Phage-Based Biocontrol of Antibiotic-Resistant Bacterium Isolated from Livestock Wastewater Treatment Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation of Antibiotic-Resistant Bacteria
2.2. Antibiotic Sensitivity
2.3. Isolation and Characterization of Bacteriophage
2.4. Biological Features of Bacteriophages
2.4.1. One-Step Growth Curve of the Isolated Phage
2.4.2. Effect of Temperature, pH, and Toxic Substances on Phage
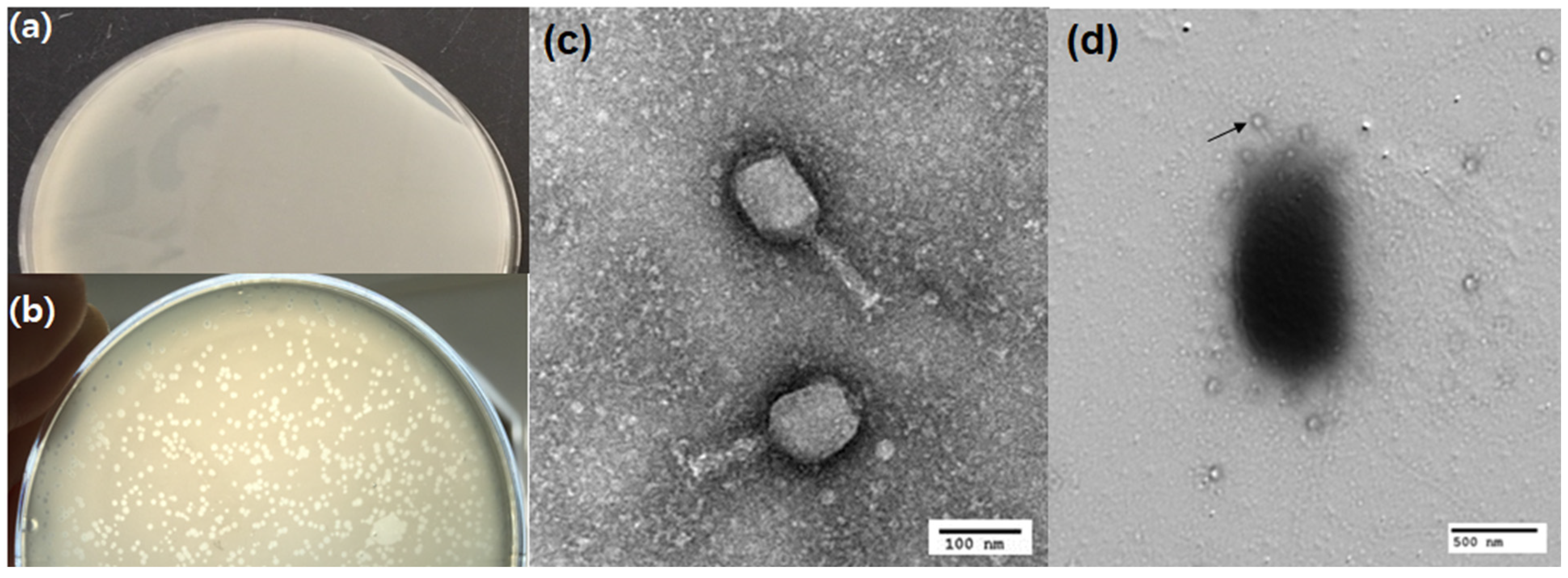
2.4.3. Morphological Study of Phage Using the Transmission Electron Microscope
2.4.4. Analysis of Phage-Host Ratio of Bacteriolysis In Vitro
2.4.5. Phage Action on Biofilm of Aeromonas spp.
2.5. Feasibility Test of Phage-Based Biocontrol in Mixed Cultures
3. Results and Discussion
3.1. Characterization of the Isolated Antibiotic-Resistant Bacteria from Livestock WWTP Effluent
3.2. Isolation and Characterization of Lytic Bacteriophage
3.2.1. Phage Morphology
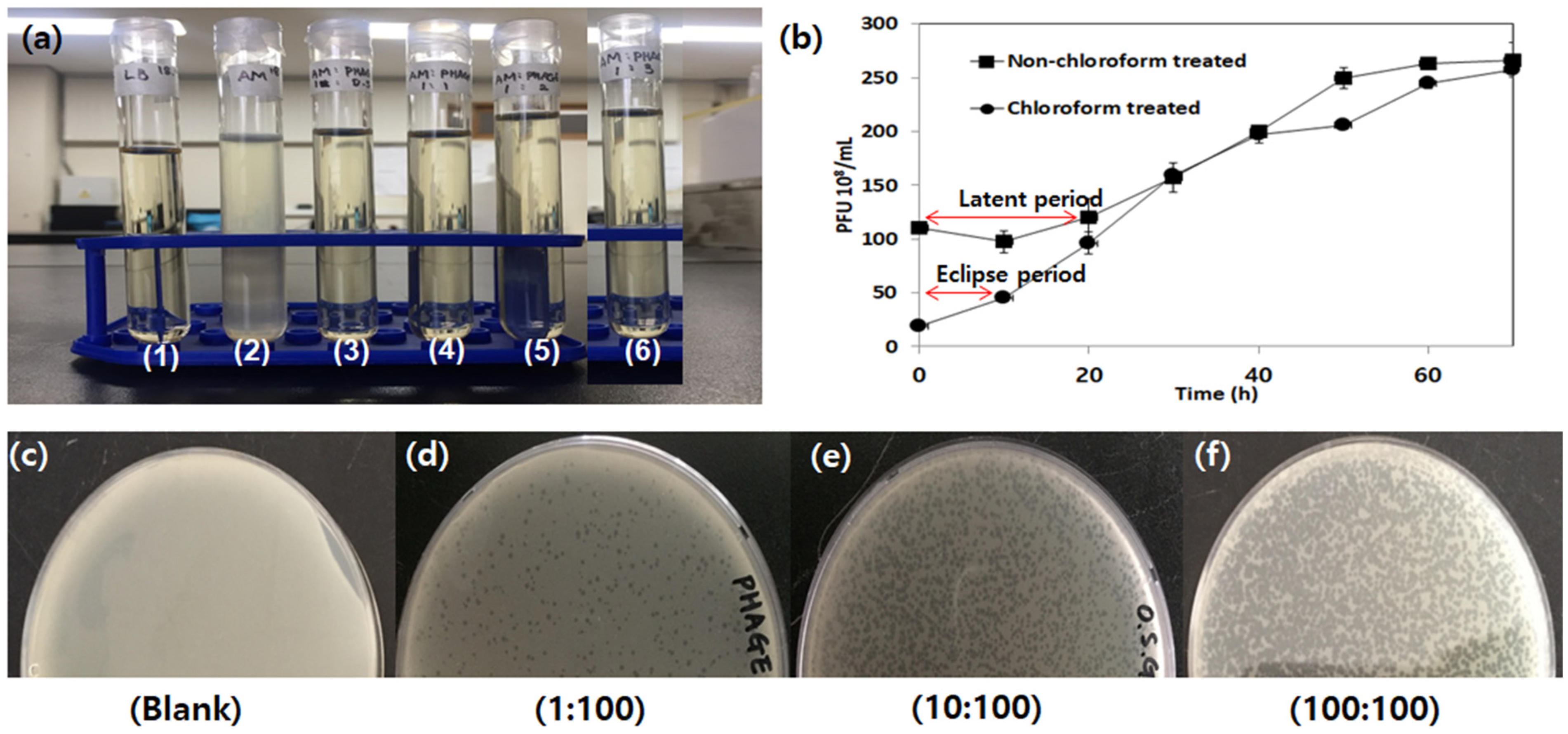
3.2.2. Phage Stability
3.2.3. One-Step Growth Curve
3.2.4. Effect of Phage-to-Host Ratio on Bacteriolysis
3.2.5. Biofilm Reduction by Lytic Aerophage
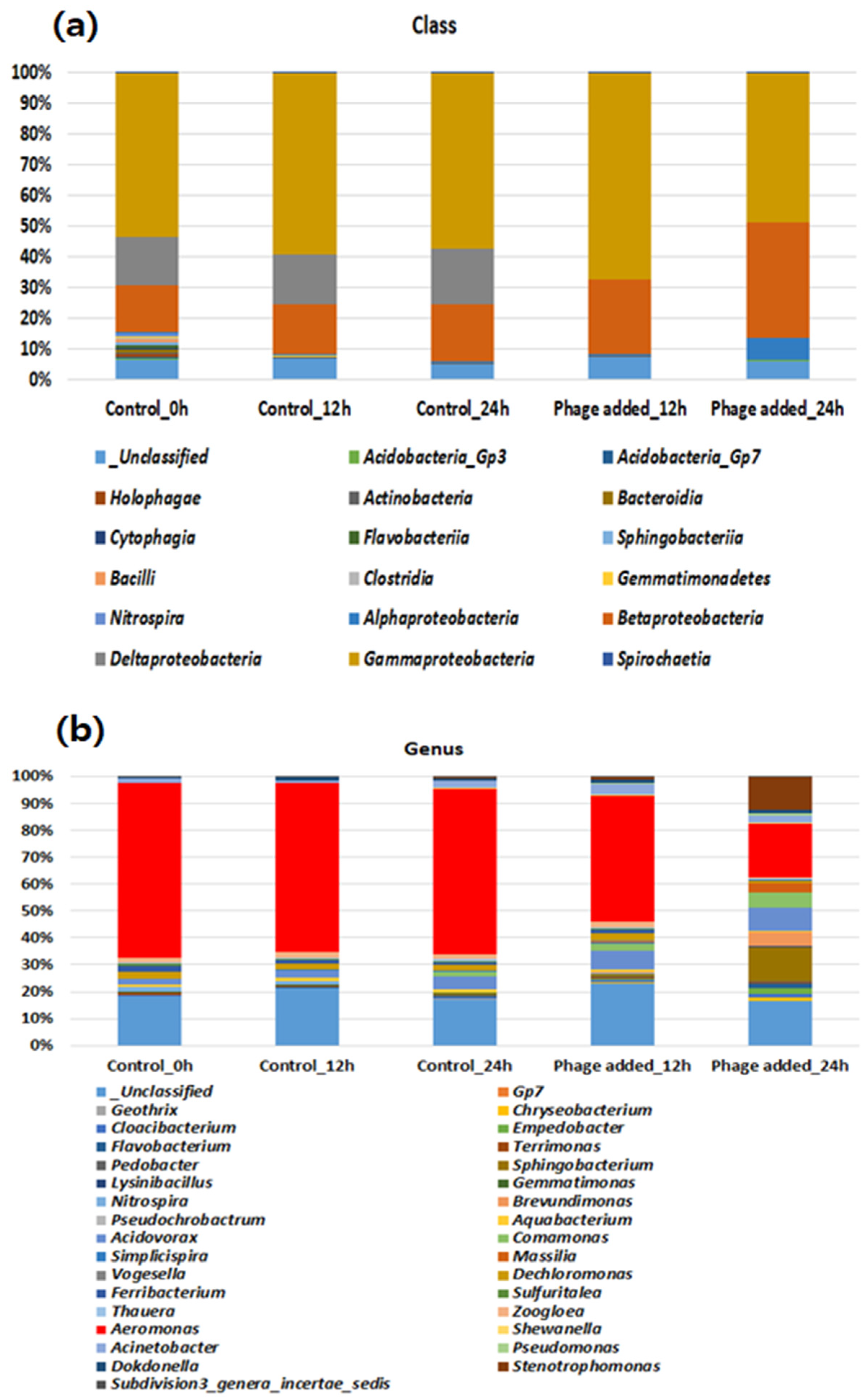
3.3. Bacterial Community Analysis in Lab-Scale Batch Reactors Controlled by Aerophage
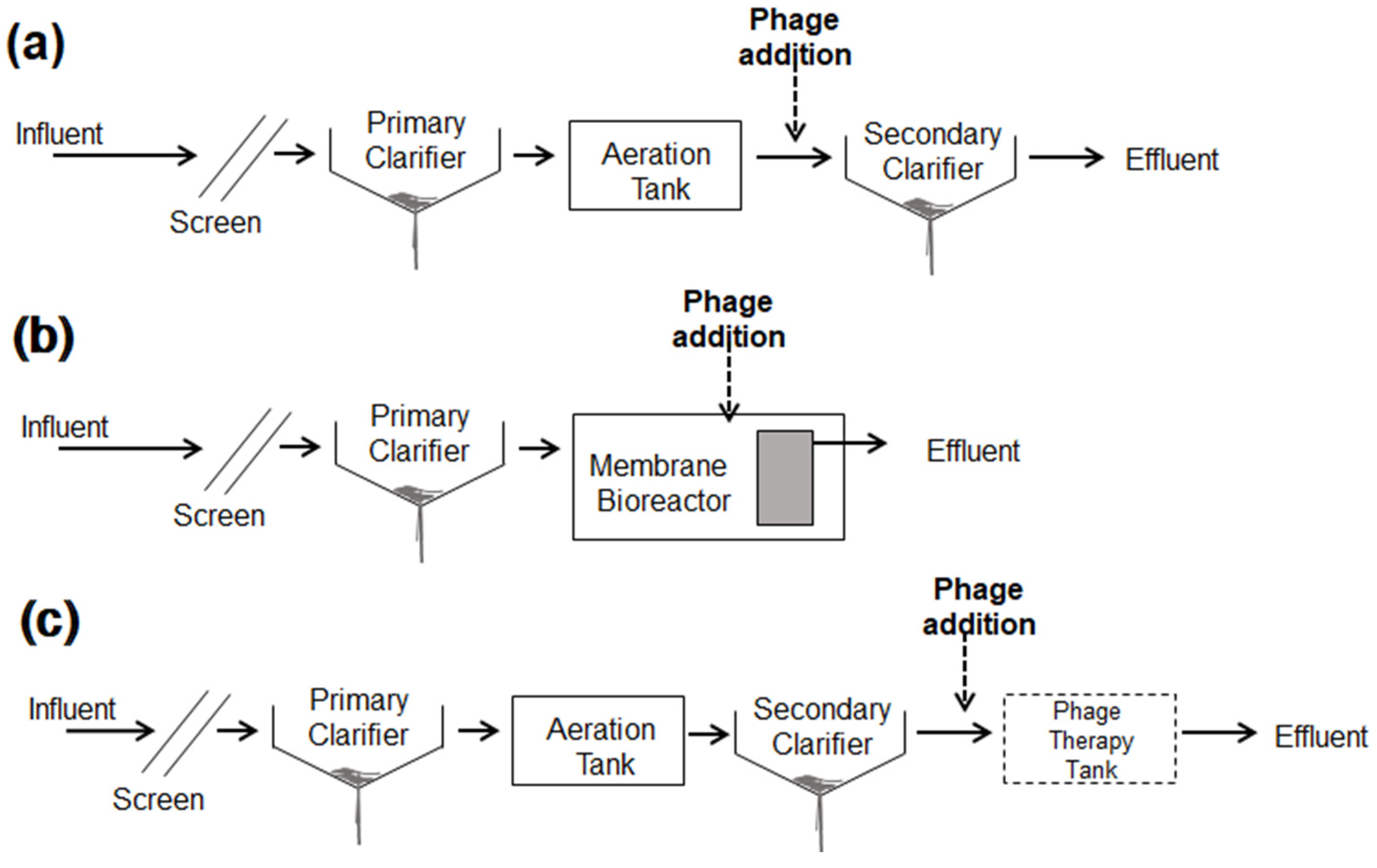
3.4. Potential Application and Limitation in Wastewater Treatment Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rohde, C.; Wittmann, J.; Kutter, E. Bacteriophages: A Therapy Concept against Multi-Drug-Resistant Bacteria. Surg. Infect. 2018, 19, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Mukherjee, R. Ameliorating the Antimicrobial Resistance Crisis: Phage Therapy. IUBMB Life 2019, 71, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, X.; Fan, X.Y.; Zhao, J.R.; Zhang, Z.X. Wastewater treatment plants as reservoirs and sources for antibiotic resistance genes: A review on occurrence, transmission and removal. J. Water Process Eng. 2022, 46, 102539. [Google Scholar] [CrossRef]
