Chemical Characteristics and Controlling Factorsof Groundwater in Chahannur Basin
Abstract
:1. Introduction
2. Materials and Methods
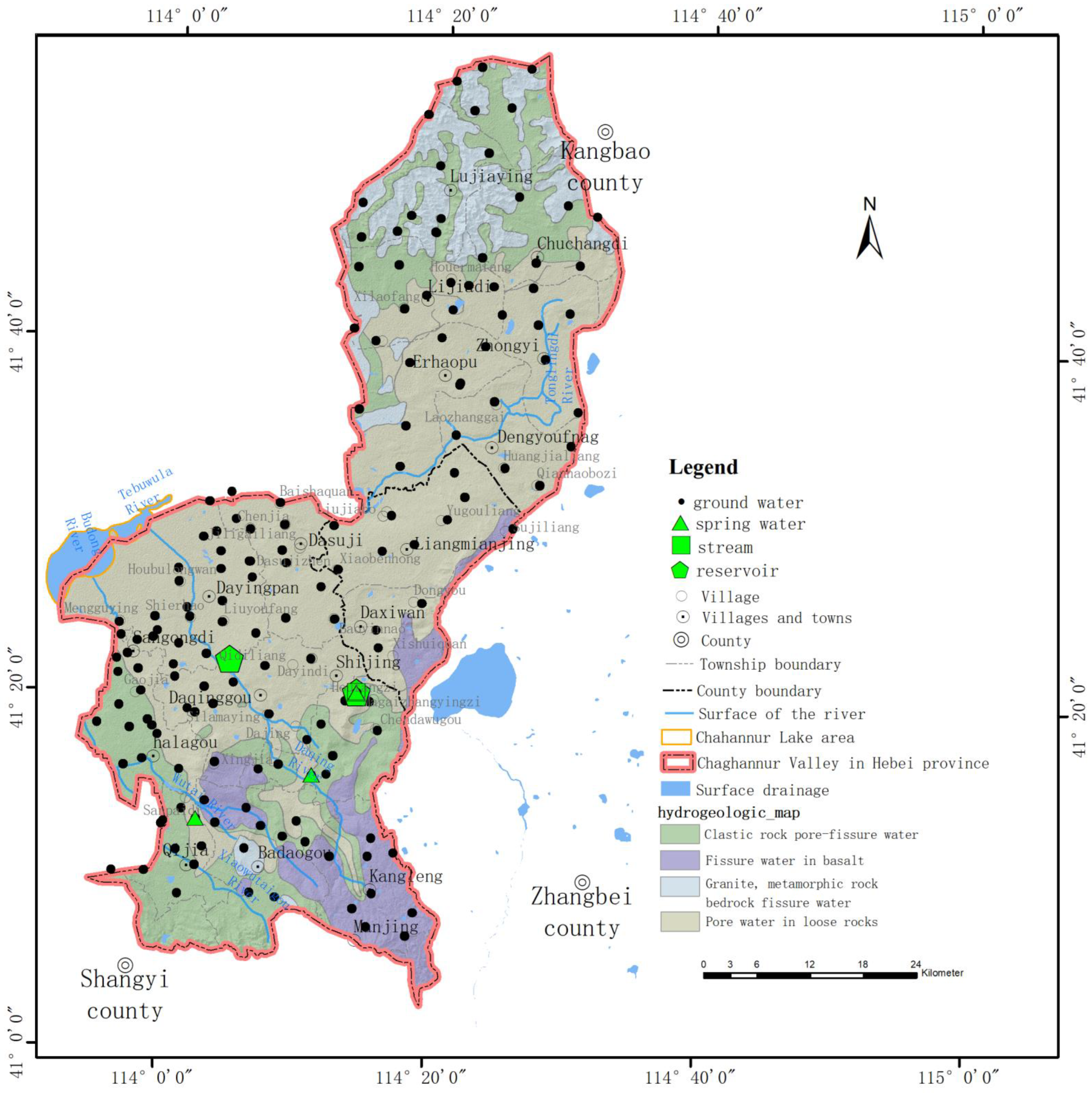
2.1. Overview of the Study Area
2.2. Sample Collection and Analysis
2.3. Analysis Method
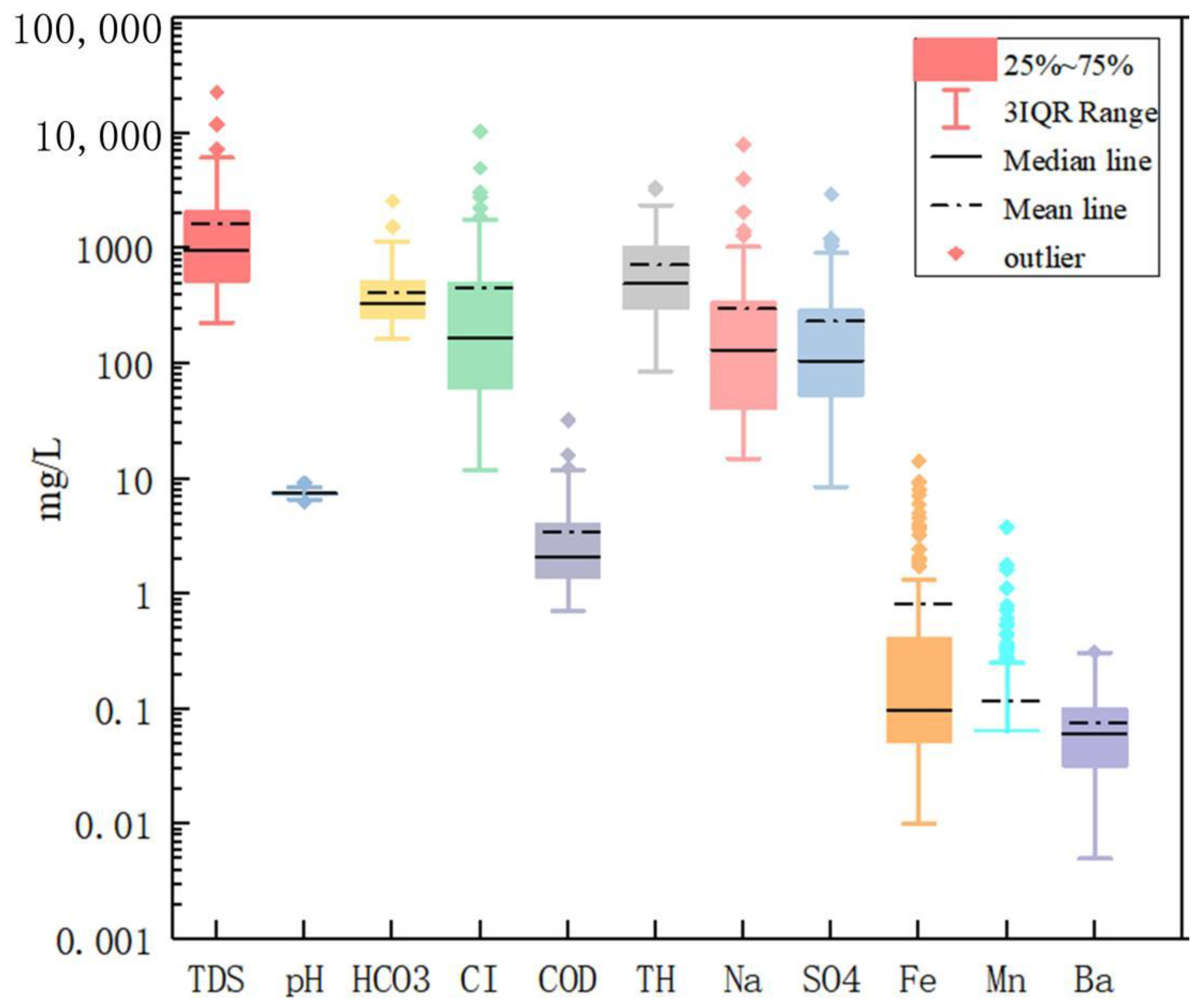
