Adsorption Characteristics and Influencing Factors of Chlorinated and Aromatic Hydrocarbons on Aquifer Medium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Kinetics of Pollutants on Aquifer Medium
2.3. Competitive Isothermal Adsorption of Pollutants on Aquifer Medium
2.4. Influencing Factors of Competitive Adsorption
2.5. Analysis Methods
3. Results and Discussion
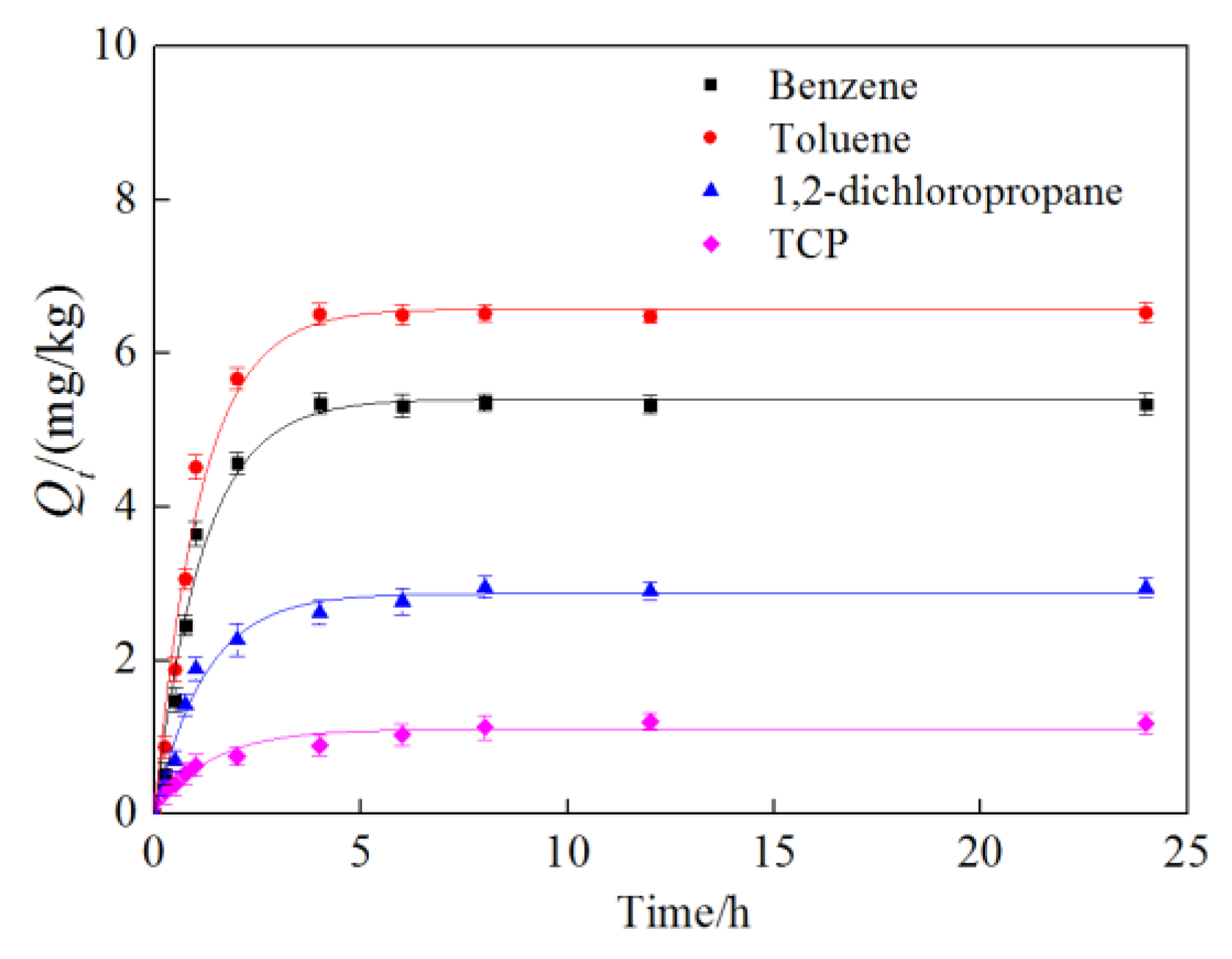
3.1. Adsorption Kinetic Characteristics
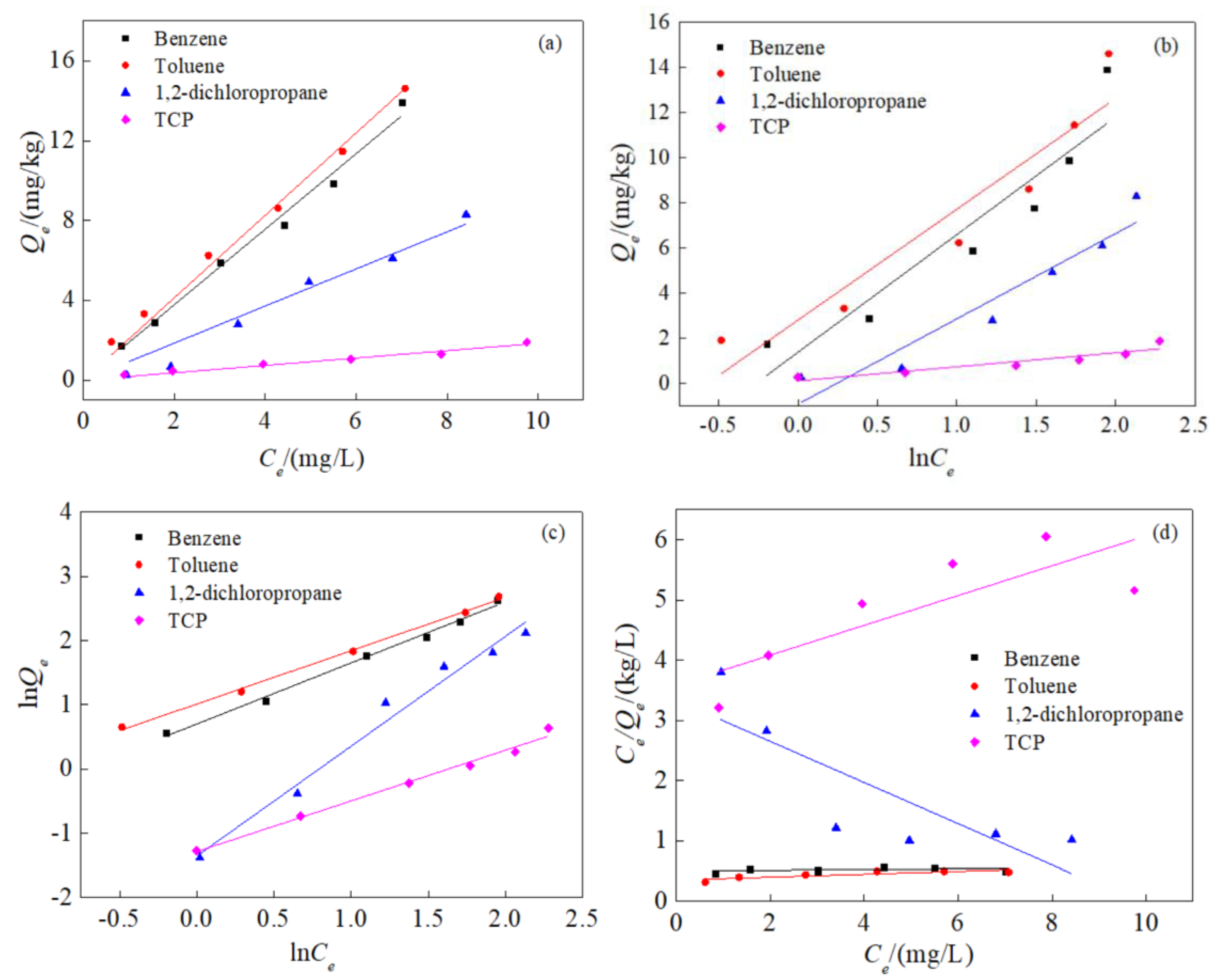
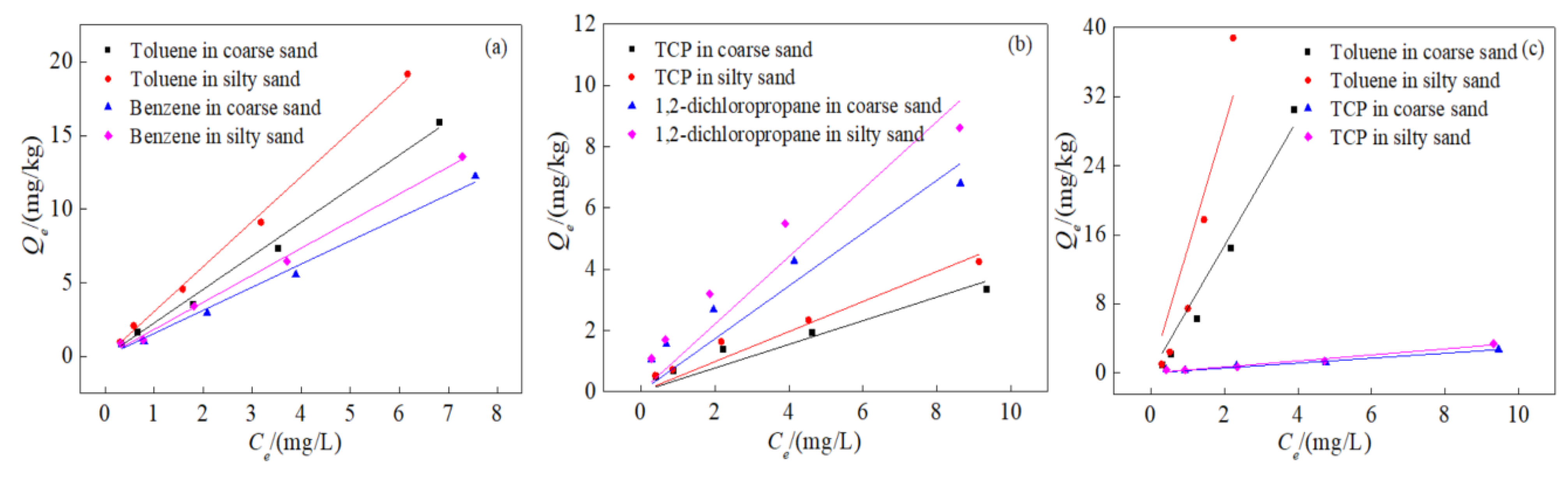
3.2. Isothermal Adsorption Characteristics
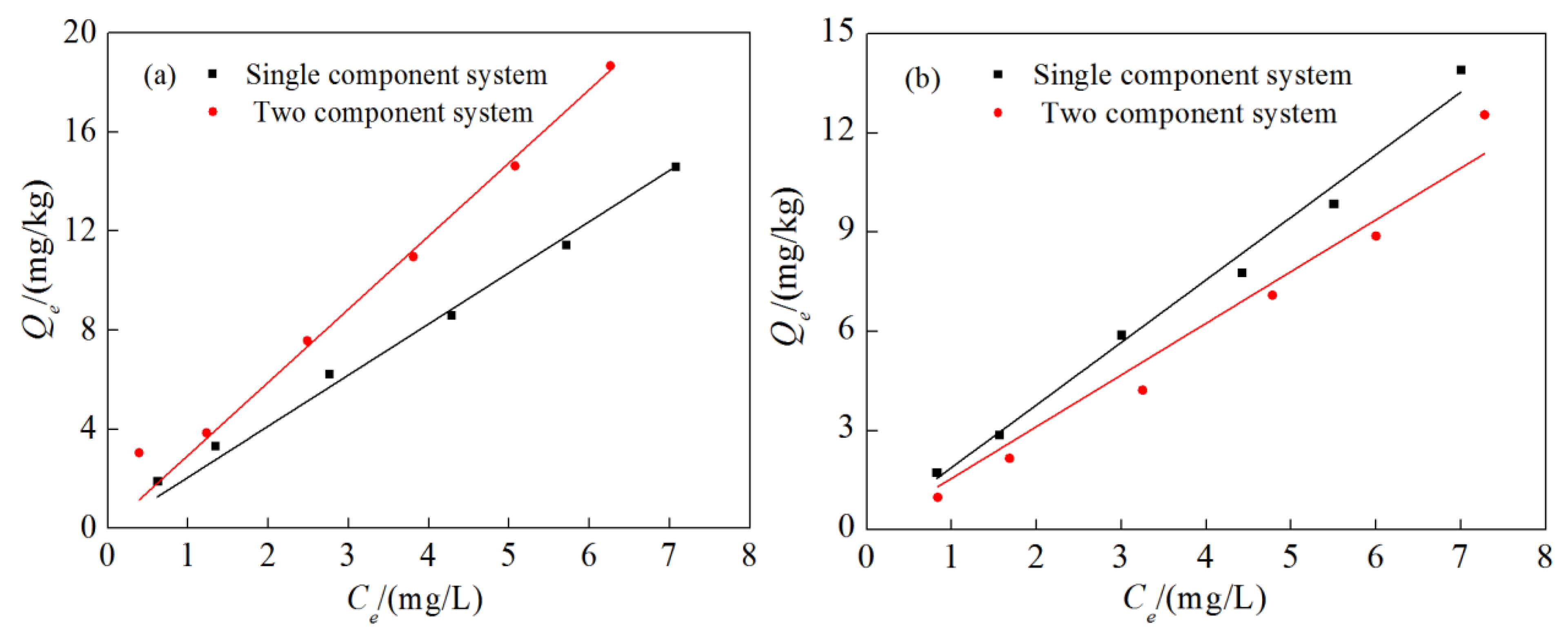
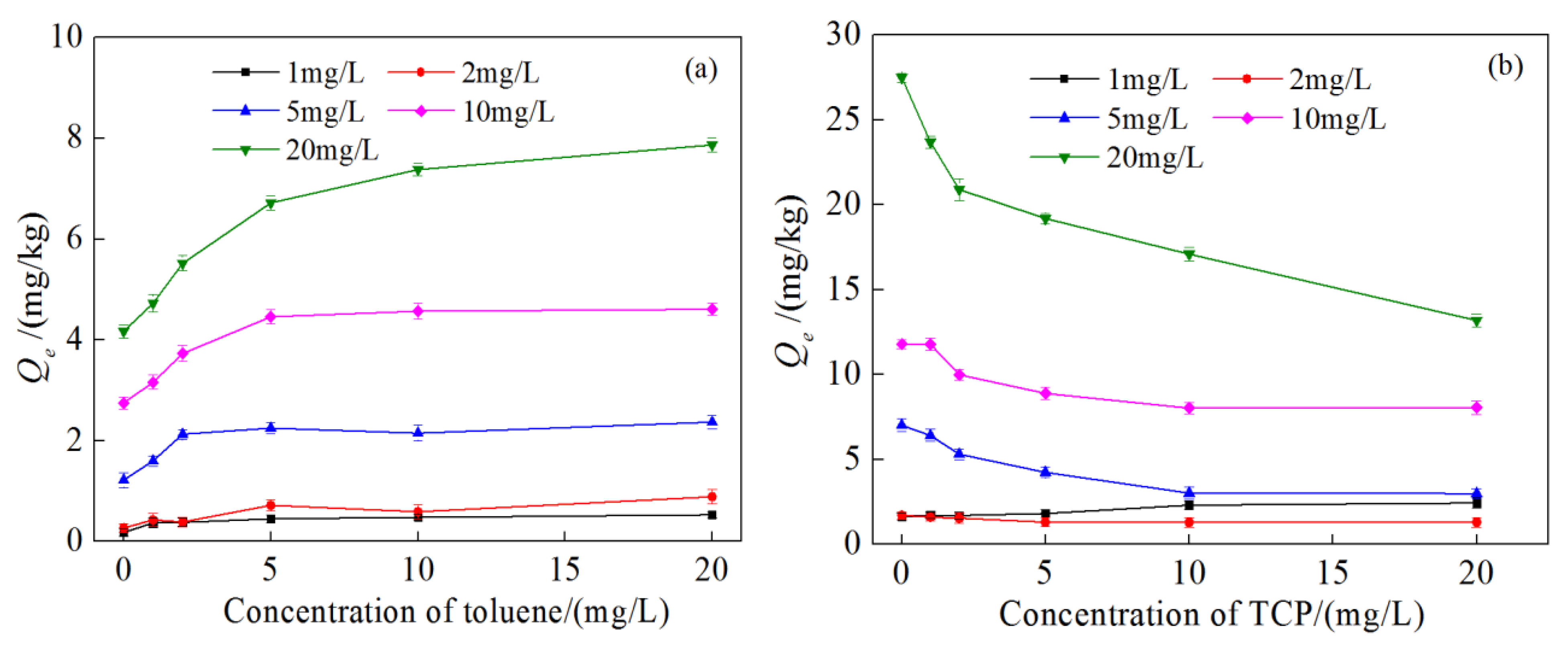
3.3. Competitive Adsorption Characteristics
3.4. Effect of Medium Particle Size on Competitive Adsorption
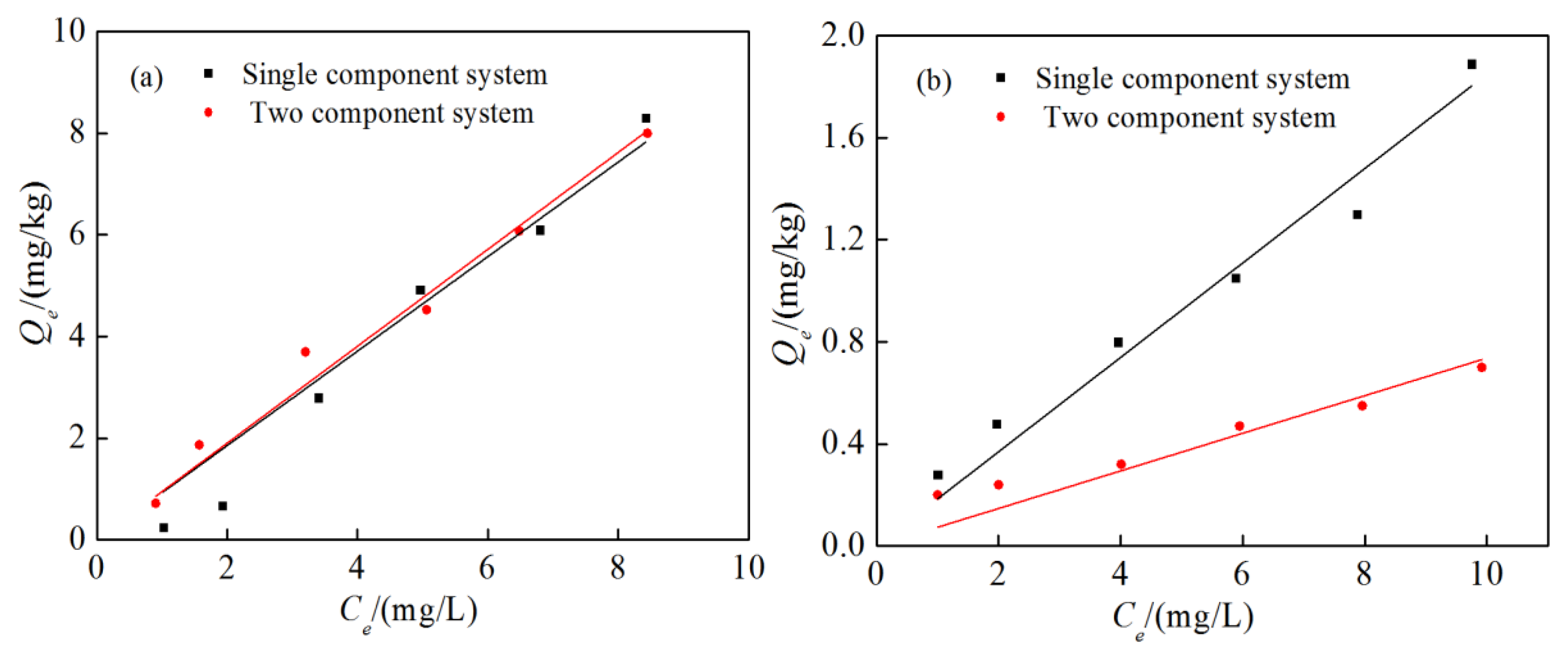
3.5. Effect of NOM on Competitive Adsorption
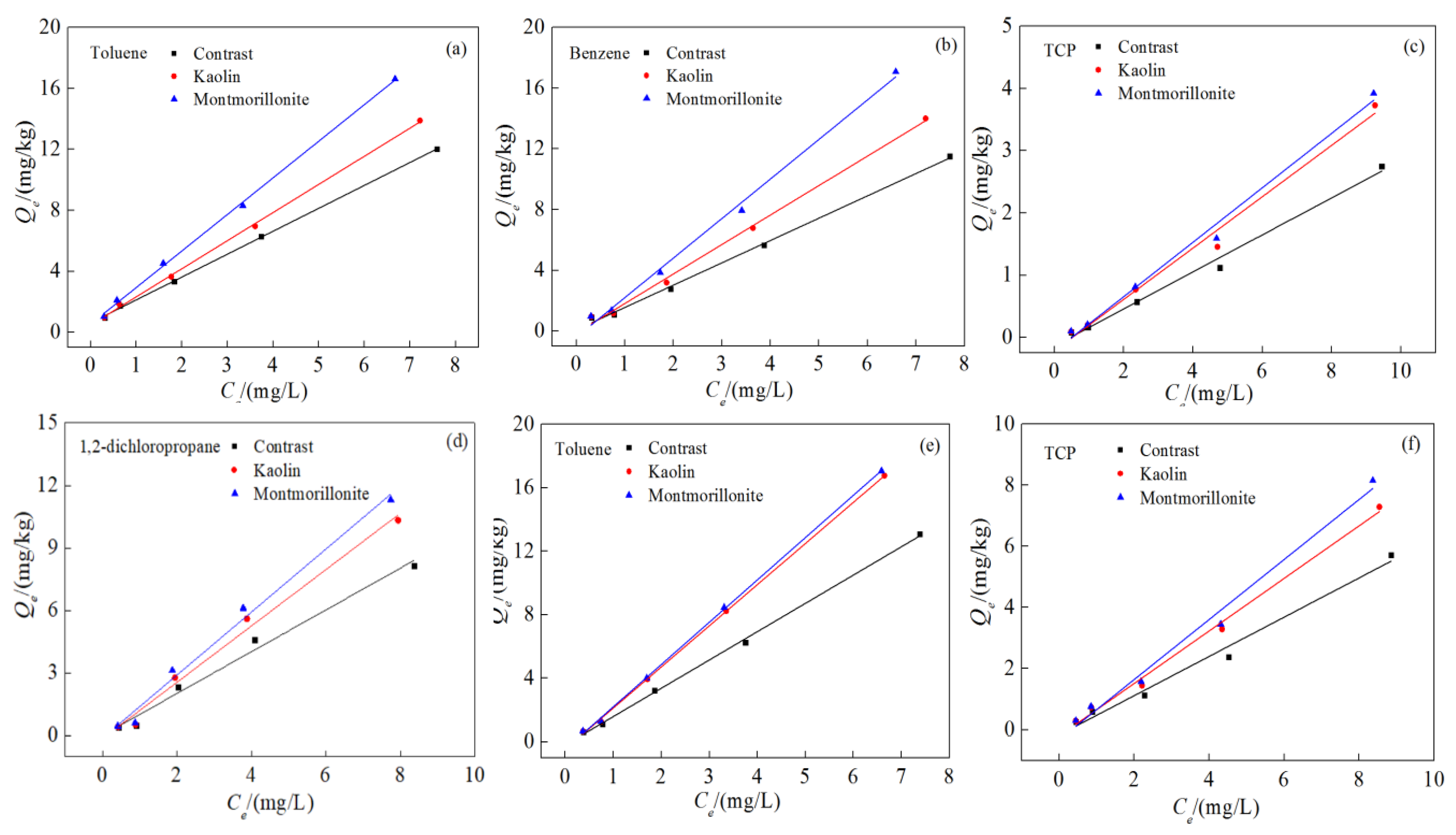
3.6. Effect of Clay Minerals on Competitive Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, B.; Huang, F.; Zhang, C.; Huang, G.; Xue, Q.; Liu, F. Pollution characteristics of aromatic hydrocarbons in the groundwater of China. J. Contam. Hydrol. 2020, 233, 103676–103686. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Bian, J.M.; Wan, H.L.; Sun, X.Q.; Li, Y.M. Probabilistic human health-risk assessment and influencing factors of aromatic hydrocarbon in groundwater near urban industrial complexes in Northeast China. Sci. Total Environ. 