Identification of Phytoplankton-Based Production of the Clam Corbicula japonica in a Low-Turbidity Temperate Estuary Using Fatty Acid and Stable Isotope Analyses
Abstract
:1. Introduction
2. Materials and Methods
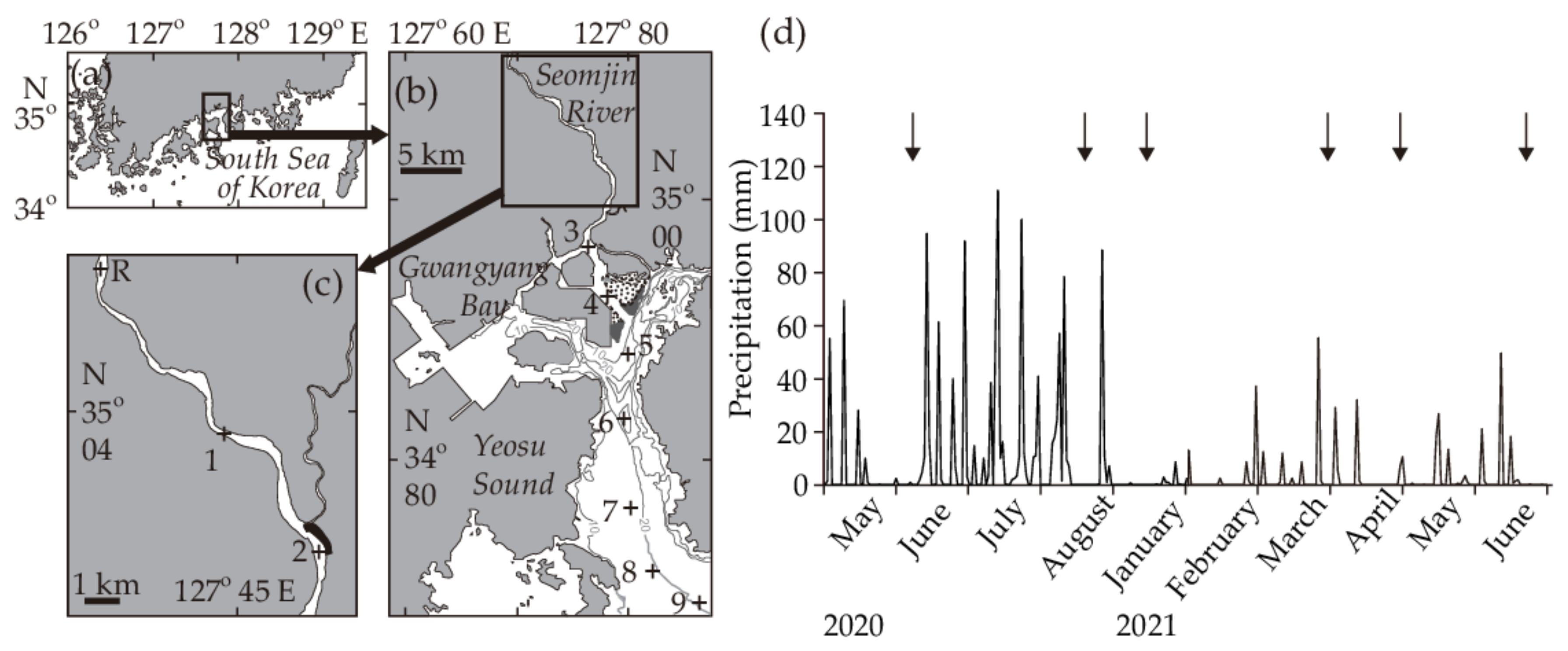
2.1. Study Area
2.2. Sample Collection and Laboratory Processing
2.3. Stable Isotope Analysis
2.4. Fatty Acid Analysis
2.5. Statistics
3. Results
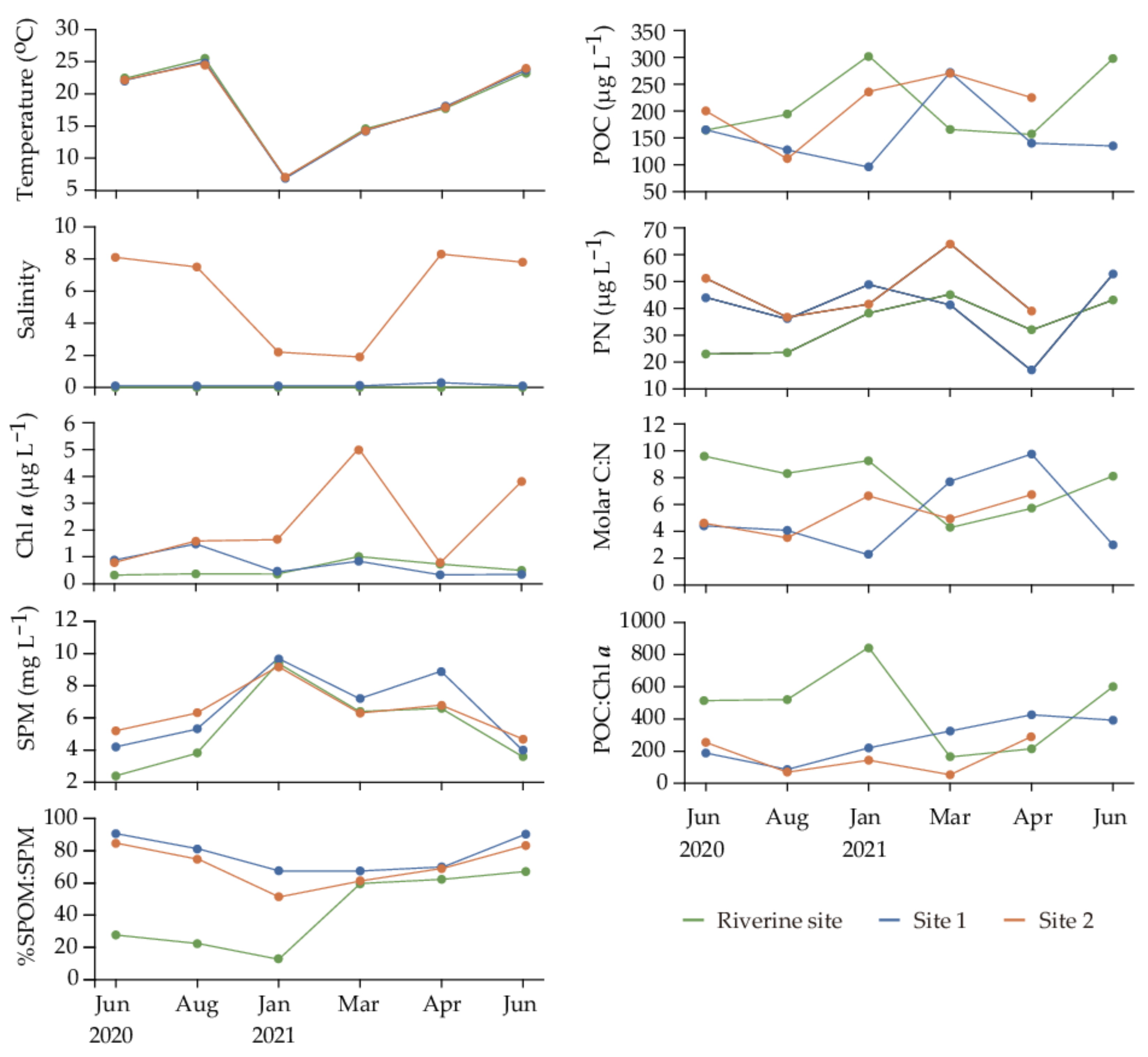
3.1. Environmental Conditions
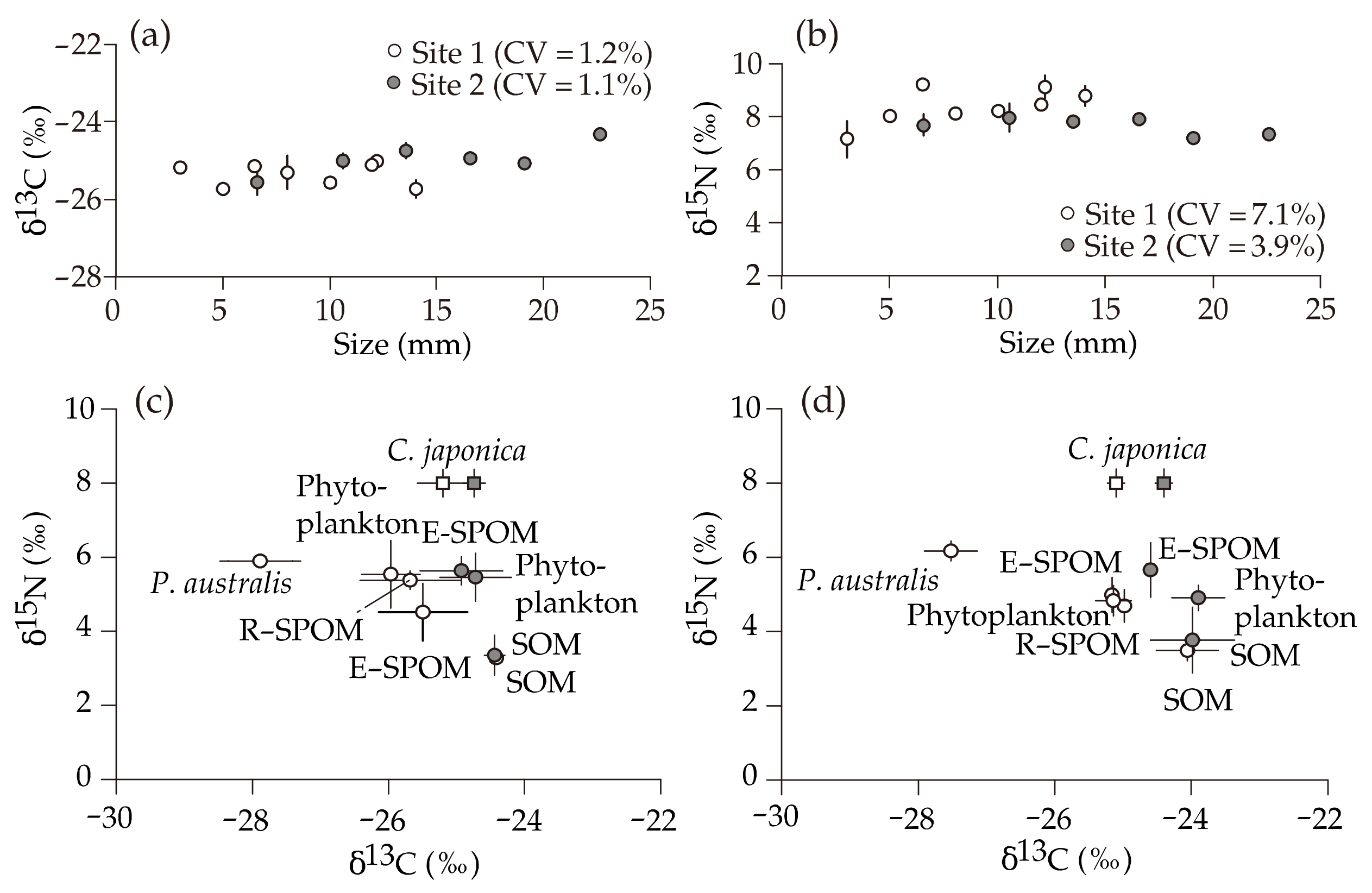
3.2. δ13C and δ15N Values of Putative Food Sources
3.3. δ13C and δ15N Values of C. japonica
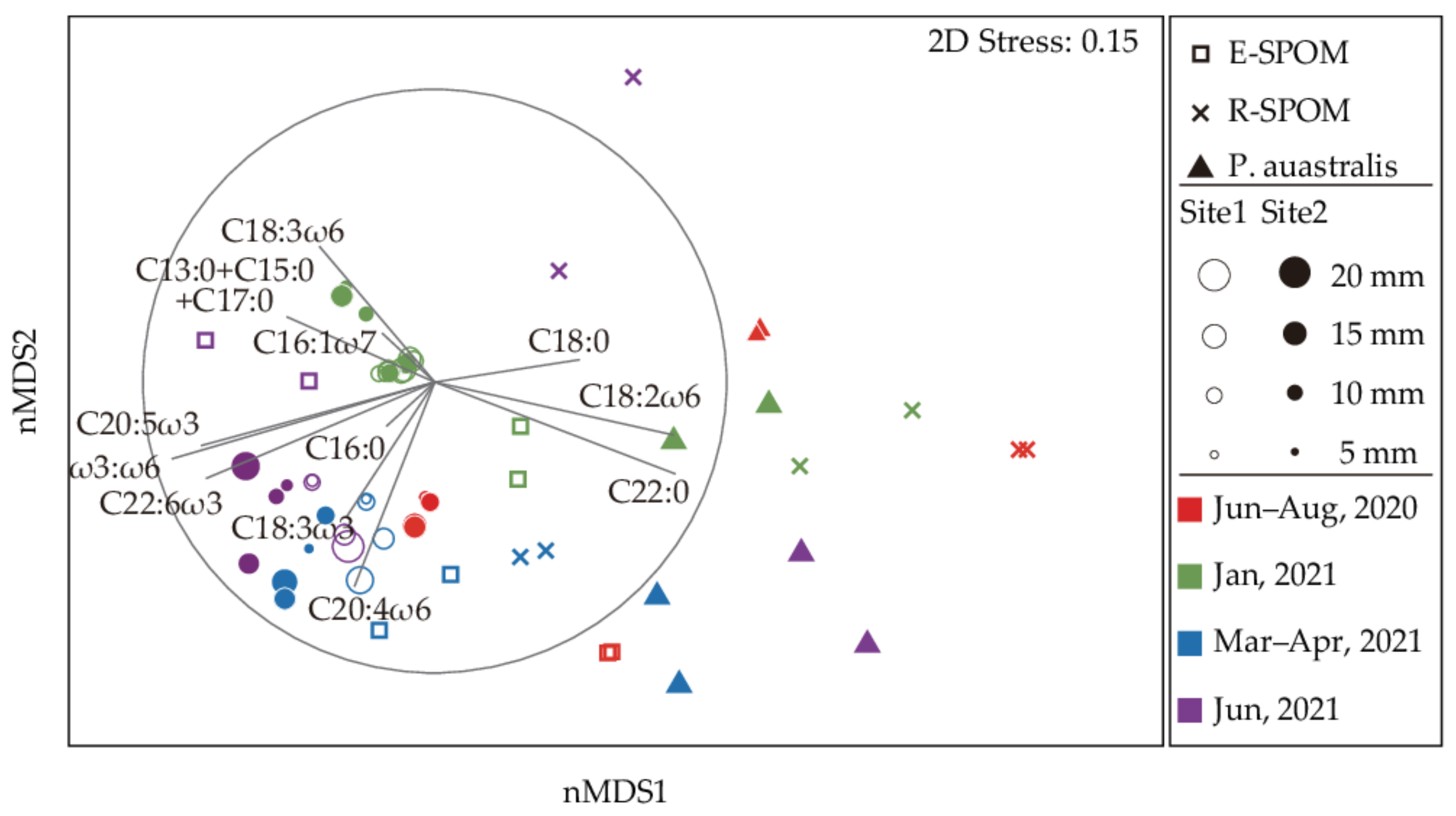
3.4. Fatty Acid Compositions of Putative Food Sources
3.5. Fatty Acid Compositions of C. japonica
4. Discussion
4.1. Origin of Suspended Particulate Organic Matter
4.2. Importance of Estuarine Phytoplankton for the Corbicula Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nakamura, M.; Yamamuro, M.; Ishikawa, M.; Nishimura, H. Role of the bivalve Corbicula japonica in the nitrogen cycle in a mesohaline lagoon. Mar. Biol. 1988, 99, 369–374. [Google Scholar] [CrossRef]
- Yamamuro, M.; Koike, I. Nitrogen metabolism of the filter-feeding bivalve Corbicula japonica and its significance in primary production of a brackish lake in Japan. Limnol. Oceanogr. 1993, 38, 997–1007. [Google Scholar] [CrossRef]
- Buttner, J.K. Biology of Corbicula in Catfish Rearing Ponds. In Proceedings of the Second International Corbicula Symposium; American Malacological Bulletin Special Edition 2. Britton, J.C., Prezant, R.S., Eds.; American Malacological Union: Hattiesburg, MS, USA, 1986; pp. 211–218. [Google Scholar]
- Lauritsen, D.D.; Mozley, S.C. Nutrient excretion by the Asiatic clam Corbicula fluminea. J. N. Am. Benthol. Soc. 1989, 8, 134–139. [Google Scholar] [CrossRef]
- Way, C.M.; Hornbach, D.J.; Miller-Way, C.A.; Payne, B.S.; Miller, A.C. Dynamics of filter feeding in Corbicula fluminea (Bivalvia: Corbiculidae). Can. J. Zool. 1990, 68, 115–120. [Google Scholar] [CrossRef]
- Phelps, H.L. The Asiatic clam (Corbicula fluminea) invasion and system-level ecological change in the Potomac River estuary near Washington, DC. Estuaries 1994, 17, 614–621. [Google Scholar] [CrossRef]
- Aldridge, D.W.; McMahon, R.F. Growth, fecundity, and bioenergetics in a natural population of the Asiatic freshwater clam, Corbicula manilensis Philippi, from North Central Texas. J. Molluscan Stu. 1978, 44, 49–70. [Google Scholar] [CrossRef]
- McMahon, R.F.; Williams, C.J. A reassessment of growth rate, life span, life cycles and population dynamics in a natural population and field caged individuals of Corbicula fluminea (Müller) (Bivalvia: Corbiculacea). In Proceeding of the Second International Corbicula Symposium; American Malacological Bulletin Special Edition 2; Prezant, R.S., Ed.; American Malacological Union: Hattiesburg, MS, USA, 1986; pp. 151–166. [Google Scholar]
- Kasai, A.; Toyohara, H.; Nakata, A.; Miura, T.; Azuma, N. Food sources for the bivalve Corbicula japonica in the foremost fishing lakes estimated from stable isotope analysis. Fish. Sci. 2006, 72, 105–114. [Google Scholar] [CrossRef]
