Abstract
The narrow pH application range and lower utilization of ferrous ions (Fe(II)) restrict the application of Fe(II)/persulfate (PS) technology. In this paper, simulated sunlight and citric acid (Cit) as a chelator were introduced in an Fe(II)/PS system to overcome the drawbacks and enhance the degradation of typical antibiotic sulfamethoxazole (SMX) in secondary wastewater. The degradation kinetics, mechanism, and influence factors of SMX in a sunlight/Fe(II)/Cit/PS system and a sunlight/Fe(II)/Cit system as a comparable system were investigated. The removal efficiency of SMX can reach 71.15% and 85.25% in the sunlight/Fe(II)/Cit system and sunlight/Fe(II)/Cit/PS system with 0.1 mM Fe(II), 0.6 mM Cit, and 1 mM PS. The increase of Fe(II) concentration in both systems proved that sunlight promoted the regeneration of Fe(II) from the ferric ion chelates. However, the Fe(II) concentration decreased after 30 min in the sunlight/Fe(II)/Cit/PS system because of the decomposition of Cit. Radical quencher experiments indicated that SO4·−, ·OH, and O2·− contributed 2.48%, 88.43%, and 6.91% to the removal of SMX, respectively. Electron paramagnetic resonance spectra also proved the formation of ·OH and O2·−. The degradation of SMX was proposed to proceed via isomerization, cleavage of S–N bond, and hydroxylation. Overall, the sunlight/Fe(II)/Cit/PS process can be used as an advanced treatment technology for antibiotics in municipal wastewater.
1. Introduction
During the last few decades, antibiotics, an important kind of emerging contaminant, have caused extensive concern due to their ubiquitous presence in the environment and potential ecological and human health risks [1,2]. Sulfonamide antibiotics were first synthesized in the 1930s and are commonly utilized to prevent and treat diseases and infections in humans and animals, as well as to foster growth in husbandry and aquaculture [3]. As the antibiotics, after administration, cannot be fully absorbed by human and animal bodies, they are excreted and discharged into the environment, resulting in the presence of SAs in wastewater, surface water, and soil [4]. In particular, wastewater effluent is a key source of antibiotics in receiving water bodies [5]. Sulfamethoxazole (SMX) is a commonly detected sulfonamide in wastewater effluent, the reported detection concentration was 0.2~7.1 μg/L in winter and 15~37.8 μg/L in Xinjiang, northwest China [6]. Moreover, the persistence of SMX and other antibiotics caused by their continuous discharge or recalcitrance may induce the spread of resistance genes and eventually pose an ecological and human health risk. Therefore, it is urgent to develop efficient advanced treatment technology to eliminate and control antibiotic pollution in secondary wastewater.
The advanced oxidation process that involves persulfate (PS) and generates highly reactive SO4·− and ·OH radicals is considered promising in the removal of antibiotics [7]. The common activation methods for PS include transition metals, thermal, carbon-based materials, and so on [8,9,10,11,12]. Among these activation methods, Ferrous ion (Fe(II)) has received the most attention because it is nontoxic, inexpensive, and environmentally friendly [13]. Previous research has proved that Fe(II) can effectively activate PS to degrade emerging contaminants, for instance, carbamazepine [14], atrazine [15], trimethoprim [16], and chloramphenicol [17]. However, the Fe(II)/PS process still contains two main drawbacks. First, the Fe(II)/PS process generally requires operating at pH values of ca. 3 to avoid the generation of Ferric ion (Fe(III)) precipitates, which can’t activate PS to generate reactive radicals and reversely produce excessive sludge [18]. Second, Fe(II) reacts with persulfate and transforms to Fe(III) quickly through Equation (1); however, Fe(II) regeneration from Fe(III) is a relatively slow process (Equation (2)). The fast transformation of Fe(II) and accumulation of Fe(III) lead to the low utility of Fe(II) and excessive sludge. Therefore, accelerating the reduction of Fe(II) from Fe(III) in an Fe(II)/PS system is very important [19].
To overcome the limitations of the Fe(II)/PS system, chelating agents such as citric acid/citrate (Cit), oxalic acid/oxalate, and ethylenediaminetetraacetic acid (EDTA) are introduced into the system to form Fe(II)/Fe(III) complexes, which can maintain solubility in a wider pH range [13,20,21]. Gao et al. [13] found that an Fe(II)/Cit/PS system can effectively degrade pyrene under neutral pH, and the maximum degradation efficiency of pyrene reached 94.4% with the molar ratio of Fe(II), citrate, PS, and pyrene of 2000/2000/2000/1 and reaction time of 720 min. Yuan et al. [22] demonstrated that an Fe(II)/Cit/PS system improved the productivity of SO4·− and the decomposition of extracellular polymeric substances, as well as the sludge dewaterability.
UV irradiation can be utilized to activate PS and peroxymonosulfate (PMS) and promote the conversion of Fe(III) to Fe(II). Ling et al. [18] developed a process that incorporates Fe(II)/Cit/UV/PMS and was used for the degradation of carbamazepine. The degradation efficiency of carbamazepine achieved up to 71% by an Fe(II)/Cit/UV/PMS process for 20 min, and significantly higher than that in the treatment system without UV. However, the introduction of UV causes excessive energy input and increases the cost of wastewater treatment. In contrast, sunlight, a clear, abundant, and economical energy, can be used as an alternative energy for UV to accelerate the Fe(II)/Fe(III) cycle. Previous research has proved that Fe(III) ligand complexes can be reduced to Fe(II) through ligand-to-metal charge transfer (LMCT) under the irradiation of sunlight [23,24]. Therefore, we propose a hypothesis that the combination of sunlight/Fe(II)/Cit/PS system has a good removal ability for the recalcitrant emerging contaminants, in which citric acid is used to form a stable soluble Fe(II)–citrate complex in wastewater and sunlight is used to drive the reduction of Fe(III) to Fe(II).
In this paper, SMX was used as the representative pollutant to study the removal and impact factors of the sunlight/Fe(II)/Cit system in eliminating the emerging contaminants in real secondary wastewater. The degradation mechanism of SMX by a sunlight/Fe(II)/Cit/PS system was explored based on the concentration changes of Fe(III)/Fe(II) and carboxylic acid, as well as the identification of the main reactive species. As Fe(III)/Cit complexes under the irradiation of sunlight can generate reactive radicals and degrade the organic matter [25], the sunlight/Fe(II)/Cit process was also investigated to make a comparison. Furthermore, the degradation products of SMX were identified and the degradation pathway of SMX by a sunlight/Fe(II)/Cit/PS system was proposed. The results can provide a scientific basis for developing advanced wastewater treatment technology for recalcitrant organic matter.