2021, 800, 149484–149495. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.H.; Zhang, Z.J.; Li, Y.S.; Guo, C.Y.; Tian, X. Quality evaluation of groundwater in the North China Plain. J. Groundw. Sci. Eng. 2015, 3, 306–315. [Google Scholar]
- Shi, J.S.; Wang, Z.; Zhang, Z.J.; Fei, Y.H.; Zhang, F.E.; Li, Y.S.; Chen, J.S.; Qian, Y. Preliminary analysis of groundwater organic pollution characteristics in North China Plain. J. Ecol. Environ. 2011, 20, 1695–1699. [Google Scholar]
- Zhang, Z.J.; Fei, Y.H.; Luo, G.Z.; Zhang, L.S.; Yang, L.Z.; Lin, J.; Wang, L.H.; Ma, Z.; Qi, J.X. Investigation and Evaluation of Groundwater Pollution in North China Plain. In Annual Compilation of Data on New Progress of Geological Science and Technology and New Achievements of Geological Prospecting in China; China Geological Education Press: Beijing, China, 2009. [Google Scholar]
- Li, X.M.; Li, Q.Q.; Jie, Y.; Huang, S.F.; Zhang, S.Y.; Ji, M. Compound pollution characteristics and ecological risk assessment of heavy metals in soil and groundwater of typical industrial site in Shanghai. Environ. Sci. 2022, 43, 5687–5697. [Google Scholar]
- Qiu, Y.F. Application of groundwater remediation technology at an organic compound contaminated site. Water Purif. Technol. 2018, 37, 235–238. [Google Scholar]
- Chiou, C.T.; Schmedding, D.W.; Manes, M. Partitioning of organic compounds in octanol-water systems. Environ. Sci. Technol. 1983, 17, 227–229. [Google Scholar] [CrossRef]
