Analysis of Calcium Carbonate Scales in Water Distribution Systems and Influence of the Electromagnetic Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples of Scale from Water Distribution Systems
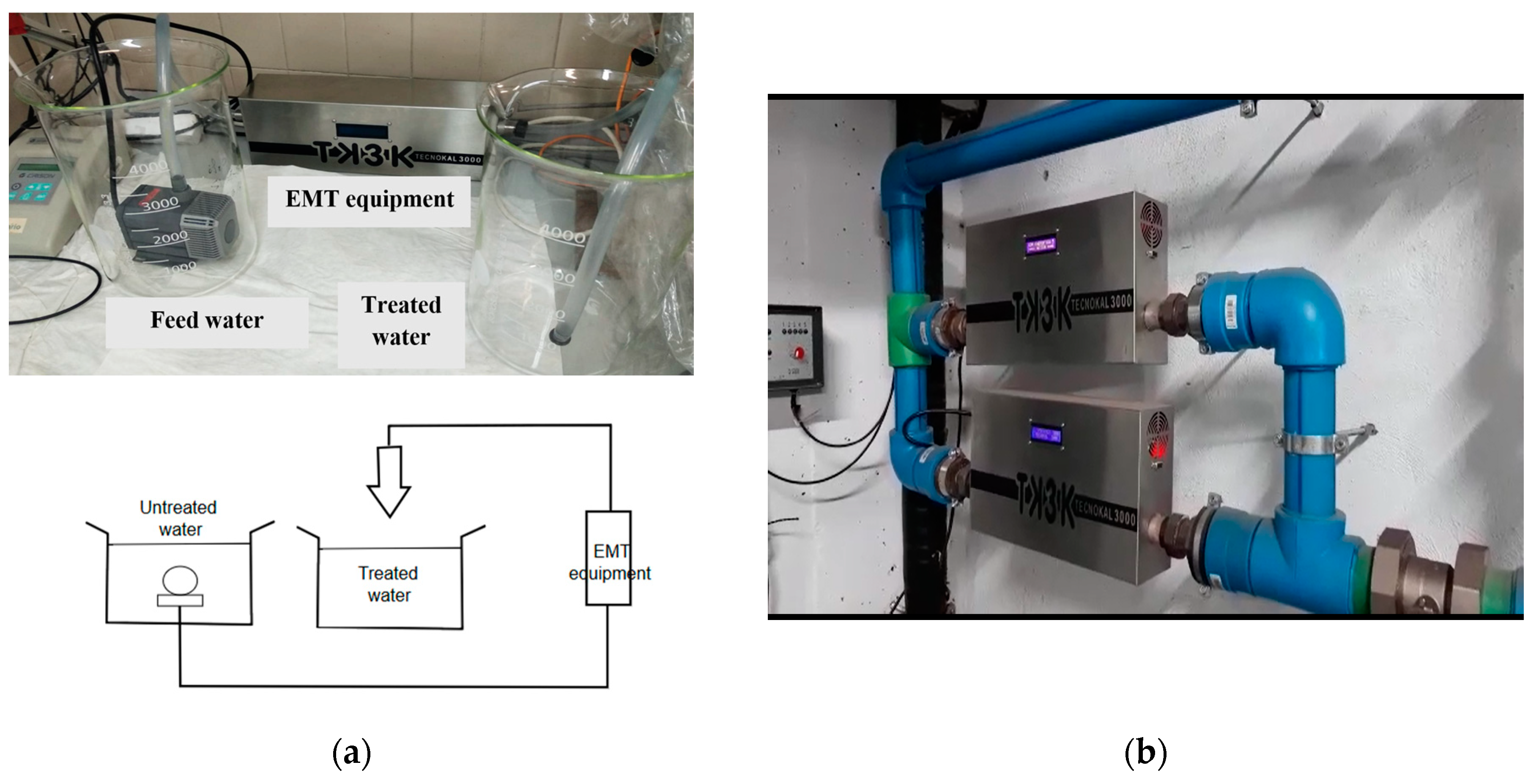
2.3. EMT Equipment
2.4. Preparation of the Synthetic Water to Obtain Precipitates
2.5. Methods of Analysis and Characterization of Precipitates
3. Results and Discussion
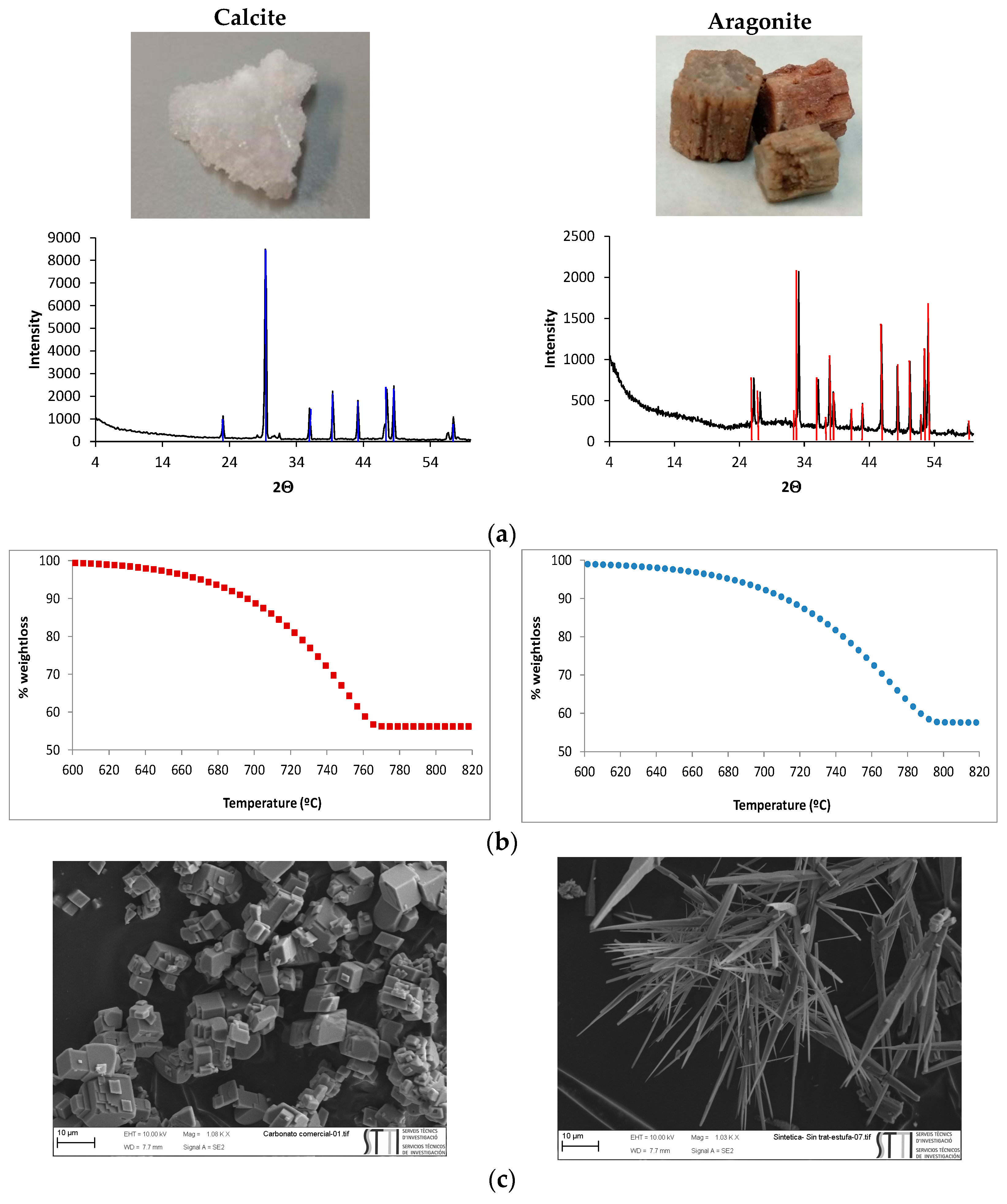
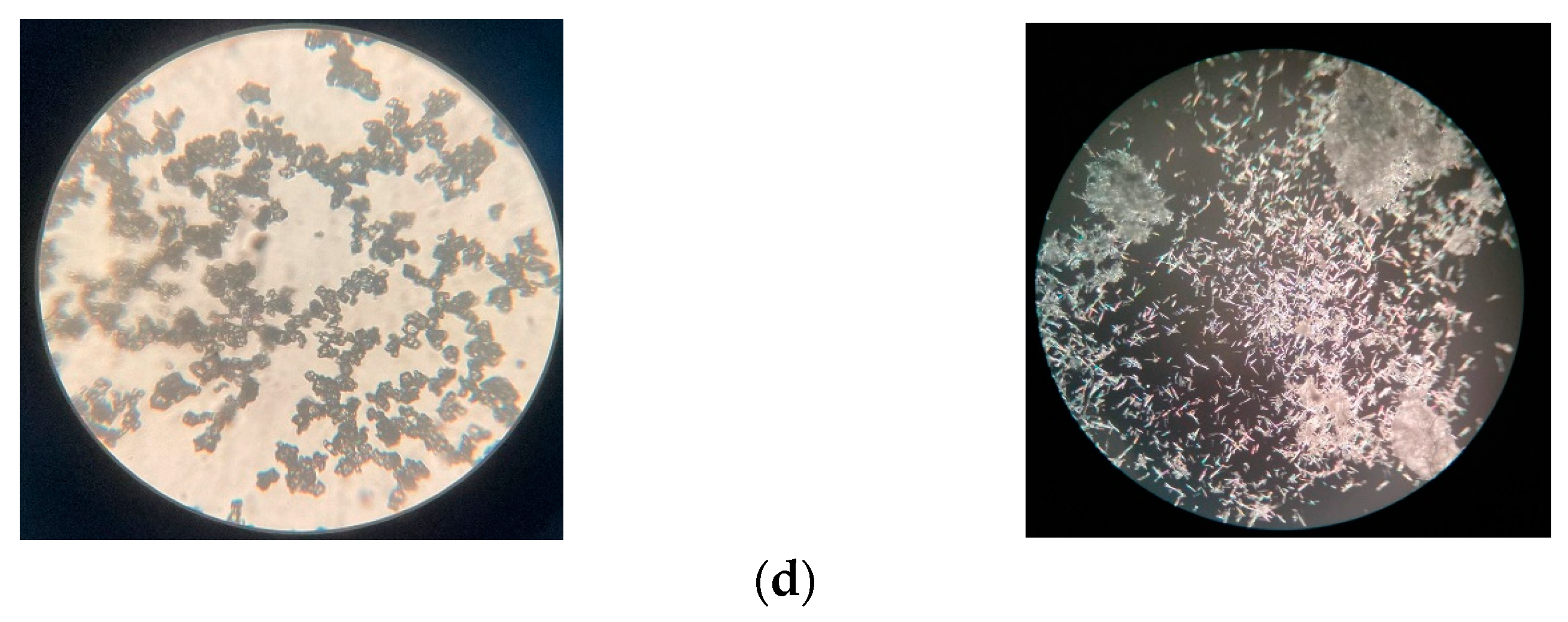
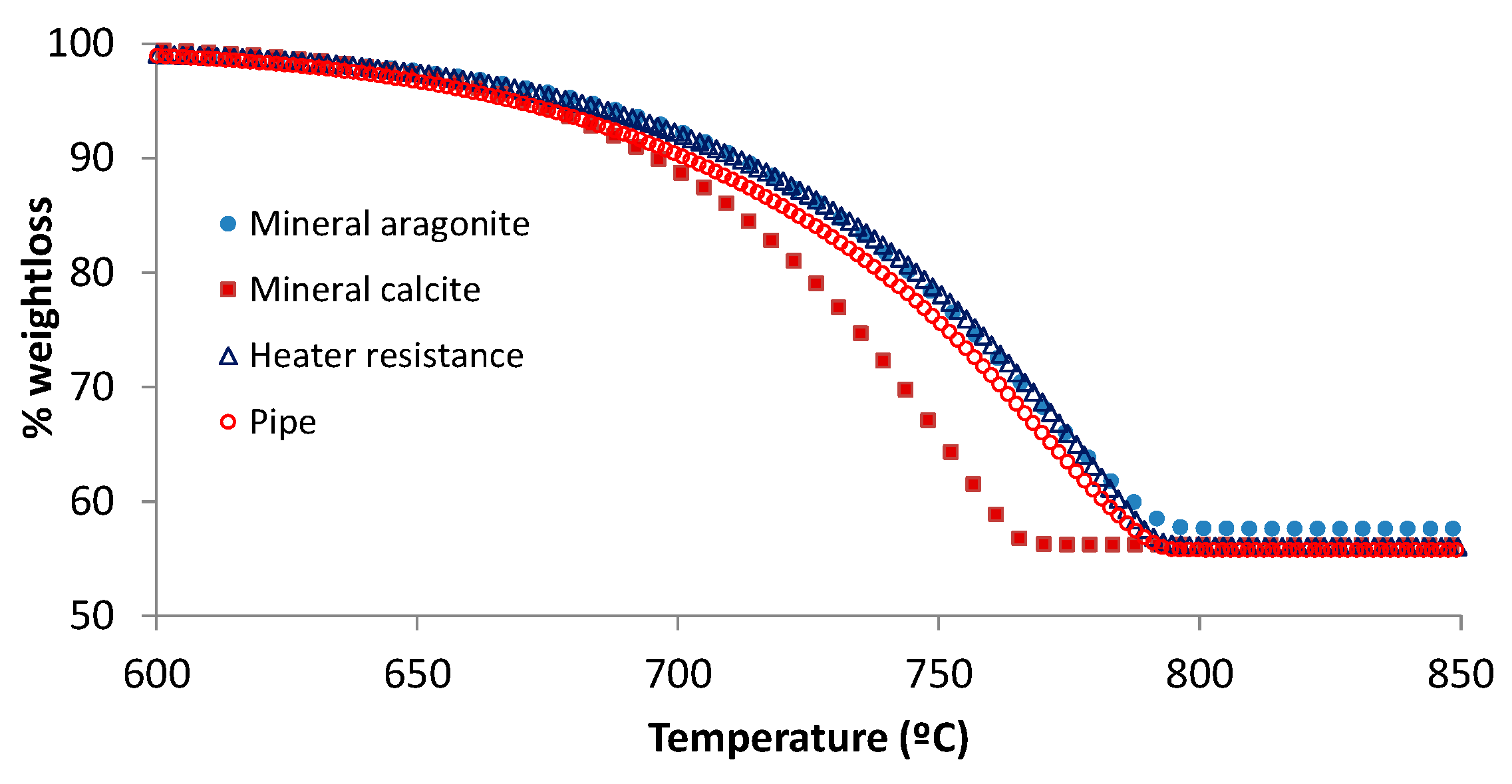
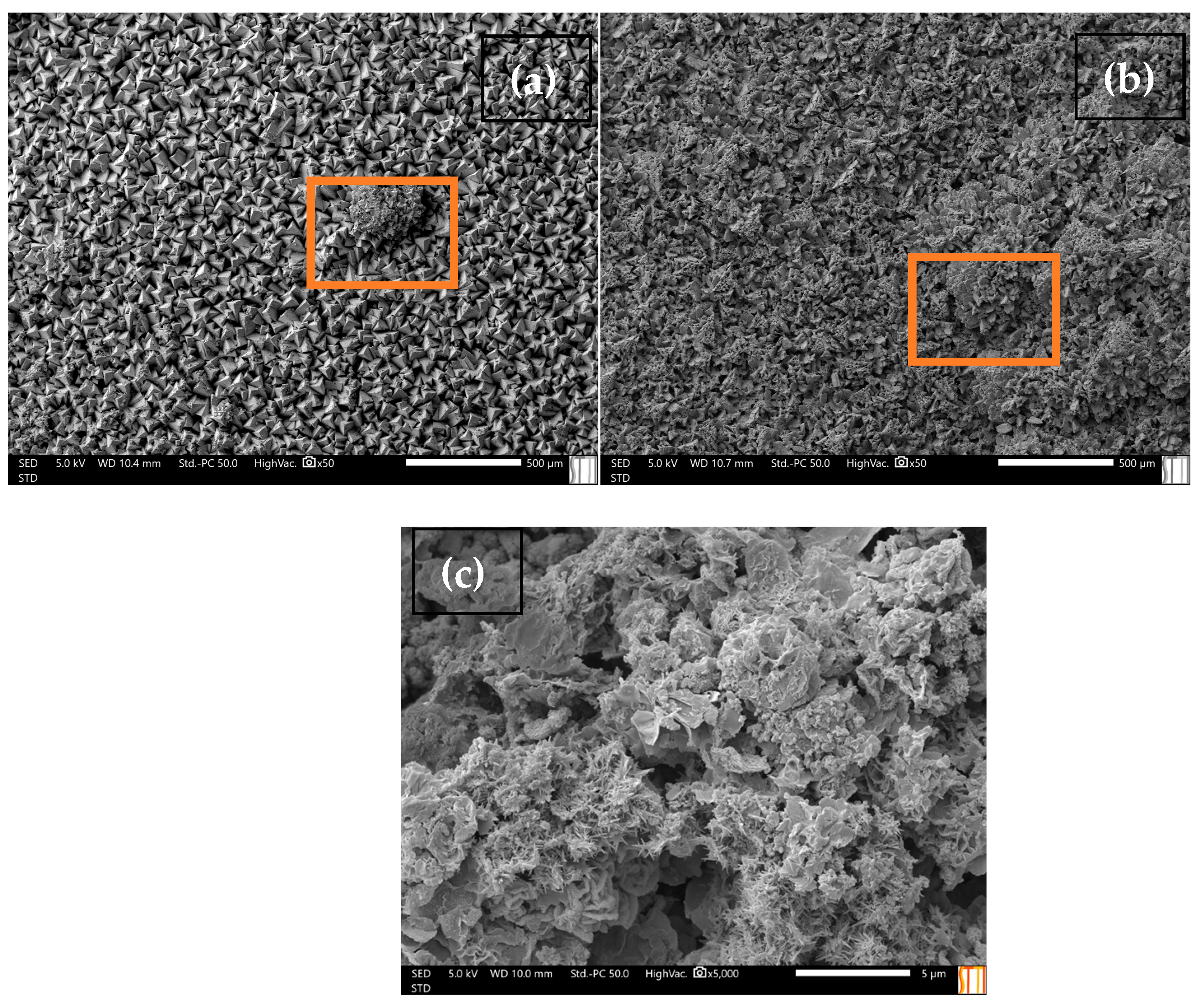
3.1. Characterization of Calcite and Aragonite Minerals
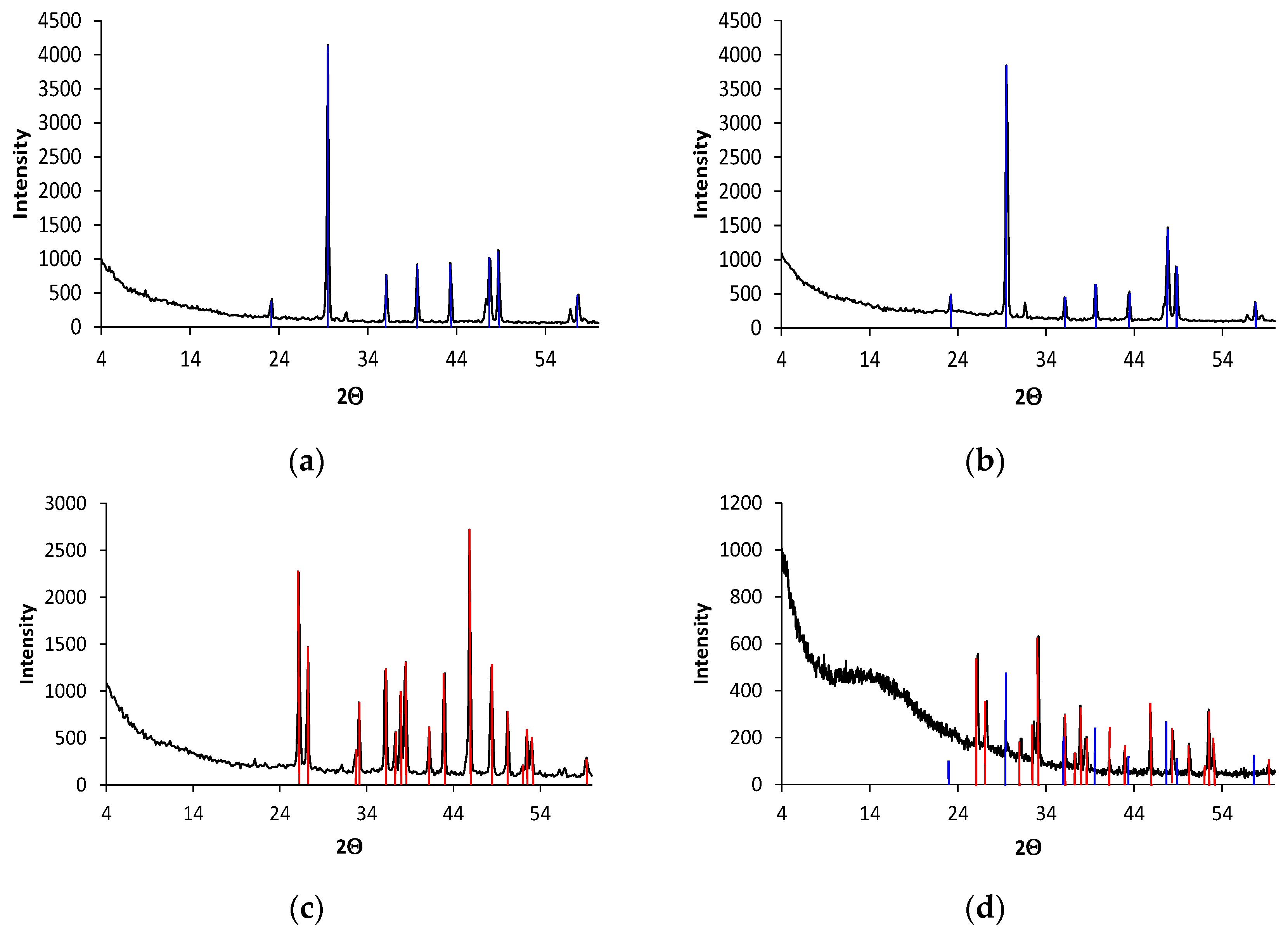
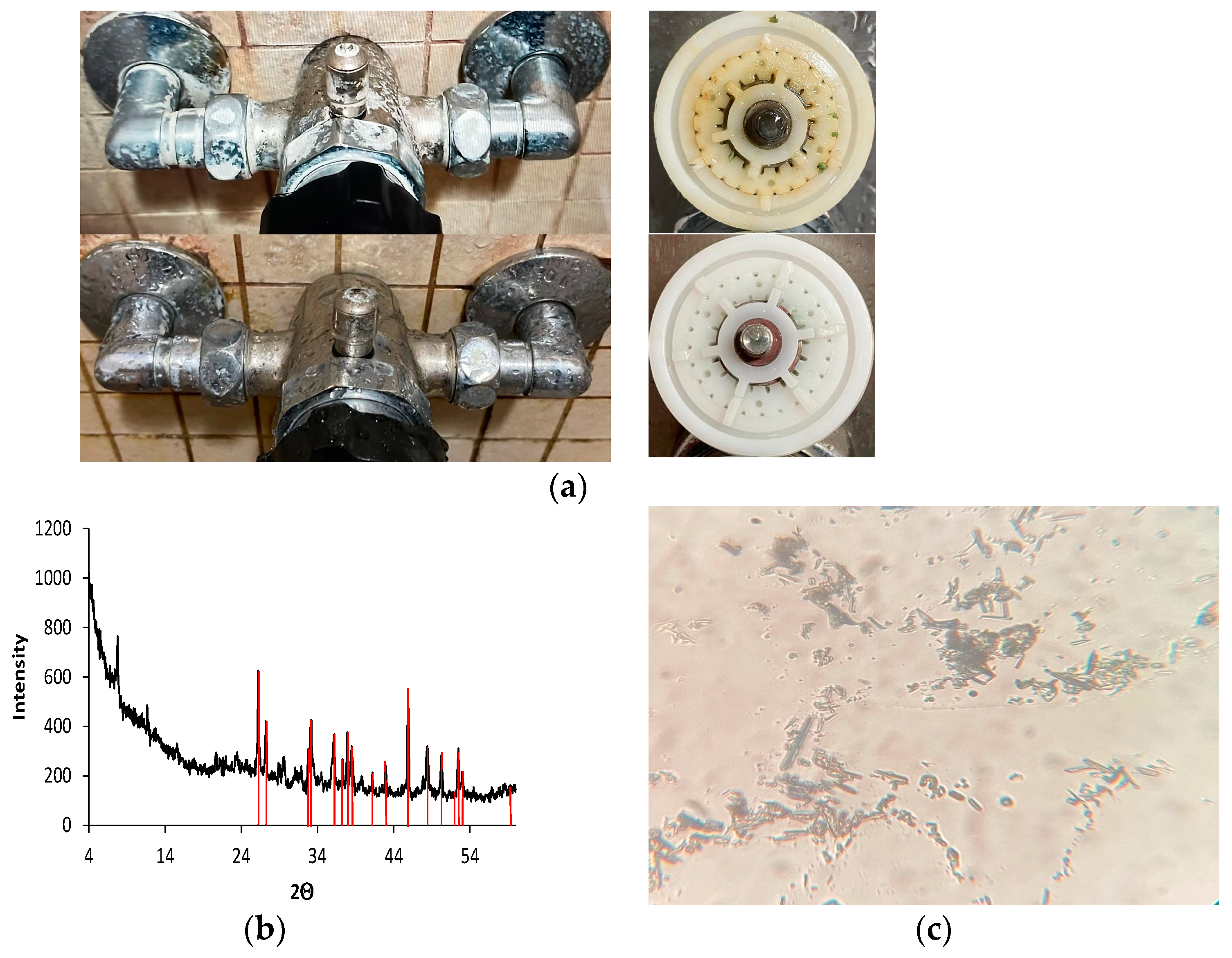
3.2. Characterization of Scales in Water Distribution Systems
3.2.1. System without EMT
3.2.2. System with EMT
3.3. Evaporation Studies to Obtain Scales with Controlled Conditions
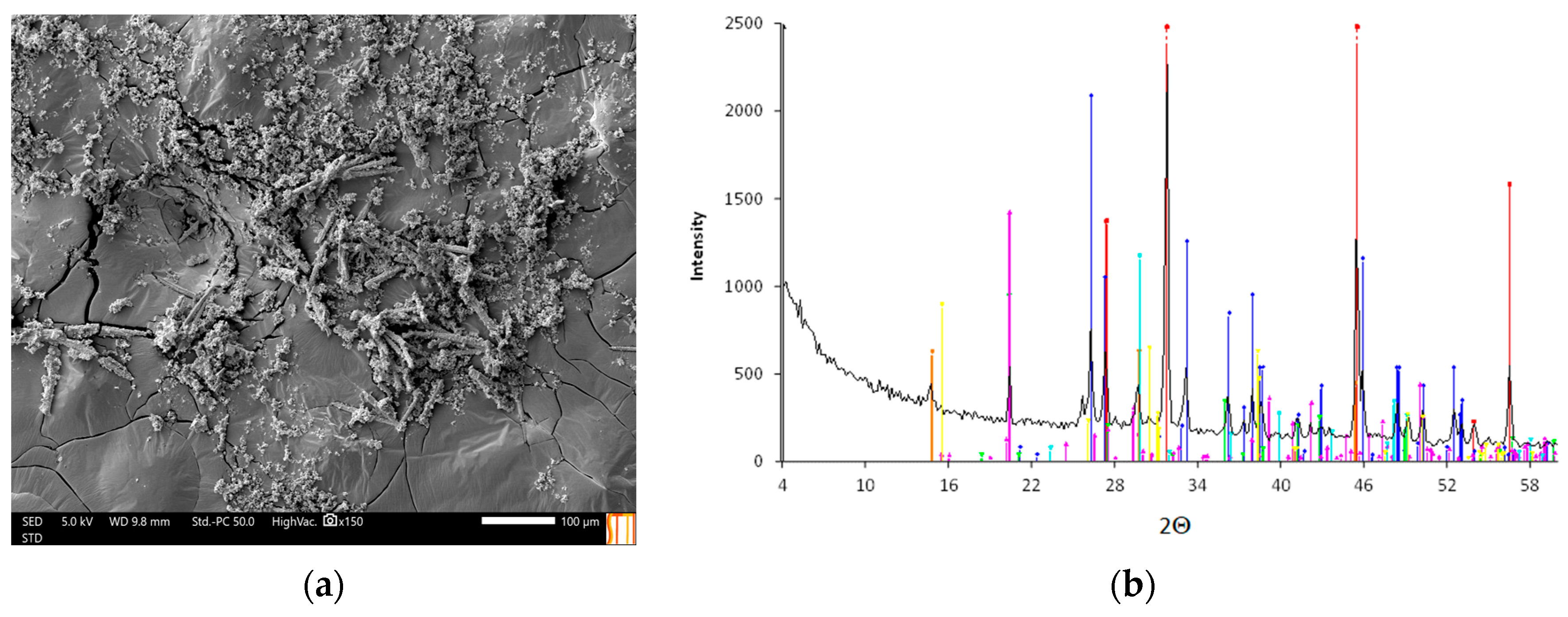
3.3.1. Analysis of Precipitates Obtained via Evaporation of Real Water
3.3.2. Analysis of Precipitates Obtained through Evaporation of Synthetic Waters. Study of the Reversion of Aragonite to Calcite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Liu, S.; Smith, K.; Cui, Y.; Wang, Z. An Overview on Corrosion of Iron and Steel Components in Reclaimed Water Supply Systems and the Mechanisms Involved. J. Clean. Prod. 2020, 276, 124079. [Google Scholar] [CrossRef]