2. Experimental Section
2.1. Chemicals
SMX (≥99.7%) was obtained from Sigma-Aldrich. Ferrous sulfate (FeSO4 ·7H2O, 99.0%), PS (K2S2O8, 99.5%), and citric acid monohydrate (C6H10O8, 99.5%) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Carbon tetrachloride (CCl4, 99%) and glacial acetic acid (C2H4O2, 99.5%) were purchased from Macklin Reagents. 5, 5-dimethyl-1-pyrrolide-N-oxide (DMPO) was purchased from Dojindo laboratories (Kumamoto, Japan). Other chemicals utilized in this study are listed in Text S1 (Supporting Information).
2.2. Secondary Wastewater Effluent
Secondary wastewater effluent was collected from the secondary clarifier following the activated sludge treatment at a sewage treatment plant in Nanjing, Jiangsu Province of China. Then, the wastewater sample was immediately transported to the laboratory and filtered using a 0.45 μm capsule filter (Millipore, Bedford, MA, USA). The wastewater was characterized by a total organic carbon (TOC) of 9.02 mg/L, pH 7.6, NO3− of 29.61 mg/L, NO2− of 0.49 mg/L, Cl− of 52.69 mg/L, and alkalinity of 2.58 mM.
2.3. Experiment Procedure
The reaction solution was first prepared in 250 mL flasks containing 100 mL of secondary wastewater effluent. A certain amount of Fe (II) and citric acid stock solution was added into the secondary wastewater effluent and stirred for 30 min under dark conditions to ensure the full complexing of Fe(II) and citric acid. The doses of Fe (II) were set as 0.05, 0.1, 0.15, and 0.2 mM, and the ratios of Fe (II) to citric acid were 1:2, 1:4, 1:6, 1:8, and 1:10, respectively. Then, a specific amount of SMX stock solution was put into the solution to reach an initial concentration of 1.0 mg/L. After that, the solution was moved to the 50 mL quartz tubes and placed in a photochemical reactor (XPA-7, Xujiang Electromechanical Plant, Nanjing, China). The simulated solar light was generated by a 500 W xenon lamp and 290 nm cutoff filters, which hinders the penetration of light lower than 290 nm. The irradiation intensity of the simulated light was 97.17 mW·cm−1 measured by a CEL-FZ-A radiometer (Beijing China Education Au-light Co., Ltd., Beijing, China). The temperature of reaction solutions was controlled at about 25 ± 2 °C by the circulating cooling water. Then, the stock solution of PS was added into the tubes to start the degradation process. After a period of reaction (0, 1, 2, 5, 10, 20, 30, 45, 60, 90, and 120 min), 0.9 mL samples were collected in 2 mL HPLC vials containing 0.3 mL of ethanol to prevent further oxidation due to any residual oxidant or free radical. The samples were filtered before the determination of SMX concentration. Meanwhile, dark/Fe(II)/PS, sunlight/Fe(II)/PS, dark/Fe(II)/Cit, sunlight/Fe(II)/Cit, and dark/Fe(II)/Cit/PS control groups were conducted as comparisons. All the experiments were conducted in duplicate.
2.4. Identification of Reactive Species
Quenching experiments were first carried out to identify the principal reactive species that are responsible for the removal of SMX by employing methanol (MeOH), tert-butyl alcohol (TBA), and CCl4 as quenchers. MeOH (150 mM), TBA (150 mM), and CCl4 (50 mM) were added to the reaction system at the beginning of the experiment. The other experimental procedure was the same as described in Section 2.3. It is known that MeOH can be used to quench both SO4·− and ·OH, and TBA is used to quench ·OH, while CCl4 is used to quench HO2· and O2·− radicals [22,26]. The contribution of reactive species can be calculated using Equations (3)–(5).
where, , , and represent the contributions of ·OH, SO4·−, HO2/O2·− to the removal of SMX, respectively. kobs is the observed first-order rate constant of SMX degradation in wastewater. kTBA, kMeOH, and represent the observed first-order rate constants of SMX removal with the addition of quenchers TBA, MeOH, and CCl4, respectively.
The active radicals produced in the sunlight/Fe(II)/Cit/PS system at 0.1 mM Fe(II), 0.6 mM Cit, 1 mM PS, and a reaction time of 5 min were trapped using DMPO (100 mM) were analyzed using Bruker EMX 10/12 equipment (Karlsruhe, Germany).
2.5. Analytical Methods
2.5.1. Determination of SMX
The concentration of SMX was measured using high-performance liquid chromatography (HPLC, Thermo Scientific, Waltham, MA, USA). The separation column was an Agilent Eclipse plus C18 column (150 mm × 4.6 mm, 5 μm). The mobile phase consisted of an acetonitrile and 0.1% formic acid solution with the proportions of 25:75 (v/v) at a flow rate of 1 mL/min. The detector wavelength was 270 nm.
2.5.2. Analysis of Fe species
The 1, 10-phenanthroline method was used to determine the concentration of dissolved total iron and Fe(II). The concentration of dissolved Fe(III) was obtained based on the difference between the concentration of dissolved total iron and Fe(II).
2.5.3. Determination of Carboxylic Acids
The concentrations of citric acid and the produced carboxylic acids were determined using HPLC at 210 nm. An Agilent ZORBAX SB-AQ column (250 mm × 4.6 mm, 5 μm) was used to separate different carboxylic acids. The mobile phase was a mixture of 0.01 mM KH2PO4 (pH 2) and methanol with a proportion of 99.5:0.5 (v/v). The flow rate was 0.6 mL/min. The pH was regulated with phosphoric acid.
2.5.4. Identification of SMX Degradation Products
After treatment for 0, 15, 30, 60, and 120 min by the sunlight/Fe(II)/Cit/PS process, the SMX-containing samples were collected for the identification of degradation products and exploration of their evolution. Before the analysis, the collected samples were concentrated 100 times using Oasis HLB solid phase cartridges (200 mg/6 mL, Waters, Milford, MA, USA). After that, the concentrated samples were analyzed using HPLC coupled with a high-resolution mass spectrometer (LTQ-Orbitrap XL MS, Thermo Scientific, Waltham, MA, USA) operating in a positive mode according to our previous publication [27]. The detailed parameters and operation conditions for the HPLC-MS spectrometer are provided in Text S2 (Supplementary Information).
