Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Biosorbents
2.3. Characterization of Biosorbents
2.4. Adsorption Experiments
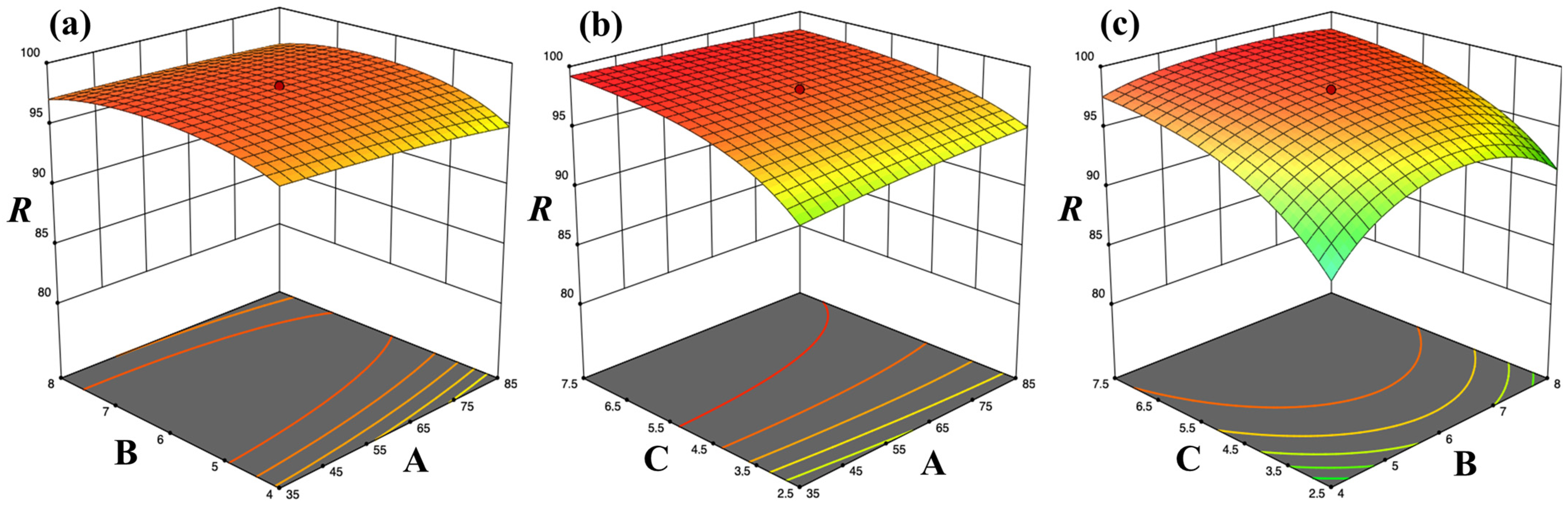
2.4.1. Optimization of Adsorption Conditions
2.4.2. Adsorption Kinetics and Isotherm Models
3. Results and Discussion
3.1. Biosorbent Characterizations
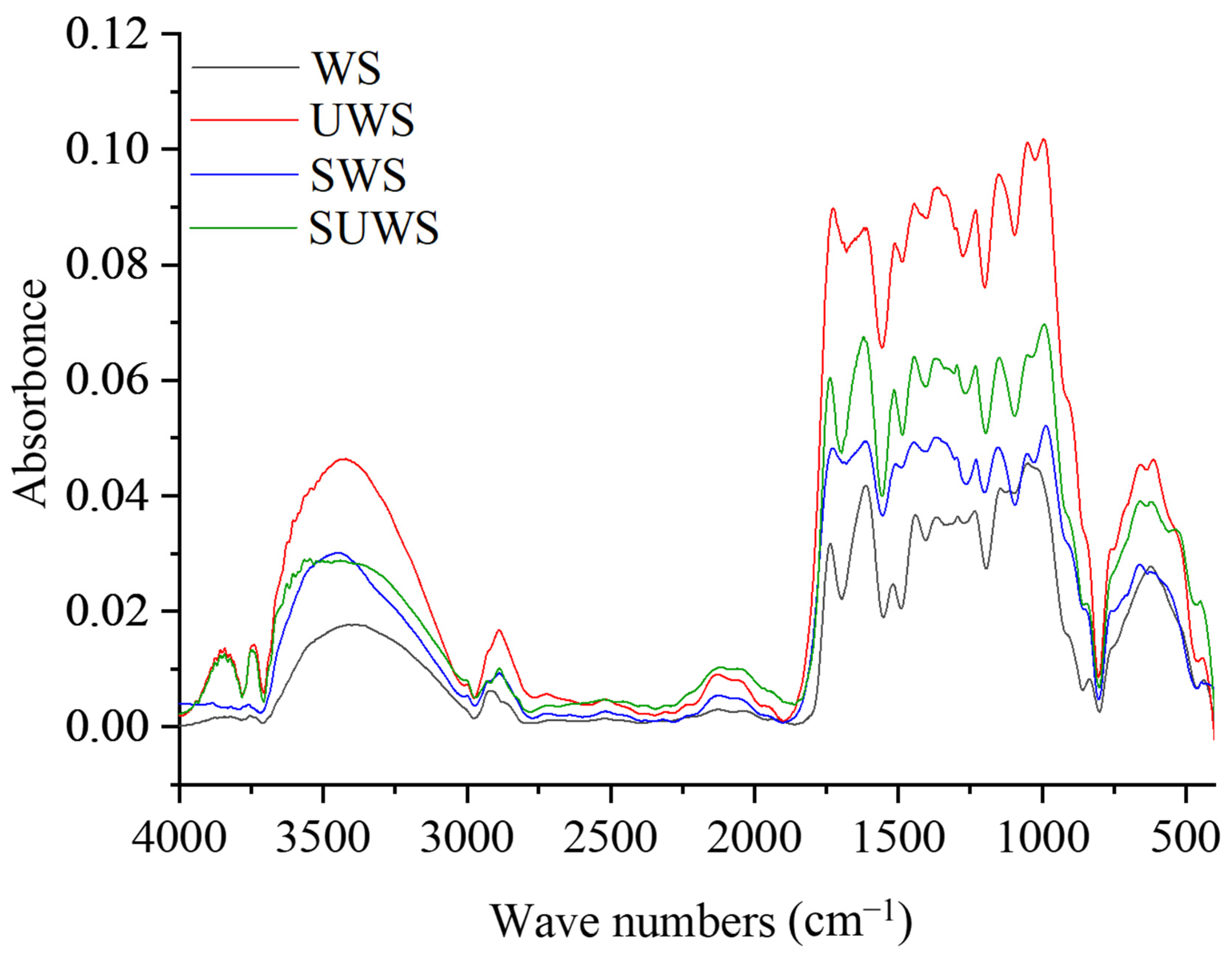
3.1.1. FTIR Analysis
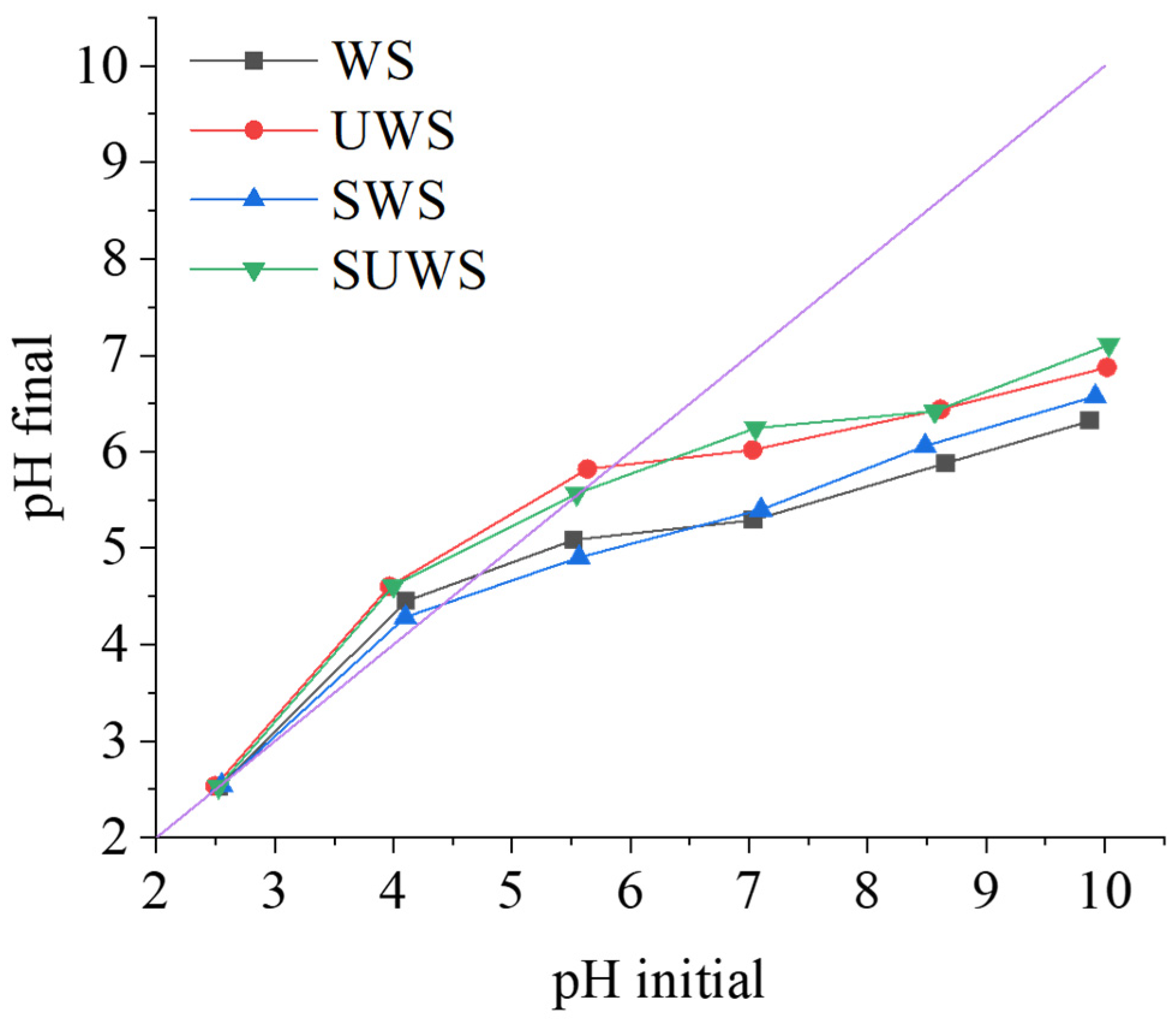
3.1.2. Determination of Zero-Point Charge
3.1.3. Elemental Analysis and SEM-EDX Analysis
3.2. Adsorption Studies
3.2.1. Effect of Operating Parameters
Effect of pH
Effect of Biosorbent Dose
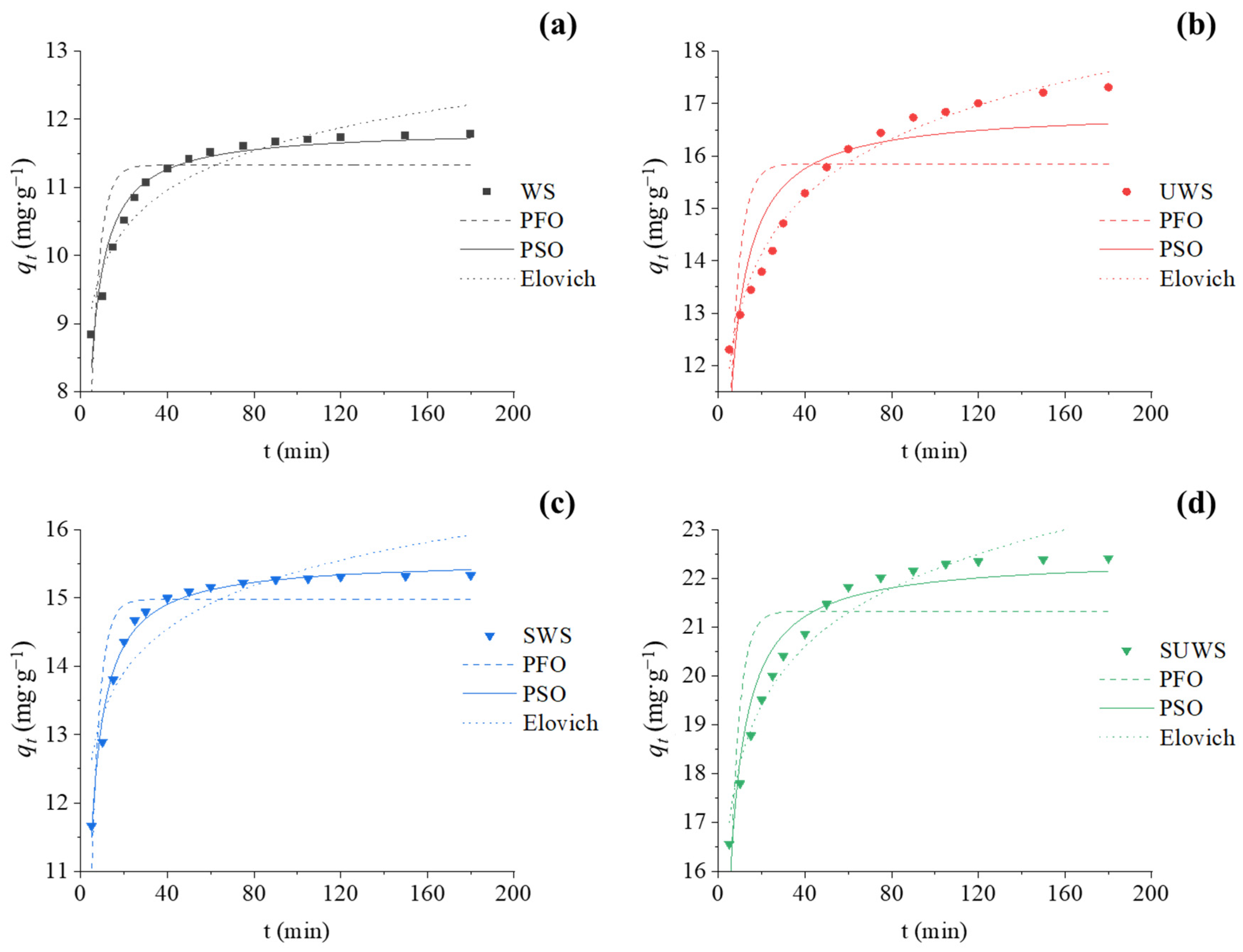
3.2.2. Adsorption Kinetics
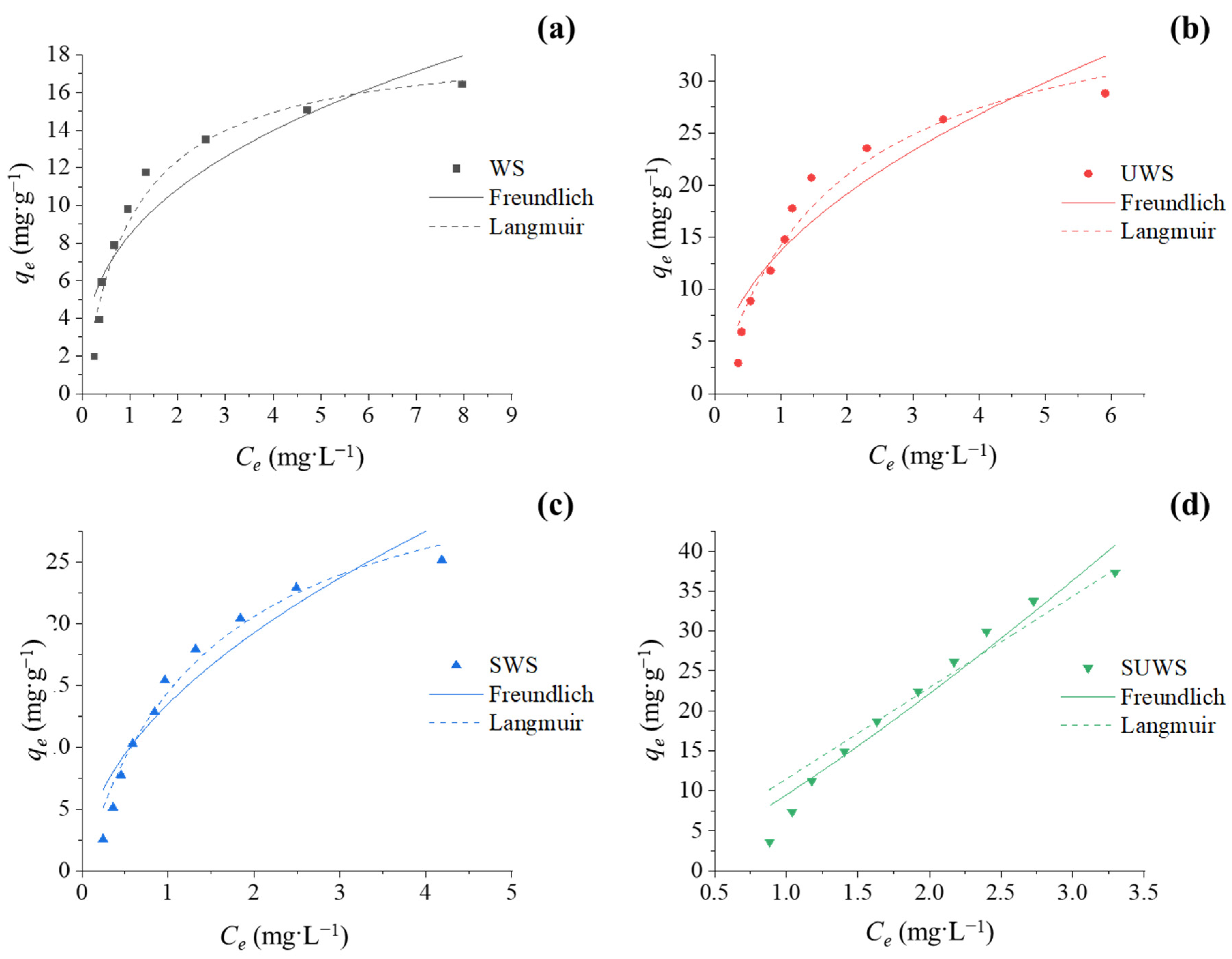
3.2.3. Adsorption Isotherms
3.3. Comparison of Various Low-Cost Biosorbents
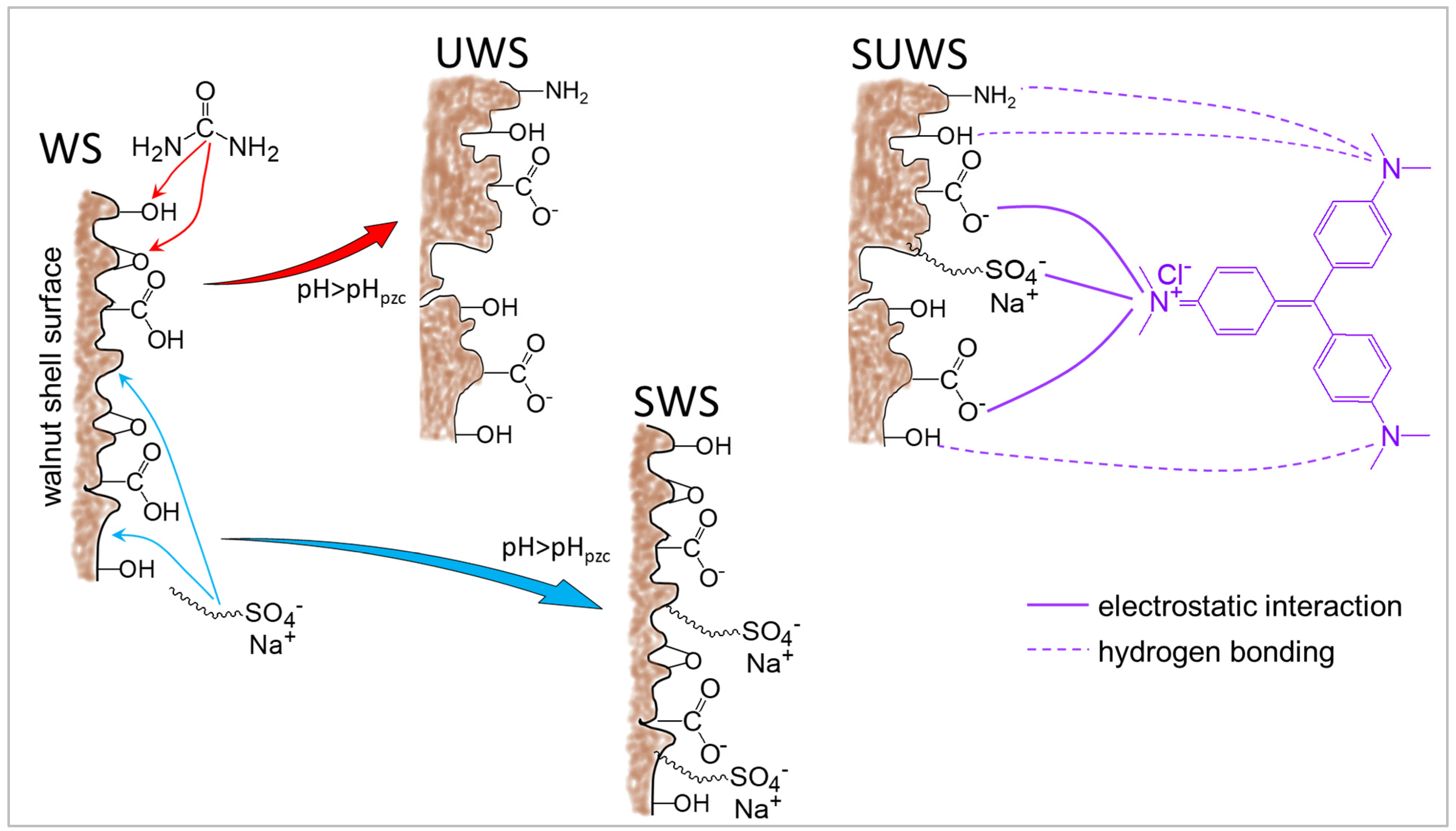
3.4. Proposed Adsorption Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| CCFD | central composite face-centered design |
| CMC | critical micellar concentration |
| CV | crystal violet |
| PFO | pseudo-first-order kinetic model |
| PSO | pseudo-second-order kinetic model |
| SDS | sodium dodecylsulfate |
| SUWS | Urea- and SDS-treated walnut shell adsorbent |
| SWS | SDS-treated walnut shell adsorbent |
| UWS | urea-treated walnut shell adsorbent |
| WS | walnut shell adsorbent |
References
- Nworie, F.S.; Nwabue, F.I.; Oti, W.; Mbam, E.; Nwali, B.U. Removal of methylene blue from aqueous solution using activated rice husk biochar: Adsorption isotherms, kinetics and error analysis. J. Chil. Chem. Soc. 2019, 64, 4365–4376. [Google Scholar] [CrossRef] [Green Version]
- Godiya, C.B.; Kumar, S.; Xiao, Y. Amine functionalized egg albumin hydrogel with enhanced adsorption potential for diclofenac sodium in water. J. Hazard. Mater. 2020, 393, 122417. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wu, X.; Tan, X.; Wang, X. Sorption of Heavy Metal Ions from Aqueous Solutions: A Review. Open Colloid Sci. J. 2011, 4, 19–31. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Martínez, M.L.; Labuckas, D.O.; Lamarque, A.L.; Maestri, D.M. Walnut (Juglans regia L.): Genetic resources, chemistry, by-products. J. Sci. Food Agric. 2010, 90, 1959–1967. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; Al-Marri, M.J. Walnut shell based adsorbents: A review study on preparation, mechanism, and application. J. Water Process Eng. 2022, 45, 102527. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Hassanpour, M.; Rowlings, D.W.; Bai, Z.; Dunn, K.; O’Hara, I.M.; Zhang, Z. Effects of lignocellulosic biomass type on nutrient recovery and heavy metal removal from digested sludge by hydrothermal treatment. J. Environ. Manag. 2022, 318, 115524. