Adsorption Performance of Different Wetland Substrates for Ammonia Nitrogen: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Determination of Physical and Chemical Properties of Substrates
2.3. Adsorption Isothermal Test of Substrates
2.3.1. Substrate Dosage Test
2.3.2. Substrate Adsorption Time Test
2.3.3. Influence Test of Initial Concentration of Ammonia Nitrogen
3. Results
3.1. Physical and Chemical Properties of Substrates
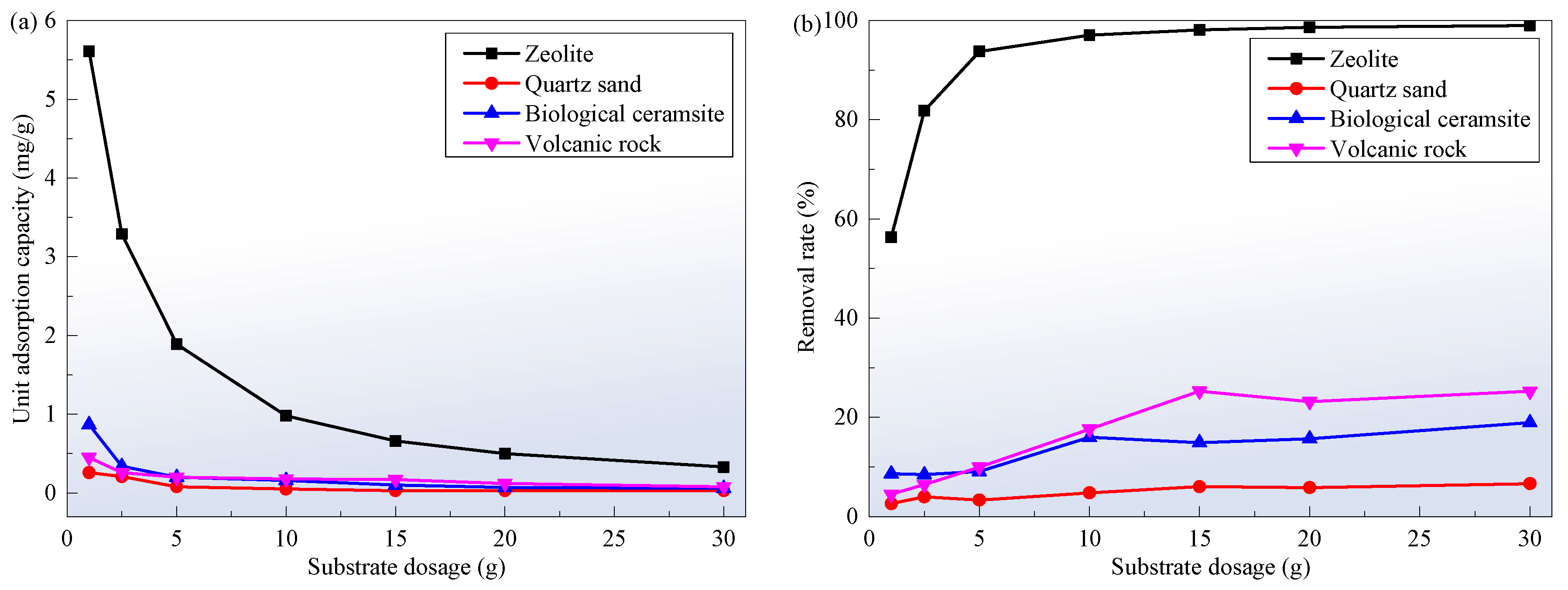
3.2. Substrate Dosage
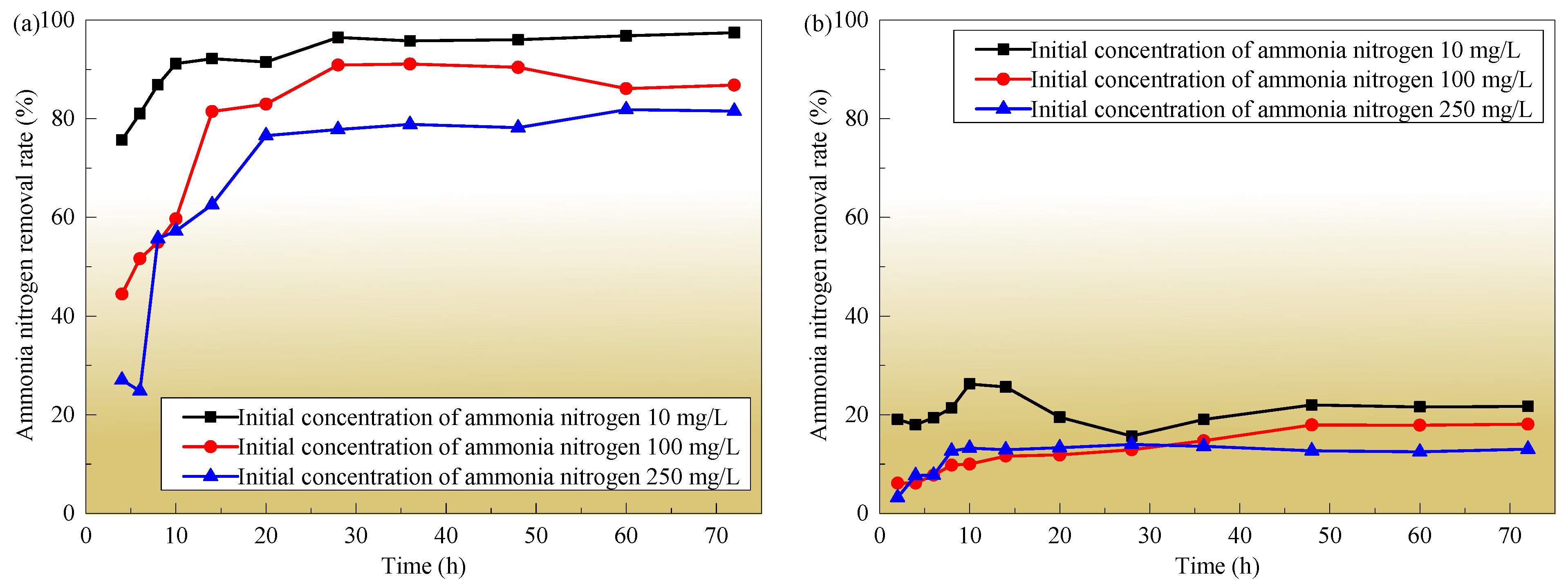
3.3. Substrate Adsorption Time
3.4. Initial Concentration of Ammonia Nitrogen
4. Discussion
4.1. Adsorption Equilibrium
4.2. Kinetic Analysis
5. Conclusions
- (1)
- The microstructure of the substrate determines the physical and chemical properties of the substrate, such as the cation exchange capacity and specific surface area. The more lamellar structures on the surface of the substrate, the better its physical and chemical properties and adsorption performance. Hence, in the future, the substrate can be modified by chemical methods to increase the lamellar structure on the surface of the substrate or select materials with many lamellar structures as the wetland substrate, which can improve the adsorption performance of the wetland substrate for ammonia nitrogen.
- (2)
- With the increase in the dosage of the four substrates, their adsorption capacity for ammonia nitrogen gradually decreases and the removal rate gradually increases. The adsorption trends for ammonia nitrogen by zeolite, quartz sand, biological ceramsite, and volcanic rock are similar in the three concentrations of the solution, that is, the removal rates all show a rapid increase in the early stage (the first 14 h) and gradually reach a stable state in the later stage (after more than 48 h). When the dosage of the substrate is constant, the higher the concentration of ammonia nitrogen in the solution, the longer it takes for the concentration of ammonia nitrogen in the substrate and solution to reach equilibrium. The higher the concentration of ammonia nitrogen in the solution, the greater the unit adsorption capacity of ammonia nitrogen by the substrate, the longer the adsorption time required to reach adsorption equilibrium, the lower the total removal rate of ammonia nitrogen, and the longer it takes for the concentration of ammonia nitrogen in the substrate and solution to reach equilibrium. The adsorption capacity of zeolite to ammonia nitrogen is much higher than that of quartz sand, biological ceramsite, and volcanic rock, so zeolite is suitable as the wetland substrate.