3. Results and Discussion
3.1. Removal of SMX by Sunlight/Fe(II)/Cit/PS System
Figure 1a illustrates the removal of SMX in secondary wastewater in Fe(II)/PS, Fe(II)/Cit, Fe(II)/Cit/PS without and with simulated sunlight at Fe(II), citric acid, and PS concentration of 0.1, 0.6, and 1 mM, respectively. No significant removal of SMX was detected in dark/Fe(II)/Cit, dark/Fe(II)/PS, and dark/Fe(II)/Cit/PS systems, with the removal efficiency of SMX of 8.28%, 9.82%, and 12.17% after 120 min, respectively. The previous publication demonstrated that an Fe(II)/PS system can remove 50.4% of SMX at an Fe(II) concentration of 50 μM, PS of 200 μM, and pH of 7.0 [28]. The lower removal of SMX in the dark/Fe(II)/PS system in this research may be attributed to the weak alkaline pH (7.6) and complex component in the wastewater. Under this circumstance, Fe(II) rapidly hydrolyses and precipitates, and cannot activate persulfate to produce active radicals, and the complex components in the wastewater may hinder the degradation of SMX. In the dark/Fe(II)/Cit/PS system, the addition of citric acid elevated the solubility of Fe, and the soluble Fe probably existed as Fe(II)–Cit or Fe(III)–Cit complexes. The lower removal of SMX indicated that Fe(II)/Fe(III)–Cit complexes couldn’t effectively activate PS in wastewater in the dark [18]. The removal of SMX in the dark/Fe(II)/Cit, dark/Fe(II)/PS, and dark/Fe(II)/Cit/PS system is probably achieved by the adsorption of Fe precipitation [29]. Sunlight slightly promoted the degradation of SMX by Fe(II)/PS. The removal of SMX by the sunlight/Fe(II)/PS system was 23.00% after 120 min. Compared with the dark/Fe(II)/PS system, the increase in SMX removal in the sunlight/Fe(II)/PS system is probably caused by the photodegradation of SMX under the irradiation of sunlight [30]. In contrast, sunlight significantly promoted the degradation of SMX in the Fe(II)/Cit system and the Fe(II)/Cit/PS system, the removal of SMX in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system was 71.15% and 85.26%, respectively. This is higher than the previous report that SMX removal of 52.3% was achieved in wastewater using an Fe(II)-activated persulfate process [31]. Previous research also reported that sunlight/Fe(III)–citrate complexes can degrade propranolol and sulfamethazine, as a sunlight/Fe(III)–citrate system can generate multiple reactive radicals (Equations (6)–(10)), with ·OH generally playing the significant role [25,32]. In a sunlight/Fe(II)/Cit/PS system, except for the reactions that occurred in the sunlight/Fe(II)/Cit system, the regenerated Fe(II) can activate PS and generate more radical species (Equation (1)), consequently enhancing the degradation of SMX in secondary wastewater.
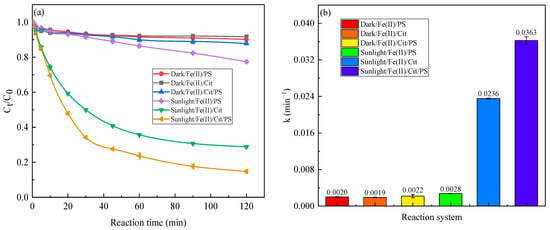
Figure 1.
Degradation efficiency (a) and pseudo-first-order rate constant (b) of SMX in different systems. Conditions: [SMX] = 1 mg/L, [Fe(II)] = 0.1 mM, [Cit] = 0.6 mM, [PS] = 1 mM, reaction time of 120 min (a) and 30 min (b).
The degradation of SMX can be classified into two stages: a rapid phase (0–30 min) and a relatively slow phase (30–120 min). The degradation of SMX in the rapid phase follows pseudo-first-order kinetics. The degradation reaction rate constant of SMX in the sunlight/Fe(II)/Cit/PS system was 0.0363 min−1, which is 18.15, 19.11, 16.50, 12.96, and 1.54 times that in the dark/Fe(II)/Cit, dark/Fe(II)/PS, dark/Fe(II)/Cit/PS, sunlight/Fe(II)/PS, and sunlight/Fe(II)/Cit systems (Figure 1b).
3.2. Changes in the Concentration of Different Fe Species and Carboxylic Acids
The intensified removal of SMX in the sunlight/Fe(II)/Cit and sunlight/Fe(II)/Cit/PS systems is strongly correlated with the regeneration of Fe(II). The concentration of different Fe species in the sunlight/Fe(II)/Cit and sunlight/Fe(II)/Cit/PS systems is displayed in Figure 2. As described in the experimental section, Fe (II) and citric acid stock solution were added into the secondary wastewater effluent and stirred for 30 min before the degradation experiment. Fe(II) can be partially oxidized to Fe(III) by oxygen during this process. Therefore, Fe(III) (0.052 mM) showed a higher initial concentration than Fe(II) (0.015 mM). The total dissolved Fe concentration was 0.067 mM, indicating that about one-third of the added Fe(II) precipitated in the weak alkaline environment. The dissolved Fe generally existed as Fe(II) or Fe(III)–Cit complexes.
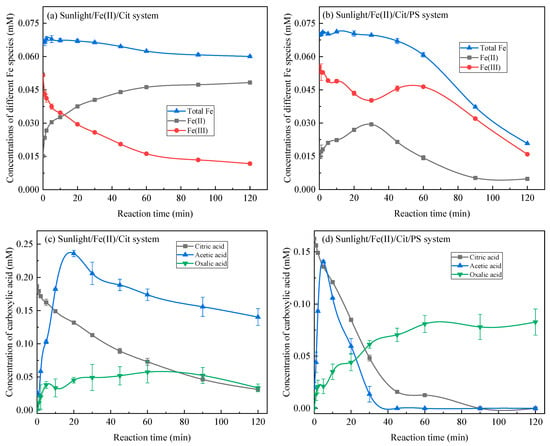
Figure 2.
Changes in the concentration of different Fe species (a,b) and carboxylic acids (c,d) in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system. Conditions: [SMX] = 1 mg/L, [Fe(II)] = 0.1 mM, [Cit] = 0.6 mM, [PS] = 1 mM.
In the sunlight/Fe(II)/Cit system, the concentration of Fe(III) decreased and Fe(II) increased with the increasing of the reaction time (Figure 2a), this is expected as Fe(II) can be reduced from Fe(III) through LMCT (Equation (6)) [25]. Notably, the increase of Fe(II) concentration was relatively fast in the first 20 min, but retarded as the reaction time was further prolonged. This can be explained by the concentration of carboxylic acid in the system (Figure 2c). During sunlight/Fe(II)/Cit treatment, citric acid can also be removed and acetic acid was formed as a primary intermediate. The concentration of acetic acid reached the highest value of 0.236 mM while citric acid remained at a relatively high concentration during the first 20 min, which maintained the quantity of Fe(III)–carboxylic acid chelates, and the regeneration of Fe(II). When the reaction time was prolonged from 20 min to 120 min, the continuous degradation of citric acid and acetic acid induced the concentration of carboxylic acid to decrease, consequently inducing the regeneration of Fe(II) rate to slow down.