- Xia, G.S.; Ball, W.P. Adsorption-partitioning uptake of nine low polarity organic chemicals on a natural sorbent. Environ. Sci. Technol. 1999, 33, 262–269. [Google Scholar] [CrossRef]
- Liu, S.J. A mathematical model for competitive adsorptions. Sep. Purif. Technol. 2015, 144, 80–89. [Google Scholar] [CrossRef]
- Baytar, O.; Şahin, Ö.; Horoz, S.; Kutluay, S. High-performance gas-phase adsorption of benzene and toluene on activated carbon: Response surface optimization, reusability, equilibrium, kinetic, and competitive adsorption studies. Environ. Sci. Pollut. Res. 2020, 27, 26191–26210. [Google Scholar] [CrossRef]
- Chen, Y.D.; Jiang, Y.P.; Zhu, Y.N.; Xia, Y.; Cheng, Y.; Huang, Y.; Liu, H. Fate and transport of ethanol-blended dissolved BTEX hydrocarbons: A quantitative tracing study of a sand tank experiment. Environ. Earth Sci. 2013, 70, 49–56. [Google Scholar] [CrossRef]
- Hussein, M.S.; Ahmed, M.J. Fixed bed and batch adsorption of benzene and toluene from aromatic hydrocarbons on 5A molecular sieve zeolite. Mater. Chem. Phys. 2016, 181, 512–517. [Google Scholar] [CrossRef]
- Qian, Y. Research on Environmental Behavior of 1,2,3-Trichloropropane in Groundwater of a Contaminated Site with Chlorinated Pollutants. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2016. [Google Scholar]