- (3)
- The adsorption data of zeolite, biological ceramsite, and volcanic rock can be well fitted by the Langmuir and Freundlich models, while the Freundlich model is more accurate than the Langmuir model in describing the isothermal characteristics of ammonia nitrogen adsorption by zeolite, quartz sand, biological ceramsite, and volcanic rock, indicating that the adsorption of ammonia nitrogen by the four substrates is principally multi-molecular layer adsorption, and the adsorption phase is heterogeneous.
- (4)
- The kinetic analyses of ammonia nitrogen adsorption by substrates are carried out by using the intra-particle diffusion model, pseudo-first-order kinetic model, and pseudo-second-order kinetic model. The adsorption of ammonia nitrogen by zeolite primarily depends on chemical adsorption, which is consistent with the high cation exchange capacity of the zeolite surface. The adsorption of ammonia nitrogen by quartz sand is the combined action of intra-particle diffusion and physical and chemical adsorption. The adsorption of ammonia nitrogen by biological ceramsite and volcanic rock is principally intra-particle diffusion, with both chemical adsorption and physical adsorption.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, D.Z.; Li, J.F.; Cao, Z.G.; Wu, B.Y.; Jiang, B.B.; Yang, Y.; Yang, J.; Chen, Y.P. Technology and Engineering Development Strategy of Water Protection and Utilization of Coal Mine in China. J. China Coal Soc. 2021, 46, 3079–3089. [Google Scholar]
- Li, Q.; Zhang, J.S.; Liang, R.H.; Wang, S.L.; Gao, J.Q.; Song, B.Z.; Huang, Z.Z.; Chu, J.H. Novel Ceramsite Substrate of Constructed Wetland: Preparation and Study on Phosphorus Removal. Environ. Sci. Technol. 2021, 44, 13–22. [Google Scholar]
- Yu, C.X.; Li, Z.Y.; Xu, Z.H.; Yang, Z.F. Lake Recovery from Eutrophication: Quantitative Response of Trophic States to Anthropogenic Influences. Ecol. Eng. 2020, 143, 105697. [Google Scholar] [CrossRef]
- Xu, D.F.; Cao, J.M.; Li, Y.X.; Howard, A.; Yu, K.W. Effect of Pyrolysis Temperature on Characteristics of Biochars Derived from Different Feedstocks: A Case Study on Ammonium Adsorption Capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.N.; Tian, W.J.; Liu, Y. Influence of Pyrolysis Temperature on Production of Digested Sludge Biochar and Its Application for Ammonium Removal from Municipal Wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Wang, Y.N.; Guo, X.C.; Lu, S.Y.; Liu, X.H.; Wang, X.H. Review of Nitrogen Removal in Low-Polluted Water by Constructed Wetlands: Performance, Mechanism, and Influencing Factors. J. Agric. Resour. Environ. 2021, 38, 722–734. [Google Scholar]
- Wang, X.; Zhu, Z.J.; Yin, M.M.; Zhang, Y.Q.; Zhang, Z.X. Application of Hybrid Constructed Wetland in Tail Water Treatment of Municipal Sewage Plant. China Water Wastewater 2020, 36, 97–101. [Google Scholar]
- Chen, X.T.; Hao, Q.J.; Xiong, Y.F.; Hu, J.; Jiang, C.S. Effects of Hematite and Biochar Addition on Wastewater Treatment Efficiency, Greenhouse Gas Emission, and Microbial Community in Subsurface Flow Constructed Wetland. Environ. Sci. 2022, 43, 1492–1499. [Google Scholar]
- Fu, G.P.; Wu, J.F.; Han, J.Y.; Zhao, L.; Chan, G.; Leong, K.F. Effects of Substrate Type on Denitrification Efficiency and Microbial Community Structure in Constructed Wetlands. Bioresour. Technol. 2020, 307, 123222. [Google Scholar] [CrossRef]
- Xu, G.M.; Li, Y.; Hou, W.H.; Wang, S.; Kong, F.L. Effects of Substrate Type on Enhancing Pollutant Removal Performance and Reducing Greenhouse Gas Emission in Vertical Subsurface Flow Constructed Wetland. J. Environ. Manag. 2021, 280, 111674. [Google Scholar] [CrossRef]
- Zhou, X.; Liang, C.L.; Jia, L.X.; Feng, L.K.; Wang, R.G.; Wu, H.M. An Innovative Biochar-Amended Substrate Vertical Flow Constructed Wetland for Low C/N Wastewater Treatment: Impact of Influent Strengths. Bioresour. Technol. 2018, 247, 844–850. [Google Scholar] [CrossRef]