In sunlight/Fe(II)/Cit/PS, the evolution of the concentration of Fe species in the first 30 min is similar to that in the sunlight/Fe(II)/Cit system but shows a different trend from 30–120 min (Figure 2b). The photoreduction of Fe(III)–citrate complexes in the first 30 min increased Fe(II) concentration and decreased Fe(III) concentration. However, the concentration of Fe(II) started to decrease once the reaction time was further increased to 120 min. This may be caused by the decomposition of citric acid in the sunlight/Fe(II)/Cit/PS system (Figure 2d). Previous research has reported that citric acid can be degraded by various advanced oxidation processes, for instance, the TiO2 photocatalytic process and the O3/H2O2/UV process [16,33]. In this study, citric acid can be removed by a sunlight/Fe(II)/Cit/PS system, with the removal reaching up to 90.26% at 45 min and 100% at 90 min, respectively (Figure 2d). Acetic acid is an important intermediate of citric acid [33], whose concentration increased to the highest level of 0.140 mM at 5 min and then declined. The further decomposition of acetic acid may generate oxalic acid, which can then be mineralized into CO2 [34]. The concentration of oxalic acid gradually increased with the reaction time and reached a plateau of ca. 0.08 mM at 60~120 min. Overall, the total carboxylic acid concentration was relatively low in the system after 30 min, which induced a decrease in the concentration of the total dissolved Fe and Fe(II) after 30 min. This is the reason that the degradation of SMX showed a relatively slow stage at 30~120 min. The decomposition and mineralization of citric acid in the sunlight/Fe(II)/Cit/PS system is favorable, doesn’t require further treatment, and won’t cause secondary pollutants in the wastewater.
3.3. Identification of Reactive Species
The reactive species responsible for SMX degradation in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system were first determined using radical-quenching experiments. TBA was employed to quench ·OH, as the second reaction rate constant of TBA with ·OH (=6 × 108 M−1s−1) was much higher than that of TBA and SO4·− (=8 × 105 M−1s−1). MeOH is selected to quench both SO4·− and ·OH because the second reaction rate constant of MeOH and ·OH ( = 9.7 × 108 M−1s−1) is comparable with that of MeOH and SO4·− ( = 2.5 × 107 M−1s−1) [35]. The difference of SMX removal in the existence of TBA and MeOH can differentiate the contribution of ·OH and SO4·−. Further, CCl4 is used to quench HO2·/O2·− since it can prevent the generation of HO2·/O2·− and can resist ·OH [26].
Figure 3a,b displays the degradation kinetics of SMX when different quenchers existed in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system, respectively. In the sunlight/Fe(II)/Cit system, the removal of SMX was obviously inhibited by TBA. The removal efficiency of SMX decreased from 71.15% without TBA to 10.29% with TBA after 120 min. Correspondingly, the elimination rate constant of SMX declined from an initial 0.0236 min−1 without TBA to only 0.0017 min−1 with TBA. This demonstrated that ·OH was the primary reactive species and contributed 92.80% of the degradation of SMX. In contrast, the degradation rate constant of SMX slightly declined to 0.0225 min−1 in the presence of CCl4, which indicated that HO2·/O2·− contributed only 4.66% to the removal of SMX.
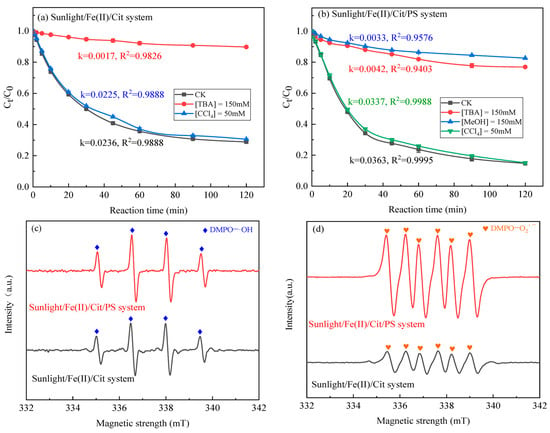
Figure 3.
Effect of radical scavengers on SMX degradation in the sunlight/Fe(II)/Cit system (a) and the sunlight/Fe(II)/Cit/PS system (b); EPR spectra of DMPO−·OH (c) and DMPO−O2·− (d) in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system. Conditions: [SMX] = 1 mg/L, [Fe(II)] = 0.1 mM, [Cit] = 0.6 mM, [PS] = 1 mM.
In the sunlight/Fe(II)/Cit/PS system, the addition of TBA, MeOH, and CCl4 resulted in the degradation rate constant of SMX decreasing from 0.0363 min−1 to 0.0042, 0.0033, and 0.0337 min−1. Accordingly, the contribution of ·OH, SO4·− and HO2·/O2·− was calculated to be 88.43%, 2.48%, and 6.91%. This demonstrated that ·OH was the primary reactive species in the degradation of SMX. However, the contribution of SO4·− to the degradation of SMX in the sunlight/Fe(II)/Cit/PS system was very small. This can be attributed to the weak alkalinity of the wastewater, in which the generated SO4·− can rapidly transform to ·OH (Equations (14) and (15)) [26].
EPR spectra using DMPO as a spin-trapping agent for SO4·−, ·OH, and O2·− were also analyzed (Figure 3c,d). The typical spectra for the spin adducts of DMPO-·OH and DMPO-O2·− were noticed in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system, proving the yield of ·OH and O2·− in the two systems. The EPR spectra intensity of DMPO-·OH and DMPO-O2·− in the sunlight/Fe(II)/Cit/PS system are a little higher than that in the sunlight/Fe(II)/Cit system, demonstrating that the yield of ·OH and O2·− are higher in the sunlight/Fe(II)/Cit/PS system. Further, the signal for DMPO-SO4·− was not observed, this may be attributed to the lower yield of SO4·−, whose signal may be covered by the strong intensity of DMPO-·OH. In summary, reactive species SO4·−, ·OH, and O2·− were produced in the sunlight/Fe(II)/Cit/PS system, and ·OH was the key radical that contributed more than 80% of the removal of SMX. This is in accordance with the previous research in the carboxylic-acid-chelated Fe(II) activated persulfate process [20,36,37].
Figure 4 displays the production mechanism of reactive radicals in the sunlight/Fe(II)/Cit/PS system. Based on the previous report, the main species of Fe(III)–Cit complex at pH 7 is [Fe3O(Cit)3H3]2−, which has a higher photolysis rate of 1.32 × 10−3 s−1 [18]. Under the irradiation of sunlight, Fe(III)–Cit complexes undergo an LMCT process and produce Fe(II) and free citrate radicals (Equation (6)). Then, the free citrate radicals react with O2, leading to the formation of O2·−/HO2· (Equations (7) and (8)). The dismutation of HO2· generates H2O2 (Equation (9)), which reacts with Fe(II) to produce ·OH via the Fenton process (Equation (10)) [25,38]. The regenerated Fe(II) can also activate PS to generate SO4·−and ·OH (Equations (1), (11) and (12)). Moreover, PS can also directly generate O2·− in an alkaline environment (Equation (13)) [39].
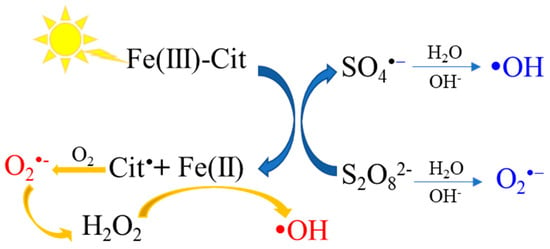
Figure 4.