- Tang, C.; Godskesen, B.; Aktor, H.; van Rijn, M.; Kristensen, J.B.; Rosshaug, P.S.; Albrechtsen, H.J.; Rygaard, M. Procedure for Calculating the Calcium Carbonate Precipitation Potential (CCPP) in Drinking Water Supply: Importance of Temperature, Ionic Species and Open/Closed System. Water 2020, 13, 42. [Google Scholar] [CrossRef]
- Blanco-Gutierrez, V.; Demourgues, A.; Jubera, V.; Gaudon, M. Eu(III)/Eu(II)-Doped (Ca0.7Sr0.3)CO3 Phosphors with Vaterite/Calcite/Aragonite Forms as Shock/Temperature Detectors. J. Mater. Chem. C Mater. 2014, 2, 9969–9977. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Cass, S. Magnetic Water Treatment. J. Magn. Magn. Mater. 2000, 209, 71–74. [Google Scholar] [CrossRef]
- Sun, M.; Jin, H.; Guo, X.; Wang, S.; Bi, J.; Ji, Z.; Zhao, Y. Precipitation and In-Situ Surface Modification of Calcium Carbonate in Synthetic Seawater: Polymorph Control, Crystallization Kinetics, and Hydrophobic Vaterite Preparation. J. Environ. Chem. Eng. 2023, 11, 110019. [Google Scholar] [CrossRef]
- Albéric, M.; Bertinetti, L.; Zou, Z.; Fratzl, P.; Habraken, W.; Politi, Y. The Crystallization of Amorphous Calcium Carbonate Is Kinetically Governed by Ion Impurities and Water. Adv. Sci. 2018, 5, 1701000. