Study of the Bunsen–Roscoe Reciprocity Law in Solar Water Disinfection (Optical Effect) for E. coli, E. faecalis and C. perfringens
Abstract
1. Introduction
1.1. Reciprocity Law in Microbial Water Disinfection
2. Materials and Methods
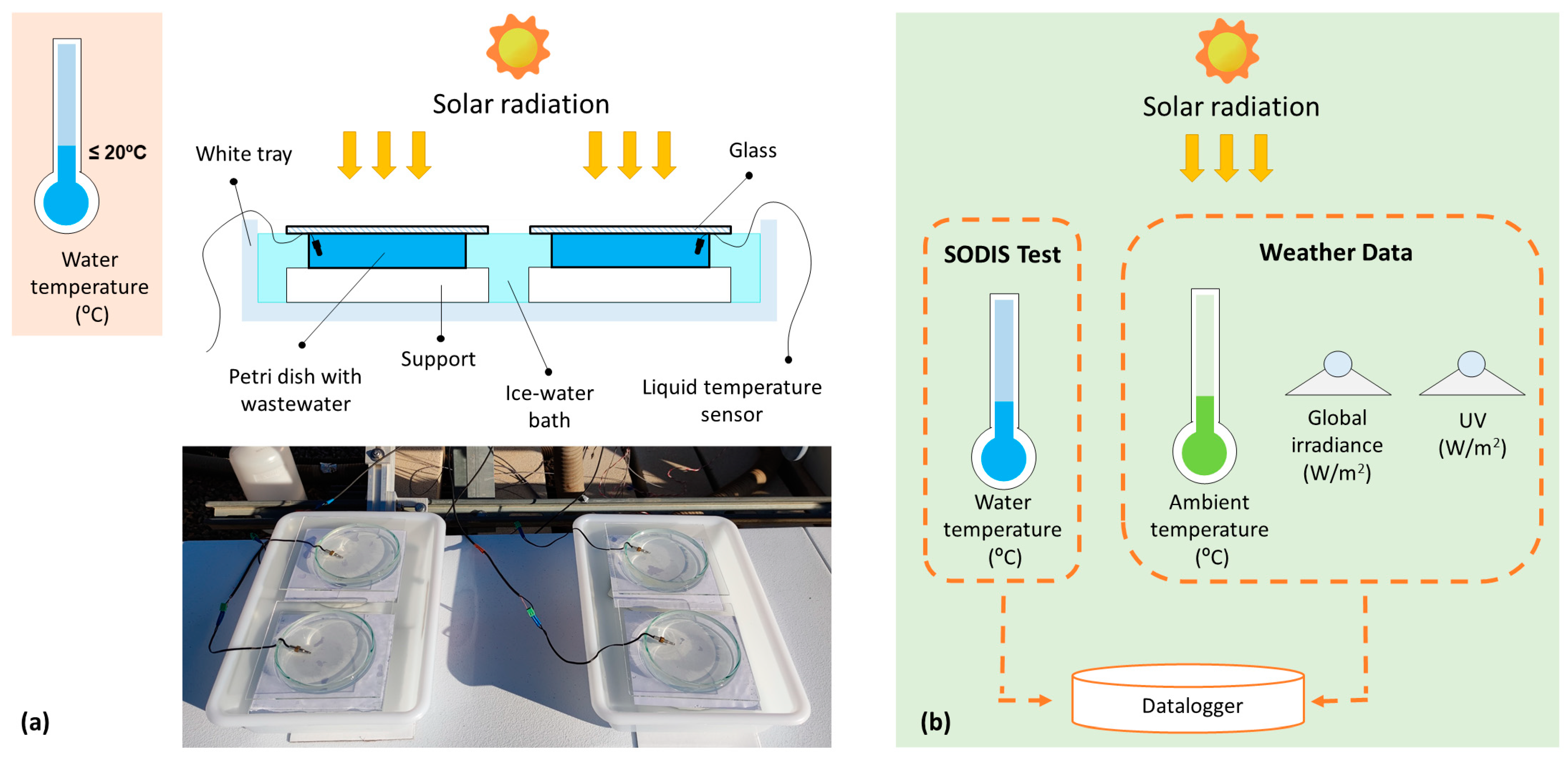
2.1. Experimental Setup