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Jahanban-Esfahlan, R.; Tabibiazar, M.; Roufegarinejad, L.; Amarowicz, R. Recent advances in the use of walnut (Juglans regia L.) shell as a valuable plant-based bio-sorbent for the removal of hazardous materials. RSC Adv. 2020, 10, 7026–7047. [Google Scholar] [CrossRef] [Green Version]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Din, M.I.; Farooq, U.; Athar, M.; Latif Mirza, M. Environmentally benevolent urea modified Saccharum bengalense as a high capacity biosorbent for removal of Pb(II) ions: Metal uptake modeling and adsorption efficiency. Desalination Water Treat. 2014, 52, 5856–5868. [Google Scholar] [CrossRef]
- Orlando, U.S.; Baes, A.U.; Nishijima, W.; Okada, M. Comparative effectivity of different types of neutral chelating agents for preparing chelated bagasse in solvent-free conditions. J. Clean. Prod. 2004, 12, 753–757. [Google Scholar] [CrossRef]
- Hu, X.; Zhu, J.; Li, J.; Wu, Q. Urea Electrooxidation: Current Development and Understanding of Ni-Based Catalysts. ChemElectroChem 2020, 7, 3211–3228. [Google Scholar] [CrossRef]
- Rong, L.; Shanwen, T.; John, T.S.I. A direct urea fuel cell—Power from fertiliser and waste. Energy Environ. Sci. 2010, 3, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Farooq, U.; Khan, M.A.; Athar, M.; Kozinski, J.A. Effect of modification of environmentally friendly biosorbent wheat (Triticum aestivum) on the biosorptive removal of cadmium(II) ions from aqueous solution. Chem. Eng. J. 2011, 171, 400–410. [Google Scholar] [CrossRef]
- Yelatonsev, D.; Mukhachev, A.; Ivanyuk, O. An Effective Biosorbent Derived from Production Waste for Water Treatment: Studying the Adsorption of Synthetic Dyes. Sci. Innov. 2021, 17, 83–96. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, G.; Guo, X.; Xu, Y. Designing a novel N-doped adsorbent with ultrahigh selectivity for CO2: Waste biomass pyrolysis and two-step activation. Biomass Convers. Biorefinery 2020, 11, 2843–2854. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Mohammady Maklavany, D.; Rashidi, A.; Jafarinejad, S. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J. Environ. Chem. Eng. 2018, 6, 6653–6663. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Z.; Zhang, G.; Zhao, P. Excellent CO2 adsorption performance of nitrogen-doped waste biocarbon prepared with different activators. J. Clean. Prod. 2020, 264, 121645. [Google Scholar] [CrossRef]
- Wang, W.; Li, D.; Xiang, P.; Zheng, Y.; Zheng, Z.; Lin, X.; He, X.; Liu, C. One-Step Pyrolysis of Nitrogen-Containing Chemicals and Biochar Derived from Walnut Shells to Absorb Polycyclic Aromatic Hydrocarbons (PAHs). Int. J. Mol. Sci. 2022, 23, 15193. [Google Scholar] [CrossRef]
- Ghasemi Kahangi, M.; Rashidi, A.; Samipoorgiri, M. Adsorption methodology: Synthesis of Nanostructured nitrogen-doped porous carbon adsorbents for perchloroethylene vapor adsorption. Anal. Method Environ. Chem. J. 2020, 3, 30–39. [Google Scholar] [CrossRef]
