1. Introduction
The transition zone of the Jianghan Plain–Dabie Mountain area represents a significant ecological functional area in China and serves as an important ecological security barrier in the middle and lower reaches of the Yangtze River [
1,
2]. Nevertheless, the ecological and geological environment of this region is relatively fragile due to the high population density and extensive exploitation of groundwater resources [
3,
4]. The development of industry and agriculture, coupled with the extensive utilisation of pesticides and chemical fertilisers, has resulted in a more pronounced agricultural non-point source pollution in the transition zone of the Jianghan Plain–Dabie Mountain area [
5]. Groundwater analysis has revealed elevated levels of pollutants, including ammonia nitrogen phenol, and organic pollutants, posing threats to both the ecological environment and human health [
6,
7].
A considerable quantity of agricultural land and animal husbandry land is situated in the transition zone of the Jianghan Plain–Dabie Mountain area. A portion of the sewage is discharged directly without undergoing any form of treatment, resulting in the detection of a number of antibiotic components in rivers and groundwater [
8,
9,
10]. The natural groundwater in the region already contains elevated levels of arsenic [
11]. Intensive industrial and agricultural activities have exacerbated contamination issues, resulting in elevated concentrations of ammonium salts in groundwater and the presence of residual pollutants from industrial chemicals [
12,
13]. The pollutants present in groundwater have the potential to adversely affect human health and safety. Concurrently, organic pollutants, such as pesticides and fertilisers, present in the surface and soil of the region, may migrate with water into deeper soil and groundwater as a consequence of the infiltration of atmospheric precipitation and groundwater seepage, thereby increasing the extent of contamination [
14]. Consequently, the prevention and control of organic pollutants have become a primary focus of ecological and environmental protection work in the transition zone of the Jianghan Plain–Dabie Mountain area.
The presence of thick layered clay soil in the upper part of the Quaternary formation in the transition zone of the Jianghan Plain–Dabie Mountain area greatly influences the migration process of water and solute in the soil [
15,
16]. Clay soil exhibits poor permeability and exchange capacity due to its special microporous structure and water-absorbing and swelling properties caused by internal clay minerals [
17]. The double electron layer effect on the surface of clay soil confers strong adsorption properties for pollutants [
18]. Therefore, low-permeability clay soil can influence the depth of pollutant migration and their contact time with the surrounding medium, thus limiting downward pollutant migration [
19]. Chemical reactions such as neutralisation, filtration, volatilisation, dispersion, and biodegradation can occur during contaminant migration in low-permeability clay soils, and these processes can also limit pollutant migration downwards [
20]. In general, under the same infiltration conditions, the thicker the clay soil, the slower the downward migration of pollutants, the lower the amount of migration, and the stronger the antifouling performance [
21].
The transport process of pollutants in layered soil is more complex and can be influenced by soil texture [
22], layer arrangement [
23,
24], soil thickness [
25], and the number of layers [
26]. Wang et al. [
27] found that the infiltration peak changes significantly when passing through soil stratification interfaces, and that both clay–sand and sand–clay interbed structures can impede the infiltration process of water. Cui et al. [
28] observed, through in-situ experiments, that layered heterogeneous structures affect the infiltration process and precipitation rate, thereby impeding water movement. Darehshouri et al. [
29] found that soil stratification significantly affects the soil hydraulic profile under evaporative conditions. Previous research has shown that the layered structure of soil significantly alters the migration process and patterns of water and solutes. However, the migration and transformation processes of water and solute in low-permeability layered clay soil in the transition zone of the Jianghan Plain–Dabie Mountain area are not well understood [
12]. Investigating the migration patterns of organic macromolecular pollutants in low-permeability layered clay and identifying the obstruction ability of low-permeability layered clay for organic macromolecular pollutants are essential for ecological environmental protection and organic pollutant prevention and control efforts in the transition zone of the Jianghan Plain–Dabie Mountain area.
Organic pollutants may volatilise or degrade when transported in soil [
30]. As an exogenous macromolecular pollutant, sodium fluorescein exhibits characteristics such as increased resistance to oxidation and degradation, greater stability, and reduced susceptibility to background interference [
31]. These attributes help mitigate the influence of degradation and volatilisation on the migration process of organic pollutants, thereby providing a clearer reflection of the adsorption and migration of organic macromolecular pollutants in soil. This allows for a more intuitive exploration of how layered clayey soil hinders the transport of organic macromolecular pollutants [
32,
33].
In this study, the Dabie Mountain Area-Jianghan Plain Rainfall, Soil moisture, and Groundwater Conversion Scientific Field Test Site was selected as the study area. Fluorescein sodium was chosen as a representative tracer for organic macromolecular pollutants. Based on field hydrogeological data, adsorption experiments and column leaching experiments were conducted to investigate the adsorption and migration behaviour of organic macromolecular pollutants in the layered soil of the study area. HYDRUS-1D software (v.4.16), combined with indoor experimental results, was used to obtain the dispersivities and dispersion coefficients of organic macromolecular pollutants migrating in the layered clay soil in the study area, aiming to explore the hindrance ability of low-permeability layered clay soil on the migration of organic macromolecular pollutants. This provides data support and a theoretical basis for future development and utilisation as well as for soil and groundwater pollution prevention and control efforts in ecologically fragile areas within the study area.