- Yamanaka, T.; Mizota, C.; Maki, Y.; Matsumasa, M. Assimilation of terrigenous organic matter via bacterial biomass as a food source for a brackish clam, Corbicula japonica (Mollusca: Bivalva). Estuar. Coast. Shelf Sci. 2013, 126, 87–92. [Google Scholar] [CrossRef]
- Reid, R.B.; McMahon, R.F.; Foighil, D.Ó.; Finnigan, R. Anterior inhalant currents and pedal feeding in bivalves. Veliger 1992, 35, 93–104. [Google Scholar]
- Kang, C.K.; Sauriau, P.G.; Richard, P.; Blanchard, G.F. Food sources of the infaunal suspension-feeding bivalve Cerastoderma edule in a muddy sandflat of Marennes-Oléron Bay, as determined by analyses of carbon and nitrogen stable isotopes. Mar. Ecol. Prog. Ser. 1999, 187, 147–158. [Google Scholar] [CrossRef]
- Rossi, F.; Herman, P.M.J.; Middelburg, J.J. Interspecific and intraspecific variation of δC and δN in deposit-and suspension-feeding bivalves (Macoma balthica and Cerastoderma edule): Evidence of ontogenetic changes in feeding mode of Macoma balthica. Limnol. Oceanogr. 2004, 49, 408–414. [Google Scholar] [CrossRef]
- Acuña, V.; Dahm, C.N. Impact of monsoonal rains on spatial scaling patterns in water chemistry of a semiarid river network. J. Geophys. Res. Biogeosci. 2007, 112, G4. [Google Scholar] [CrossRef]
- Dekar, M.P.; Magoulick, D.D.; Huxel, G.R. Shifts in the trophic base of intermittent stream food webs. Hydrobiologia 2009, 635, 263–277. [Google Scholar] [CrossRef]
- Kim, B.; Choi, K.; Kim, C.; Lee, U.H.; Kim, Y.H. Effects of the summer monsoon on the distribution and loading of organic carbon in a deep reservoir, Lake Soyang, Korea. Water Res. 2000, 34, 3495–3504. [Google Scholar] [CrossRef]
- Fry, B.; Sherr, E.B. δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 1984, 27, 13–47. [Google Scholar] [CrossRef]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta. 1978, 42, 495–506. [Google Scholar] [CrossRef]
- France, R.L. Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol. Oceanogr. 1995, 40, 1310–1313. [Google Scholar] [CrossRef]
- Dalsgaard, J.; John, M.S.; Kattner, G.; Müller-Navarra, D.; Hagen, W. Fatty acid trophic markers in the pelagic marine environment. Adv. Mar. Biol. 2003, 46, 225–340. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Zanden, M.J.V.; Rasmussen, J.B. Primary consumer δ15N and δ13C and the trophic position of aquatic consumers. Ecology 1999, 80, 1395–1404. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Shin, P.K.; Yip, K.M.; Xu, W.Z.; Wong, W.H.; Cheung, S.G. Fatty acid as markers to demonstrating trophic relationships among diatoms, rotifers, and green-lipped mussels. J. Exp. Mar. Biol. Ecol. 2008, 357, 75–84. [Google Scholar] [CrossRef]
- Kelly, J.R.; Scheibling, R.E. Fatty acids as dietary tracers in benthic food webs. Mar. Ecol. Prog. Ser. 2012, 446, 1–22. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, F.; Yan, X. Stable isotopes and fatty acids as dietary tracers of intertidal bivalves. Fish. Sci. 2013, 79, 749–756. [Google Scholar] [CrossRef]
- Guo, F.; Lee, S.Y.; Kainz, M.J.; Brett, M.T. Fatty acids as dietary biomarkers in mangrove ecosystems: Current status and future perspective. Sci. Total Environ. 2020, 739, 139907. [Google Scholar] [CrossRef]