- Bartczak, P.; Wawrzkiewicz, M.; Borysiak, S.; Jesionowski, T. Efficient Biosorbents for Removal of Hazardous Textile Dye, C.I. Basic Blue from Aqueous Solutions. Processes 2022, 10, 586. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sureshkumar, M.V. Removal of chromium(VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour. Technol. 2008, 99, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Oei, B.C.; Ibrahim, S.; Wang, S.; Ang, H.M. Surfactant modified barley straw for removal of acid and reactive dyes from aqueous solution. Bioresour. Technol. 2009, 100, 4292–4295. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.A.; Ebrahim, S.E.; Alwared, A.I. Flotation and Sorptive-Flotation Methods for Removal of Lead Ions from Wastewater Using SDS as Surfactant and Barley Husk as Biosorbent. J. Chem. 2013, 2013, 413948. [Google Scholar] [CrossRef]
- Jain, S.N.; Gogate, P.R. Acid Blue 113 removal from aqueous solution using novel biosorbent based on NaOH treated and surfactant modified fallen leaves of Prunus Dulcis. J. Environ. Chem. Eng. 2017, 5, 3384–3394. [Google Scholar] [CrossRef]
- Ansari, R.; Seyghali, B.; Mohammad-khah, A.; Zanjanchi, M.A. Application of Nano Surfactant Modified Biosorbent as an Efficient Adsorbent for Dye Removal. Sep. Sci. Technol. 2012, 47, 1802–1812. [Google Scholar] [CrossRef]
- Azizi, A.; Krika, F.; Krika, A. Efficient anionic surfactant treatment of cork for cationic dye removal from aqueous media. Glob. NEST J. 2021, 23, 218–225. [Google Scholar] [CrossRef]
- Is, F.; Bonusa, N.H.; Ilmi, L.Y.; Budi, H. Enhanced adsorption capacity of peanut shell toward rhodamine B via sodium dodecyl sulfate modification. Rasayan J. Chem. 2018, 11, 1166–1176. [Google Scholar] [CrossRef]
- Bois, R.; van Hecke, E.; Pezron, I.; Nesterenko, A. Screening of Surfactant Foaming Properties Using the Gas-Sparging Method: Design of an Optimal Protocol. J. Surfactants Deterg. 2020, 23, 359–369. [Google Scholar] [CrossRef]
- Shouman, M.A.; Fathy, N.A.; Khedr, S.A.; Attia, A.A. Comparative Biosorption Studies of Hexavalent Chromium Ion onto Raw and Modified Palm Branches. Adv. Phys. Chem. 2013, 2013, 159712. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Garg, U.K.; Kaur, M.P.; Garg, V.K.; Sud, D. Removal of Nickel(II) from aqueous solution by adsorption on agricultural waste biomass using a response surface methodological approach. Bioresour. Technol. 2008, 99, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.K.; Kaur, M.P.; Sud, D.; Garg, V.K. Removal of hexavalent chromium from aqueous solution by adsorption on treated sugarcane bagasse using response surface methodological approach. Desalination 2009, 249, 475–479. [Google Scholar] [CrossRef]