The formation of reactive species in sunlight/Fe(II)/Cit/PS system.
3.4. Influencing Factors on the Removal of SMX
3.4.1. Influence of Fe(II) Concentration
Figure 5 depicts the effects of Fe(II) concentration on the removal of SMX in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system with Fe(II)/Cit molar ratio of 1:6 and PS concentration of 1 mM. In both systems, higher Fe(II) concentration within 0.05~0.2 mM facilitates higher SMX removal, but the increasing trend of SMX degradation rate was somewhat different. In the sunlight/Fe(II)/Cit system, the removal of SMX significantly increased from 32.4% to 71.1%, and the degradation rate constant of SMX in the first 30 min was enhanced from 0.0055 min−1 to 0.0236 min−1, when the Fe(II) concentration increased from 0.05 mM to 0.1 mM. However, the removal of SMX reached a plateau when the Fe(II) concentration increased to 0.15 and 0.2 mM. The degradation rate of SMX was 88.2% and 90.2%, while the degradation rate constant of SMX was 0.0294 min−1 and 0.0305 min−1 at Fe(II) concentrations of 0.15 and 0.2 mM, respectively. This suggested that a very high concentration of Fe(II) is not beneficial for the degradation of SMX, which can be explained from two aspects. First, overdosage of Fe(II) can react with hydroxyl radicals (Equation (14)) and subsequently affect the degradation of SMX [22]. Second, citric acid, as a low molecular weight organic matter, can also consume reactive species [40].
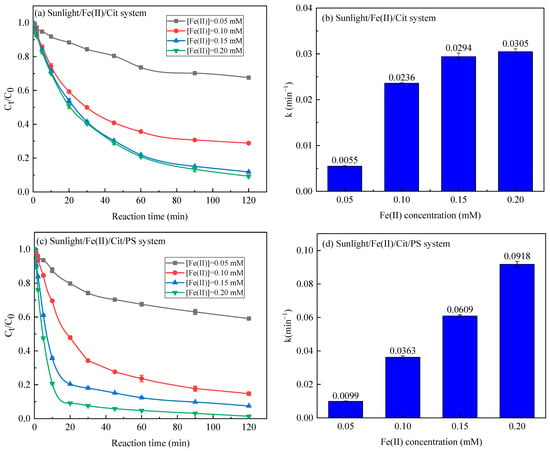
Figure 5.
Degradation efficiency and reaction kinetic rate of SMX in the sunlight/Fe(II)/Cit system (a,b) and the sunlight/Fe(II)/Cit/PS system (c,d) with varying Fe(II) concentration. Conditions: [SMX] = 1 mg/L, [Fe(II)]/[Cit] = 1:6, [PS] = 1 mM.
The removal of SMX in the sunlight/Fe(II)/Cit/PS system is obviously higher than that in the sunlight/Fe(II)/Cit system. The elimination of SMX in sunlight/Fe(II)/Cit/PS system was fast during the first 20–30 min and retarded at 30–120 min, especially at higher Fe(II) concentrations. This is caused by the lower dissolved Fe(II) and carboxylic acid in the later stage, which is discussed above. Compared with the sunlight/Fe(II)/Cit system, the degradation rate constant of SMX in the first 30 min significantly increased from 0.0099 min−1 to 0.0918 min−1 when the Fe(II) concentration increased from 0.05 mM to 0.2 mM. This indicates that a higher concentration of Fe(II) effectively activated PS and resulted in a higher yield of reactive species. Notably, the removal efficiency of SMX was close and all higher than 85% after a reaction time of 120 min at Fe(II) concentration of 0.1~0.2 mM. As a higher concentration of Fe(II) produces more sludge, which needs to be further treated, we prefer to use the Fe(II) concentration of 0.1 mM.
3.4.2. Influence of Citric Acid Concentration
Figure 6 depicts the effect of Cit on the removal of SMX in the sunlight/Fe(II)/Cit system and the sunlight/Fe(II)/Cit/PS system with 0.1 mM Fe(II) and 1 mM PS. In the sunlight/Fe(II)/Cit system, the degradation rate constant of SMX elevated from 0.0053 min−1 to 0.0304 min−1 when citric acid increased from 0.2 mM to 0.8 mM. This is consistent with previous research [41] and indicates that a higher concentration of Fe–Cit complexes produces more reactive species. However, when the citric acid concentration increased to 1 mM, the degradation rate constant of SMX decreased to 0.0203 min−1. This is reasonable because overdosage of citric acid competes with SMX for reactive species, and consequently affects the removal of SMX.
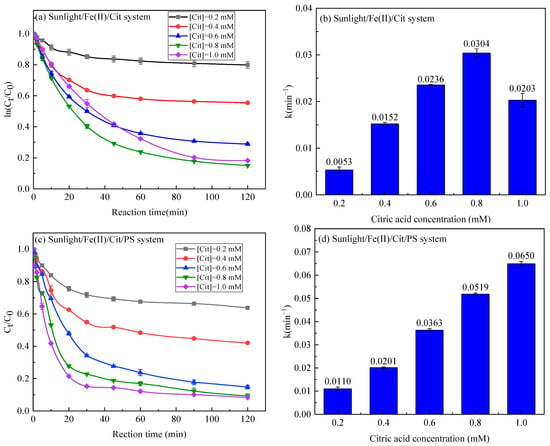
Figure 6.
Degradation efficiency and reaction kinetic rate of SMX in the sunlight/Fe(II)/Cit system (a,b) and the sunlight/Fe(II)/Cit/PS system (c,d) with varying citric acid concentration. Conditions: [SMX] = 1 mg/L, [Fe(II)] = 0.1 mM, [PS] = 1 mM.
In the sunlight/Fe(II)/Cit/PS system, the degradation of SMX increased with the increase of citric acid concentration, with the degradation rate constant increasing from 0.0110 min−1 to 0.0650 min−1 when the citric acid concentration increased from 0.2 mM to 1 mM. In particular, compared with the sunlight/Fe(II)/Cit system, the degradation of SMX at 1 mM of citric acid wasn’t inhibited. This proved that a high concentration of citric acid assured the solubility of Fe(II), and the effective activation of PS generated more reactive species in the sunlight/Fe(II)/Cit/PS system. Citric acid also consumed the reactive species; however, the degradation of SMX was not affected due to the higher quantity of reactive species in the sunlight/Fe(II)/Cit/PS system.