2. Materials and Methods
2.1. Study Area
The Dabie Mountain Area-Jianghan Plain Rainfall, Soil moisture, and Groundwater Conversion Scientific Field Test Site is situated in Xiaogang Town, Xiaogang City, Hubei Province (113°70′ E, 31°10′ N). The field test site is located on the secondary terrace of the transitional zone between the northern Jianghan Plain and the Dabie Mountains along the Huan River. This area features a subtropical monsoon climate, with the regional topography gradually declining from north to south. The steep ridge at the forefront of the terrace is approximately 1–1.5 m high, transitioning smoothly towards the inner edge to form a relatively flat second terrace of the Huan River. The main exposed stratum in the research area is the Upper Pleistocene of the Quaternary System (
), underlain by the Paleogene Yuntai Mountain Formation bedrock (Ey). The Upper Pleistocene (
) exhibits a distinct binary structure, with the upper part consisting of a thicker layer of low-permeability clay soil, and the lower part comprising sandy loam and gravel layers (
Figure 1). Groundwater exists in the lower gravel layers in two forms: pore-phreatic water and pore-confined water. Dynamic changes in the aquifer water levels within the research area reveal a close connection between the Quaternary pore water and the pore-fracture water of the red beds. The Quaternary pore water continuously recharges the red bed pore-fracture water, sharing similar dynamic variations, and being significantly influenced by precipitation. Both primarily receive infiltration recharge from atmospheric precipitation, exhibiting seasonal dynamic changes. The overlaying low-permeability clay layer and sudden shifts in the stratigraphic structure significantly impact precipitation infiltration and aquifer recharge [
34,
35,
36,
37].
2.2. Soil Samples
Based on the geological stratification revealed by drilling at the field test site area, 5 undisturbed soil samples from different depths were selected for the experiment: 2.0 m silty sub-clay soil, 5.0 m silty clay soil, 6.5 m silty sub-clay soil, 13.5 m sandy sub-clay soil, and 14.2 m sandy sub-sandy soil. The particle size distribution data of the soil samples are presented in
Table 1, and the mineral composition of the soil samples is shown in
Table 2. In the clay soil samples from the research area, the mineral composition is predominantly composed of clay minerals, including illite, kaolinite, and illite-montmorillonite interlayer clay. In contrast, the mineral composition of the sandy soil samples primarily consists of granular minerals such as quartz, k-feldspar, and plagioclase. The soil samples were dried at 45 °C, ground, and passed through a 2 mm sieve for subsequent use in static adsorption experiments and column leaching experiments.
2.3. Adsorption Experiments
Kinetic adsorption experiments and isotherm adsorption experiments were conducted on the 5 soil samples from the study area to investigate the adsorption characteristics of fluorescein sodium by the soils. In the kinetic adsorption experiments, 5 g of dried and sieved soil samples was placed in 50 mL PET bottles, to which 25 mL of a 5 g/L fluorescein sodium solution was added. The bottles were then placed in a constant temperature oscillator and oscillated at 25 °C and 200 rpm for durations of 0.25 h, 0.5 h, 1 h, 3 h, 5 h, 8 h, 12 h, and 24 h. In the isotherm adsorption experiments, 5 g of dried and sieved soil samples was placed in 50 mL PET bottles, to which 25 mL of fluorescein sodium solution at concentrations of 1 g/L, 5 g/L, 10 g/L, 50 g/L, 100 g/L, 125 g/L, and 150 g/L was added. The bottles were then fully oscillated in a constant temperature oscillator at 25 °C and 200 rpm for 4 h.
After oscillation, the samples were centrifuged at 4000 rpm for 10 min to obtain the supernatant. Subsequently, the supernatant was then filtered through a 0.45 μm filter and diluted before measuring the concentration of fluorescein sodium using a fluorescence spectrophotometer (GGUN-FL30, Albillia Sàrl, Auvernier, Switzerland). The adsorption amount of fluorescein sodium by the soils in the research area was calculated using the following formula [
38]:
where
q (mg/g) is the adsorption capacity of soil samples for fluorescein sodium;
V (L) is the volume of fluorescein sodium solution;
m (g) is the mass of soil samples;
C0 and
Ce (mg/L) are the concentration of fluorescein sodium at initial time and equilibrium time, respectively.
To describe the adsorption characteristics of soil samples for fluorescein sodium, the pseudo-second-order kinetic model, Langmuir isotherm model, and Freundlich isotherm model were used for fitting the experimental adsorption data. The expressions for these models are as follows:
Pseudo-second-order kinetic model [
39]:
Langmuir isotherm model [
40]:
Freundlich isotherm model [
41]:
where
qe is the equilibrium adsorption capacity (mg/g);
qt is the adsorbed concentration at time t (mg/g);
K2 is the pseudo-second-order constant;
qe is the equilibrium adsorption capacity (mg/g);
qm is the maximum adsorption capacity (mg/g);
KL is the Langmuir isotherm constant;
Ce is the concentration of fluorescein sodium at equilibrium (g/L);
Kf is the Freundlich isotherm constant;
n is the adsorption intensity parameter.