- Kauppinen, A.; Siponen, S.; Pitkanen, T.; Holmfeldt, K.; Pursiainen, A.; Torvinen, E.; Miettinen, I.T. Phage biocontrol of Pseudomonas Aeruginosa in water. Viruses 2021, 13, 928. [Google Scholar] [CrossRef]
- Ahn, Y.; Choi, J. Bacterial communities and antibiotic resistance communities in a full scale hospital wastewater treatment plant by high-throughput pyrosequencing. Water 2016, 8, 580. [Google Scholar] [CrossRef]
- Mohan Raj, J.R.; Karunasagar, I. Phages amid Antimicrobial Resistance. Crit. Rev. Microbiol. 2019, 45, 701–711. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total. Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Liu, S.S.; Qu, H.M.; Yang, D.; Hu, H. Chlorine disinfection increases both intracellular and extracellular antibiotic resistance genes in a full-scale wastewater treatment. Water Res. 2018, 136, 131–136. [Google Scholar] [CrossRef]
- Zhang, Z.; LI, B.; Li, N.; Sardar, M.F.; Song, T.; Zhu, C.; Lv, X.; Li, H. Effects of UV disinfection on phenotypes and genotypes of antibiotic-resistant bacteria in secondary effluent from a municipal wastewater treatment plant. Water Res. 2019, 157, 546–554. [Google Scholar] [CrossRef]
- Yuan, Q.B.; Guo, M.T.; Yang, J. Fate of antibiotic resistant bacteria and genes during wastewater chlorination: Implication for antibiotic resistance control. PLoS ONE 2015, 10, e0119403. [Google Scholar] [CrossRef] [PubMed]