- Bibi, R.; Kang, H.Y.; Kim, D.; Jang, J.; Kundu, G.K.; Kim, Y.K.; Kang, C.K. Dominance of autochthonous phytoplankton-derived particulate organic matter in a low-turbidity temperate estuarine embayment, Gwangyang Bay, Korea. Front. Mar. Sci. 2020, 7, 580260. [Google Scholar] [CrossRef]
- Kim, B.J.; Ro, Y.J.; Jung, K.Y.; Park, K.S. Numerical modeling of circulation characteristics in the Kwangyang estuarine system. JKSCOE 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Kang, H.Y.; Kim, C.; Kim, D.; Lee, Y.J.; Park, H.J.; Kundu, G.K.; Kim, Y.K.; Bibi, R.; Jang, J.; Lee, K.H.; et al. Identifying patterns in the multitrophic community and food-web structure of a low-turbidity temperate estuarine bay. Sci. Rep. 2020, 10, 16637. [Google Scholar] [CrossRef]
- Kang, C.K.; Lee, Y.W.; Choy, E.J.; Shin, J.K.; Seo, I.S.; Hong, J.S. Microphytobenthos seasonality determines growth and reproduction in intertidal bivalves. Mar. Ecol. Prog. Ser. 2006, 315, 113–127. [Google Scholar] [CrossRef]
- Holm-Hassen, O.; Lorenzen, C.J.; Holms, R.W.; Strickland, J.D.H. Fluorometric determination of chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of tota1 lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.; Waldock, M.J. The effect of algal and artificial diets on the growth and fatty acid composition of Crassostrea gigas spat. J. Mar. Biolog. Assoc. U.K. 1981, 61, 431–448. [Google Scholar] [CrossRef]
- Waldock, M.J.; Holland, D.L. Fatty acid metabolism in young oysters, Crassostrea gigas: Polyunsaturated fatty acids. Lipids 1984, 19, 332–336. [Google Scholar] [CrossRef]
- Brett, M.T.; Müller-Navarra, D.C.; Persson, J. Crustacean zooplankton fatty acid composition. In Lipids in Aquatic Ecosystems; Arts, M.T., Brett, M.T., Kainz, M.J., Eds.; Springer: New York, NY, USA, 2009; pp. 115–146. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- De’ath, G. Multivariate regression trees: A new technique for modeling species-environment relationships. Ecology 2002, 83, 1105–1117. [Google Scholar] [CrossRef]
- Budge, S.M.; Iverson, S.J.; Bowen, W.D.; Ackman, R.G. Among-and within-species variability in fatty acid signatures of marine fish and invertebrates on the Scotian Shelf, Georges Bank, and southern Gulf of St. Lawrence. Can. J. Fish. Aquat. Sci. 2002, 59, 886–898. [Google Scholar] [CrossRef]
- Doi, H.; Matsumasa, M.; Toya, T.; Satoh, N.; Mizota, C.; Maki, Y.; Kikuchi, E. Spatial shifts in food sources for macrozoobenthos in an estuarine ecosystem: Carbon and nitrogen stable isotope analyses. Estuar. Coast. Shelf Sci. 2005, 64, 316–322. [Google Scholar] [CrossRef]
- Kasai, A.; Nakata, A. Utilization of terrestrial organic matter by the bivalve Corbicula japonica estimated from stable isotope analysis. Fish. Sci. 2005, 71, 151–158. [Google Scholar] [CrossRef]
- Kang, C.K.; Lee, W.C.; Park, J.I.; Choi, W.J.; Kim, Y.S.; Lee, P.Y. Isotopic determination of terrestrial food sources for a brackish water clam Corbicula japonica PRIME in an estuarine system of Youngil Bay, Korea. J. Korean Soc. Oceanogr. 2000, 35, 56–64. [Google Scholar]
- Kim, C.; Kang, H.Y.; Lee, Y.J.; Yun, S.G.; Kang, C.K. Isotopic variation of macroinvertebrates and their sources of organic matter along an estuarine gradient. Estuar. Coast. 2020, 433, 496–511. [Google Scholar] [CrossRef]