2.2. Control of Climatic Conditions and Electrical Parameters
2.3. Microbiologic Analysis
2.4. Physicochemical Analysis
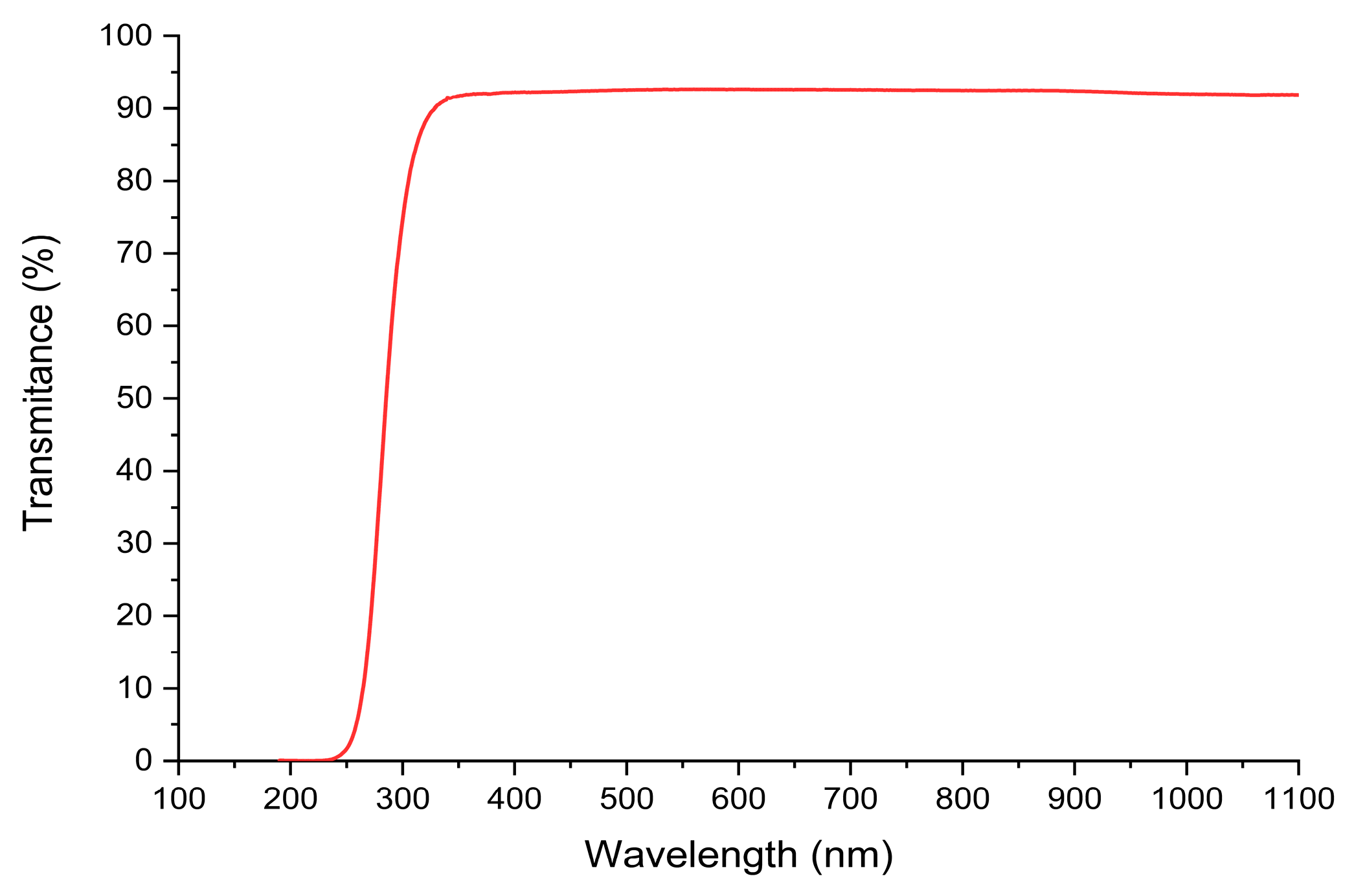
3. Results and Discussion
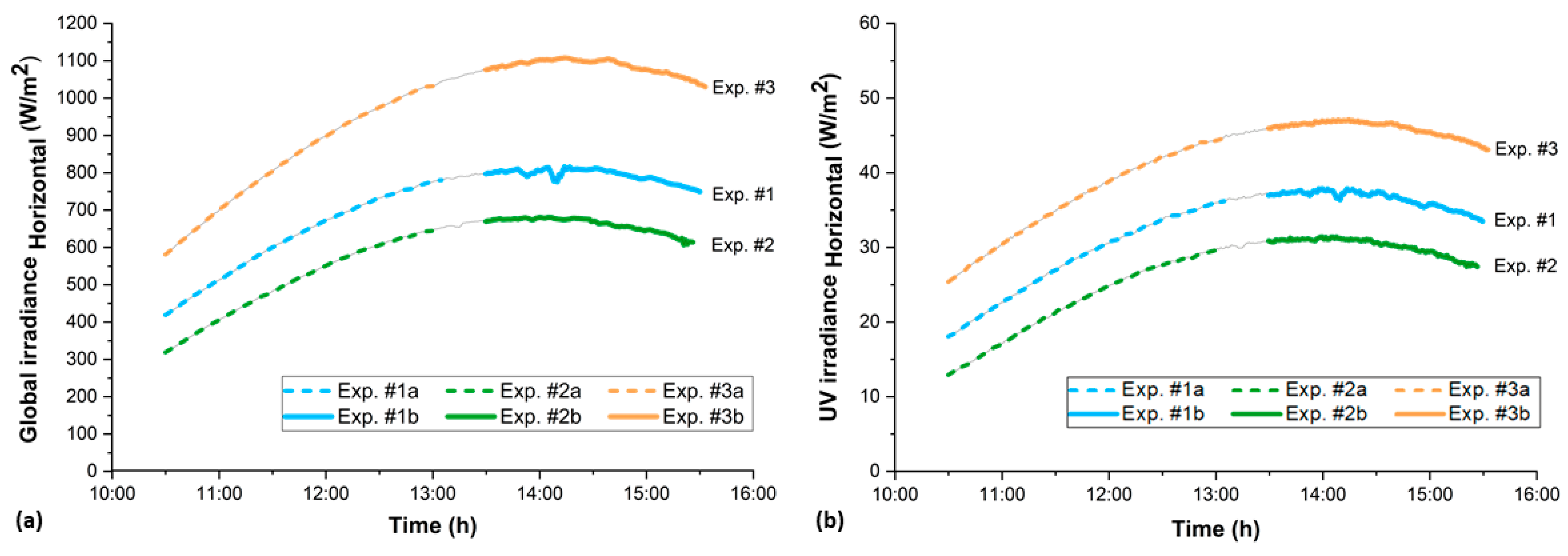
3.1. Weather Conditions
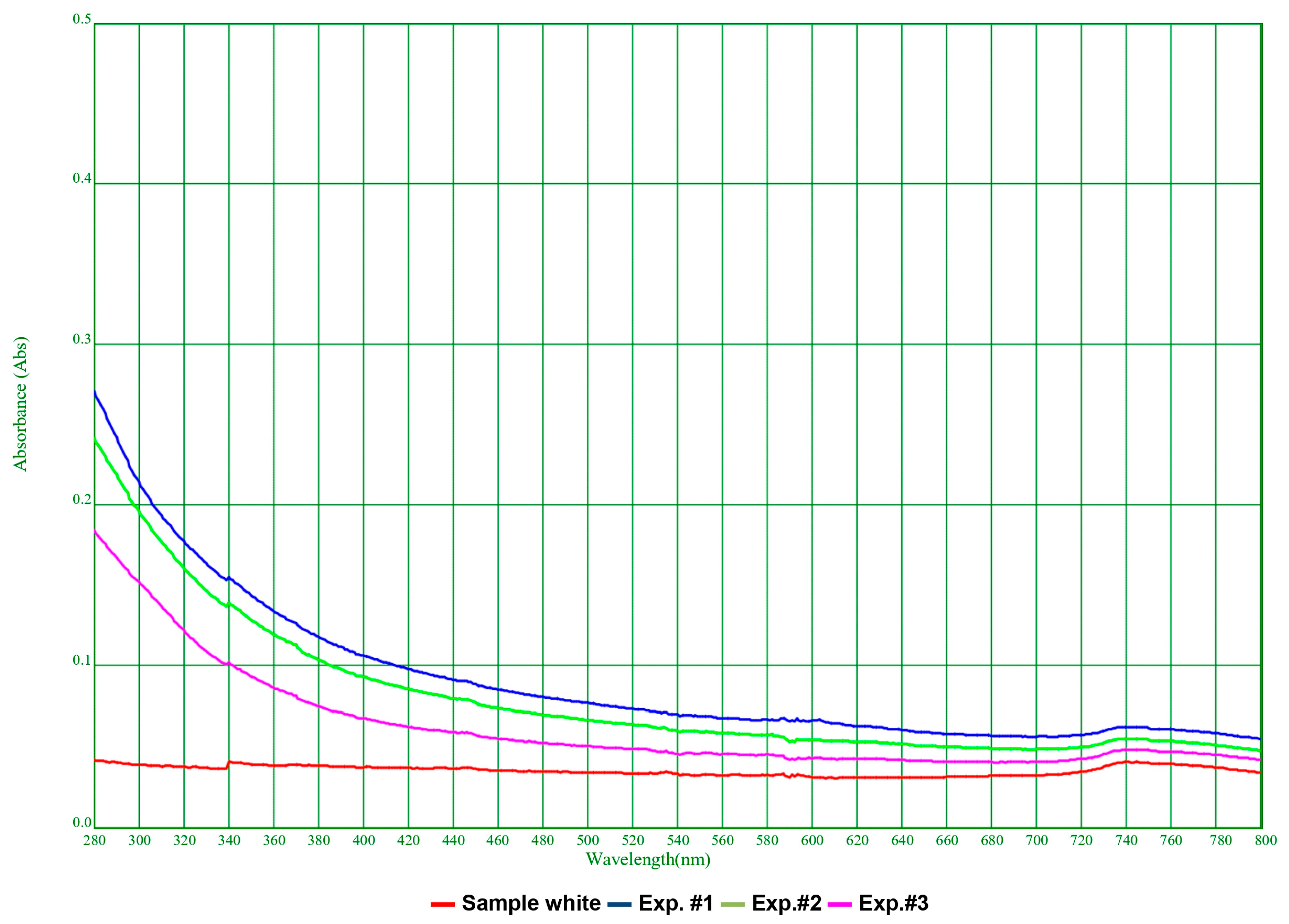