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The Kinetics and Mechanisms of Amorphous Calcium Carbonate (ACC) Crystallization to Calcite, via Vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Martínez Moya, S.; Botella, N.B. Review of Techniques to Reduce and Prevent Carbonate Scale. Prospecting in Water Treatment by Magnetism and Electromagnetism. Water 2021, 13, 2365. [Google Scholar] [CrossRef]
- Gilart, F.; Deas, D.; Ferrer, D.; López, P.; Ribeaux, G.; Castillo, J. High Flow Capacity Devices for Anti-Scale Magnetic Treatment of Water. Chem. Eng. Process. Process Intensif. 2013, 70, 211–216. [Google Scholar] [CrossRef]
- Mascolo, M.C. Effect of Magnetic Field on Calcium Carbonate Precipitated in Natural Waters with Prevalent Temporary Hardness. J. Water Process Eng. 2021, 41, 102087. [Google Scholar] [CrossRef]
- Foster, A.R.; Stark, E.R.; Ikner, L.A.; Pepper, I.L. Bench Scale Investigation of the Effects of a Magnetic Water Treatment Device in Pool Systems on Chlorine Demand. J. Water Process Eng. 2022, 50, 103198. [Google Scholar] [CrossRef]
- Saquete-Ferrándiz, M.D.; Boluda-Botella, N.; Martínez-Moya, S.; García-Quiles, J. Forced Precipitation Experiments for Study of the Electromagnetic Treatment of Water. Chem. Eng. Technol. 2023, 46, 1156–1162. [Google Scholar] [CrossRef]
- Rouina, M.; Kariminia, H.R.; Mousavi, S.A.; Shahryari, E. Effect of Electromagnetic Field on Membrane Fouling in Reverse Osmosis Process. Desalination 2016, 395, 41–45. [Google Scholar] [CrossRef]