- Siham, H.; Ahmed, E.-S.; Nourhan, Z.; Marwa, E.-A. Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- Tang, R.; Dai, C.; Li, C.; Liu, W.; Gao, S.; Wang, C. Removal of Methylene Blue from Aqueous Solution Using Agricultural Residue Walnut Shell: Equilibrium, Kinetic, and Thermodynamic Studies. J. Chem. 2017, 2017, 8404965. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ren, H. Comparative study of the photo-discoloration of moso bamboo (Phyllostachys pubescens Mazel) and two wood species. Appl. Surf. Sci. 2008, 254, 7029–7034. [Google Scholar] [CrossRef]
- Sohan, S. Chemical, Structural and Elemental Characterization of Biosorbents Using FE-SEM, SEM-EDX, XRD/XRPD and ATR-FTIR Techniques. J. Chem. Eng. Process Technol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Salman, M.; Athar, M.; Farooq, U.; Nazir, H.; Noor, A.; Nazir, S. Microwave-assisted urea-modified sorghum biomass for Cr (III) elimination from aqueous solutions. Korean J. Chem. Eng. 2013, 30, 1257–1264. [Google Scholar] [CrossRef]
- Nazerian, M.; Kashi, H.R.; Rudi, H.; Papadopoulos, A.N.; Vatankhah, E.; Foti, D.; Kermaniyan, H. Comparison of the Estimation Ability of the Tensile Index of Paper Impregnated by UF-Modified Starch Adhesive Using ANFIS and MLR. J. Compos. Sci. 2022, 6, 341. [Google Scholar] [CrossRef]
- Chiang, T.C.; Hamdan, S.; Osman, M.S. Urea Formaldehyde Composites Reinforced with Sago Fibres Analysis by FTIR, TGA, and DSC. Adv. Mater. Sci. Eng. 2016, 2016, 5954636. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Mei, J.; Zhang, L. High-added-value biomass-derived composites by chemically coupling post-consumer plastics with agricultural and forestry wastes. J. Clean. Prod. 2021, 284, 124768. [Google Scholar] [CrossRef]
- Is, F.; Sahroni, I.; Dahlyani, M.S.E.; Oktaviyani, A.M.N.; Nurillahi, R. Surfactant-modified Salacca zalacca skin as adsorbent for removal of methylene blue and Batik’s wastewater. Mater. Today Proc. 2021, 44, 3211–3216. [Google Scholar] [CrossRef]
- Al-Degs, Y.S.; El-Barghouthi, M.I.; El-Sheikh, A.H.; Walker, G.M. Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dye. Pigment. 2008, 77, 16–23. [Google Scholar] [CrossRef]
- Yao, Y.; Bergeron, A.D.; Davaritouchaee, M. Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew. Energy 2018, 115, 139–148. [Google Scholar] [CrossRef]
- Lim, L.; Priyantha, N.; Zaidi, N.; Jamil, U.A.N.; Hei Ing, C.; Zehra, T.; Liyandeniya, A. Chemical modification of artocarpus odoratissimus skin for enhancement of their adsorption capacities toward toxic malachite green dye. J. Mater Environ. Sci. 2016, 7, 3211–3224. [Google Scholar]