- Kotay, S.M.; Datta, T.; Choi, J.; Goel, R. Biocontrol of biomass bulking caused by Haliscomenobacter hydrossis using a newly isolated lytic bacteriophage. Water Res. 2010, 45, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.S.; Choi, J.; Motlagh, A.M.; Mukherji, S.T.; Goel, R. Bacteriophage therapy for membrane biofouling in membrane bioreactors and antibiotic-resistant bacterial biofilms. Biotechnol. Bioeng. 2015, 112, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Choi, J.; Ahn, Y.H. Biofouling reduction in a MBR by the application of a lytic phage on a modified nanocomposite membrane. Environ. Sci. Water Res. Technol. 2018, 4, 1624–1638. [Google Scholar] [CrossRef]
- Withey, S.; Cartmell, E.; Avery, L.M.; Stephenson, T. Bacteriophages—Potential for application in wastewater treatment processes. Sci. Total Environ. 2005, 339, 1–18. [Google Scholar] [CrossRef]
- Meerbergen, K.; Willems, K.A.; Dewil, R.; Impe, J.V.; Appels, L.; Lievens, B. Isolation and screening of bacterial isolates from wastewater treatment plants to decolorize Azo dyes. J. Biosci. Bioeng. 2018, 125, 448–456. [Google Scholar] [CrossRef]
- Numberger, D.; Ganzert, L.; Zoccarato, L.; Muhldorfer, K.; Sauer, S.; Grossart, H.S.; Greenwood, A.D. Characterization of bacterial communities in wastewater with enhanced taxonomic resolution by full-Length 16S rRNA sequencing. Sci. Rep. 2019, 9, 9673. [Google Scholar] [CrossRef]
- Pallavali, R.R.; Degati, V.L.; Lomada, D.; Reddy, M.C.; Durbaka, V.R.P. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS ONE 2017, 12, e0179245. [Google Scholar] [CrossRef]
- Pallavali, R.R.; Degati, V.L.; Reddy, N.V.R.; Durbaka, V.R.P. Isolation and Characterization of a lytic bacteriophage (VB_PAnP_PADP4) against MDR- Pseudomonas Aeruginosa isolated from septic wound infections. Afr. J. Biotechnol. 2019, 18, 325–333. [Google Scholar]