- Stojiljkovic, D.T.; Mitić, N.C.; Šmelcerović, A.A.; Kalićanin, B.M.; Tasić-Kostov, M.Z.; Djurović-Petrovi, M.D. Effect of Variable Frequency Electromagnetic Field on Deposit Formation in Installations with Geothermal Water in Sijarinjska Spa (Serbia). Therm. Sci. 2011, 15, 643–648. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, J.; Jia, Y. Experimental Study on the Scale Inhibition Effect of the Alternating Electromagnetic Field on CaCO3 Fouling on the Heat Exchanger Surface in Different Circulating Cooling Water Conditions. Int. J. Therm. Sci. 2023, 192, 108388. [Google Scholar] [CrossRef]
- Zhao, J.D.; Liu, Z.A.; Zhao, E.J. Combined Effect of Constant High Voltage Electrostatic Field and Variable Frequency Pulsed Electromagnetic Field on the Morphology of Calcium Carbonate Scale in Circulating Cooling Water Systems. Water Sci. Technol. 2014, 70, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Latva, M.; Inkinen, J.; Rämö, J.; Kaunisto, T.; Mäkinen, R.; Ahonen, M.; Matilainen, J.; Pehkonen, S. Studies on the Magnetic Water Treatment in New Pilot Scale Drinking Water System and in Old Existing Real-Life Water System. J. Water Process Eng. 2016, 9, 215–224. [Google Scholar] [CrossRef]
- Botello-Zubiate, M.E.; Alvarez, A.; Martínez-Villafañe, A.; Almeraya-Calderon, F.; Matutes-Aquino, J.A. Influence of Magnetic Water Treatment on the Calcium Carbonate Phase Formation and the Electrochemical Corrosion Behavior of Carbon Steel. J. Alloys Compd. 2004, 369, 256–259. [Google Scholar] [CrossRef]