3.4.3. Influence of PS Concentration
Figure 7 depicts the removal of SMX and degradation rate constant of SMX in the sunlight/Fe(II)/Cit/PS system with varying PS concentration at 0.1 mM Fe(II) and 0.6 mM citric acid. The degradation of SMX increased with increasing of PS concentration, with the degradation rate constant of SMX of 0.0259–0.0450 min−1 at 0.5–4 mM PS. The activation of PS can produce reactive species SO4·− and ·OH; therefore, it is expected that the elevated PS concentration would show a positive effect on SMX degradation [42]. However, the SMX degradation rate constant only increased a little when PS concentration increased from 1 mM to 4 mM, this suggested that Fe(II) is an important limitation factor with overdosage of PS. Further, excessive PS dosage of PS will consume reactive species, which to some extent results in the minor improvement in the SMX degradation rate.
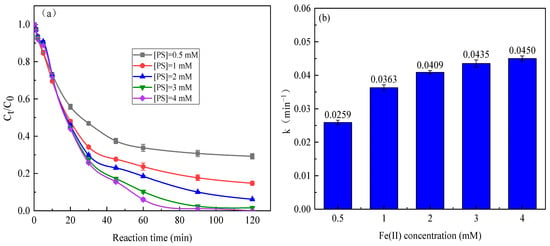
Figure 7.
Degradation efficiency (a) and reaction kinetic rate (b) of SMX in the sunlight/Fe(II)/Cit/PS system with varying PS concentration.
3.5. Degradation Products of SMX
The degradation products of SMX in the sunlight/Fe(II)/Cit/PS system were further analyzed using HPLC high resolution mass spectroscopy. Four main degradation products of SMX were identified, and the detailed information, including chemical formula, accurate mass weight, and ring double bond equivalent (RDB), was listed in Table S1 (Supporting information). The mass error between the experimental and theoretical mass was lower than 5 ppm, indicating the reliability of the chemical formula of SMX and their degradation products. The structure of the degradation products was elucidated based on their MS/MS spectrum and previous publications.
Correspondingly, possible degradation pathways were proposed for the degradation of SMX by the sunlight/Fe(II)/Cit/PS system (Figure 8). In degradation pathway I, the cleavage of the S–N bond of SMX via the hydrolysis of the sulfonamide bond generated the degradation product TP99 (3-amino-5-methylisoxazole). TP99 has been reported to be generated in various treatment technologies of SMX, for instance, photodegradation [27], catalytic ozonation [43], chlorination [44], and peroxymonosulfate activation by immobilized CoFe2O4 network [45]. In degradation pathway II, an SMX isomer denoted TP254 was formed via isoxazole ring rearrangement [46]. TP254 has the same m/z value and fragmentation ions but a different elution time as SMX. The SMX isomer was reported in the direct photolysis of SMX [46,47]. In degradation pathway III, the aromatic ring of SMX was attacked by reactive species such as ·OH and formed two hydroxylated products, TP270 and TP286. The characteristic fragment ion of TP270 (m/z 172.0071) and TP 286 (m/z 188.0019) were 16 Da and 32 Da higher than the characteristic fragment ion of SMX (m/z 156.0120), respectively (Figure S1, Supplementary Materials). This indicates that the hydroxyl replacement occurred on C of the aromatic ring. TP270 was observed in the catalytic ozonation of SMX [43], photocatalytic degradation of SMX [48], and PMS activation by 2D ZIF-L arrays supported on zinc foam degradation of SMX [49]. Wang et al. [50] observed both TP270 and TP286 in the degradation of SMX by PMS activated by iron and sulfur co-doped graphite carbon nitride.
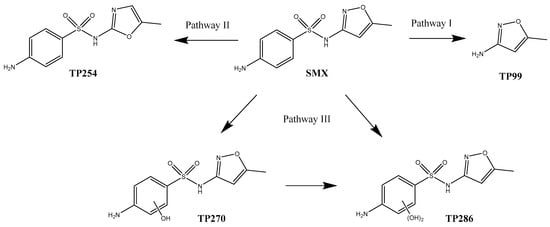
Figure 8.
Degradation pathway of SMX by sunlight/Fe(II)/Cit/PS process.
As there are no standards for the degradation products of SMX, we semi-quantify the yield of degradation products of SMX by dividing their peak area by the initial peak area of SMX (A/ASMX0) (Figure S2, Supporting Information). It should be noted that the yield of TP270 was the most predominant, reaching 0.158 after reaction for 60 min and then decreasing to 0.131 at 120 min. In contrast, the yield of TP254, TP286, and TP99 were relatively low, reaching 0.020, 0.006, and 0.005 at a reaction time of 120 min. Overall, the degradation products of SMX in the sunlight/Fe(II)/Cit/PS process were mainly formed through isomerization, cleavage of the S–N bond, and hydroxylation. The hydroxylated product TP270 was the main degradation product, which proved the main reactive species is ·OH and was consistent with the previous discussion.
4. Conclusions
This study proposed a novel advanced treatment technology, the sunlight/Fe(II)/Cit/PS process, in which citric acid was used as a chelate agent to form Fe(II)/Fe(III)–Cit complexes, and sunlight was introduced to promote the regeneration of Fe(II) of Fe(III)–Cit complexes through an LMCT process. The removal efficiency of SMX can reach up to 85.26% in the sunlight/Fe(II)/Cit/PS system with Fe(II) concentration of 0.1 mM, Cit concentration of 0.6 mM, and PS concentration of 1 mM. The Fe(II) concentration increased during the first 30 min, which enhanced the activation of PS and the degradation of SMX. However, the Fe(II) concentration decreased at 30–120 min because of the degradation of Cit in the system. This is favorable concerning the chemical oxygen demand of the effluent of the wastewater. A radical quencher experiment demonstrated that SO4·−, ·OH, and O2·− were produced in the sunlight/Fe(II)/Cit/PS system, with ·OH playing the most important contribution to the degradation of SMX. EPR spectra proved the presence of ·OH and O2·−, while the signal of SO4·− may be covered by the strong intensity of ·OH. Three degradation pathways were proposed, including isomerization, cleavage of S–N bond, and hydroxylation. This study can broaden our understanding of the degradation mechanism of SMX in the sunlight/Fe(II)/Cit/PS system, which could be a promising advanced treatment for antibiotics in secondary wastewater.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16111564/s1, Figure S1: The MS/MS spectra of SMX and its degradation products. (A) SMX, (B) TP254, (C) TP270, (D) TP 286; Figure S2: Residual SMX and yield of the degradation products of SMX during sunlight/Fe(II)/Cit/PS treatment. Table S1: Elemental composition and mass accuracy of SMX and its transformation products. Text S1: Chemicals; Text S2: Identification the degradation products of SMX.