- Kasurinen, J.; Spruit, C.M.; Wicklund, A.; Pajunen, M.I.; Skurnik, M. Screening of bacteriophage encoded toxic proteins with a next generation sequencing-based assay. Viruses 2021, 13, 750. [Google Scholar] [CrossRef]
- Skurnik, M.; Pajunen, M.; Kiljunen, S. Biotechnological challenges of phage therapy. Biotechnol. Lett. 2007, 29, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, Y.; Lu, C.; Liu, Y. Isolation and characterization of bacteriophages against virulent Aeromonas hydrophila. BMC Microbiol. 2020, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Torabi, L.R.; Naghavi, N.S.; Doudi, M.; Monajemi, R. Efficacious antibacterial potency of novel bacteriophages against Esbl-producing Klebsiella Pneumoniae isolated from burn wound infections. Iran. J. Microbiol. 2021, 13, 678–690. [Google Scholar] [PubMed]
- Pallavali, R.R.; Degati, V.L.; Narala, V.R.; Velpula, K.K.; Yenuhu, S. Lytic bacteriophages against bacterial biofilms formed by multidrug-resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus isolated from burn wounds. Phage 2021, 2, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Surachat, K.; Singkhamanan, K. Isolation and characterization of a novel autographiviridae phage and its combined effect with tigecycline in controlling multidrug-resistant Acinetobacter baumannii-Associated skin and soft tissue infections. Viruses 2022, 14, 194. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Rhee, C.; Park, S.G.; Kim, D.W.; Yu, S.I.; Shin, J.; Hwang, S.; Shin, S.G. Tracking microbial community shifts during recovery process in overloaded anaerobic digesters under biological and non-biological supplementation strategies. Bioresour. Technol. 