2.4. Column Leaching Experiments
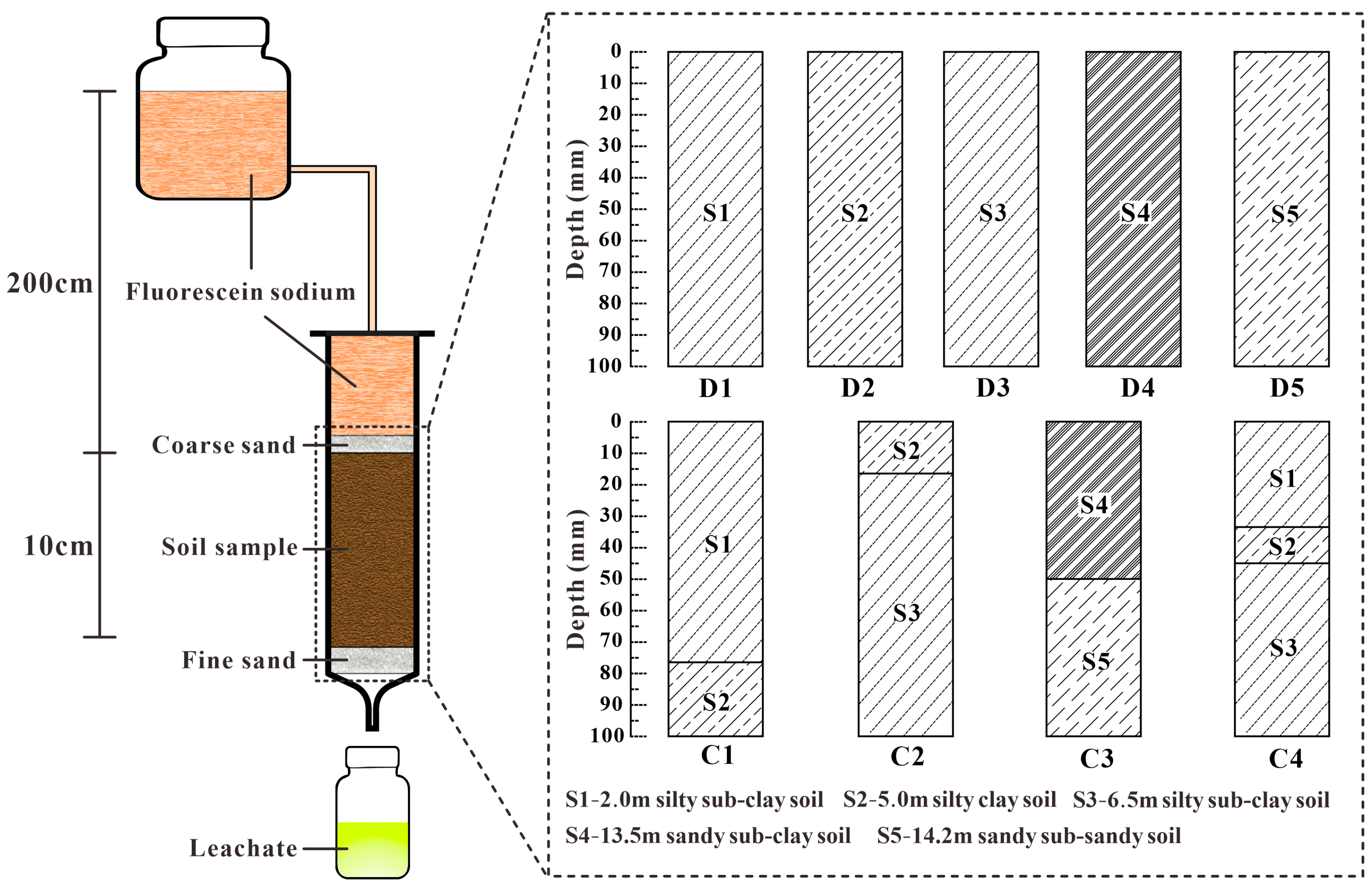
In the column leaching experiment, 9 soil columns were prepared using samples from 5 types of soil: 2.0 m of silty sub-clay soil, 5.0 m of silty clay soil, 6.5 m of silty sub-clay soil, 13.5 m of sandy sub-clay soil, and 14.2 m of sandy sub-sandy soil (
Figure 2). The 5 single-layer uniform soil columns designated D1–D5 were prepared using the aforementioned five soil samples, while the four-layered soil columns designated C1–C4 were prepared using these five soil samples in proportions that reflect the actual stratigraphic sequence, layer thickness, and bulk density of the soil at the experimental site. Nine acrylic tubes with an inner diameter of 40 mm and a height of 148 mm were selected as the leaching columns. The inner walls of the columns were roughened to ensure tight contact between the soil samples and the columns, and the columns were rinsed with deionised water before soil filling. A 100-mesh nylon cloth was placed at the bottom of each column, followed by a layering process from bottom to top: 15 mm of fine sand, 100 mm of the soil sample under test, and finally, 10 mm of coarse sand. The soil was compacted during the filling process to ensure the bulk density of the soil sample was consistent with that of the actual soil layers at the experimental site. While the columns had been filled, the top was sealed with sealing gel, and a hose was connected to deliver the fluorescein sodium solution.