Author Contributions
Conceptualization, W.L.; methodology, W.L.; formal analysis, Y.Z. (Yuhao Zhou); investigation, X.C. and Y.Z. (Yan Zhu); data curation, Y.Z. (Yan Zhu) and G.T.; writing—original draft preparation, X.C. and W.L.; writing—review and editing, W.L. and J.H.; supervision, W.L.; funding acquisition, W.L. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
We sincerely appreciate the financial support of the National Natural Science Foundation of China (No. 32171628), the Carbon peak Carbon Neutrality Science and Technology Innovation Special Fund of Jiangsu Province (BK20220016), and Qinglan Project of Jiangsu Province of China (2022).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.; Li, W.; Liu, K.; Guo, Y.; Ding, C.; Han, J. Global review of macrolide antibiotics in the aquatic environment: Sources, occurrence, fate, ecotoxicity, and risk assessment. J. Hazard. Mater. 2022, 439, 129628. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Shukla, P.; Giri, B.S.; Chowdhary, P.; Chandra, R.; Gupta, P.; Pandey, A. Prevalence and hazardous impact of pharmaceutical and personal care products and antibiotics in environment: A review on emerging contaminants. Environ. Res. 2021, 194, 110664. [Google Scholar] [CrossRef] [PubMed]
- Doretto, K.M.; Peruchi, L.M.; Rath, S. Sorption and desorption of sulfadimethoxine, sulfaquinoxaline and sulfamethazine antimicrobials in Brazilian soils. Sci. Total Environ. 2014, 476–477, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Cui, H.; Jia, X.; Huang, X. Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review. Sci. Total Environ. 2022, 820, 153178. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ji, M.; Zhai, H.; Guo, Y.; Liu, Y. Occurrence of antibiotics and antibiotic resistance genes in WWTP effluent-receiving water bodies and reclaimed wastewater treatment plants. Sci. Total Environ. 2021, 796, 148919. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Ailijiang, N.; Mamat, A.; Chang, J.; Zhang, Q.; Liu, Y.; Li, N. Occurrence of antibiotics in the different biological treatment processes, reclaimed wastewater treatment plants and effluent-irrigated soils. J. Environ. Chem. Eng. 2022, 10, 107715. [Google Scholar] [CrossRef]
- Wu, Z.; Gong, S.; Liu, J.; Shi, J.; Deng, H. Progress and problems of water treatment based on UV/persulfate oxidation process for degradation of emerging contaminants: A review. J. Water Process Eng. 2024, 58, 104870. [Google Scholar] [CrossRef]
- Qiu, Q.; Li, G.; Dai, Y.; Xu, Y.; Bao, P. Removal of antibiotic resistant microbes by Fe(II)-activated persulfate oxidation. J. Hazard. Mater. 2020, 396, 122733. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Q.; Lou, Y.; Liu, G.; Li, S.; Chen, L.; Yuan, B.; Zou, D.; Chen, J. Efficient degradation of Nizatidine by a Fe(II)/persulfate system activated with Zero-valent iron. Chem. Eng. Res. Des. 2023, 193, 447–459. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Ioannidi, A.A.; Mantzavinos, D.; Frontistis, Z. Heat-activated persulfate for the degradation of micropollutants in water: A comprehensive review and future perspectives. J. Environ. Manag. 2022, 318, 115568. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, J.; Dong, L.; Liu, B.; Xing, D.; Yang, S.; Wu, X.; Wang, Q.; Fan, J.; Feng, L.; et al. Removal of antibiotic resistant bacteria and antibiotic resistance genes in wastewater effluent by UV-activated persulfate. J. Hazard. Mater. 2020, 388, 122070. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, F.; Jian, H.; Zhen, K.; Zhang, P.; Tang, X.; Fu, Z.; Xu, W.; Wang, C.; Sun, H. Pyrene degradation in an aqueous system using ferrous citrate complex activated persulfate over a wide pH range. J. Environ. Chem. Eng. 2021, 9, 106733. [Google Scholar] [CrossRef]
- Rao, Y.F.; Qu, L.; Yang, H.; Chu, W. Degradation of carbamazepine by Fe(II)-activated persulfate process. J. Hazard. Mater. 2014, 268, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Shi, Z.; Zhou, S. Modeling of Fe(II)-activated persulfate oxidation using atrazine as a target contaminant. Sep. Purif. Technol. 2016, 169, 59–65. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Trimethoprim degradation by Fenton and Fe(II)-activated persulfate processes. Chemosphere 2018, 191, 97–105. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Li, M.; Wang, X.; Bi, W.; Dong, W. Degradation of chloramphenicol by persulfate activated by Fe2+ and zerovalent iron. Chem. Eng. J. 2015, 279, 507–515. [Google Scholar] [CrossRef]
- Ling, L.; Zhang, D.; Fan, C.; Shang, C. A Fe(II)/citrate/UV/PMS process for carbamazepine degradation at a very low Fe(II)/PMS ratio and neutral pH: The mechanisms. Water Res. 2017, 124, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, S.; Bai, X.; Jin, X.; Shi, X.; Han, J.; Gao, Y.; Jin, P. Efficient Fe(III) reduction and persulfate activation induced by ligand-to-metal charge transfer under visible light enhances degradation of organics. Chem. Eng. J. 2022, 446, 137052. [Google Scholar] [CrossRef]
- Han, D.; Wan, J.; Wang, Y.; Li, Y.; Li, D.; Guan, Z. New insights into the role of organic chelating agents in Fe(II) activated persulfate processes. Chem. Eng. J. 2015, 269, 425–433. [Google Scholar] [CrossRef]
- Silva, G.D.; Marson, E.O.; Batista, L.L.; Ueira-Vieira, C.; Starling, M.C.V.M.; Trovó, A.G. Contrasting the performance of photo-Fenton at neutral pH in the presence of different organic iron-complexes using hydrogen peroxide or persulfate as oxidants for naproxen degradation and removal of antimicrobial activity. Process Saf. Environ. Prot. 2021, 147, 798–807. [Google Scholar] [CrossRef]
- Yuan, D.; Li, X.; Xiong, S.; Cui, J.; Zhou, J.; Kou, Y. Improving sludge dewaterability via Fe2+ chelated citrate activated peroxydisulfate oxidation. J. Environ. Sci. 2022, 125, 223–233. [Google Scholar] [CrossRef]
- Voelker, B.M.; Morel, F.M.M.; Sulzberger, B. Iron redox cycling in surface waters: Effects of humic substances and light. Environ. Sci. Technol. 1997, 31, 1004–1011. [Google Scholar] [CrossRef]
- Yin, R.; Chen, Y.; Hu, J.; Lu, G.; Zeng, L.; Choi, W.; Zhu, M. Complexes of Fe(III)-organic pollutants that directly activate Fenton-like processes under visible light. Appl. Catal. B Environ. 2021, 283, 119663. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Wang, Z.; Xue, M.; Zhu, X.; Tao, T. Photodegradation of propranolol by Fe(III)-citrate complexes: Kinetics, mechanism and effect of environmental media. J. Hazard. Mater. 2011, 194, 202–208. [Google Scholar] [CrossRef]