- Im, D.H.; Suh, H.L. Resource utilization of three copepods along salinity gradient in the Seomjin River estuary, revealed by stable isotope analysis. Ocean Sci. J. 2021, 56, 106–116. [Google Scholar] [CrossRef]
- Riera, P.; Richard, P. Isotopic determination of food sources of Crassostrea gigas along a trophic gradient in the estuarine bay of Marennes-Oléron. Estuar. Coast. Shelf Sci. 1996, 42, 347–360. [Google Scholar] [CrossRef]
- Deegan, L.A.; Garritt, R.H. Evidence for spatial variability in estuarine food webs. Mar. Ecol. Prog. Ser. 1997, 147, 31–47. [Google Scholar] [CrossRef]
- Connolly, R.M.; Gorman, D.; Guest, M.A. Movement of carbon among estuarine habitats and its assimilation by invertebrates. Oecologia 2005, 144, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Howe, E.R.; Simenstad, C.A. Using stable isotopes to discern mechanisms of connectivity in estuarine detritus-based food webs. Mar. Ecol. Prog. Ser. 2015, 518, 13–29. [Google Scholar] [CrossRef]
- Michener, R.H.; Kaufman, L. Stable isotope ratios as tracers in marine aquatic food webs: An update. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R.H., Lajtha, K., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 238–282. [Google Scholar]
- Kang, S.; Kim, J.H.; Kim, D.; Song, H.; Ryu, J.S.; Ock, G.; Shin, K.H. Temporal variation in riverine organic carbon concentrations and fluxes in two contrasting estuary systems: Geum and Seomjin, South Korea. Mar. Pollut. Bull. 2019, 33, 105126. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Kang, Y.H.; Kim, J.K.; Kang, H.Y.; Kang, C.K. Year-to-year variation of phytoplankton blooms in an anthropogenically polluted and complex estuary: A novel paradigm for river discharge influence. Mar. Pollut. Bull. 2020, 161, 111756. [Google Scholar] [CrossRef]
- Savoye, N.; Aminot, A.; Tréguer, P.; Fontugne, M.; Naulet, N.; Kerouel, R. Dynamics of particulate organic matter d15N and d13C during spring phytoplankton blooms in a macrotidal ecosystem (Bay of Seine, France). Mar. Ecol. Prog. Ser. 2003, 255, 27–41. [Google Scholar] [CrossRef]
- Liénart, C.; Savoye, N.; Bozec, Y.; Breton, E.; Conan, P.; David, V.; Feunteun, E.; Grangeré, K.; Kerhervé, P.; Lebreton, B.; et al. Dynamics of particulate organic matter composition in coastal systems: A spatio-temporal study at multi-systems scale. Prog. Oceanogr. 2017, 156, 221–239. [Google Scholar] [CrossRef]
- Napolitano, G.E.; Pollero, R.J.; Gayoso, A.M.; Macdonald, B.A.; Thompson, R.J. Fatty acids as trophic markers of phytoplankton blooms in the Bahia Blanca estuary (Buenos Aires, Argentina) and in Trinity Bay (Newfoundland, Canada). Biochem. Syst. Ecol. 1997, 25, 739–755. [Google Scholar] [CrossRef]
- Parrish, C.C.; Abrajano, T.A.; Budge, S.M.; Helleur, R.J.; Hudson, E.D.; Pulchan, K.; Ramos, C. Lipid and phenolic biomarkers in marine ecosystems: Analysis and applications. In The Handbook of Environmental Chemistry Part D. Marine Chemistry; Wangersky, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 5, pp. 193–223. [Google Scholar]
- Viso, A.C.; Marty, J.C. Fatty acids from 28 marine microalgae. Phytochemistry 1993, 34, 1521–1533. [Google Scholar] [CrossRef]