- Martínez-Moya, S. Tratamiento de Agua Por Electromagnetismo: Influencia En La Precipitación de Carbonato Cálcico y Efectos En Otras Aplicaciones. Ph.D. Thesis, Universidad de Alicante, Alicante, Spain, 2023. [Google Scholar]
- Altiner, M.; Yildirim, M. Production and Characterization of Synthetic Aragonite Prepared from Dolomite by Eco-Friendly Leaching–Carbonation Process. Adv. Powder Technol. 2017, 28, 553–564. [Google Scholar] [CrossRef]
- Pérez-Huerta, A.; Laiginhas, F. Preliminary Data on the Nanoscale Chemical Characterization of the Inter-Crystalline Organic Matrix of a Calcium Carbonate Biomineral. Minerals 2018, 8, 223. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikandan, E.; Jayachandran, M. Enhanced Polymer Induced Precipitation of Polymorphous in Calcium Carbonate: Calcite Aragonite Vaterite Phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Kezuka, Y.; Kawai, K.; Eguchi, K.; Tajika, M. Fabrication of Single-Crystalline Calcite Needle-Like Particles Using the Aragonite–Calcite Phase Transition. Minerals 2017, 7, 133. [Google Scholar] [CrossRef]
- Farhadi-Khouzani, M.; Chevrier, D.M.; Zhang, P.; Hedin, N.; Gebauer, D. Water as the Key to Proto-Aragonite Amorphous CaCO3. Angew. Chem. Int. Ed. 2016, 55, 8117–8120. [Google Scholar] [CrossRef]