- World Health Organization (WHO). 1,2,3-Trichloropropane. Concise International Chemical Assessment Document; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Merrill, J.P.; Suchomel, E.J.; Varadhan, S.; Asher, M.; Kane, L.Z.; Hawley, E.L.; Deeb, R.A. Development and validation of technologies for remediation of 1,2,3-Trichloropropane in groundwater. Curr. Pollut. Rep. 2019, 5, 228–237. [Google Scholar] [CrossRef]
- Li, H.; Han, Z.T.; Ma, C.X.; Gui, J.Y. Comparison of 1,2,3-Trichloropropane reduction and oxidation by nanoscale zero-valent iron, zinc and activated persulfate. J. Groundw. Sci. Eng. 2015, 3, 156–163. [Google Scholar]
- Yin, S.H. Study on Sorption of Volatile Orgainc Contaminats by Natural Soil and Sediments. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2006. [Google Scholar]
- Zhang, H.; Yang, Y.T.; Ma, J.H.; Li, R.F. Adsorption equilibrium and kinetics of toluene on hierarchical mordenite. J. Fuel Chem. Technol. 2018, 46, 710–716. [Google Scholar]
- Wang, W. Research on the Migration and Transformation Mechanism of Petroleum Characteristic Contaminants in Shallow Groundwater. Ph.D. Thesis, Jilin University, Changchun, China, 2012. [Google Scholar]
- Ma, Y.J.; Bi, E.P.; Chen, H.H. Sorption behavior of benzene and toluene to soils. Acta Petrol. Mineral. 2011, 30, 1105–1110. [Google Scholar]
- Schwab, A.P.; Splichal, P.A.; Banks, M.K. Adsorption of atrazine and alachlor to aquifer material and soil. Water Air Soil Pollut. 2006, 177, 119–134. [Google Scholar] [CrossRef]