The column leaching experiments were conducted using the constant head saturated infiltration method, with the water head set at 2 m. Initially, the columns were completely saturated from bottom to top with deionised water using the peristaltic pump, and after the liquid level above the columns stabilised, the pore water velocity for each column was calculated. Subsequently, the water supply was switched from bottom to top. A continuous top-end injection of 5 g/L fluorescein sodium solution at the fixed head of 2 m was initiated using a Mariotte bottle. The peristaltic pump continuously supplied fluorescein sodium solution to the Mariotte bottle to maintain a stable liquid level at 2 m. After the start of the leaching experiments, the leachate was collected at the bottom of each column using PET bottles, filtered through a 0.45 μm filter, diluted, and then the fluorescein sodium concentration was measured using a fluorescence photometer (GGUN-FL30, Albillia Sàrl, Auvernier, Switzerland). The experiments were considered complete when the concentration of fluorescein sodium in the leachate collected from below the column stabilised and its relative concentration approached 1, indicating that the fluorescein sodium solution had fully penetrated through the column.
2.5. Numerical Simulation of Solute Transport
A one-dimensional solute transport model was established based on laboratory soil column leaching experiments. This model utilises HYDRUS-1D software (v.4.16) to simulate the migration process of fluorescein sodium, representing organic macromolecular pollutants, as it enters the soil column with water in both homogeneous and layered soils. By employing model inversion, the dispersivities and dispersion coefficients of fluorescein sodium within the soil samples of the study area were determined. Model parameters were configured to align with the actual conditions of the experimental soil columns. By utilising the particle size distribution data of the experimental soils, crucial parameters including residual water content, saturated water content, air entry value, and saturated hydraulic conductivity required for the model were obtained using the Network Prediction system within HYDRUS-1D software (v.4.16). Additionally, the parameters
KL and
LF, representing the soil’s adsorption capacity, were adopted from those obtained in the adsorption experiments. The one-dimensional dispersion equation employed in the solute transport simulation is as follows [
42]:
Upper boundary condition:
Lower boundary condition:
where
D is the dispersion coefficient (cm
2/d);
S is the adsorption capacity of the unit soil for fluorescein sodium (g/L);
C is the concentration of fluorescein sodium in the solution (g/L);
C0 is the background concentration of fluorescein sodium in the column liquid phase (g/L);
Ci is the concentration of fluorescein sodium in the leachate (5 g/L);
N is the effective porosity of the soil;
v is the percolation velocity of the solution in the soil (cm/d).
3. Results and Discussion
3.1. Soil Adsorption Characteristics of Fluorescein Sodium
3.1.1. Kinetic Adsorption Characteristics
Through kinetic adsorption experiments, the relationship between the adsorbed concentration of soils in the research area for fluorescein sodium and adsorption time was explored, with results shown in
Figure 3. It can be observed that the adsorbed concentration of the soils for fluorescein sodium gradually increases over time, with a higher adsorption rate in the initial stage of the adsorption process. The adsorption rate in the initial stage of the adsorption process of the soils for fluorescein sodium was ranked as S2 > S1 > S5 > S4 > S3. As the adsorption process approached equilibrium, the adsorption rate exhibited a gradual decline, while the adsorption capacity exhibited a tendency towards stabilisation.
The pseudo-second-order kinetic model was employed to fit the results of kinetic adsorption [
43], and the fitting results are presented in
Table 3. The equilibrium adsorption capacity (
qe,cal) calculated from the pseudo-second-order kinetic equation is close to the experimental value (
qe,exp), with the highest fitting correlation coefficient reaching 0.999. This indicates that the pseudo-second-order kinetic model accurately describe the kinetic adsorption process of fluorescein sodium by soils in the research area. The pseudo-second-order constants (
K2) for the five types of soil are ranked as S4 > S5 > S1 > S2 > S3. A higher
K2 value indicates that adsorption equilibrium can be attained more quickly. It is evident that sandy soil achieves adsorption equilibrium faster than clay soil. The equilibrium adsorption capacities (
qe) of the five soil types are ranked as follows: S2 > S1 > S3 > S4 > S5. Notably, clay soil exhibits a significantly higher equilibrium adsorption capacity for fluorescein sodium compared to sandy soil. The equilibrium adsorption capacity of soil for fluorescein sodium is observed to increase in proportion to the rise in clay content. This trend is closely linked to the presence of more adsorption sites in clayey soils. Apart from S3, which is silty sub-clay soil, the adsorption rate of fluorescein sodium by clay soil is significantly higher than that by sandy soil.