3.2. Physicochemical Tests
3.3. Control of the Thermal Effect in SODIS
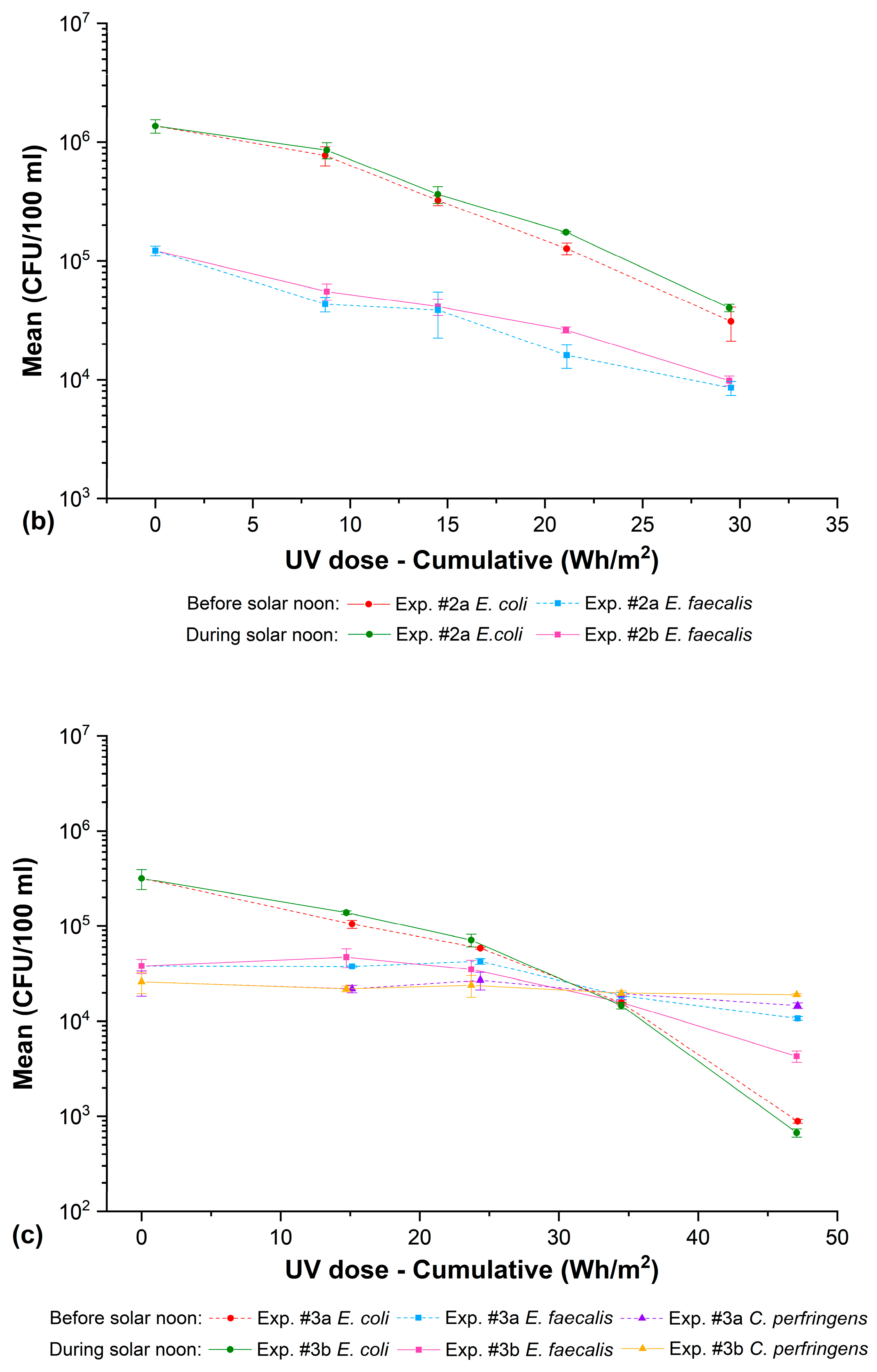
3.4. Solar Disinfection: Exclusively UV (Optical Effect)
- (1)
- The significant influence of temperature on solar disinfection (thermal effect), which is a key factor.