- Desvilettes, C. Dynamique des Acides Gras dans la Chalne Trophique Phytoplancton-Zooplancton-Larves de Brochet et Evolution des Constituants Lipidiques Chez le Brochet (Esox lucius L.) aux Premiers Stades de vie. Ph.D. Thesis, Université Clermont-Ferrand 2, Clermont-Ferrand, France, 1994. [Google Scholar]
- Zhukova, N.V. Fatty acids of marine mollusks: Impact of diet, bacterial symbiosis and biosynthetic potential. Biomolecules 2019, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Desvilettes, C.H.; Bourdier, G.; Breton, J.C.; Combrouze, P. Fatty acids as organic markers for the study of trophic relationships in littoral cladoceran communities of a pond. J. Plankton Res. 1994, 16, 643–659. [Google Scholar] [CrossRef]
- Teshima, S.; Kanazawa, A.; Koshio, S.; Mukai, H.; Yamasaki, S.; Hirata, H. Fatty acid details for bivalves, Tapes philippinarum and Corbicula japonica, and marine types of algae Nannochloropsis sp. and Chlorella sp. Mem. Fac. Fish. Kagoshima Univ. 1990, 39, 137–149. [Google Scholar]
- Shin, W.S.; Fujibayashi, M.; Nishimura, O. Contribution of potential organic matter sources to the macrobenthos nutrition in the Natori estuarine tidal flats, Japan. Russ. J. Mar. Biol. 2014, 40, 396–404. [Google Scholar] [CrossRef]
- Basen, T.; Martin-Creuzburg, D.; Rothhaupt, K.O. Role of essential lipids in determining food quality for the invasive freshwater clam Corbicula fluminea. J. N. Am. Benthol. Soc. 2011, 30, 653–664. [Google Scholar] [CrossRef]
- Baptista, M.; Repolho, T.; Maulvault, A.L.; Lopes, V.M.; Narciso, L.; Marques, A.; Bandarra, N.; Rosa, R. Temporal dynamics of amino and fatty acid composition in the razor clam Ensis siliqua (Mollusca: Bivalvia). Helgol. Mar. Res. 2014, 68, 465–482. [Google Scholar] [CrossRef]
- Cook, M.E. Diet-induced immunosuppression. In Poultry Immunology (Poultry Science Symposium); Davision, T.F., Morris, T.R., Payne, L.N., Eds.; Carfax Publishing: Abingdon, UK, 1996; Volume 24, pp. 317–325. [Google Scholar]
- Stanley-Samuelson, D.W. The biological significance of prostaglandins and related eicosanoids in invertebrates. Am. Zool. 1994, 34, 589–598. [Google Scholar] [CrossRef]
- Shin, W.S.; Fujibayashi, M.; Nomura, M.; Nakano, K.; Nishimura, O. Fatty acid composition between Nuttallia olivacea and Hediste spp. in the Nanakita Estuary, Japan: Estimation of food sources. J. Water Environ. Technol. 2012, 10, 11–22. [Google Scholar] [CrossRef]
- Lee, J.B.; Shin, Y.J.; Lee, J.H.; Choi, Y.M.; Lee, D.W.; Cha, H.K. Estimation of potential fishery yield for Corbicula japonica in the Seomjin River, Korea. Korean J. Malacol. 2012, 28, 91–99. [Google Scholar] [CrossRef]
- Canuel, E.A.; Cloern, J.E.; Ringelberg, D.B.; Guckert, J.B.; Rau, G.H. Molecular and isotopic tracers used to examine sources of organic matter and its incorporation into the food webs of San Francisco Bay. Limnol. Oceanogr. 1995, 40, 67–81. [Google Scholar] [CrossRef]
- Antonio, E.S.; Kasai, A.; Ueno, M.; Kurikawa, Y.; Tsuchiya, K.; Toyohara, H.; Ishishi, Y.; Yokoyama, H.; Yamashita, Y. Consumption of terrestrial organic matter by estuarine molluscs determined by analysis of their stable isotopes and cellulase activity. Estuar. Coast. Shelf Sci. 2010, 86, 401–407. [Google Scholar] [CrossRef]