- Deng, S.J.; Chen, J.Q.; Chang, J.J. Application of Biochar as an Innovative Substrate in Constructed Wetlands/Biofilters for Wastewater Treatment: Performance and Ecological Benefits. J. Clean. Prod. 2021, 293, 126156. [Google Scholar] [CrossRef]
- Chen, H.Y.; Duan, Y.L. Contrast of Adsorption Property and Influencing Factors of Different Biochar for Ammonia Nitrogen Removal in Water. Water Purif. Technol. 2022, 41, 71–78. [Google Scholar]
- Xiao, Q.L.; Xiao, L.P.; Peng, H.; Zhu, J.; Jian, Y.; Jia, J.L.; Ji, X.H. Nitrogen and Phosphorus Adsorption Capacity of Different Substrate Combinations. Res. Environ. Sci. 2022, 35, 1277–1287. [Google Scholar]
- Zhao, D.Y.; Zhang, S.; Zhao, S.N.; Zhao, Y.J.; Tian, W.D.; Liu, J.J. Screening of Ammonia Nitrogen Removal Schemes from Construct Wetland Matrix and Dynamic Analysis of Influencing Factors. J. Water Resour. Water Eng. 2018, 29, 121–126. [Google Scholar]
- Hu, S.M. Research on Modified Zeolite Applied in Constructed Wetland to Treat Rural Domestic Sewage; Shenyang Jianzhu University: Shenyang, China, 2023. [Google Scholar]
- Lou, H.J.; Liu, W.; Hu, Y.J.; Zhang, G.N.; Wu, H.J.; Zhao, Y.J. Determination of Ammonia Nitrogen in Water by Salicylic Acid Spectrophotometry. Chem. Anal. Meterage 2020, 29, 25–29. [Google Scholar]
- Hayashi, K.; Nishimura, S.; Yagi, K. Ammonia Volatilization from a Paddy Field Following Applications of Urea: Rice Plants Are Both an Absorber and an Emitter for Atmospheric Ammonia. Sci. Total Environ. 2008, 390, 485–494. [Google Scholar] [CrossRef]
- Shan, W.P.; Song, H. Catalysts for the Selective Catalytic Reduction of NOx with NH3 at Low Temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Lu, S.Y.; Jin, X.C.; Yu, G. Nitrogen Removal Mechanism of Constructed Wetland. Acta Ecol. Sin. 2006, 26, 2670–2677. [Google Scholar]
- Zhang, J.; Zhou, Q.; He, R. Mechanism of Nitrogen and Phosphorus Removal in Free-Water Surface Constructed Wetland. Ecol. Environ. 2004, 13, 98–101. [Google Scholar]
- Vymazal, J.; Brix, H.; Cooper, P.; Haberl, R.; Perfler, R.; Laber, J. Removal Mechanisms and Types of Constructed Wetlands; Backhuys Publishers: Leiden, The Netherlands, 1998; pp. 17–66. [Google Scholar]
- Zhang, H.Y.; Huang, Z.H.; Wang, J.; Zhang, J.; Meng, C.R.; Wei, C.Z. Effects of Different Acidifiers on PH and Phosphorus Availability in Calcareous Soil. Soil Fertil. Sci. China 2019, 1, 145–150. [Google Scholar]
- Suo, G.F.; Lv, H.H.; Wang, Y.Y.; Liu, Y.X.; He, L.L.; Yang, S.M. Study on the Adsorption Properties of Nitrogen by Different Biochars. J. Agro-Environ. Sci. 2018, 37, 1193–1202. [Google Scholar]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a Few Heavy Metals on Natural and Modified Kaolinite and Montmorillonite: A Review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Wang, Z.; Li, W.P.; Yu, L.H.; Yang, W.H.; Lv, W.X.; Wang, G.Q. Selection of the Optimum Ratio Matrix for Removal of Ammonia Nitrogen and Phosphorus in Eco-Ditch and Its Influencing Factors in Cold and Arid Areas. J. Soil Water Conserv. 2020, 34, 262–267. [Google Scholar]
- Liu, M.H.; Wu, S.B.; Chen, L.; Dong, R.J. How Substrate Influences Nitrogen Transformations in Tidal Flow Constructed Wetlands Treating High Ammonium Wastewater? Ecol. Eng. 2014, 73, 478–486. [Google Scholar] [CrossRef]
- Su, J.Z. Study on the Performance of Biochar Adsorption of Nano-Plastic and the Synergistic Removal of Ammonia Nitrogen by Modified Bioretention Cell; Nanchang University: Nanchang, China, 2023. [Google Scholar]
- Chen, S.J.; Huang, D.; Gao, Y.N.; Meng, Z.G.; Zhang, G.Q. Study on Nitrogen and Phosphorus Removal Performance of Constructed Wetland Modular Substrate. Technol. Water Treat. 2023. Available online: https://link.cnki.net/urlid/33.1127.P.20231213.1650.004 (accessed on 14 December 2023).