2021, 340, 125614. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Toya, R.; Sasaki, Y.; Uemura, R.; Sueyoshi, M. Indications and patterns of antimicrobial use in pig farms in the southern Kyushu, Japan: Large amount of tetracyclines used to treat respiratory disease in post-weaning and fattening pigs. J. Vet. Med. Sci. 2021, 83, 322–328. [Google Scholar] [CrossRef]
- Sawyer, R.T. Leech Biology and Behaviour: Feeding, Biology, Ecology and Systematic; Clarendon Press: Oxford, UK, 1986; Volume II. [Google Scholar]
- Ghenghesh, K.S.; El-Mohammady, H.; Levin, S.Y.; Zorgani, A.; Tawil, K. Antimicrobial resistance profile of Aeromonas species isolated from Libya. Libyan J. Med. 2013, 8, 21320. [Google Scholar] [CrossRef]
- Aravena-Roman, M.; Inglis, T.J.J.; Henderson, B.; Riley, T.V.; Chang, B.J. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob. Agents Chemother. 2012, 56, 1110–1112. [Google Scholar] [CrossRef]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtboke, D.I. Protective effects of bacteriophages against Aeromonas hydrophila species causing motile Aeromonas Septicemia (MAS) in striped catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Shiha, S.; Grewal, R.K.; Roy, S. Modeling bacteria-phage interactions and its implications for phage therapy. Adv. Appl. Microbiol. 2018, 103, 103–141. [Google Scholar]
- Khairnar, L.; Pal, P.; Chandekar, R.H.; Paunikar, W.N. Isolation and characterization of bacteriophages infecting Nocardioforms in wastewater treatment plant. Biotechnol. Res. Int. 2014, 4, 151952. [Google Scholar] [CrossRef]
- Liu, M.; Gill, J.J.; Young, R.; Summer, E.J. Bacteriophages of wastewater foaming-associated filamentous Gordonia reduce host levels in raw activated sludge. Sci. Rep. 2015, 5, 13754. [Google Scholar] [CrossRef] [PubMed]