- Napolitano, G.E. Fatty acids as trophic and chemical markers in freshwater ecosystems. In Lipids in Freshwater Ecosystems; Wetzel, R.G., Ed.; Springer: New York, NY, USA, 1999; pp. 21–44. [Google Scholar]
- Erwin, J. Comparative biochemistry of fatty acids in eukaryotic microorganisms. In Lipids and Biomembranes of Eukaryotic Microorganisms; Erwin, J., Ed.; Academic Press: Waltham, MA, USA, 1973; pp. 141–143. [Google Scholar]
- Pond, D.W.; Bell, M.V.; Harris, R.P.; Sargent, J.R. Microplanktonic polyunsaturated fatty acid markers: A mesocosm trial. Estuar. Coast. Shelf Sci. 1998, 46, 61–67. [Google Scholar] [CrossRef]
- Gugger, M.; Lyra, C.; Suominen, I.; Tsitko, I.; Humbert, J.F.; Salkinoja-Salonen, M.S.; Sivonen, K. Cellular fatty acids as chemotaxonomic markers of the genera Anabaena, Aphanizomenon, Microcystis, Nostoc and Planktothrix (cyanobacteria). Int. J. Syst. Evol. Microbiol. 2002, 52, 1007–1015. [Google Scholar] [CrossRef]
- Mansour, M.P.; Volkman, J.K.; Jackson, A.E.; Blackburn, S.I. The fatty acid and sterol composition of five marine dinoflagellates. J. Phycol. 1999, 35, 710–720. [Google Scholar] [CrossRef]
- Budge, S.M.; Parrish, C.C.; McKenzie, C.H. Fatty acid composition of phytoplankton, settling particulate matter and sediments at a sheltered bivalve aquaculture site. Mar. Chem. 2001, 76, 285–303. [Google Scholar] [CrossRef]
- Shorland, F.B. The distribution of fatty acids in plant lipids. In Chemical Plant Taxonomy; Swain, T., Ed.; Academic Press: Waltham, MA, USA, 1963; pp. 253–311. [Google Scholar]
- Budge, S.M.; Parrish, C.C. Lipid biogeochemistry of plankton, settling matter and sediments in Trinity Bay, Newfoundland. II. Fatty acids. Org. Geochem. 1998, 29, 1547–1559. [Google Scholar] [CrossRef]
- Najdek, M.; Debobbis, D.; Mioković, D.; Ivančić, I. Fatty acid and phytoplankton compositions of different types of mucilaginous aggregates in the northern Adriatic. J. Plankton Res. 2002, 24, 429–441. [Google Scholar] [CrossRef]
- Volkman, J.K.; Eglinton, G.; Corner, E.D. Sterols and fatty acids of the marine diatom Biddulphia sinensis. Phytochemistry 1980, 19, 1809–1813. [Google Scholar] [CrossRef]
- Shilla, D.; Routh, J. Using biochemical and isotopic tracers to characterise organic matter sources and their incorporation into estuarine food webs (Rufiji delta, Tanzania). Chem. Ecol. 2017, 33, 893–917. [Google Scholar] [CrossRef]
- Alfaro, A.C.; Thomas, F.; Sergent, L.; Duxbury, M. Identification of trophic interactions within an estuarine food web (northern New Zealand) using fatty acid biomarkers and stable isotopes. Estuar. Coast. Shelf Sci. 2006, 70, 271–286. [Google Scholar] [CrossRef]
- Martino, R.C.; Cruz, G.M.D. Proximate composition and fatty acid content of the mangrove oyster Crassostrea rhizophorae along the year seasons. Braz. Arch. Biol. Technol. 2004, 47, 955–960. [Google Scholar] [CrossRef]
- Bachok, Z.; Meziane, T.; Mfilinge, P.L.; Tsuchiya, M. Fatty acid markers as an indicator for temporal changes in food sources of the bivalve Quidnipagus palatum. Aquat. Ecosyst. Health Manag. 2009, 12, 390–400. [Google Scholar] [CrossRef]