- Zhang, K.F.; He, J.T.; Liu, M.L.; Qu, X.Y.; Zhang, J. The effects of different contents of organic carbon on the adsorption of trichlorinated hydrocarbon in soil. Acta Petrol. Mineral. 2009, 28, 649–652. [Google Scholar]
- Ping, L.; Luo, Y.; Wu, L.; Qian, W.; Song, J.; Christie, P. Phenanthrene adsorption by soils treated with humic substances under different pH and temperature conditions. Env. Geochem. Health 2006, 28, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Garba, J.; Samsuri, A.W.; Othman, R.; Hamdani, M.S.A. Adsorption-desorption and leaching potential of glyphosate and aminomethylphosphonic acid in acidic Malaysian soil amended with cow dung and rice husk ash. Env. Monit Assess 2018, 190, 676–690. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, X.Z.; Xie, J.C.; Zhao, Z.C.; Hou, Y.L.; Liu, F.; Song, Q.W.; Wang, Y.Q.; Lu, L. Comprehensive experiment of adsorption of BTEX in groundwater by different porous media. Exp. Technol. Manag. 2021, 38, 89–95. [Google Scholar]
- Lou, B.F. The Competitive Adsorption Effect and Its Influencing Factors of Organic Pollutants on sediments. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2004. [Google Scholar]
- Lee, S.W.; Cheon, J.K.; Park, H.J.; Lee, M.G. Adsorption characteristics of binary vapors among acetone, MEK, benzene, and toluene. Korean J. Chem. Eng. 2018, 25, 1154–1159. [Google Scholar] [CrossRef]