| Phage/Host Ratio | Bacteriolytic Activity (% Live Cell) | Optical Density at 600 nm |
|---|---|---|
| Blank | 96.9 ± 0.7 | 0.25 ± 0.03 |
| 0.01:100 | 79.9 ± 0.5 | 0.26 ± 0.02 |
| 0.1:100 | 44.3 ± 0.3 | 0.18 ± 0.01 |
| 1:100 | 5.9 ± 0.5 | 0.06 ± 0.007 |
| 10:100 | 0.9 ± 0.3 | 0.004 ± 0.00 |
| 100:100 | 0.9 ± 0.2 | 0.002 ± 0.00 |
| COD (mg/L) | NH3-N (mg/L) | PO43−-P (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h | 12 h | 24 h | 0 h | 12 h | 24 h | 0 h | 12 h | 24 h | |
| Batch reactor without lytic phage | 352 ± 3.5 | 50 ± 0.0 | 0 ± 0.0 | 26.2 ± 0.0 | 5.2 ± 0.0 | 3.3 ± 0.0 | 4.3 ± 0.0 | 7.5 ± 0.0 | 7.2 ± 0.2 |
| Batch reactor with lytic phage | 352 ± 3.5 | 52 ±0.0 | 0 ± 0.0 | 26.2 ± 0.0 | 5.2 ± 0.0 | 3.2 ± 0.0 | 4.3 ± 0.0 | 7.8 ± 0.0 | 7.8 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pallavali, R.; Shin, D.; Choi, J. Phage-Based Biocontrol of Antibiotic-Resistant Bacterium Isolated from Livestock Wastewater Treatment Plant. Water 2023, 15, 1616. https://doi.org/10.3390/w15081616
Pallavali R, Shin D, Choi J. Phage-Based Biocontrol of Antibiotic-Resistant Bacterium Isolated from Livestock Wastewater Treatment Plant. Water. 2023; 15(8):1616. https://doi.org/10.3390/w15081616
Chicago/Turabian StylePallavali, Rojarani, Donghyeok Shin, and Jeongdong Choi. 2023. "Phage-Based Biocontrol of Antibiotic-Resistant Bacterium Isolated from Livestock Wastewater Treatment Plant" Water 15, no. 8: 1616. https://doi.org/10.3390/w15081616
APA StylePallavali, R., Shin, D., & Choi, J. (2023). Phage-Based Biocontrol of Antibiotic-Resistant Bacterium Isolated from Livestock Wastewater Treatment Plant. Water, 15(8), 1616. https://doi.org/10.3390/w15081616