- Bachok, Z.; Mfilinge, P.L.; Tsuchiya, M. The diet of the mud clam Geloina coaxans (Mollusca, Bivalvia) as indicated by fatty acid markers in a subtropical mangrove forest of Okinawa, Japan. J. Exp. Mar. Biol. Ecol. 2003, 292, 187–197. [Google Scholar] [CrossRef]

| Food Sources | Site | P (within Source) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | Site | Season | Interaction | |||
| δ13C (‰) | |||||||
| E-SPOM | −25.3 ± 0.2 b,c (4) | −24.7 ± 0.3 c,d (4) | 0.034 * | 0.136 | 0.999 | ||
| SOM | –24.2 ± 0.2d (8) | 0.885 | 0.058 | 0.769 | |||
| Phytoplankton | June | −26.0 ± 0.3 b (2) | −24.7 ± 0.1 c,d (2) | 0.002 * | 0.007 * | 0.999 | |
| August | −25.1 ± 0.2 b,c (2) | −23.9 ± 0.3 d (2) | |||||
| P. australis | −27.7 ± 0.2 a (4) | 0.327 | |||||
| R-SPOM | −25.3 ± 0.4 b,c (4) | 0.141 | |||||
| One-way ANOVA (among sources) | F8,23 = 42.00; p < 0.001 * | ||||||
| δ15N (‰) | |||||||
| E-SPOM | 4.8 ± 0.2 i (4) | 5.7 ± 0.6 j,k (4) | 0.022 * | 0.368 | 0.411 | ||
| SOM | 3.5 ± 0.2 h (8) | 0.452 | 0.219 | 0.654 | |||
| Phytoplankton | 5.2 ± 0.3 i,j (8) | 0.999 | 0.064 | 0.763 | |||
| P. australis | 6.0 ± 0.2 k (4) | 0.149 | |||||
| R-SPOM | 5.0 ± 0.3 i,j (4) | 0.076 | |||||
| One-way ANOVA (among sources) | F5,26 = 25.00; p < 0.001 * | ||||||
| Factor | δ13C | δ15N | ||||||
|---|---|---|---|---|---|---|---|---|
| df | MS | F | p | df | MS | F | p | |
| Site | 1 | 1.729 | 47.14 | <0.001 * | 1 | 0.160 | 2.054 | 0.167 |
| Season | 1 | 0.191 | 5.215 | 0.033 * | 1 | 0.257 | 3.297 | 0.084 |
| Interaction | 1 | 0.098 | 2.675 | 0.118 | 1 | 0.030 | 0.379 | 0.545 |
| Residual | 20 | 0.037 | 20 | 0.071 | ||||
| Site 1 | Site 2 | Overall mean | ||||||
| June | −25.2 ± 0.3 a (8) | −24.7 ± 0.1 b (4) | 8.0 ± 0.3 (24) | |||||
| August | −25.1 ± 0.1 a (8) | −24.4 ± 0.1 b (4) | ||||||
| Group | Factors | df | Sums of sqs | Mean sqs | Pseudo-F | p (perm) |
|---|---|---|---|---|---|---|
| Food sources | Season | 4 | 3462 | 1154 | 17.75 | <0.001 * |
| Source type | 2 | 5307 | 2653 | 40.81 | <0.001 * | |
| Interaction | 8 | 4256 | 709.3 | 10.91 | <0.001 * | |
| Residual | 20 | 780.2 | 65.01 | |||
| Residual | 1 | 7.114 | 7.951 | |||
| Pairwise “Season × Source type” for pairs of levels of factor “Source type” | ||||||
| Within “Season” | df | Sums of sqs | Mean sqs | Pseudo-F | p (perm) | |
| August 2020 | E-SPOM vs. R-SPOM | 1 | 848 | 848 | 770.8 | <0.001 * |
| 2 | 2.200 | 1.100 | ||||
| E-SPOM vs. P. australis | 1 | 1414 | 1413 | 15.03 | <0.001 * | |
| 2 | 0.181 | 0.094 | ||||
| R-SPOM vs. P. australis | 1 | 1011 | 1010 | 883.7 | <0.001 * | |
| 2 | 2.289 | 1.144 | ||||
| January 2021 | E- SPOM vs. R-SPOM | 1 | 863.4 | 863.4 | 4.772 | <0.001 * |
| 2 | 361.5 | 180.9 | ||||
| E-SPOM vs. P. australis | 1 | 813.8 | 813.8 | 5.591 | <0.001 * | |
| 2 | 291.1 | 145.57 | ||||
| R-SPOM vs. P. australis | 1 | 898.7 | 898.7 | 15.86 | 0.339 | |
| 2 | 113.4 | 56.68 | ||||
| Mar–April 2021 | E- SPOM vs. R-SPOM | 1 | 364.4 | 364.4 | 14.71 | <0.001 * |
| 2 | 49.55 | 24.78 | ||||
| E-SPOM vs. P. australis | 1 | 1471 | 1470 | 86.70 | <0.001 * | |
| 2 | 33.93 | 16.96 | ||||
| R-SPOM vs. P. australis | 1 | 533.2 | 533.2 | 28.92 | <0.001 * | |
| 2 | 36.88 | 18.44 | ||||
| June 2021 | E- SPOM vs. R-SPOM | 1 | 2175 | 2175 | 19.99 | <0.001 * |
| 2 | 217.6 | 108.8 | ||||