- (2)
- The use of synthetic water samples (with greater control over variables and a more controlled and reproducible study) versus natural samples (exhibiting the complexity and variability of the real environment).
- (3)
- The importance of differences in the intensity and spectrum of UV radiation emitted. For example, UV LEDs generally emit UV radiation in a specific wavelength range, and its intensity can be controlled, whereas solar radiation contains a broader mix of wavelengths, and its intensity varies according to climatic conditions and geographical location.
- (4)
- The fact that bacteria may be affected differently under UV radiation, depending on the wavelength applied and the type of specific species. Some bacterial species may be less sensitive to certain wavelengths and more sensitive to others. This is due to the individual characteristics of each species and the defence and repair mechanisms of the genetic material, which may also be influenced by environmental factors such as the availability of nutrients or the intensity of UV radiation.
4. Summary and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Test | Sample | Time (h) | UV Dose | Mean (CFU/100 mL) | Log10 Reduction (N/N0) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Real | Duration | (Wh/m2) | (KJ/m2) | E. coli | E. faecalis | C. perfringens | E. coli | E. faecalis | C. perfringens | ||
| Exp. #1a | * M0 | 10:30:19 | 0.00 | 0.00 | 0.00 | 610,000 ± 36,100 | - | 37,300 ± 6030 | 0.00 | - | 0.00 |
| M1 | 11:30:19 | 1.00 | 11.49 | 41.36 | 313,000 ± 68,100 | - | 30,700 ± 3210 | 0.29 | - | 0.08 | |
| M2 | 12:00:19 | 1.50 | 18.73 | 67.42 | 159,000 ± 12,000 | - | 30,000 ± 3610 | 0.58 | - | 0.09 | |
| M3 | 12:30:19 | 2.00 | 26.79 | 96.43 | 70,300 ± 13,600 | - | 26,300 ± 6810 | 0.94 | - | 0.15 | |
| M4 | 13:05:19 | 2.58 | 37.02 | 133.26 | 6230 ± 1100 | - | 27,700 ± 8020 | 1.99 | - | 0.13 | |
| Exp. #1b | * M5 | 13:30:19 | 0.00 | 0.00 | 0.00 | 610,000 ± 36,100 | - | 37,300 ± 6030 | 0.00 | - | 0.00 |
| M6 | 14:05:19 | 0.58 | 11.22 | 40.37 | 153,000 ± 8190 | - | 38,000 ± 4000 | 0.60 | - | 0.00 | |
| M7 | 14:29:19 | 0.97 | 18.67 | 67.20 | 88,300 ± 4510 | - | 35,000 ± 10,400 | 0.84 | - | 0.03 | |
| M8 | 14:55:19 | 1.40 | 26.60 | 95.74 | 47,300 ± 10,200 | - | 33,000 ± 2650 | 1.11 | - | 0.05 | |
| M9 | 15:31:19 | 2.02 | 37.06 | 133.40 | 5300 ± 624 | - | 29,300 ± 5690 | 2.06 | - | 0.11 | |
| Exp. #2a | * M10 | 10:30:44 | 0.00 | 0.00 | 0.00 | 1,370,000 ± 181,000 | 122,000 ± 11,400 | - | 0.00 | 0.00 | - |
| M11 | 11:30:44 | 1.00 | 8.72 | 31.39 | 773,000 ± 142,000 | 43,300 ± 6030 | - | 0.25 | 0.45 | - | |
| M12 | 12:00:44 | 1.50 | 14.51 | 52.23 | 323,000 ± 30,600 | 38,700 ± 16,200 | - | 0.63 | 0.50 | - | |
| M13 | 12:30:44 | 2.00 | 21.11 | 76.00 | 127,000 ± 14,400 | 16,100 ± 3610 | - | 1.03 | 0.88 | - | |
| M14 | 13:05:44 | 2.58 | 29.54 | 106.33 | 31,000 ± 9850 | 853 ± 1170 | - | 1.65 | 2.16 | - | |
| Exp. #2b | * M15 | 13:30:44 | 0.00 | 0.00 | 0.00 | 1,370,000 ± 181,000 | 122,000 ± 11,400 | - | 0.00 | 0.00 | - |
| M16 | 14:03:44 | 0.55 | 8.80 | 31.67 | 857,000 ± 134,000 | 55,300 ± 8620 | - | 0.20 | 0.34 | - | |
| M17 | 14:25:44 | 0.92 | 14.50 | 52.21 | 363,000 ± 58,600 | 41,300 ± 6510 | - | 0.58 | 0.47 | - | |
| M18 | 14:51:44 | 1.35 | 21.08 | 75.87 | 174,000 ± 2890 | 26,300 ± 1530 | - | 0.90 | 0.67 | - | |
| M19 | 15:26:44 | 1.93 | 29.45 | 106.01 | 40,300 ± 2890 | 9830 ± 929 | - | 1.53 | 1.09 | - | |
| Exp. #3a | * M20 | 10:30:59 | 0.00 | 0.00 | 0.00 | 317,000 ± 75,700 | 38,000 ± 6560 | 25,700 ± 6430 | 0.00 | 0.00 | 0.00 |
| M21 | 11:30:59 | 1.00 | 15.14 | 55.53 | 105,000 ± 10,400 | 37,700 ± 577 | 22,000 ± 2000 | 0.48 | 0.00 | 0.07 | |
| M22 | 12:00:59 | 1.50 | 24.36 | 88.86 | 58,300 ± 1530 | 42,700 ± 2890 | 27,000 ± 5570 | 0.74 | 0.00 | 0.00 | |
| M23 | 12:30:59 | 2.00 | 34.48 | 125.40 | 15,700 ± 231 | 18,600 ± 1620 | 19,600 ± 1430 | 1.31 | 0.31 | 0.12 | |
| M24 | 13:05:59 | 2.58 | 47.15 | 171.10 | 887 ± 40.4 | 10,700 ± 473 | 14,700 ± 1000 | 2.55 | 0.55 | 0.24 | |
| Exp. #3b | * M25 | 13:30:59 | 0.00 | 0.00 | 0.00 | 317,000 ± 75,700 | 38,000 ± 6560 | 25,700 ± 6430 | 0.00 | 0.00 | 0.00 |
| M26 | 14:08:59 | 0.63 | 14.73 | 54.43 | 138,000 ± 6110 | 47,300 ± 10,600 | 22,300 ± 1530 | 0.36 | 0.00 | 0.06 | |
| M27 | 14:31:59 | 1.02 | 23.70 | 86.72 | 71,000 ± 10,800 | 35,300 ± 8740 | 22,400 ± 6080 | 0.65 | 0.03 | 0.06 | |
| M28 | 14:59:59 | 1.48 | 34.47 | 125.46 | 14,700 ± 1190 | 15,300 ± 3210 | 19,900 ± 1140 | 1.33 | 0.40 | 0.11 | |
| M29 | 15:33:59 | 2.05 | 47.08 | 170.78 | 665 ± 66.6 | 4300 ± 600 | 19,100 ± 493 | 2.68 | 0.95 | 0.13 | |
References
- UN Department of Economic and Social Affairs; World Bank. Making Every Drop Count. An Agenda for Water Action: High-Level Panel on Water Outcome Document. 2018. Available online: https://reliefweb.int/report/world/making-every-drop-count-agenda-water-action-high-level-panel-water-outcome-document-14 (accessed on 9 December 2023).