- Li, D.; Li, Y. Application of Zeolite in Water Treatment. Water Wastewater Eng. 1998, 24, 60–63. [Google Scholar]
- Tang, D.Y.; Zheng, Z.; Lin, Z.R.; Tao, C.L.; Gao, H.; Lu, H. Adsorption of Ammonium-Nitrogen from Low Concentration Wastewater by Natural Zeolite. Environ. Sci. Technol. 2010, 33, 206–209. [Google Scholar]
- Liu, L.; Wu, S.B.; Guo, J.B.; Cheng, B.; Dong, R.J.; Pang, C.L. Adsorption of Ammonia Nitrogen in Effluent from Pig Manure Biogas Plant by Zeolite. J. Agro-Environ. Sci. 2011, 30, 2130–2135. [Google Scholar]
- Halim, A.A.; Aziz, H.A.; Johari, M.A.M.; Ariffin, K.S. Comparison Study of Ammonia and COD Adsorption on Zeolite, Activated Carbon and Composite Materials in Landfill Leachate Treatment. Desalination 2010, 262, 31–35. [Google Scholar] [CrossRef]
- Qiu, F.G.; Sun, Y.; Chen, L.X. Characteristics of Aluminum-Based Water Treatment Residual (WTR) and Its Adsorption Performance on Ammonium and Phosphate. Environ. Eng. 2016, 34, 54–59. [Google Scholar]
- Zhu, L.L.; Qiu, L.P.; Zhang, Y.Y.; Cheng, H.T.; Liu, P.P. Ammonia Nitrogen Adsorption onto Volcanic Rocks, Haydites and Zeolites at Low Temperatures. J. Univ. Jinan Sci. Technol. 2016, 30, 287–292. [Google Scholar]
- Liu, Y.; Liu, X.H.; Zhang, Y.R.; Wang, W.L. Adsorption Properties of Low Concentration Ammonia Nitrogen Wastewater by Three Constructed Wetland Fillers. Environ. Chem. 2018, 37, 1118–1127. [Google Scholar]
- Chen, C.; Luo, H.; Cao, X.Y.; Jing, Z.Q. Mechanism of Enhanced Nitrogen and Phosphorus Removal from Rainwater Runoff by Different Modified Zeolites. Water Purif. Technol. 2020, 39, 141–149. [Google Scholar]
- He, L.W.Z.; Chen, Y.; Zhao, Y.T.; Li, Y.J.; Yang, S.S. Adsorption Properties of Ammonia Nitrogen by Alkali-Hydrothermal Water Treatment Sludge Adsorbent. Technol. Water Treat. 2022, 48, 82–87. [Google Scholar]
- Liu, J.; Zhu, Z.Q.; Zhu, Y.N.; Yan, Q.M.; He, H.; Zhang, L.H.; Huang, X.N. Adsorption Characteristics of Phosphate in Water by the Porous Biomorph-Genetic Composite of Fe/C with Ginkgo Shell Template. Res. Environ. Sci. 2019, 32, 1239–1249. [Google Scholar]