3. Results and Discussion
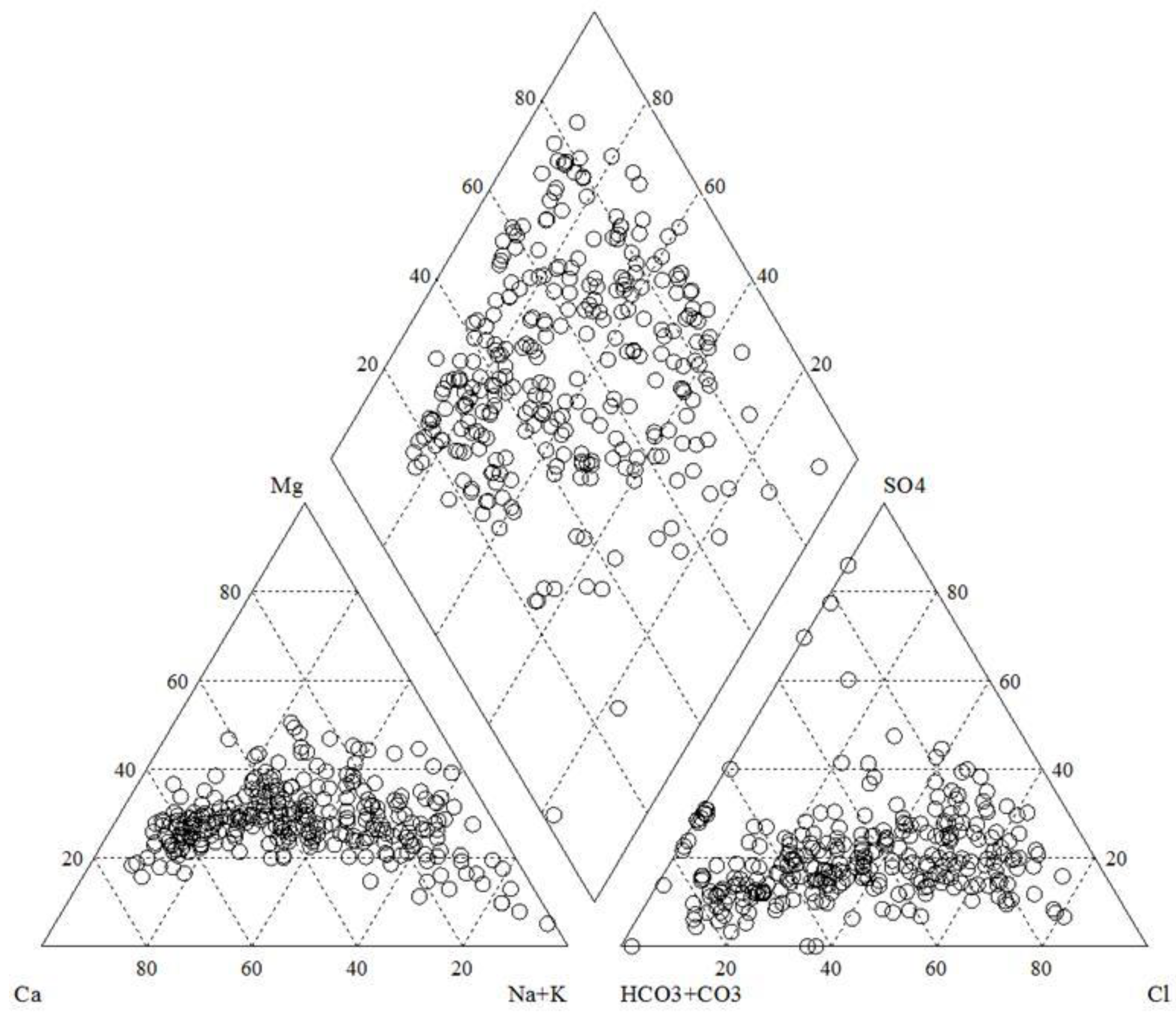
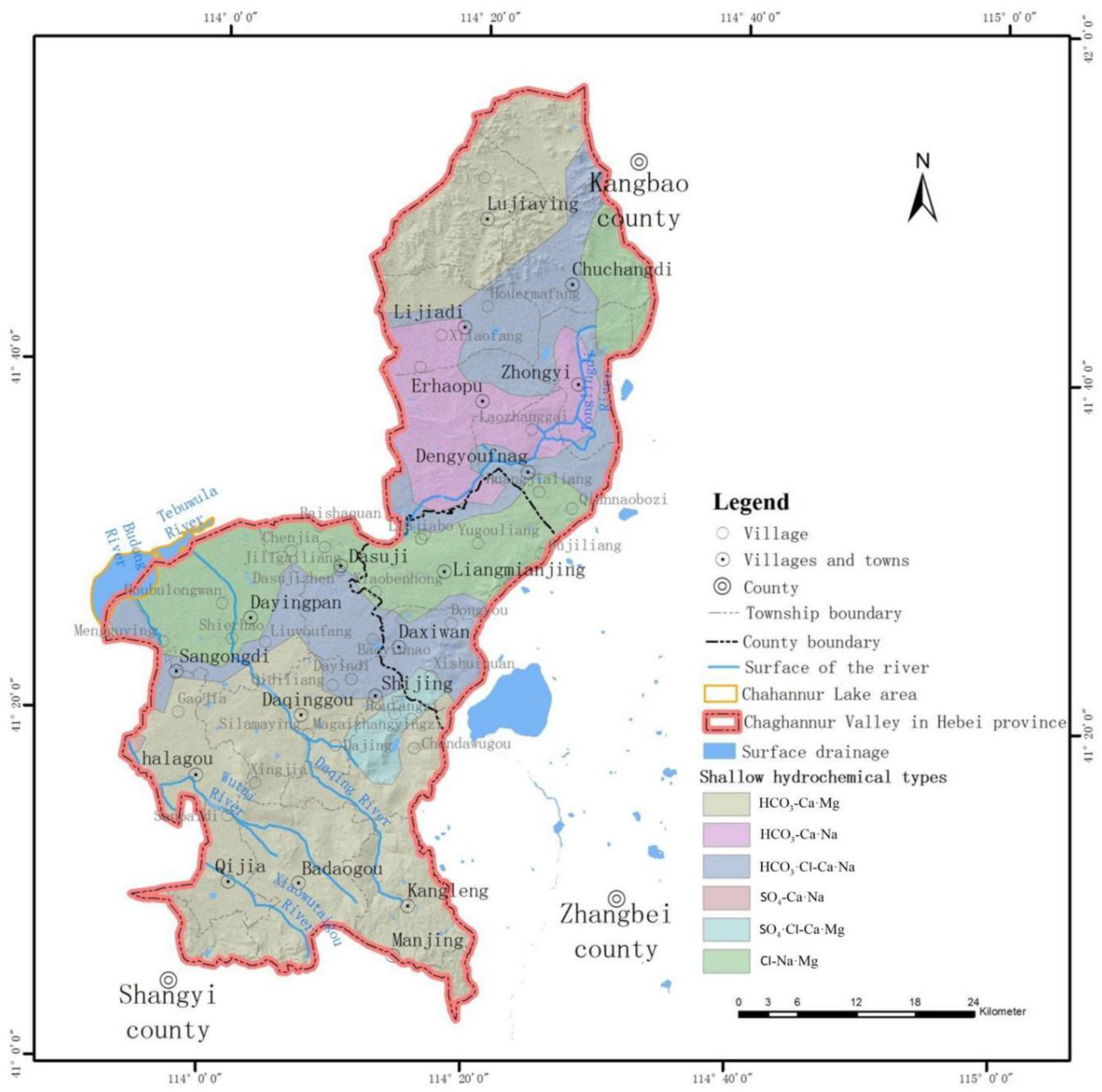
3.1. Chemical Types of Groundwater in the Watershed