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B.A. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Alfaro, S.; Rueda-Márquez, J.J.; Perales, J.A.; Manzano, M.A. Combining sun-based technologies (microalgae and solar disinfection) for urban wastewater regeneration. Sci. Total Environ. 2018, 619–620, 1049–1057. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The problem of drinking water access: A review of disinfection technologies with an emphasis on solar treatment methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Martínez-garcía, A.; Vincent, M.; Rubiolo, V.; Domingos, M. Assessment of a pilot solar V-trough reactor for solar water disinfection. Chem. Eng. J. 2020, 399, 125719. [Google Scholar] [CrossRef]
- Meierhofer, R.; Wegelin, M. Solar Disinfection of Water: A Guide for the Application of SODIS; EAWAG/SANDEC: Duebendorf, Switzerland, 2002. [Google Scholar]
- Wegelin, M.; Canoninca, S.; Mechsner, K.; Fleischmann, T.; Pesaro, F.; Mtsler, A. Solar water disinfection: Scope of the process and analysis of radiation experiments. J. Water SRT-Aqua 1994, 43, 154–169. [Google Scholar]
- Joyce, T.M.; McGuigan, K.G.; Elmore-Meegan, M.; Conroy, R.M. Inactivation of fecal bacteria in drinking water by solar heating. Appl. Environ. Microbiol. 1996, 62, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Mcguigan, K.G.; Joyce, T.M.; Conroy, R.M.; Gillespie, J.B. Solar disinfection of drinking water contained in transparent plastic bottles: Characterizing the bacterial inactivation process. J. Appl. Microbiol. 1998, 84, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. The antagonistic and synergistic effects of temperature during solar disinfection of synthetic secondary effluent. J. Photochem. Photobiol. A Chem. 2014, 280, 14–26. [Google Scholar] [CrossRef]
- Vivar, M.; Pichel, N.; Fuentes, M.; López-Vargas, A. Separating the UV and thermal components during real-time solar disinfection experiments: The effect of temperature. Sol. Energy 2017, 146, 334–341. [Google Scholar] [CrossRef]
- Bunsen, R.W.; Roscoe, H.E. Photochemische Untersuchungen. Ann. Phys. 1863, 193, 529–562. [Google Scholar] [CrossRef]
- Martin, J.W.; Chin, J.W.; Nguyen, T. Reciprocity law experiments in polymeric photodegradation: A critical review. Prog. Org. Coat. 2003, 47, 292–311. [Google Scholar] [CrossRef]
- Pousty, D.; Hofmann, R.; Gerchman, Y.; Mamane, H. Wavelength-dependent time–dose reciprocity and stress mechanism for UV-LED disinfection of Escherichia coli. J. Photochem. Photobiol. B Biol. 2021, 217, 112129. [Google Scholar] [CrossRef]
- Kamel, A.; Palacios, A.; Fuentes, M.; Vivar, M. Analysing the Reciprocity Law for UV-LEDs in Water. Water 2023, 15, 352. [Google Scholar] [CrossRef]
- Rinc, A.; Pulgarin, C. Field solar E. coli inactivation in the absence and presence of TiO2: Is UV solar dose an appropriate parameter for standardization of water solar disinfection? Sol. Energy 2004, 77, 635–648. [Google Scholar] [CrossRef]
- Giannakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pulgarin, C. Temperature-dependent change of light dose effects on E. coli inactivation during simulated solar treatment of secondary effluent. Chem. Eng. Sci. 2015, 126, 483–487. [Google Scholar] [CrossRef]
- Berney, M.; Weilenmann, H.; Simonetti, A.; Egli, T. Efficacy of solar disinfection of Escherichia coli, Shigella flexneri, Salmonella Typhimurium and Vibrio cholerae. J. Appl. Microbiol. 2006, 101, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Bosshard, F.; Berney, M.; Scheifele, M.; Weilenmann, H.; Egli, T. Solar disinfection (SODIS) and subsequent dark storage of Salmonella typhimurium and Shigella flexneri monitored by flow cytometry. Microbiology 2009, 155, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Estación Depuradora de Aguas Residuales (EDAR) de Linares. Proceso de Depuración. Available online: https://www.linaqua.es/ciclo-del-agua/depuracion (accessed on 2 January 2024).
- UNE-EN ISO 8199:2018; Water Quality—General Requirements and Guidance for Microbiological Examinations by Culture. International Standard: Geneva, Switzerland, 2018.