- Nowicki, P.; Pietrzak, R.; Wachowska, H. Sorption properties of active carbons obtained from walnut shells by chemical and physical activation. Catal. Today 2010, 150, 107–114. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Singh, K.P.; Gupta, S.; Singh, A.K.; Sinha, S. Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J. Hazard. Mater. 2011, 186, 1462–1473. [Google Scholar] [CrossRef]
- Önal, Y.; Akmil-Başar, C.; Eren, D.; Sarıcı-Özdemir, Ç.; Depci, T. Adsorption kinetics of malachite green onto activated carbon prepared from Tunçbilek lignite. J. Hazard. Mater. 2006, 128, 150–157. [Google Scholar] [CrossRef]
- Cao, J.-S.; Lin, J.-X.; Fang, F.; Zhang, M.-T.; Hu, Z.-R. A new absorbent by modifying walnut shell for the removal of anionic dye: Kinetic and thermodynamic studies. Bioresour. Technol. 2014, 163, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L. Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front. Environ. Sci. Eng. China 2009, 3, 320–324. [Google Scholar] [CrossRef]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 186, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Juang, R.-S. Equilibrium and kinetic studies on the adsorption of surfactant, organic acids and dyes from water onto natural biopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 35–46. [Google Scholar] [CrossRef]
- Lima, E.C.; Sher, F.; Guleria, A.; Saeb, M.R.; Anastopoulos, I.; Tran, H.N.; Hosseini-Bandegharaei, A. Is one performing the treatment data of adsorption kinetics correctly? J. Environ. Chem. Eng. 2021, 9, 104813. [Google Scholar] [CrossRef]
- Brandani, S. Kinetics of liquid phase batch adsorption experiments. Adsorption 2021, 27, 353–368. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.-C.; Tseng, R.-L. High adsorption capacity NaOH-activated carbon for dye removal from aqueous solution. J. Hazard. Mater. 2008, 152, 1256–1267. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; McKay, G. Elovich equation and modified second-order equation for sorption of cadmium ions onto bone char. J. Chem. Technol. Biotechnol. 2000, 75, 963–970. [Google Scholar] [CrossRef]
- Shao, L.; Chen, H.; Li, Y.; Li, J.; Chen, G.; Wang, G. Pretreatment of corn stover via sodium hydroxide–urea solutions to improve the glucose yield. Bioresour. Technol. 2020, 307, 123191. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Chiu, W.-T.; Wang, C.-C. Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresour. Technol. 2005, 96, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.E.; Murguía, M.C. Biosorption of an anionic dye by peanut shell modified with gemini surfactants: A study on the stability of the modification and the removal efficiency. J. Mol. Liq. 2020, 317, 114262. [Google Scholar] [CrossRef]
- Kumar, R.; Ahmad, R. Biosorption of hazardous crystal violet dye from aqueous solution onto treated ginger waste (TGW). Desalination 2011, 265, 112–118. [Google Scholar] [CrossRef]