3.1.2. Isotherm Adsorption Characteristics
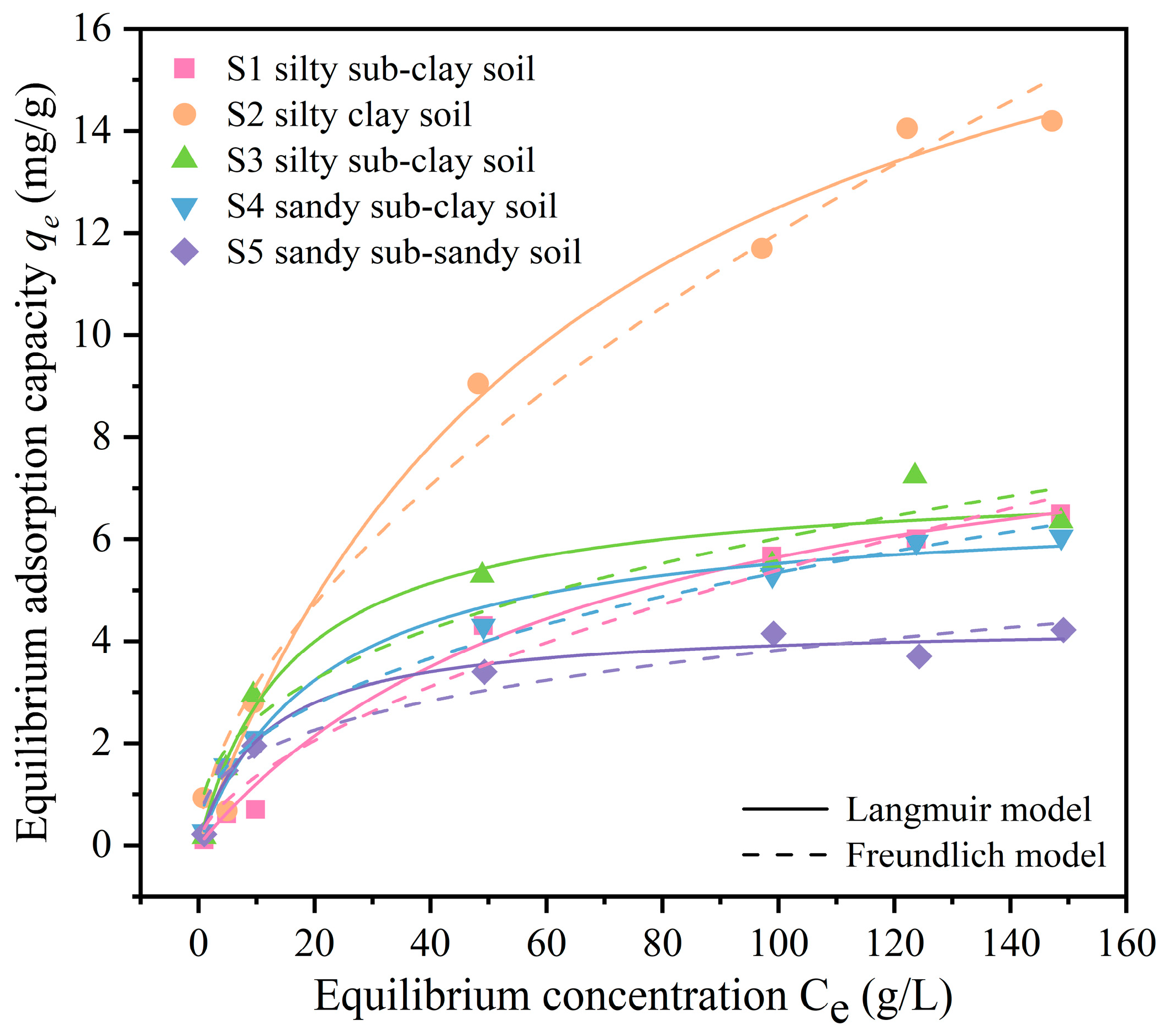
The results of the isotherm adsorption experiments were fitted using both the Langmuir and Freundlich models to investigate the adsorption characteristics of the soil samples from the research area for fluorescein sodium. This analysis aimed to determine the adsorption parameters
KL and
KF for subsequent solute transport simulation studies. The fitting results are illustrated in
Figure 4, and the adsorption parameters are presented in
Table 4.
The Langmuir model, frequently employed to describe the adsorption process, assumes that the adsorbate forms a monolayer on the surface of the adsorbent, beyond which no further adsorption occurs [
44]. The fitting results of the Langmuir model indicate a strong correlation between the adsorption of fluorescein sodium by soil samples in the study area and the Langmuir model, with correlation coefficients (
R2) ranging from 0.979 to 0.991. This suggests that the adsorption pattern of fluorescein sodium by soils in the study area follows a monolayer adsorption mode, attributed to the abundance of adsorption sites on the surfaces of clay minerals in the soil [
45]. There are significant differences in the maximum adsorption capacity of fluorescein sodium among different types of soil in the research area. For instance, S2 silty clay soil exhibits a maximum adsorption capacity of 20.762 mg/g, whereas S5 sandy sub-sandy soil only reaches 4.351 mg/g. The overall trend of maximum adsorption capacity of soils in the study area is as follows: silty clay soil > silty sub-clay soil > sandy sub-clay soil > sandy sub-sandy soil. It is evident that clay soils demonstrate significantly higher maximum adsorption capacities for fluorescein sodium compared to sandy soils. Furthermore, the maximum adsorption capacity of soil for fluorescein sodium is observed to increase with an increase in the clay fraction content of the soil. For instance, both S1 and S3 are silty sub-clay soils, yet the maximum adsorption capacity of fluorescein sodium by S1 exceeds that of S3. This difference in adsorption capacity can be attributed to the higher organic matter content within S1, which is shallower compared to S3, consequently resulting in a greater adsorption capacity [
46].