- Nie, M.; Yan, C.; Xiong, X.; Wen, X.; Yang, X.; Dong, W. Degradation of chloramphenicol using a combination system of simulated solar light, Fe2+ and persulfate. Chem. Eng. J. 2018, 348, 455–463. [Google Scholar] [CrossRef]
- Li, W.; Ding, C.; Korshin, G.; Li, J.; Cheng, H. Effect of chlorination on the characteristics of effluent organic matter and the phototransformation of sulfamethoxazole in secondary wastewater. Chemosphere 2022, 295, 133193. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhou, Y.; Wang, Z.; Li, J.; Zhang, X.; Fu, C.; Du, X.; Wang, Z.; Qiu, W. Accelerate sulfamethoxazole degradation and detoxification by persulfate mediated with Fe2+ & dithionite: Experiments and DFT calculation. J. Hazard. Mater. 2022, 436, 129254. [Google Scholar]
- Tan, Y.; Cheng, Z.; Liu, Y.; Gao, X.; Liu, S.; Shen, Z. Quantum parameter analysis of the adsorption mechanism by freshly formed ferric hydroxide for synthetic dye and antibiotic wastewaters. Chemosphere 2021, 280, 130577. [Google Scholar] [CrossRef]
- Bahnmüller, S.; von Gunten, U.; Canonica, S. Sunlight-induced transformation of sulfadiazine and sulfamethoxazole in surface waters and wastewater effluents. Water Res. 2014, 57, 183–192. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J. Comparative study on sulfamethoxazole degradation by Fenton and Fe(II)-activated persulfate process. RSC Adv. 2017, 7, 48670–48677. [Google Scholar] [CrossRef]
- Ouyang, Z.; Yang, C.; He, J.; Yao, Q.; Zhang, B.; Wang, H.; Jiang, Y.; Zhou, J.; Deng, Y.; Liu, Y.; et al. Homogeneous photocatalytic degradation of sulfamethazine induced by Fe(III)-carboxylate complexes: Kinetics, mechanism and products. Chem. Eng. J. 2020, 402, 126122. [Google Scholar] [CrossRef]
- Quici, N.; Morgada, M.E.; Gettar, R.T.; Bolte, M.; Litter, M.I. Photocatalytic degradation of citric acid under different conditions: TiO2 heterogeneous photocatalysis against homogeneous photolytic processes promoted by Fe(III) and H2O2. Appl. Catal. B Environ. 2007, 71, 117–124. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, D.; Chu, W.; Li, M.; Lu, X. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Chen, L.; Li, X.; Guan, Y.; Xie, P.; Pan, C. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine. Environ. Sci. Technol. 2013, 47, 11685–11691. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gu, X.; Lu, S.; Xue, Y.; Zhang, X.; Hu, M.; Qiu, Z.; Sui, Q. Degradation of phenanthrene in aqueous solution by a persulfate/percarbonate system activated with CA chelated-Fe(II). Chem. Eng. J. 2018, 333, 122–131. [Google Scholar] [CrossRef]
- Dong, H.; Qiang, Z.; Hu, J.; Sans, C. Accelerated degradation of iopamidol in iron activated persulfate systems: Roles of complexing agents. Chem. Eng. J. 2017, 316, 288–295. [Google Scholar] [CrossRef]
- Ou, X.; Quan, X.; Chen, S.; Zhang, F.; Zhao, Y. Photocatalytic reaction by Fe(III)–citrate complex and its effect on the photodegradation of atrazine in aqueous solution. J. Photochem. Photobiol. A Chem. 2008, 197, 382–388. [Google Scholar] [CrossRef]
- Venâncio, J.P.F.; Rodrigues, C.S.D.; Nunes, O.C.; Madeira, L.M. Application of iron-activated persulfate for municipal wastewater disinfection. J. Hazard. Mater. 2022, 426, 127989. [Google Scholar] [CrossRef]
- Chen, L.; Dong, X.; Feng, R.; Li, W.; Ding, D.; Cai, T.; Jiang, C. Oxalic acid enhanced ferrous/persulfate process for the degradation of triclosan in soil: Efficiency, mechanism and a column study. Chem. Eng. J. 2023, 473, 144961. [Google Scholar] [CrossRef]
- Guo, J.; Du, Y.; Lan, Y.; Mao, J. Photodegradation mechanism and kinetics of methyl orange catalyzed by Fe(III) and citric acid. J. Hazard. Mater. 2011, 186, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Huang, D.; Wu, Y.; Dong, W. Enhanced activation of persulfate by Fe(III) and catechin without light: Reaction kinetics, parameters and mechanism. J. Hazard. Mater. 2021, 413, 125420. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Sun, Z.; Chen, Y.; Zhang, H.; Sun, Y.; Lu, D.; Ma, J. Catalytic ozonation of sulfamethoxazole using low-cost natural silicate ore supported Fe2O3: Influencing factors, reaction mechanisms and degradation pathways. RSC Adv. 2023, 13, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, L.; Wang, M.; Zhang, Q.; Zhang, Y.; Li, Y. The influence of bromide and iodide ions on the sulfamethoxazole (SMX) halogenation during chlorination. Sci. Total Environ. 2022, 848, 157687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ge, L.; Yan, W.; Yang, S.; Wang, G.; Miao, D.; Jin, P. Peroxymonosulfate activation by immobilized CoFe2O4 network for the degradation of sulfamethoxazole. J. Environ. Chem. Eng. 2022, 10, 107781. [Google Scholar] [CrossRef]
- Su, T.; Deng, H.; Benskin, J.P.; Radke, M. Biodegradation of sulfamethoxazole photo-transformation products in a water/sediment test. Chemosphere 2016, 148, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Willach, S.; Lutze, H.V.; Eckey, K.; Löppenberg, K.; Lüling, M.; Wolbert, J.B.; Kujawinski, D.M.; Jochmann, M.A.; Karst, U.; Schmidt, T.C. Direct Photolysis of Sulfamethoxazole Using Various Irradiation Sources and Wavelength Ranges—Insights from Degradation Product Analysis and Compound-Specific Stable Isotope Analysis. Environ. Sci. Technol. 2018, 52, 1225–1233. [Google Scholar] [CrossRef]
- Akbari, M.Z.; Xu, Y.; Liang, C.; Lu, Z.; Shen, S.; Peng, L. Synthesis of ZnO@VC for enhancement of synergic photocatalytic degradation of SMX: Toxicity assessment, kinetics and transformation pathway determination. Chem. Eng. Process.-Process Intensif. 2023, 193, 109544. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Gao, B.; Hu, B.; Zhou, S.; Liu, B.; Jiang, W.; Liu, C.; Che, G. 2D ZIF-L arrays supported on zinc foam to activate peroxymonosulfate for degrading sulfamethoxazole through both radical and non-radical pathways. Sep. Purif. Technol. 2024, 330, 125656. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Wang, J. Iron and sulfur co-doped graphite carbon nitride (FeOy/S-g-C3N4) for activating peroxymonosulfate to enhance sulfamethoxazole degradation. Chem. Eng. J. 2020, 382, 122836. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).