- UNE-EN ISO 9308-1:2014; Water Quality—Enumeration of Escherichia coli and Coliform bacteria. Part 1: Membrane Filtration Method for Waters with Low Bacterial Background Flora. International Standard: Geneva, Switzerland, 2014.
- UNE-EN ISO 7899-2:2000; Water quality—Detection and Enumeration of Intestinal Enterococci. Part 2: Membrane Filtration Method. International Standard: Geneva, Switzerland, 2000.
- UNE-EN ISO 14189:2013; Water Quality—Enumeration of Clostridium Perfringens—Method Using Membrane Filtration. International Standard: Geneva, Switzerland, 2013.
- Albrecht, J.A. Clostridium Perfringens; University of Nebraska-Food Saftey: Lincoln, NE, USA, 2005. [Google Scholar]
- Bad Bug Book, Handbook of Foodborne Pathogenic Microorganisms and Natural Toxins; Food and Drug Administration (FDA), U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2012.
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stakl, D.A.; Brock, T. Brock Biology of Microorganisms, 14th ed.; Pearson: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Sichel, C.; Tello, J.; de Cara, M.; Fernández-Ibáñez, P. Effect of UV solar intensity and dose on the photocatalytic disinfection of bacteria and fungi. Catalysis Today 2007, 129, 152–160. [Google Scholar] [CrossRef]
- Ubomba-Jaswa, E.; Navntoft, C.; Inmaculada, M.; Fernandez-Ibáñez; McGuigan, K.G. Solar disinfection of drinking water (SODIS): An investigation of the effect of UV-A dose on inactivation efficiency. Photochem. Photobiol. Sci. 2009, 8, 587–595. Available online: https://link.springer.com/article/10.1039/b816593a (accessed on 13 December 2023). [CrossRef] [PubMed]
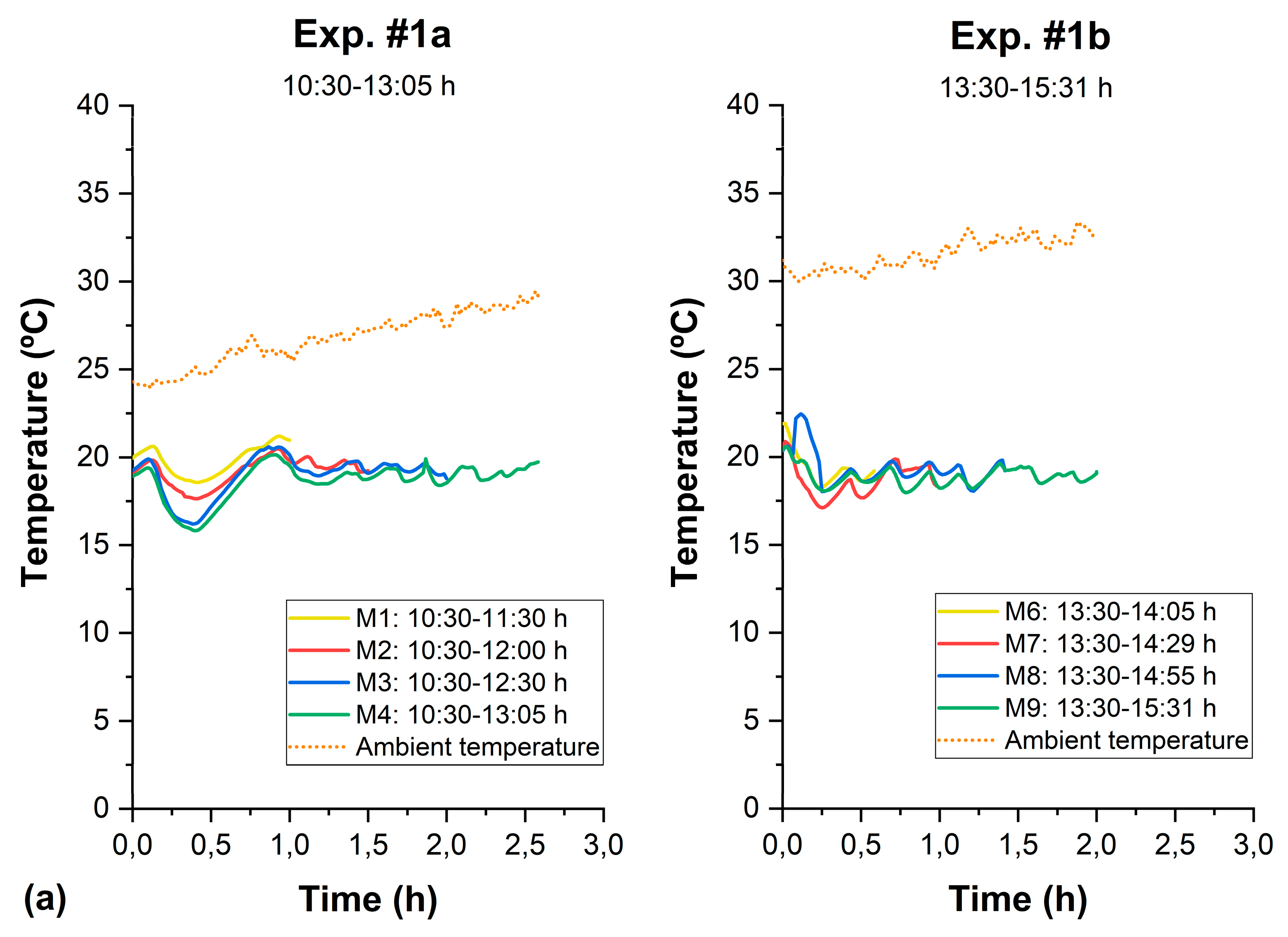
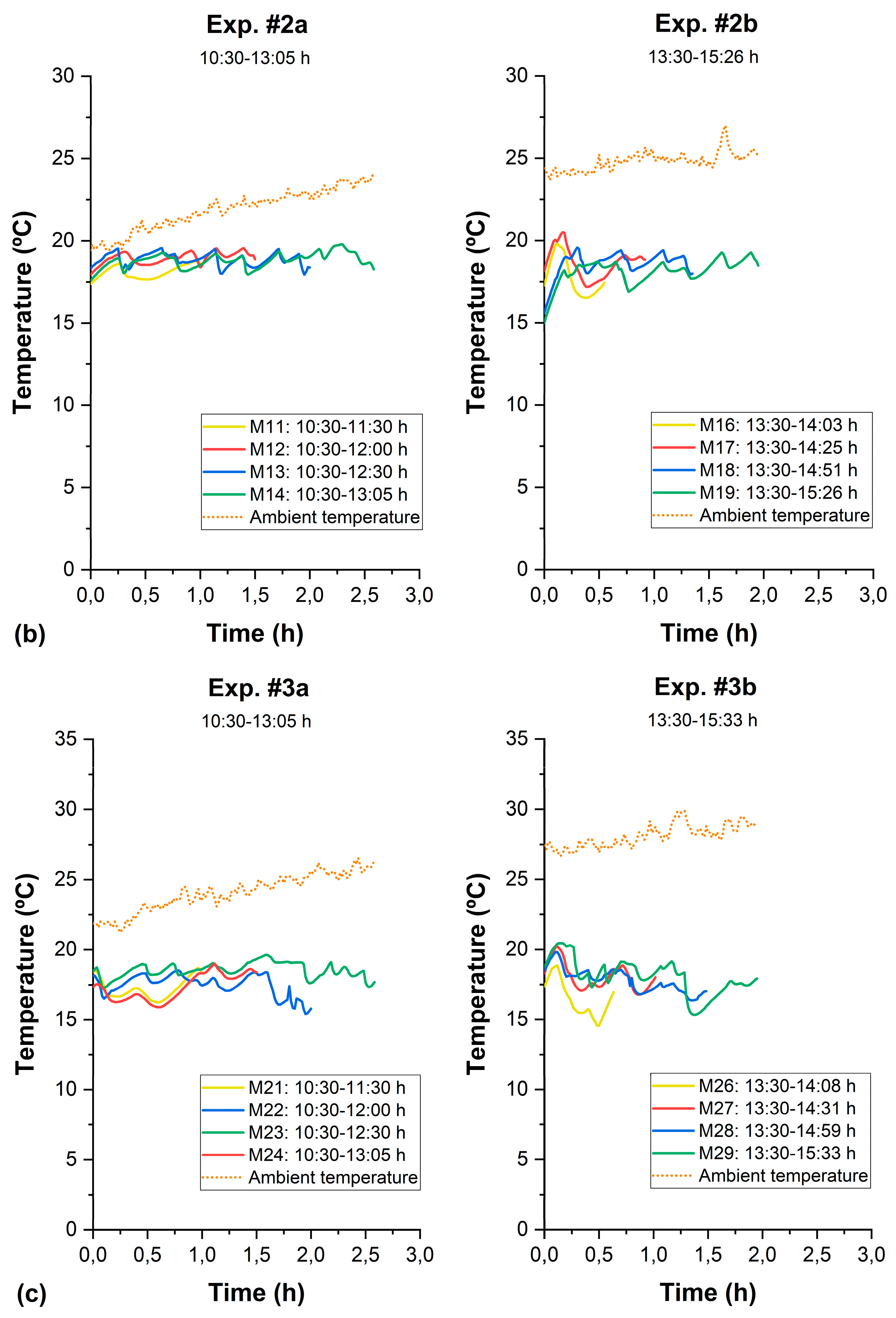

| Test | Sample | Time (h) | Girradiance (W/m2) (280–3000 nm) | UVirradiance (W/m2) (280–400 nm) | UV Dose | Log10 Reduction (N/N0) | pH | Turbidity (NTU) | Conductivity (μS/cm) | COD (mg/L) | BOD5 (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Real | Exp. | (Wh/m2) | (KJ/m2) | E. coli | E. faecalis | C. perfringens | |||||||||
| Exp. #1a | * M0 | 10:30:19 | 0.00 | 419.35 | 18.07 | 0.00 | 0.00 | 0.00 | - | 0.00 | 7.77 | 8.70 | 955 | 44 | 19 |
| M1 | 11:30:19 | 1.00 | 600.52 | 27.00 | 11.49 | 41.36 | 0.29 | - | 0.08 | 7.87 | 8.20 | 957 | - | - | |
| M2 | 12:00:19 | 1.50 | 672.76 | 30.58 | 18.73 | 67.42 | 0.58 | - | 0.09 | 7.95 | 7.61 | 958 | - | - | |