- Gong, R.; Zhu, S.; Zhang, D.; Chen, J.; Ni, S.; Guan, R. Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: Kinetic and thermodynamic profile. Desalination 2008, 230, 220–228. [Google Scholar] [CrossRef]
- Parab, H.; Sudersanan, M.; Shenoy, N.; Pathare, T.; Vaze, B. Use of Agro-Industrial Wastes for Removal of Basic Dyes from Aqueous Solutions. CLEAN Soil Air Water 2009, 37, 963–969. [Google Scholar] [CrossRef]
- Silveira, M.B.; Pavan, F.A.; Gelos, N.F.; Lima, E.C.; Dias, S.L.P. Punica granatum Shell Preparation, Characterization, and Use for Crystal Violet Removal from Aqueous Solution. CLEAN Soil Air Water 2014, 42, 939–946. [Google Scholar] [CrossRef]
- Ahmad, R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J. Hazard. Mater. 2009, 171, 767–773. [Google Scholar] [CrossRef]
- Smitha, T.; Santhi, T.; Prasad, A.L.; Manonmani, S. Cucumis sativus used as adsorbent for the removal of dyes from aqueous solution. Arab. J. Chem. 2017, 10, S244–S251. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.S.; Liu, X.; Wen, L.; Zhou, Y.; Jiang, Y.; Li, Z. Comparison of Basic Dye Crystal Violet Removal from Aqueous Solution by Low-Cost Biosorbents. Sep. Sci. Technol. 2008, 43, 3712–3731. [Google Scholar] [CrossRef]
- Saha, P.D.; Chakraborty, S.; Chowdhury, S. Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpus heterophyllus (jackfruit) leaf powder. Colloids Surf. B Biointerfaces 2012, 92, 262–270. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.-S.; Lee, D.-J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mater. 2002, 92, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.; Pham, T.T.; Phan, M.N.; Ngo, T.M.V.; Dang, V.D.; Vu, C.M. Adsorption characteristics of anionic surfactant onto laterite soil with differently charged surfaces and application for cationic dye removal. J. Mol. Liq. 2020, 301, 112456. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, C.; Zhang, Y.; Wu, H. Synthesis and Characterization of Cellulose Carbamate from Wood Pulp, Assisted by Supercritical Carbon Dioxide. BioResources 2013, 8, 1398–1408. [Google Scholar] [CrossRef]
- Tardy, B.L.; Yokota, S.; Ago, M.; Xiang, W.; Kondo, T.; Bordes, R.; Rojas, O.J. Nanocellulose–surfactant interactions. Curr. Opin. Colloid Interface Sci. 2017, 29, 57–67. [Google Scholar] [CrossRef]
- Putri, K.N.A.; Keereerak, A.; Chinpa, W. Novel cellulose-based biosorbent from lemongrass leaf combined with cellulose acetate for adsorption of crystal violet. Int. J. Biol. Macromol. 2020, 156, 762–772. [Google Scholar] [CrossRef]

| Factors | Ranges in Five Levels | |||||
|---|---|---|---|---|---|---|
| −1.682 | −1 | 0 | +1 | +1.682 | ||
| CV concentration (mg·L−1) | A | 18.0 | 35 | 60 | 85 | 102.1 |
| pH | B | 2.6 | 4 | 6 | 8 | 9.4 |