- Abedi ZAssadi, A.; Farahmandkia, Z.; Mehrasebi, M.R. The effect of natural organic compounds on the adsorption of toluene and ethylene benzene on MWCNT. J. Env. Health Sci. Eng. 2019, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.K.; Zhang, Z.X.; Wang, P.; Wang, Y.; Zhang, Z.; Han, Z.; Ma, L. Transformation of ammonium nitrogen and response characteristics of nitrifying functional genes in tannery sludge contaminated soil. J. Groundw. Sci. Eng. 2022, 10, 223–232. [Google Scholar]
- Tang, L.; Gudda, F.O.; Wu, C.X.; Ling, W.T.; El-Ramady, H.; Mosa, A.; Wang, J. Contributions of partition and adsorption to polycyclic aromatic hydrocarbons sorption by fractionated soil at different particle sizes. Chemosphere 2022, 301, 134715–134725. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Karanfil, T. The effects of dissolved natural organic matter on the adsorption of synthetic organic chemicals by activated carbons and carbon nanotubes. Water Res. 2011, 45, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Apul, O.G.; Wang, Q.; Zhou, Y.; Karanfil, T. Adsorption of aromatic organic contaminants by graphene nanosheets: Comparison with carbon nanotubes and activated carbon. Water Res. 2013, 47, 1648–1654. [Google Scholar] [CrossRef]
- Liu, W.J. Aging and Bioavailability of Polycyclic Aromatic Hydrocarbons in Organo-Mineral Complexes with Different Bridging Cations. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2020. [Google Scholar]