| M3 | 12:30:19 | 2.00 | 732.60 | 33.77 | 26.79 | 96.43 | 0.94 | - | 0.15 | 7.73 | 9.34 | 961 | - | - | |
| M4 | 13:05:19 | 2.58 | 780.63 | 36.19 | 37.02 | 133.26 | 1.99 | - | 0.13 | 7.92 | 8.90 | 955 | - | - | |
| Exp. #1b | * M5 | 13:30:19 | 0.00 | 797.17 | 36.98 | 0.00 | 0.00 | 0.00 | - | 0.00 | 7.77 | 8.70 | 955 | 44 | 19 |
| M6 | 14:05:19 | 0.58 | 813.92 | 37.63 | 11.22 | 40.37 | 0.60 | - | 0.00 | 8.19 | 7.82 | 555 | - | - | |
| M7 | 14:29:19 | 0.97 | 809.40 | 37.21 | 18.67 | 67.20 | 0.84 | - | 0.03 | 8.81 | 9.98 | 942 | - | - | |
| M8 | 14:55:19 | 1.40 | 785.57 | 35.52 | 26.60 | 95.74 | 1.11 | - | 0.05 | 8.26 | 9.58 | 949 | - | - | |
| M9 | 15:31:19 | 2.02 | 747.74 | 33.41 | 37.06 | 133.40 | 2.06 | - | 0.11 | 8.24 | 8.85 | 835 | - | - | |
| Exp. #2a | * M10 | 10:30:44 | 0.00 | 319.16 | 12.94 | 0.00 | 0.00 | 0.00 | 0.00 | - | 7.70 | 8.61 | 951 | 54 | 23 |
| M11 | 11:30:44 | 1.00 | 482.48 | 21.24 | 8.72 | 31.39 | 0.25 | 0.45 | - | 7.91 | 8.09 | 945 | - | - | |
| M12 | 12:00:44 | 1.50 | 551.28 | 24.84 | 14.51 | 52.23 | 0.63 | 0.50 | - | 7.84 | 8.30 | 955 | - | - | |
| M13 | 12:30:44 | 2.00 | 606.58 | 27.58 | 21.11 | 76.00 | 1.03 | 0.88 | - | 7.74 | 8.64 | 942 | - | - | |
| M14 | 13:05:44 | 2.58 | 653.13 | 29.87 | 29.54 | 106.33 | 1.65 | 2.16 | - | 7.74 | 8.75 | 947 | - | - | |
| Exp. #2b | * M15 | 13:30:44 | 0.00 | 669.83 | 30.88 | 0.00 | 0.00 | 0.00 | 0.00 | - | 7.70 | 8.61 | 951 | 54 | 23 |
| M16 | 14:03:44 | 0.55 | 678.54 | 31.07 | 8.80 | 31.67 | 0.20 | 0.34 | - | 7.93 | 8.20 | 928 | - | - | |
| M17 | 14:25:44 | 0.92 | 676.42 | 31.13 | 14.50 | 52.21 | 0.58 | 0.47 | - | 7.94 | 8.38 | 566 | - | - | |
| M18 | 14:51:44 | 1.35 | 654.73 | 29.82 | 21.08 | 75.87 | 0.90 | 0.67 | - | 7.99 | 8.52 | 942 | - | - | |
| M19 | 15:26:44 | 1.93 | 614.52 | 27.69 | 29.45 | 106.01 | 1.53 | 1.09 | - | 8.01 | 8.47 | 907 | - | - | |
| Exp. #3a | * M20 | 10:30:59 | 0.00 | 569.78 | 25.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.64 | 4.77 | 921 | 50 | 22.5 |
| M21 | 11:30:59 | 1.00 | 754.35 | 34.87 | 15.14 | 55.53 | 0.48 | 0.00 | 0.07 | 7.92 | 3.98 | 919 | - | - | |
| M22 | 12:00:59 | 1.50 | 828.41 | 39.00 | 24.36 | 88.86 | 0.74 | 0.00 | 0.00 | 7.95 | 3.79 | 916 | - | - | |
| M23 | 12:30:59 | 2.00 | 893.16 | 42.09 | 34.48 | 125.40 | 1.31 | 0.31 | 0.12 | 7.96 | 4.28 | 918 | - | - | |
| M24 | 13:05:59 | 2.58 | 950.09 | 44.75 | 47.15 | 171.10 | 2.55 | 0.55 | 0.24 | 8.09 | 4.88 | 911 | - | - | |