- Adavi, K.; Molaei Dehkordi, A. Synthesis and Polymorph Controlling of Calcite and Aragonite Calcium Carbonate Nanoparticles in a Confined Impinging-Jets Reactor. Chem. Eng. Process. Process Intensif. 2021, 159, 108239. [Google Scholar] [CrossRef]
- Ghiasi, M.; Abdollahy, M.; Khalesi, M.R.; Ghiasi, E. Control of the Morphology, Specific Surface Area and Agglomeration of Precipitated Calcium Carbonate Crystals through a Multiphase Carbonation Process. CrystEngComm 2020, 22, 1970–1984. [Google Scholar] [CrossRef]
- Mavromatis, V.; Montouillout, V.; Noireaux, J.; Gaillardet, J.; Schott, J. Characterization of Boron Incorporation and Speciation in Calcite and Aragonite from Co-Precipitation Experiments under Controlled PH, Temperature and Precipitation Rate. Geochim. Cosmochim. Acta 2015, 150, 299–313. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, C.; Wu, L.; Zhang, Q.; Zhu, L.; Zhao, R. Influence of Alternating Electromagnetic Field and Ultrasonic on Calcium Carbonate Crystallization in the Presence of Magnesium Ions. J. Cryst. Growth 2018, 499, 67–76. [Google Scholar] [CrossRef]
- Piyadasa, C.; Yeager, T.R.; Gray, S.R.; Stewart, M.B.; Ridgway, H.F.; Pelekani, C.; Orbell, J.D. The Influence of Electromagnetic Fields from Two Commercially Available Water-Treatment Devices on Calcium Carbonate Precipitation. Environ. Sci. 2017, 3, 566–572. [Google Scholar] [CrossRef]