| E-SPOM vs. P. australis | 1 | 2394 | 2394 | 31.82 | <0.001 * | |
| 2 | 150.5 | 75.25 | ||||
| R-SPOM vs. P. australis | 1 | 1558 | 1558 | 10.36 | 0.328 | |
| 2 | 300.8 | 150.4 | ||||
| FA | June 2020 (2) | August (2) | January 2021 (12) | April 2021 (8) | June 2021 (8) | p |
|---|---|---|---|---|---|---|
| C13:0 + C15:0 + C17:0 | 5.4 ± 0.7 b | 2.9 ± 0.0 b | 2.0 ± 0.9 a | 1.7 ± 0.5 a | 3.7 ± 0.8 b | 0.001 * |
| C16:0 | 40.0 ± 0.4 c | 29.4 ± 0.1 bc | 18.8 ± 2.7 a | 21.3 ± 3.5 a | 24.2 ± 6.9 ab | 0.019 * |
| C18:0 | 13.2 ± 0.1 c | 5.8 ± 0.1 ac | 8.0 ± 1.1 a | 6.5 ± 0.4 b | 7.6 ± 2.6 ab | 0.005 * |
| C22:0 | — | — | — | 0.2 ± 0.3 | 0.1 ± 0.2 | 0.228 |
| ∑ SFA | 67.0 ± 0.1 c | 47.1 ± 0.9 bc | 44.6 ± 7.7 ab | 41.3 ± 3.2 a | 54.8 ± 3.9c | 0.010 * |
| C16:1ω7 | 7.4 ± 0.0 c | 9.2 ± 0.2 c | 4.3 ± 0.7 b | 3.5 ± 3.3 abc | 1.8 ± 1.4 a | 0.008 * |
| ∑ MUFA | 16.1 ± 0.2 ab | 27.2 ± 0.7 bc | 25.4 ± 3.6 b | 17.9 ± 3.3 a | 14.3 ± 1.6 ac | 0.004 * |
| C18:2ω6 | 1.7 ± 0.0 bc | 3.7 ± 0.1 c | 1.5 ± 0.7 b | 1.7 ± 1.1 b | 0.4 ± 0.5 a | 0.004 * |
| C18:3ω3 | 2.4 ± 0.0 ab | 1.7 ± 0.0 a | — | 2.7 ± 0.5 b | 1.6 ± 0.9 a | 0.021 * |
| C18:3ω6 | 0.7 ± 0.1 a | 0.3 ± 0.0 a | 8.9 ± 1.6 c | 1.6 ± 0.1 b | 1.8 ± 0.8 ab | <0.001 * |
| C20:4ω6 | 2.3 ± 0.1 | 3.8 ± 0.1 | — | 3.3 ± 0.4 | 3.4 ± 0.9 | 0.143 |
| C20:5ω3 | 3.7 ± 0.1 a | 8.8 ± 0.0 ab | 6.9 ± 1.2 b | 12.6 ± 2.3 c | 7.1 ± 3.3 b | 0.001 * |
| C22:6ω3 | 3.4 ± 0.1 a | 4.6 ± 0.1 a | 7.9 ± 1.2 b | 16.4 ± 4.9 c | 13.9 ± 2.6 c | <0.001 * |
| ∑ PUFA | 15.8 ± 0.1 a | 25.6 ± 0.2 ab | 29.9 ± 4.2 b | 40.8 ± 5.1 c | 30.9 ± 4.2 b | <0.001 * |
| ω3:ω6 ratio | 2.0 ± 0.1 a | 1.9 ± 0.0 a | 1.9 ± 0.3 a | 4.8 ± 1.2 b | 3.9 ± 1.2 b | <0.001 * |
| Group | Factors | df | Sums of sqs | Mean sqs | Pseudo-F | p (perm) |
|---|---|---|---|---|---|---|
| C. japonica | Month | 4 | 4487.1 | 1121.8 | 20.22 | <0.001 * |
| Site | 1 | 58.6 | 58.6 | 1.0612 | 0.354 | |
| Month × Site | 4 | 210.41 | 52.603 | 0.94819 | 0.500 | |
| Size (Month × Site) | 21 | 1187.8 | 56.561 | 7.9506 | 0.128 | |
| Residual | 1 | 7.114 | 7.114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.; Kim, C.; Jang, J.; Kim, D.; Kang, C.-K. Identification of Phytoplankton-Based Production of the Clam Corbicula japonica in a Low-Turbidity Temperate Estuary Using Fatty Acid and Stable Isotope Analyses. Water 2023, 15, 1670. https://doi.org/10.3390/w15091670
Seo D, Kim C, Jang J, Kim D, Kang C-K. Identification of Phytoplankton-Based Production of the Clam Corbicula japonica in a Low-Turbidity Temperate Estuary Using Fatty Acid and Stable Isotope Analyses. Water. 2023; 15(9):1670. https://doi.org/10.3390/w15091670
Chicago/Turabian StyleSeo, Dongkyu, Changseong Kim, Jaebin Jang, Dongyoung Kim, and Chang-Keun Kang. 2023. "Identification of Phytoplankton-Based Production of the Clam Corbicula japonica in a Low-Turbidity Temperate Estuary Using Fatty Acid and Stable Isotope Analyses" Water 15, no. 9: 1670. https://doi.org/10.3390/w15091670
APA StyleSeo, D., Kim, C., Jang, J., Kim, D., & Kang, C.-K. (2023). Identification of Phytoplankton-Based Production of the Clam Corbicula japonica in a Low-Turbidity Temperate Estuary Using Fatty Acid and Stable Isotope Analyses. Water, 15(9), 1670. https://doi.org/10.3390/w15091670