| Biosorbent dose (g·L−1) | C | 0.8 | 2.5 | 5 | 7.5 | 9.2 |
| Independent Factors | Adsorption (%) R | ||||||
|---|---|---|---|---|---|---|---|
| CV Concentration A | pH B | Biosorbent Dose C | WS | UWS | SWS | SUWS | |
| 1 | −1 | −1 | −1 | 93.2 | 86.3 | 88.8 | 84.0 |
| 2 | +1 | −1 | −1 | 81.3 | 88.2 | 89.8 | 91.9 |
| 3 | −1 | +1 | −1 | 88.8 | 76.0 | 93.9 | 91.3 |
| 4 | +1 | +1 | −1 | 83.3 | 88.1 | 91.6 | 92.0 |
| 5 | −1 | −1 | +1 | 98.6 | 97.8 | 98.2 | 98.7 |
| 6 | +1 | −1 | +1 | 95.9 | 94.0 | 96.5 | 95.9 |
| 7 | −1 | +1 | +1 | 98.5 | 98.9 | 98.5 | 98.7 |
| 8 | +1 | +1 | +1 | 97.8 | 95.1 | 98.5 | 98.7 |
| 9 | −1.682 | 0 | 0 | 97.9 | 98.2 | 97.8 | 98.5 |
| 10 | +1.682 | 0 | 0 | 90.0 | 97.8 | 93.8 | 96.7 |
| 11 | 0 | −1.682 | 0 | 80.5 | 83.2 | 88.8 | 88.5 |
| 12 | 0 | +1.682 | 0 | 96.0 | 92.8 | 92.3 | 91.3 |
| 13 | 0 | 0 | −1.682 | 73.1 | 83.1 | 85.6 | 88.5 |
| 14 | 0 | 0 | +1.682 | 98.2 | 98.2 | 98.0 | 98.9 |
| 15 | 0 | 0 | 0 | 95.1 | 94.3 | 97.8 | 98.1 |
| 16 | 0 | 0 | 0 | 95.1 | 94.3 | 97.8 | 98.1 |
| 17 | 0 | 0 | 0 | 95.1 | 94.3 | 97.8 | 98.1 |
| 18 | 0 | 0 | 0 | 95.1 | 94.3 | 97.8 | 98.1 |
| 19 | 0 | 0 | 0 | 95.1 | 94.3 | 97.8 | 98.1 |
| 20 | 0 | 0 | 0 | 95.1 | 94.3 | 97.8 | 98.1 |
| WS | UWS | SWS | SUWS | |||||
|---|---|---|---|---|---|---|---|---|
| pH Initial | pH Final | pH Initial | pH Final | pH Initial | pH Final | pH Initial | pH Final | |
| 2.5 | 2.5 | 2.5 | 2.5 | 2.6 | 2.6 | 2.5 | 2.5 | |
| 4.1 | 4.5 | 4.0 | 4.6 | 4.1 | 4.3 | 4.0 | 4.6 | |
| 5.5 | 5.1 | 5.6 | 5.8 | 5.6 | 4.9 | 5.6 | 5.6 | |
| 7.0 | 5.3 | 7.0 | 6.0 | 7.1 | 5.4 | 7.1 | 6.3 | |
| 8.7 | 5.9 | 8.6 | 6.4 | 8.5 | 6.1 | 8.6 | 6.4 | |
| 9.9 | 6.3 | 10.0 | 6.9 | 9.9 | 6.6 | 10.0 | 7.1 | |
| pHpzc | 4.7 ± 0.1 | 5.9 ± 0.1 | 4.4 ± 0.1 | 5.6 ± 0.1 | ||||
| Biosorbent | WS | UWS | SWS | SUWS |
|---|---|---|---|---|
| C (%) | 46.04 ± 0.03 | 47.67 ± 0.83 | 46.40 ± 0.26 | 47.48 ± 0.14 |
| H (%) | 5.49 ± 0.08 | 5.61 ± 0.17 | 5.49 ± 0.03 | 5.78 ± 0.15 |
| N (%) | 0.58 ± 0.01 | 2.73 ± 0.65 | 0.36 ± 0.06 | 2.16 ± 0.23 |
| S (%) | 0 | 0 | 0.05 ± 0.01 | 0.11 ± 0.01 |
| Biosorbent | WS | UWS | SWS | SUWS |
|---|---|---|---|---|
| Coefficients of best-fitting model equations for coded response variables A, B and C * | ||||
| 0.0001 | 0.0005 | −0.0002 | −0.0001 | |
| −0.04 | −0.08 | −0.12 | −0.15 | |
| −0.02 | −0.01 | −0.04 | −0.04 | |
| 0.0034 | 0.0004 | 0.0006 | 0.0012 | |
| 0.03 | 0.04 | 0.00 | 0.01 | |
| −0.000 | −0.007 | −0.001 | −0.004 | |
| −0.06 | −0.03 | 0.02 | 0.02 | |
| 0.29 | 0.80 | 1.42 | 1.72 | |
| 0.36 | 0.62 | 0.75 | 0.88 | |
| 2.08 | −0.97 | −3.57 | −4.97 | |
| Fit statistics ** | ||||
| R2 | 0.93 | 0.95 | 0.93 | 0.96 |
| F-value | 15.12 | 19.52 | 14.47 | 23.32 |
| p-value | 0.0001 | <0.0001 | 0.0001 | <0.0001 |
| Adeq precision | 13.12 | 15.57 | 11.36 | 14.14 |