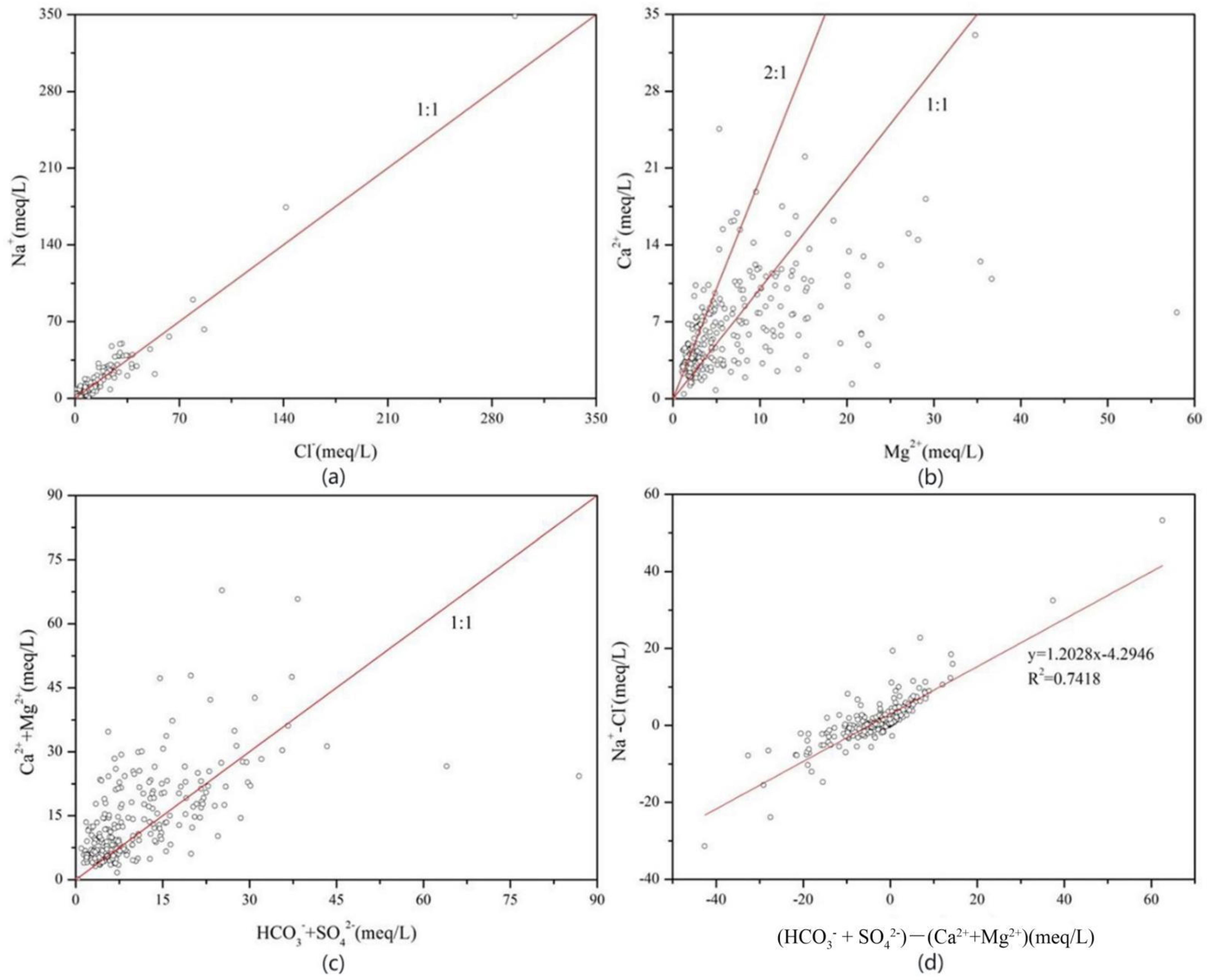
3.2. Cause Analysis of Hydrochemical Types
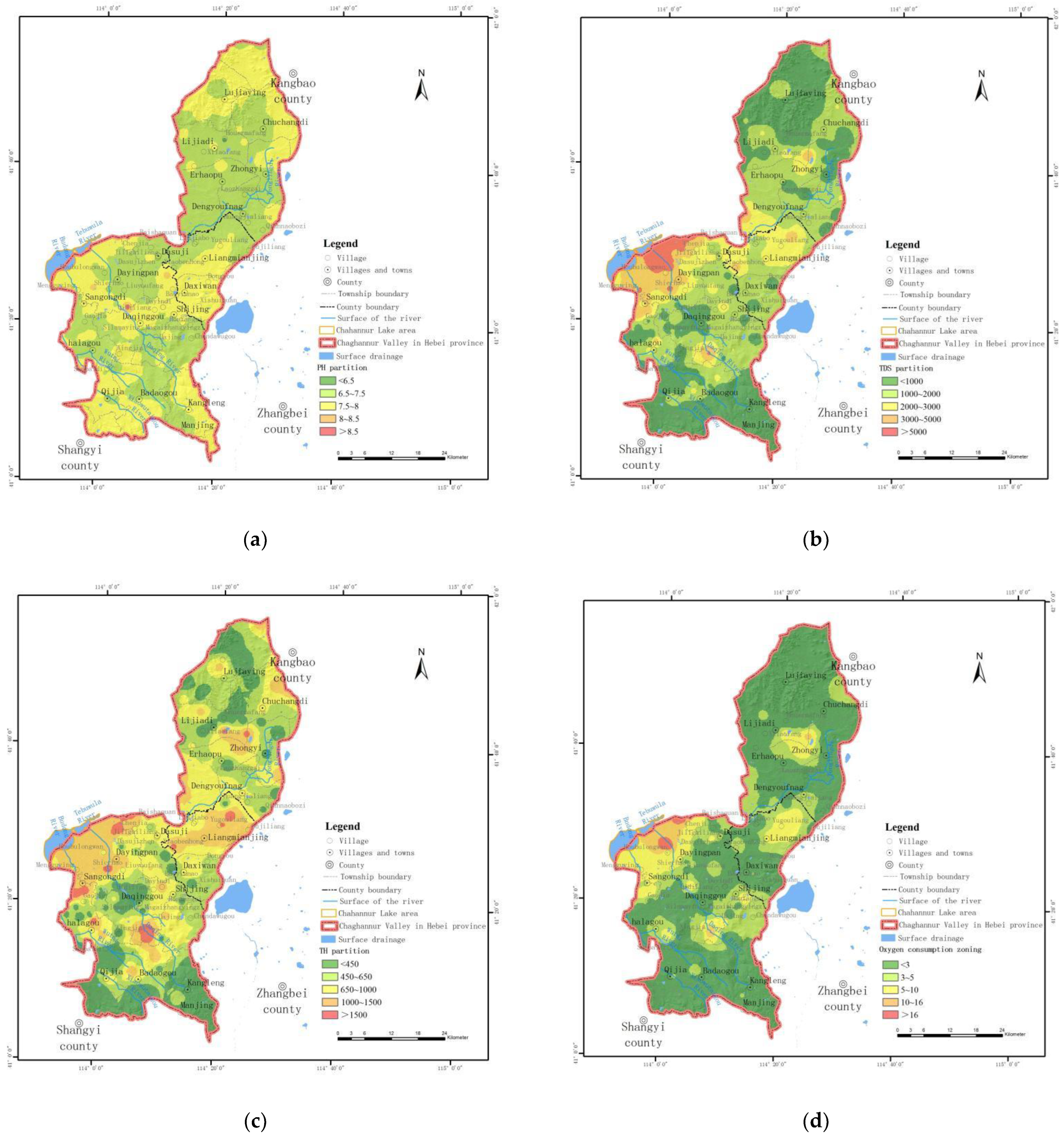