- Murphy, E.M.; Zachara, J.M.; Smith, S.C. Influence of mineral-bound humic substances on the sorption of hydrophobic organic compounds. Environ. Sci. Technol. 1990, 24, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption of aromatic compounds by carbonaceous adsorbents: A comparative study on granular activated carbon, activated carbon fiber, and carbon nanotubes. Environ. Sci. Technol. 2010, 44, 6377–6383. [Google Scholar] [CrossRef]
- Zhou, Y.; Apul, O.G.; Karanfil, T. Adsorption of halogenated aliphatic contaminants by graphene nanomaterials. Water Res. 2015, 79, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.J. Sorption and Transport of Typical Gasoline Components in Saturated Porous Media. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2012. [Google Scholar]
- Novikau, R.; Lujaniene, G. Adsorption behaviour of pollutants: Heavy metals, radionuclides, organic pollutants, on clays and their minerals (raw, modified and treated): A review. J. Environ. Manag. 2022, 309, 114685–114707. [Google Scholar] [CrossRef] [PubMed]

| Soil Type | Medium Size (mm) | TOC (%) | pH | Cation Exchange Capacity (CEC, mol/kg) |
|---|---|---|---|---|
| Un−sieved sand | - | 0.22 | 7.15 | 9.82 |
| Silty sand | 0.075~0.1 | 0.27 | 7.10 | 10.21 |
| Fine sand | 0.1~0.25 | 0.25 | 7.20 | 10.10 |
| Coarse sand | 0.5~1 | 0.19 | 7.18 | 9.24 |
| Pollutants | Henry | Temkin | Freundlich | Langmuir | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Kd (L/kg) | R2 | K1 | K2 | R2 | Kf | n | R2 | KL | R2 | |