The Freundlich model, commonly utilised to describe adsorption characteristics on heterogeneous surfaces [
47]. The fitting results of the Freundlich model indicate that the adsorption of fluorescein sodium by soil samples from the research area correlates with the Freundlich model, with correlation coefficients (
R2) ranging from 0.919 to 0.984, lower than those of the Langmuir model. The adsorption intensity parameter,
n, can be utilised to determine the adsorption characteristics of the adsorbent [
48]. For the five types of soil samples from the research area, 1/
n is less than 1, and
n falls within the range of 1 to 10, indicating that the adsorption of fluorescein sodium by the soils follows a normal and favourable adsorption process. Notably, the 1/
n value for S5 sandy sub-sandy soil is significantly lower than that for clay soil, suggesting that the adsorption process is more likely to occur in sandy sub-sandy soil compared to clay soil in the research area.
The adsorption experiment results indicate that the clay minerals and organic matter present in the clay soil of the study area offer more adsorption sites, thereby leading to a significantly higher adsorption capacity for fluorescein sodium compared to sandy soil. Although sandy soil demonstrates a more readily occurring adsorption process and a faster rate of adsorption for fluorescein sodium, the fewer adsorption sites in sandy soil compared to clay soil lead to weaker adsorption performance. Consequently, it is evident that organic macromolecular pollutants migrating within the zone of aeration in the study area may be absorbed by the clay soil present in that zone, thereby impeding their transport process.
3.2. Characteristics of Fluorescein Sodium Breakthrough Curves of Soil Columns
To investigate the transport characteristics of fluorescein sodium in the soil samples of the research area, column leaching experiments were conducted to simulate the saturated infiltration process of fluorescein sodium. The transport process of fluorescein sodium in five types of soils at the experimental site was investigated using homogeneous soil columns. Furthermore, layer-packed soil columns, filled proportionally based on the actual stratigraphy of the experimental site, were employed to simulate the transport process of fluorescein sodium within the layered soils of the site. Breakthrough curves, which represent the relationship between the relative concentration of the leachate solute (C/C
0) and the corresponding time, provide an intuitive depiction of solute transport dynamics and concentration trends during the transport process [
49].
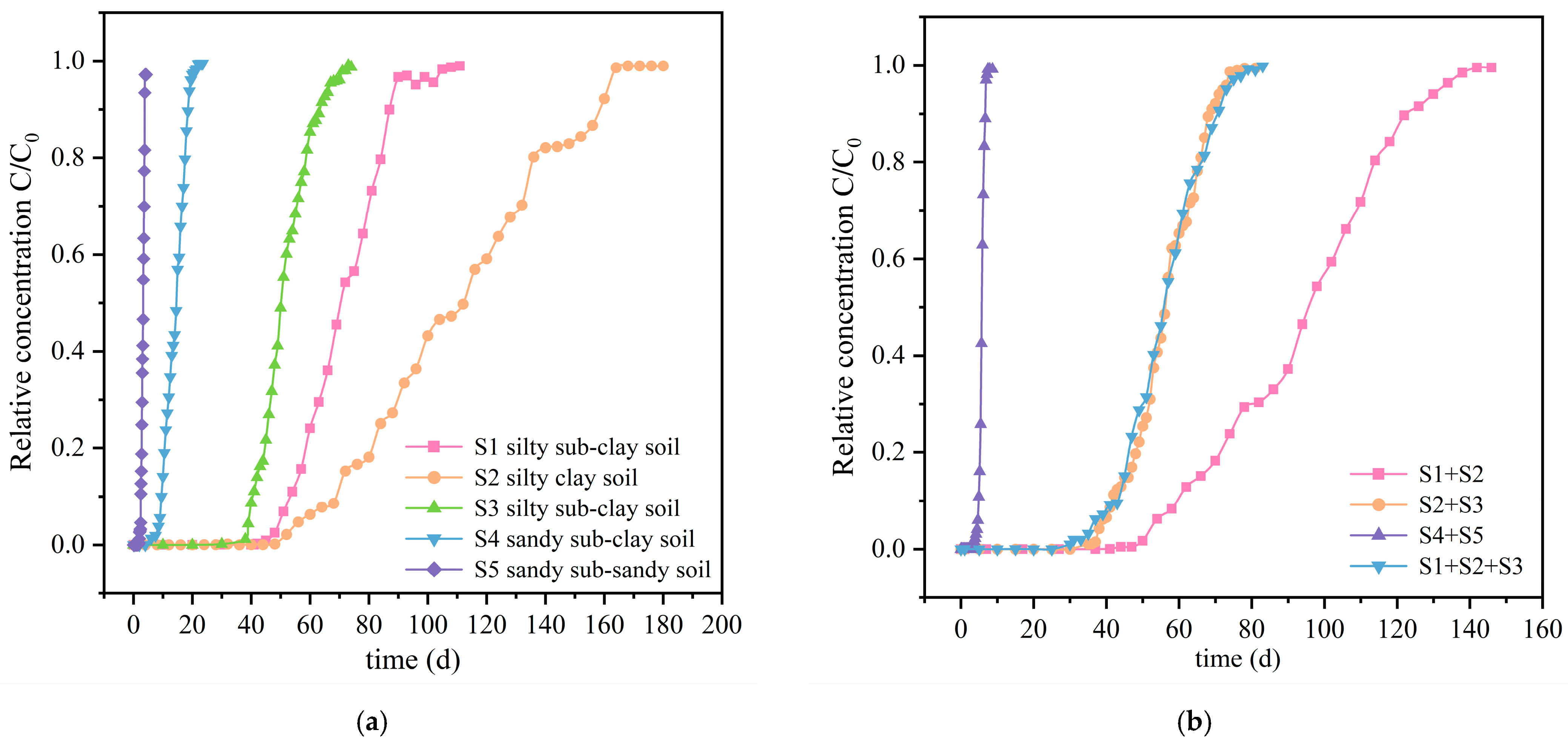
The breakthrough curves obtained from the column leaching experiments are shown in
Figure 5. Following the commencement of the experiments, the time at which fluorescein sodium first appears in the leachate is defined as the initial breakthrough time. When the relative concentration of fluorescein sodium in the leachate stabilises and approaches 1, this time is defined as the complete breakthrough time. The difference between the initial and complete breakthrough times is referred to as the breakthrough duration. The breakthrough parameters of the column leaching experiments are presented in
Table 5.