- Chiou, M.S.; Li, H.Y. Equilibrium and Kinetic Modeling of Adsorption of Reactive Dye on Cross-Linked Chitosan Beads. J. Hazard. Mater. 2002, 93, 233–248. [Google Scholar] [CrossRef]
- Yeddou, N.; Bensmaili, A. Kinetic Models for the Sorption of Dye from Aqueous Solution by Clay-Wood Sawdust Mixture. Desalination 2005, 185, 499–508. [Google Scholar] [CrossRef]

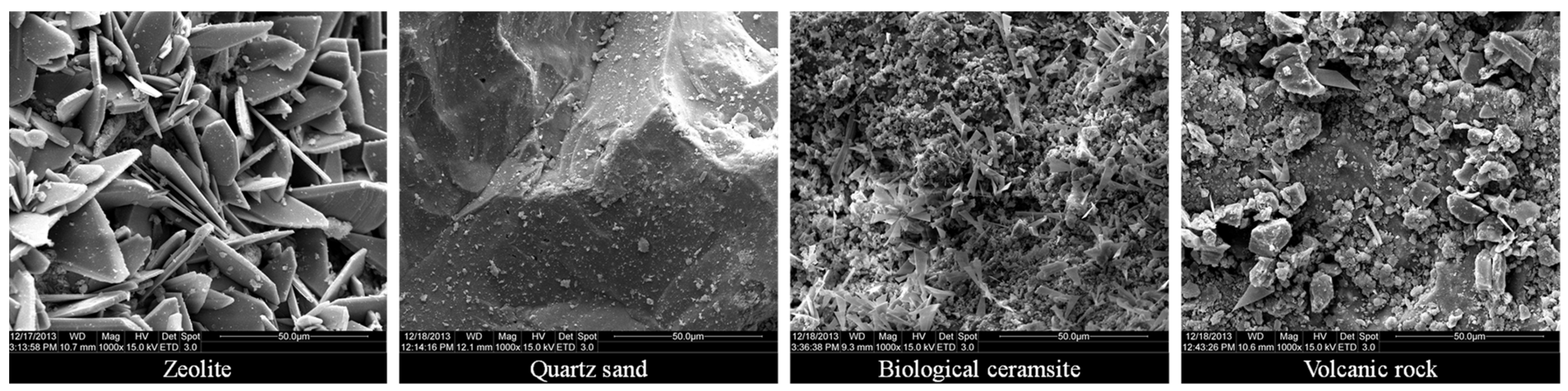




| Material | Density (m3/g) | Cation Exchange Capacity (coml/kg) | Specific Surface Area (m2/g) | Total Pore Volume (mL/g) | Average Pore Diameter (nm) | pH |
|---|---|---|---|---|---|---|
| Zeolite | 2.05–2.21 | 4.17–4.41 | 16.59 | 0.0612 | 14.76 | 7.09 |
| Quartz sand | 2.51–2.75 | 0.17–0.53 | 0.12 | 0.0010 | 3.47 | 7.30 |
| Biological ceramsite | 1.64–1.70 | 0.57–0.69 | 0.71 | 0.0161 | 6.88 | 7.37 |
| Volcanic rock | 1.94–2.00 | 0.17–0.75 | 0.35 | 0.0006 | 90.86 | 7.31 |
| Substrate Dosage (g) | Zeolite | Quartz Sand | Biological Ceramsite | Volcanic Rock | ||||
|---|---|---|---|---|---|---|---|---|
| Unit Adsorption Capacity (mg/g) | Removal Rate (%) | Unit Adsorption Capacity (mg/g) | Removal Rate (%) | Unit Adsorption Capacity (mg/g) | Removal Rate (%) | Unit Adsorption Capacity (mg/g) | Removal Rate (%) | |
| 1.0 | 5.61 | 56.32 | 0.26 | 2.62 | 0.87 | 8.65 | 0.45 | 4.51 |
| 2.5 | 3.29 | 81.83 | 0.21 | 3.99 | 0.34 | 8.47 | 0.26 | 6.47 |
| 5.0 | 1.89 | 93.76 | 0.08 | 3.34 | 0.20 | 9.09 | 0.20 | 9.96 |
| 10 | 0.98 | 97.05 | 0.05 | 4.78 | 0.16 | 15.99 | 0.18 | 17.66 |
| 15 | 0.66 | 98.10 | 0.03 | 6.07 | 0.10 | 14.90 | 0.17 | 25.29 |
| 20 | 0.50 | 98.66 | 0.03 | 5.85 | 0.07 | 15.67 | 0.12 | 23.18 |
| 30 | 0.33 | 98.99 | 0.03 | 6.65 | 0.06 | 18.94 | 0.08 | 25.26 |
| Substrate | Index | Ammonia Nitrogen Concentration (mg/L) | ||
|---|---|---|---|---|
| 10 | 100 | 250 | ||
| Zeolite | Adsorption saturation time (h) | 10 | 14 | 14 |
| Quartz sand | 10 | 10 | 10 | |
| Biological ceramsite | 14 | 16 | 16 | |
| Volcanic rock | 10 | 14 | 14 | |
| Zeolite | Removal rate (%) | 91.16 | 81.48 | 76.60 |
| Quartz sand | 26.22 | 11.624 | 13.24 | |
| Biological ceramsite | 32.32 | 19.53 | 19.65 | |
| Volcanic rock | 35.88 | 15.82 | 11.33 | |