| Parameters | Biosorbent | ||||
|---|---|---|---|---|---|
| WS | UWS | SWS | SUWS | ||
| Experimental | qt,exp (mg·g−1) | 11.78 | 17.31 | 15.33 | 22.42 |
| Pseudo-first-order | qt,cal (mg·g−1) | 11.33 | 15.85 | 14.97 | 21.33 |
| k1 (min−1) | 0.242 | 0.219 | 0.259 | 0.237 | |
| R2 | 0.64 | 0.42 | 0.79 | 0.58 | |
| Pseudo-second-order | qt,cal (mg·g−1) | 11.86 | 16.88 | 15.57 | 22.43 |
| k2 (g·mg−1·min−1) | 0.040 | 0.021 | 0.036 | 0.019 | |
| R2 | 0.95 | 0.82 | 0.99 | 0.93 | |
| Elovich | qt,cal (mg·g−1) | 21.22 | 17.61 | 15.93 | 23.21 |
| α (mg·g−1·min−1) | 10,249.4 | 609.8 | 172,774.4 | 6351.0 | |
| β (g·mg−1) | 1.296 | 0.633 | 1.088 | 0.577 | |
| R2 | 0.91 | 0.98 | 0.82 | 0.96 | |
| Parameters | Biosorbent | ||||
|---|---|---|---|---|---|
| WS | UWS | SWS | SUWS | ||
| Experimental | qe,exp (mg·g−1) | 16.89 | 28.82 | 25.16 | 37.34 |
| Freundlich | qe,cal (mg·g−1) | 22.92 | 32.36 | 28.15 | 40.78 |
| kF (L·g−1) | 8.44 | 13.68 | 13.48 | 9.49 | |
| bF | 0.363 | 0.485 | 0.514 | 1.22 | |
| R2 | 0.84 | 0.86 | 0.90 | 0.95 | |
| Langmuir | qe,cal (mg·g−1) | 17.62 | 30.43 | 26.43 | 37.75 |
| kL (L·g−1) | 18.07 | 22.18 | 24.29 | 11.45 | |
| (L·mg−1) | 0.961 | 0.560 | 0.680 | 0.000 | |
| R2 | 0.91 | 0.94 | 0.96 | 0.93 | |
| Biosorbent | (mg·g−1) | Reference |
|---|---|---|
| Sugarcane fiber | 10.44 | Parab et al., 2009 [65] |
| Orange peels | 14.3 | Annadurai, 2002 [71] |
| WS | 22.92 | Present study |
| Pinus bark powder | 32.78 | Ahmad, 2009 [67] |
| Cucumis sativus peels treated with H2SO4 | 35.33 | Smitha et al., 2017 [68] |
| SUWS | 37.75 | Present study |
| Rice bran | 42.25 | Wang et al., 2008 [69] |
| Jackfruit leaf powder | 43.39 | Das et al., 2012 [70] |
| Rice husk treated with NaOH | 44.87 | Chakraborty et al., 2011 [47] |
| Pomegranate fruit shell powder | 50.21 | Silveira et al., 2014 [66] |
| Coir pith | 65.53 | Parab et al., 2009 [65] |
| Esterified wheat straw | 227.27 | Gong et al., 2008 [64] |
| Ginger waste treated with H2SO4-ZnCl2 | 277.7 | Kumar & Rais, 2011 [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shkliarenko, Y.; Halysh, V.; Nesterenko, A. Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal. Water 2023, 15, 1536. https://doi.org/10.3390/w15081536
Shkliarenko Y, Halysh V, Nesterenko A. Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal. Water. 2023; 15(8):1536. https://doi.org/10.3390/w15081536
Chicago/Turabian StyleShkliarenko, Yuliana, Vita Halysh, and Alla Nesterenko. 2023. "Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal" Water 15, no. 8: 1536. https://doi.org/10.3390/w15081536
APA StyleShkliarenko, Y., Halysh, V., & Nesterenko, A. (2023). Adsorptive Performance of Walnut Shells Modified with Urea and Surfactant for Cationic Dye Removal. Water, 15(8), 1536. https://doi.org/10.3390/w15081536