| Benzene | 1.8920 | 0.9965 | 5.2066 | 1.3958 | 0.9531 | 5.0130 | 1.0502 | 0.9923 | 0.0113 | −0.0905 |
| Toluene | 2.0658 | 0.9969 | 4.9083 | 2.8177 | 0.9182 | 10.2754 | 1.2039 | 0.9967 | 0.0673 | 0.6824 |
| 1,2−dichloropropane | 0.9292 | 0.9809 | 3.7784 | −0.9096 | 0.9526 | 0.0440 | 0.5842 | 0.9743 | −0.1024 | 0.5979 |
| TCP | 0.1849 | 0.9900 | 0.6223 | 0.1205 | 0.9594 | 0.0516 | 1.2668 | 0.9859 | 0.0673 | 0.5858 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Han, Z.; Kong, X.; Wang, Y.; Song, L. Adsorption Characteristics and Influencing Factors of Chlorinated and Aromatic Hydrocarbons on Aquifer Medium. Water 2023, 15, 1539. https://doi.org/10.3390/w15081539
Li H, Han Z, Kong X, Wang Y, Song L. Adsorption Characteristics and Influencing Factors of Chlorinated and Aromatic Hydrocarbons on Aquifer Medium. Water. 2023; 15(8):1539. https://doi.org/10.3390/w15081539
Chicago/Turabian StyleLi, Hui, Zhantao Han, Xiangke Kong, Yanyan Wang, and Le Song. 2023. "Adsorption Characteristics and Influencing Factors of Chlorinated and Aromatic Hydrocarbons on Aquifer Medium" Water 15, no. 8: 1539. https://doi.org/10.3390/w15081539
APA StyleLi, H., Han, Z., Kong, X., Wang, Y., & Song, L. (2023). Adsorption Characteristics and Influencing Factors of Chlorinated and Aromatic Hydrocarbons on Aquifer Medium. Water, 15(8), 1539. https://doi.org/10.3390/w15081539





