 1 | 0.933 | 0.810 | 0.911 | |||||||
| SO42− | 1 | 0.939 | 0.793 | ||||||||
| CaCO3 | 1 | 0.636 | |||||||||
| NO3− | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arıman, S.; Soydan-Oksal, N.G.; Beden, N.; Ahmadzai, H. Assessment of Groundwater Quality through Hydrochemistry Using Principal Components Analysis (PCA) and Water Quality Index (WQI) in Kızılırmak Delta, Turkey. Water 2024, 16, 1570. https://doi.org/10.3390/w16111570
Arıman S, Soydan-Oksal NG, Beden N, Ahmadzai H. Assessment of Groundwater Quality through Hydrochemistry Using Principal Components Analysis (PCA) and Water Quality Index (WQI) in Kızılırmak Delta, Turkey. Water. 2024; 16(11):1570. https://doi.org/10.3390/w16111570
Chicago/Turabian StyleArıman, Sema, Nazire Göksu Soydan-Oksal, Neslihan Beden, and Hayatullah Ahmadzai. 2024. "Assessment of Groundwater Quality through Hydrochemistry Using Principal Components Analysis (PCA) and Water Quality Index (WQI) in Kızılırmak Delta, Turkey" Water 16, no. 11: 1570. https://doi.org/10.3390/w16111570
APA StyleArıman, S., Soydan-Oksal, N. G., Beden, N., & Ahmadzai, H. (2024). Assessment of Groundwater Quality through Hydrochemistry Using Principal Components Analysis (PCA) and Water Quality Index (WQI) in Kızılırmak Delta, Turkey. Water, 16(11), 1570. https://doi.org/10.3390/w16111570