- Jimoh, O.A.; Ariffin, K.S.; Hussin, H.B.; Temitope, A.E. Synthesis of Precipitated Calcium Carbonate: A Review. Carbonates Evaporites 2018, 33, 331–346. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, X.; Johnson, D.; Lin, L.; Wang, H.; Xu, P. Effectiveness and Mechanisms of Electromagnetic Field on Reverse Osmosis Membrane Scaling Control during Brackish Groundwater Desalination. Sep. Purif. Technol. 2022, 280, 119823. [Google Scholar] [CrossRef]
- Dobersek, D.; Goricanec, D. An Experimentally Evaluated Magnetic Device’s Efficiency for Water-Scale Reduction on Electric Heaters. Energy 2014, 77, 271–278. [Google Scholar] [CrossRef]
- Chibowski, E.; Szcześ, A. Magnetic Water Treatment—A Review of the Latest Approaches. Chemosphere 2018, 203, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, J.; Zhao, E.; Liu, X.; Jia, Y.; Ying, C.; Lin, L. Effect of Electric Field and Mg2+ Doping on Calcium Carbonate Scaling Shown in Experiments and First Principle Calculations. Water Supply 2020, 20, 3251–3265. [Google Scholar] [CrossRef]
- Al Helal, A.; Soames, A.; Gubner, R.; Iglauer, S.; Barifcani, A. Influence of Magnetic Fields on Calcium Carbonate Scaling in Aqueous Solutions at 150 °C and 1 Bar. J. Colloid. Interface Sci. 2018, 509, 472–484. [Google Scholar] [CrossRef]





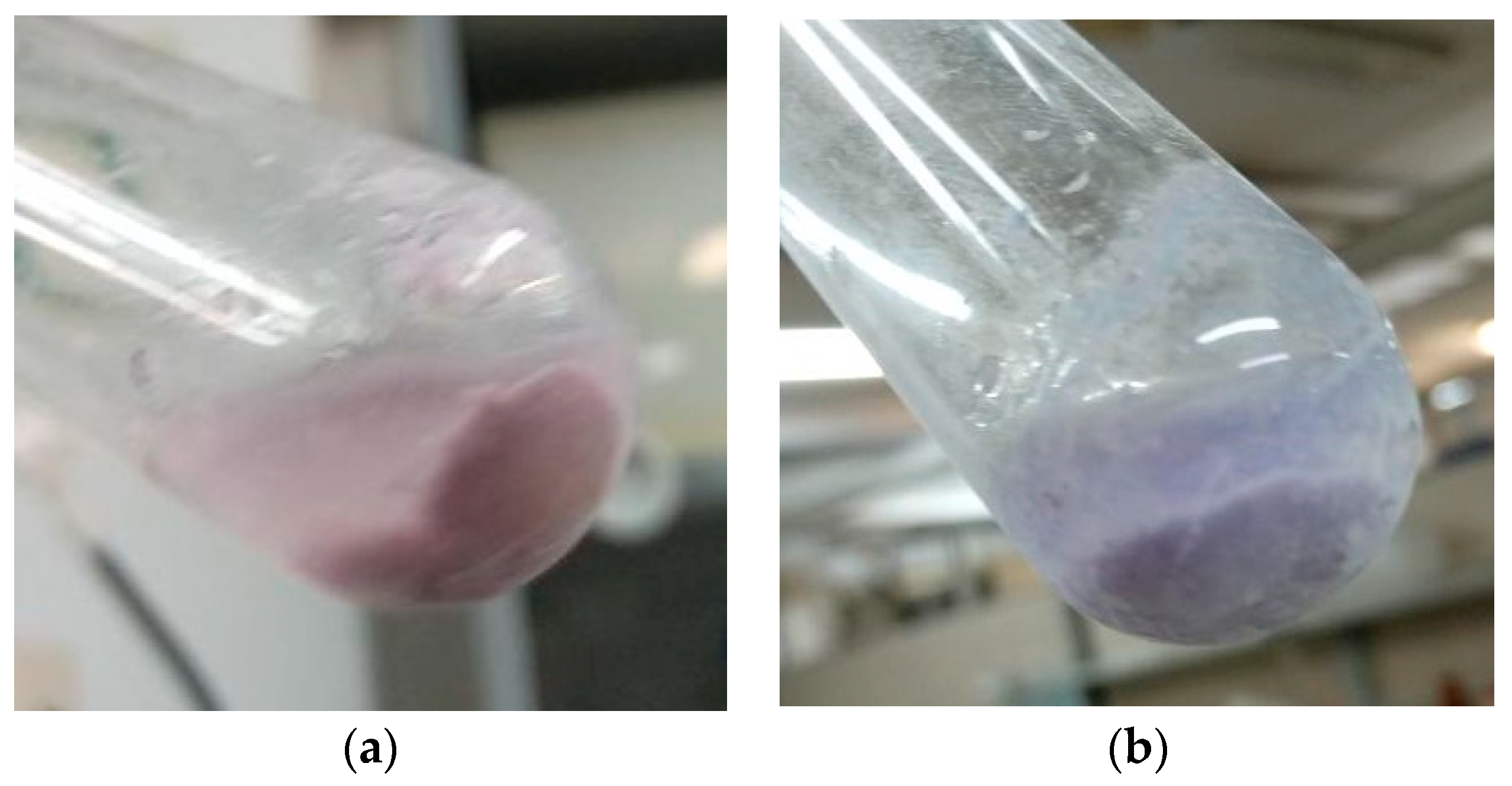
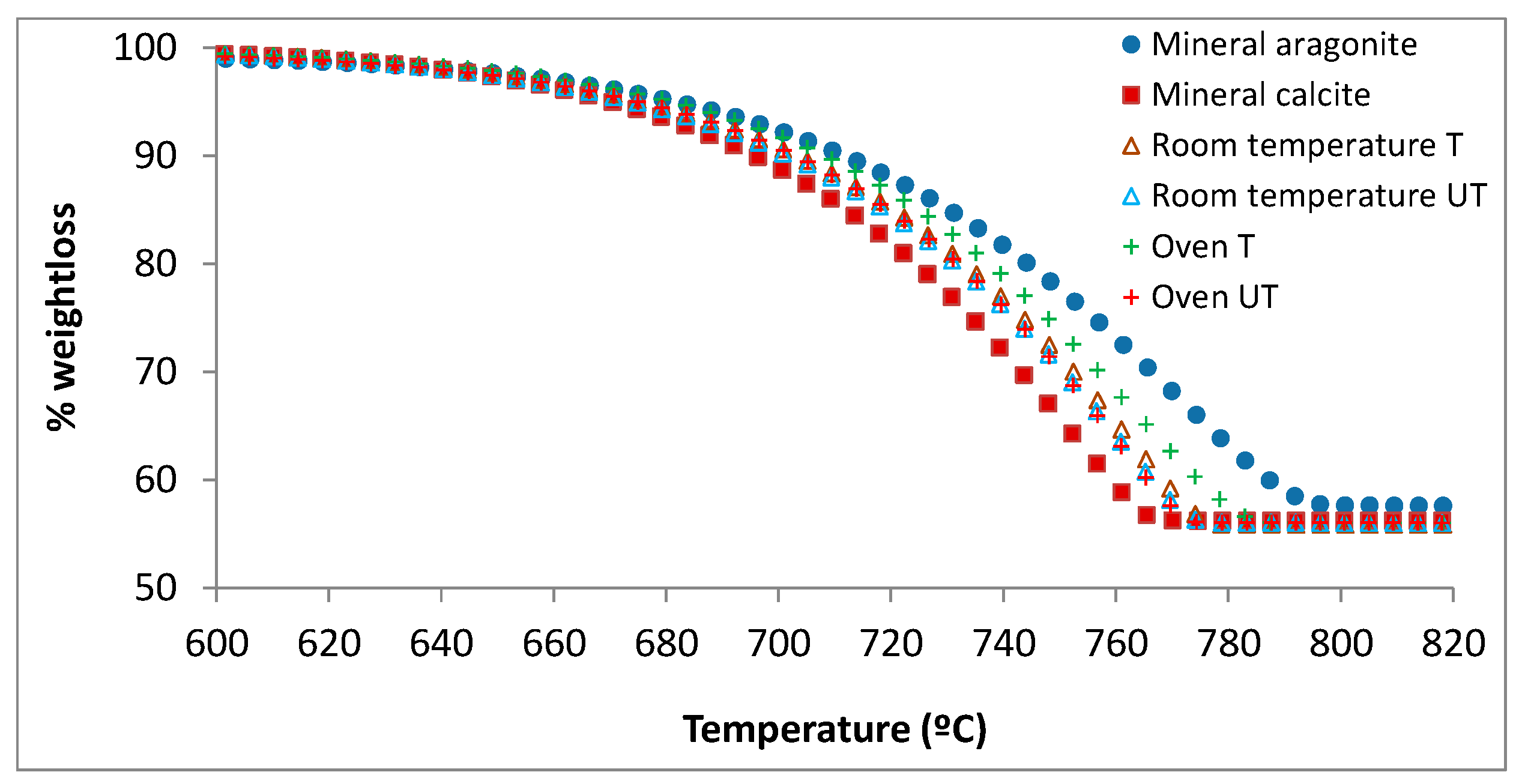
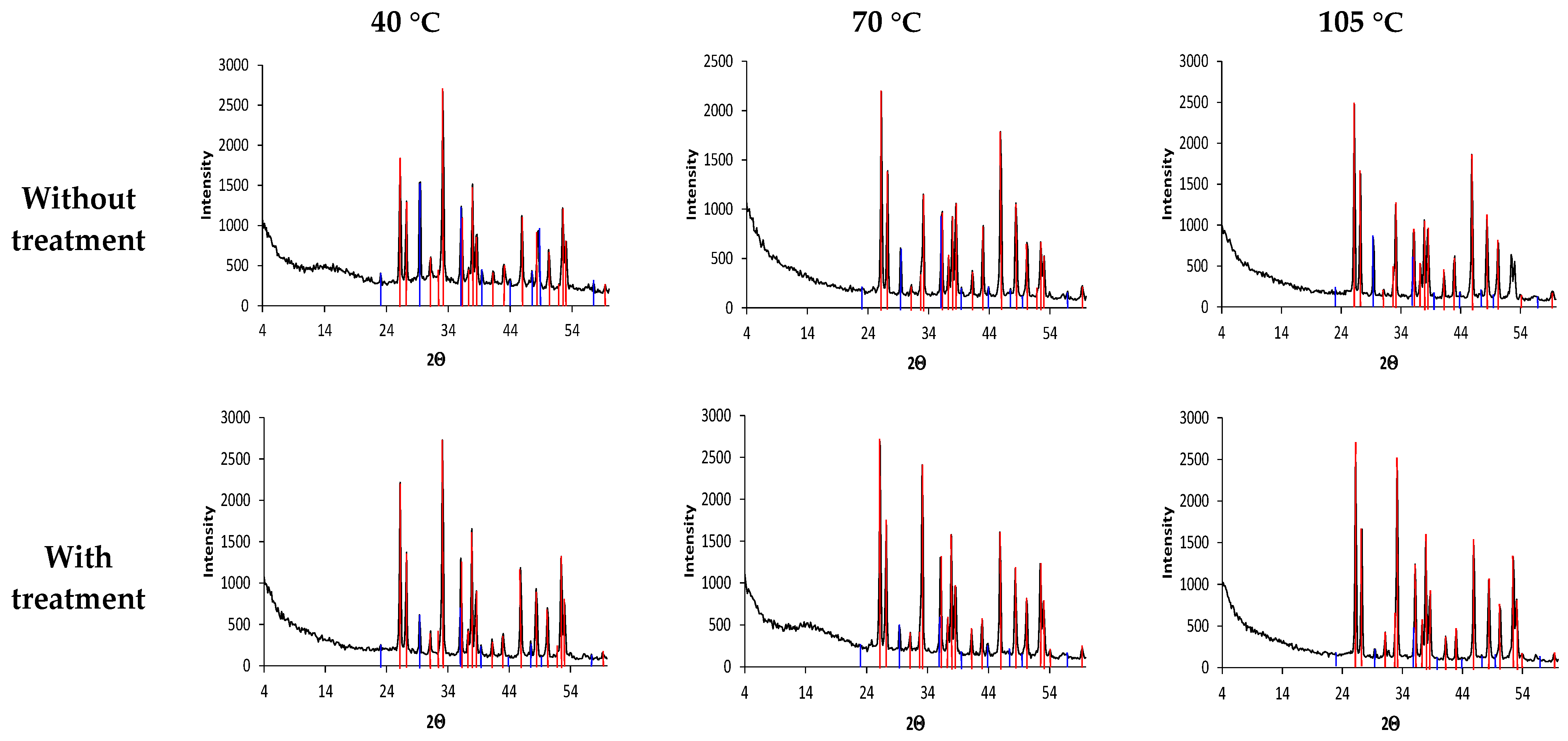

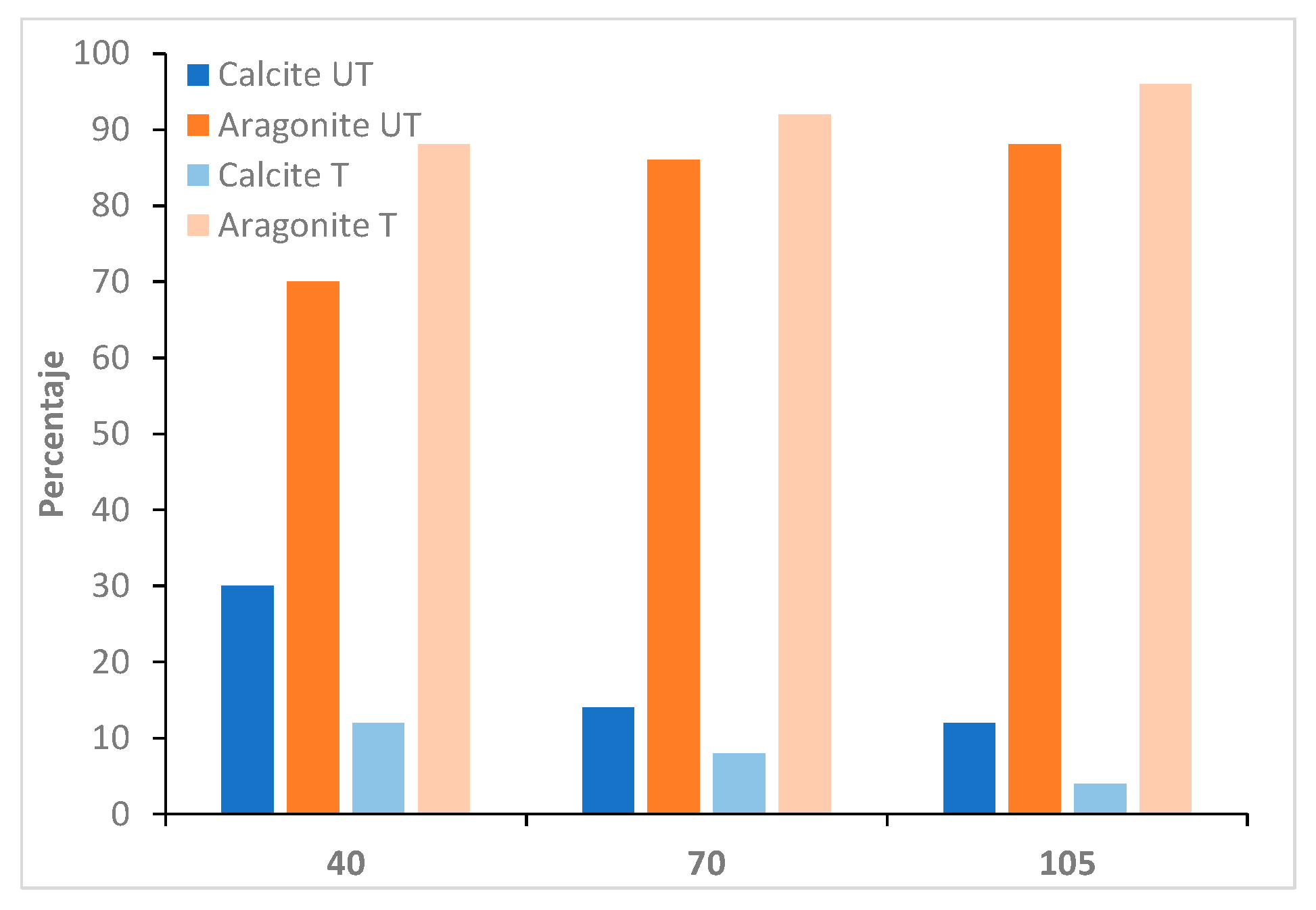


| Parameters | Tap Water |
|---|---|
| pH | 8.26 ± 0.05 |
| Conductivity (µS/cm) | 970 ± 10 |