| Isotheram | Zeolite | Quartz Sand | Biological Ceramsite | Volcanic Rock |
|---|---|---|---|---|
| Langmuir | ||||
| R2 | 0.924 | 0.758 | 0.978 | 0.870 |
| Qm (mg/g) | 3.85 | 0.12 | 0.31 | 0.21 |
| K | 6.18 × 10−2 | 6.11 × 10−3 | 5.25 × 10−3 | 1.31 × 10−2 |
| Equilibrium isotherms | ||||
| Freundlich | ||||
| R2 | 0.997 | 0.992 | 0.997 | 0.967 |
| 1/n | 0.558 | 0.682 | 0.792 | 0.592 |
| F | 0.343 | 1.864 × 10−3 | 2.693 × 10−3 | 7.439 × 10−3 |
| Equilibrium isotherms | Qe = 0.343Ce0.558 | Qe = 1.864 × 10−3Ce0.682 | Qe = 2.693 × 10−3Ce0.792 | Qe = 7.439 × 10−3Ce0.592 |
| Kinetic Model | Constants | Adsorbent | |||
|---|---|---|---|---|---|
| Ammoniacal Nitrogen | Zeolite | Quartz Sand | Biological Ceramics | Volcanic Rocks | |
| intra-particle | R2 | 0.740 | 0.963 | 0.895 | 0.885 |
| U | 0.154 | 0.013 | 0.031 | 0.018 | |
| C | 0.759 | 0.026 | −0.003 | 0.010 | |
| 1st-order | |||||
| R2 | 0.657 | 0.945 | 0.813 | 0.861 | |
| K1 | 0.042 | 2.323 | 1.369 | 1.369 | |
| Qe (mg/g) | 0.959 | 0.960 | 0.982 | 1.809 | |
| 2nd-order | |||||
| R2 | 0.997 | 0.945 | 0.848 | 0.841 | |
| K2 | 0.140 | −23.419 | −442.008 | −52.952 | |
| Qe (mg/g) | 1.893 | 0.022 | 0.048 | 0.030 | |
| experimental Qe (mg/g) | 1.863 | 0.123 | 0.237 | 0.149 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Liu, M.; Zhou, J.; Chen, L.; Cai, M. Adsorption Performance of Different Wetland Substrates for Ammonia Nitrogen: An Experimental Study. Water 2024, 16, 174. https://doi.org/10.3390/w16010174
Li P, Liu M, Zhou J, Chen L, Cai M. Adsorption Performance of Different Wetland Substrates for Ammonia Nitrogen: An Experimental Study. Water. 2024; 16(1):174. https://doi.org/10.3390/w16010174
Chicago/Turabian StyleLi, Peng, Minghui Liu, Jiaxing Zhou, Li Chen, and Meifeng Cai. 2024. "Adsorption Performance of Different Wetland Substrates for Ammonia Nitrogen: An Experimental Study" Water 16, no. 1: 174. https://doi.org/10.3390/w16010174
APA StyleLi, P., Liu, M., Zhou, J., Chen, L., & Cai, M. (2024). Adsorption Performance of Different Wetland Substrates for Ammonia Nitrogen: An Experimental Study. Water, 16(1), 174. https://doi.org/10.3390/w16010174