| Exp. #3b | * M25 | 13:30:59 | 0.00 | 973.07 | 45.96 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 7.64 | 4.77 | 921 | 50 | 22.5 |
| M26 | 14:08:59 | 0.63 | 996.81 | 46.88 | 14.73 | 54.43 | 0.36 | 0.00 | 0.06 | 7.90 | 5.23 | 918 | - | - | |
| M27 | 14:31:59 | 1.02 | 991.40 | 46.47 | 23.70 | 86.72 | 0.65 | 0.03 | 0.06 | 7.91 | 4.37 | 917 | - | - | |
| M28 | 14:59:59 | 1.48 | 975.28 | 45.44 | 34.47 | 125.46 | 1.33 | 0.40 | 0.11 | 7.90 | 4.34 | 910 | - | - | |
| M29 | 15:33:59 | 2.05 | 935.55 | 43.10 | 47.08 | 170.78 | 2.68 | 0.95 | 0.13 | 7.98 | 4.60 | 1026 | - | - | |
| Bacteria | Experiment | Turbidity (NTU) Average | kultraviolet | R2 |
|---|---|---|---|---|
| E. coli | #1a | 8.55 | 0.044 ± 0.005 | 0.997 |
| #1b | 8.99 | 0.050 ± 0.003 | 0.999 | |
| C. perfringens | #1a | 8.55 | 0.027 ± 0.002 | 1 |
| #1b | 8.99 | 0.039 ± 5.662 × 10−4 | 1 | |
| E. coli | #2a | 8.48 | 0.051 ± 0.003 | 0.999 |
| #2b | 8.44 | 0.046 ± 0.004 | 0.999 | |
| E. faecalis | #2a | 8.48 | 0.040 ± 0.002 | 1 |
| #2b | 8.44 | 0.035 ± 0.001 | 1 | |
| E. coli | #3a | 4.34 | 0.045 ± 0.005 | 0.995 |
| #3b | 4.66 | 0.046 ± 0.006 | 0.993 | |
| E. faecalis | #3a | 4.34 | 0.008 ± 0.002 | 0.999 |
| #3b | 4.66 | 0.014 ± 0.004 | 0.996 | |
| C. perfringens | #3a | 4.34 | 0.004 ± 9.949 × 10−4 | 1 |
| #3b | 4.66 | 0.003 ± 3.744 × 10−4 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, J.; Palacios, A.M.; Fuentes, M.; Vivar, M. Study of the Bunsen–Roscoe Reciprocity Law in Solar Water Disinfection (Optical Effect) for E. coli, E. faecalis and C. perfringens. Water 2024, 16, 1406. https://doi.org/10.3390/w16101406
Torres J, Palacios AM, Fuentes M, Vivar M. Study of the Bunsen–Roscoe Reciprocity Law in Solar Water Disinfection (Optical Effect) for E. coli, E. faecalis and C. perfringens. Water. 2024; 16(10):1406. https://doi.org/10.3390/w16101406
Chicago/Turabian StyleTorres, Julia, Ana María Palacios, Manuel Fuentes, and Marta Vivar. 2024. "Study of the Bunsen–Roscoe Reciprocity Law in Solar Water Disinfection (Optical Effect) for E. coli, E. faecalis and C. perfringens" Water 16, no. 10: 1406. https://doi.org/10.3390/w16101406
APA StyleTorres, J., Palacios, A. M., Fuentes, M., & Vivar, M. (2024). Study of the Bunsen–Roscoe Reciprocity Law in Solar Water Disinfection (Optical Effect) for E. coli, E. faecalis and C. perfringens. Water, 16(10), 1406. https://doi.org/10.3390/w16101406