The overall breakthrough duration of fluorescein sodium in the soils of the study area can be ranked as follows: S2 > S1 > S3 > S4 > S5. For instance, the breakthrough duration for S2, a silty clay soil, extends to 116 days, whereas for S5, a sandy sub-sandy soil, it is merely 2.6 days. Notably, the breakthrough duration of fluorescein sodium in clay soil significantly exceeds that in sandy soil and demonstrates an increasing trend with higher clay content. This indicates that clay soil in the study area can hinder the migration of fluorescein sodium to a certain extent. The breakthrough duration of fluorescein sodium in the soil is directly proportional to the maximum adsorption capacity of the soil and inversely proportional to the hydraulic conductivity of the soil. Thus, it can be observed that clay soil primarily hinders the migration of fluorescein sodium due to its inherent low permeability and strong adsorption capacity. The lower hydraulic conductivity of clay soil indicates a reduced effective porosity within the soil medium, leading to an increased volume of stagnant water within the saturated medium. Consequently, the displacement effect of mechanical dispersion on solute is diminished. Furthermore, the lower hydraulic conductivity prolongs the contact time between fluorescein sodium and soil particles, thereby increasing the amount of fluorescein sodium adsorbed in the soil [
50].
The breakthrough duration of fluorescein sodium in layered soil columns falls within the range between the longest and shortest breakthrough duration of fluorescein sodium in homogeneous soil columns corresponding to the soils filled in the columns. Furthermore, the breakthrough duration of fluorescein sodium increases as the adsorption capacity of the soils within the layered soil columns enhances and the solution flow rate within the columns decreases. The breakthrough duration of fluorescein sodium in the column composed of three types of clay soil (S1 + S2 + S3) is significantly longer (58 days) compared to the column composed of two types of sandy soil (S4 + S5), which has a breakthrough duration of 5.6 days. This indicates that the layered clay soil in the study area can effectively impede the migration of fluorescein sodium. This effect is not only attributed to the low permeability and strong adsorption capacity of clay soil, which hinder the transport of fluorescein sodium, but also to the lateral movement of moisture at different soil interfaces within layered clay soil. This lateral movement may also impose certain hindrances on the migration of fluorescein sodium [
20,
51].
3.3. The Hindering Effect of Layered Clay Soil on the Migration of Organic Macromolecular Pollutants
Based on the results of adsorption experiments and soil column leaching experiments, the migration process of organic macromolecular pollutants in both homogeneous and layered soil columns was simulated using HYDRUS-1D software (v.4.16). The dispersion parameters of these columns, acquired through inversion, are presented in
Table 6.
In homogeneous soils, the overall trend of dispersivities and average seepage velocity during the migration process of organic macromolecular pollutants is S2 < S1 < S3 < S4 < S5. Similarly, the dispersion coefficients of these pollutants follow the same pattern. Moreover, the dispersion coefficients of clay soils are significantly lower than those of sandy soils, and they decrease with the increase in clay fraction. In S2 silty clay soil, the dispersion coefficient of organic macromolecular pollutants is only 0.0038 cm2/d, whereas in S5 sandy sub-sandy soil, the dispersion coefficient can reach 4.724 cm2/d, representing a difference of four orders of magnitude. This difference is attributed to the stronger adsorption capacity of clay soil for organic macromolecular pollutants compared to sandy soil. Additionally, clay soil has fewer effective pores, leading to more tortuous pathways for the transport of water and solute. Notably, the seepage velocity of organic macromolecular pollutants in clay soil is markedly lower than that in sandy soil. The reduced seepage velocity extends the contact time between the solute and soil particles, facilitating greater adsorption of organic macromolecular pollutants by the soil. Consequently, the migration of organic macromolecular pollutants is significantly impeded.
When compared to homogeneous soils, the dispersivities of organic macromolecular pollutants decrease in layered clay soil, accompanied by a corresponding alteration in dispersion coefficients. However, the magnitude of change in dispersivities is smaller than that of the dispersion coefficients. In soil column C1, the dispersivity of S1 silty sub-clay soil is 0.147 cm, compared to 0.210 cm in the corresponding homogeneous soil column D1. Additionally, the dispersion coefficient of S1 in soil column C1 is 0.0088 cm2/d, whereas in soil column D1, it is 0.025 cm2/d. This is due to the fact that in the laboratory column leaching experiments, the migration distance of solute in layered soil is relatively short and the seepage velocity is slow, resulting in a smaller spatial scale effect of dispersivities and minimal changes in dispersivities.