| Ca2+ (mg/L) | 75 ± 1 |
| Mg2+ (mg/L) | 30 ± 1 |
| Na+ (mg/L) | 100 ± 5 |
| K+ (mg/L) | 3 ± 0.1 |
| HCO3− (mg/L) | 165 ± 5 |
| SO42− (mg/L) | 85 ± 1 |
| Cl− (mg/L) | 215 ± 5 |
| NO3− (mg/L) | 7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boluda-Botella, N.; Saquete, M.D.; Martínez-Moya, S.; Morales-Paredes, C.A.; Rodríguez-Díaz, J.M. Analysis of Calcium Carbonate Scales in Water Distribution Systems and Influence of the Electromagnetic Treatment. Water 2024, 16, 1554. https://doi.org/10.3390/w16111554
Boluda-Botella N, Saquete MD, Martínez-Moya S, Morales-Paredes CA, Rodríguez-Díaz JM. Analysis of Calcium Carbonate Scales in Water Distribution Systems and Influence of the Electromagnetic Treatment. Water. 2024; 16(11):1554. https://doi.org/10.3390/w16111554
Chicago/Turabian StyleBoluda-Botella, Nuria, María Dolores Saquete, Sergio Martínez-Moya, Carlos Augusto Morales-Paredes, and Joan Manuel Rodríguez-Díaz. 2024. "Analysis of Calcium Carbonate Scales in Water Distribution Systems and Influence of the Electromagnetic Treatment" Water 16, no. 11: 1554. https://doi.org/10.3390/w16111554
APA StyleBoluda-Botella, N., Saquete, M. D., Martínez-Moya, S., Morales-Paredes, C. A., & Rodríguez-Díaz, J. M. (2024). Analysis of Calcium Carbonate Scales in Water Distribution Systems and Influence of the Electromagnetic Treatment. Water, 16(11), 1554. https://doi.org/10.3390/w16111554