In the column leching experiments, the migration distance of pollutants in clayed soil is shorter and the average seepage velocity is lower, so the spatial scale of the dispersivity is smaller and the layered structure of the soil has less influence on the dispersivity. Lateral transport of water from different soil interfaces in layered soil can cause large changes in the average seepage velocity of the soil column, leading to a large effect of the layered structure of the soil on the dispersion coefficient. However, the lateral movement of moisture at different soil interfaces within layered structures can induce significant variations in the average seepage velocity of the soil column, thereby leading to pronounced changes in the dispersion coefficient [
52]. Consequently, the primary influence of layered soil on dispersion is the alteration of the average seepage velocity of water and solute transport. It can be demonstrated that as the average flow velocity decreases, the dispersion coefficient also decreases, and thereby enhancing the inhibitory effect of layered soils on pollutants. As the average seepage velocity decreases, the dispersion coefficient diminishes accordingly, thereby enhancing the obstructive influence of layered soil on pollutants.
The clay soils in the study area, ranging from 0.2 m to 12.5 m deep, exhibit dispersion coefficients significantly lower than the sandy soils below, ranging from 13 m to 14 m. This indicates that the thick layers of subsoil clay intercalated with clay in the study area exert a distinct control over the vertical transport of solute. The overlying layered clay soils in the study area effectively impede the downward migration of organic macromolecular pollutants to the underlying aquifer through their inherent low permeability and capacity for pollutant adsorption. Additionally, the lower permeability of clay soils enables soil particles to have longer contact times with organic macromolecular pollutants compared to sandy soils, which allows for more pollutant adsorption. Moreover, the lateral movement of moisture at different soil interfaces in layered soils extends the time for organic pollutants to reach the underlying aquifer. Scientists have shown that organic pollutants in low-permeability clay soils can continue to migrate downwards under the force of gravity into the deeper parts of the soil over time, posing a threat to the soil and even to the underlying aquifers, which has led to low-permeability clay soils sometimes being considered as a long-term “source of contamination” [
53,
54]. From the dispersion parameters of the soil in the study area, it can be seen that although the low-permeability layered clay soil can hinder the downward migration of organic macromolecular pollutants, it can only delay the time of pollutant entry into the deep soil and aquifer, and the organic macromolecular pollutants still migrate slowly downward in the layered clay soil. Therefore, the low-permeability clayey soils in the study area can be used as a short-term barrier to protect the deep soil and groundwater, but on a longer time scale, the organic macromolecular pollutants in the low-permeability layered clay soil will still pose a threat to the deep soil and groundwater.
Organic macromolecular pollutants are diverse and have different properties. For example, phenol is volatile [
55], polycyclic aromatic hydrocarbons (PAHs) are readily adsorbed by soil [
56], and the pesticide CFP produces water-soluble TCPs that cause contamination to spread in water [
57]. Some organic macromolecular pollutants may volatilise, degrade, or interact with the soil as they migrate through the soil. These processes have the potential to hinder the transport of organic macromolecular pollutants in the soil. Fluorescein sodium is stable, hardly degrades when migrating through the layered clay soil in the study area, and does not easily interact with the soil. Therefore, the use of fluorescein sodium to investigate the hindering effect of low-permeability layered clay soil in the study area on organic macromolecular contaminants does not reflect the complex interaction between pollutants and soil. As a result, the dispersion coefficients obtained in this study may be slightly higher than the dispersion coefficients of organic macromolecular pollutants during their actual migration in the layered clay soil of the study area.
4. Conclusions
In this study, fluorescein sodium was used as a representative to investigate the adsorption and transport patterns of organic macromolecular pollutants in layered clay soil in the transition zone of the Jianghan Plain–Dabie Mountain area. The breakthrough duration of fluorescein sodium in clay soil significantly exceeds that in sandy soil and shows a direct correlation with clay content while being inversely related to soil hydraulic conductivity. This duration is directly proportional to the maximum adsorption capacity of the soil and inversely proportional to its hydraulic conductivity. In layered soil columns, the breakthrough duration of fluorescein sodium falls within the range observed in homogeneous soil columns, suggesting that the layered structure influences its penetration. Furthermore, the dispersion coefficient of organic macromolecular pollutants in clay soil is notably lower than in sandy soil and decreases with increasing clay content. Consequently, compared to homogeneous soils, layered clay soil exhibits reduced dispersivity of organic macromolecular pollutants, accompanied by alterations in the dispersion coefficient.
The layered clay soil in the study area effectively hinders the downward migration of organic macromolecular pollutants to the underlying aquifer due to its inherent low permeability and adsorption capacity for pollutants. Additionally, the lateral movement of water at interfaces between different soil layers in layered soil prolongs the time taken for organic macromolecular pollutants to reach the underlying aquifer. However, on longer time scales, organic macromolecular pollutants continue to migrate slowly in low-permeability layered clay soil, posing a threat to deeper soils and groundwater. Therefore, the use of low-permeability layered clay soil as a natural protective barrier for deep soil and groundwater in the transition zone of the Jianghan Plain–Dabie Mountain area is not a long-term solution. For the future development, use, and environmental protection of this region, the emission of pollutants should be reduced, and environmentally friendly and efficient measures should be sought to remediate the contaminated shallow soils.