3.3. Spatial Distribution Characteristics of Groundwater Indexes in Watershed
- Spatial distribution characteristics of pH
- Spatial distribution characteristics of TDS
- Spatial distribution characteristics of total hardness (TH)
- Spatial distribution characteristics of chemical oxygen demand (COD)
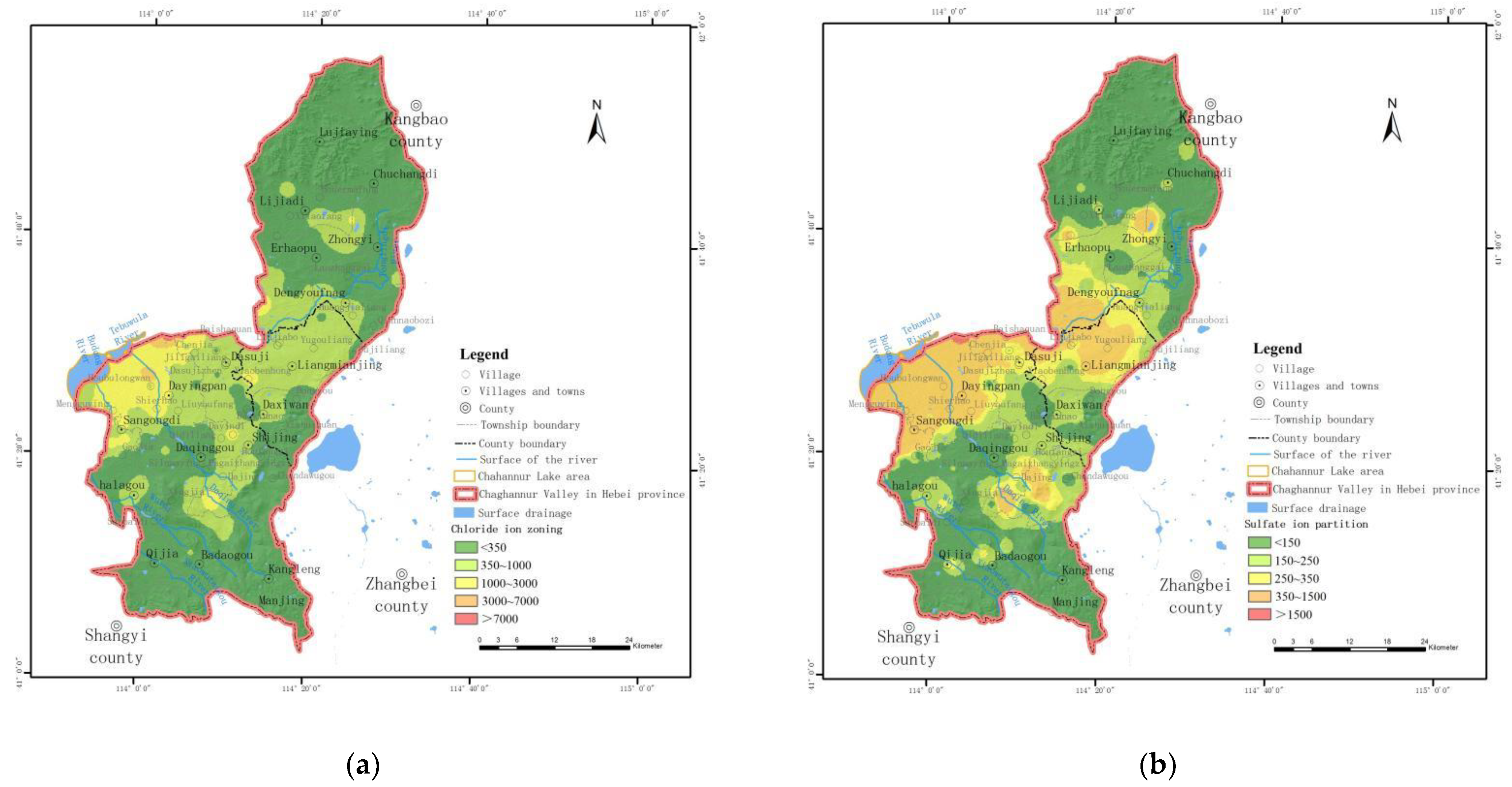
- Spatial distribution characteristics of chloride (Cl−)
- Spatial distribution characteristics of sulfate (SO42−)
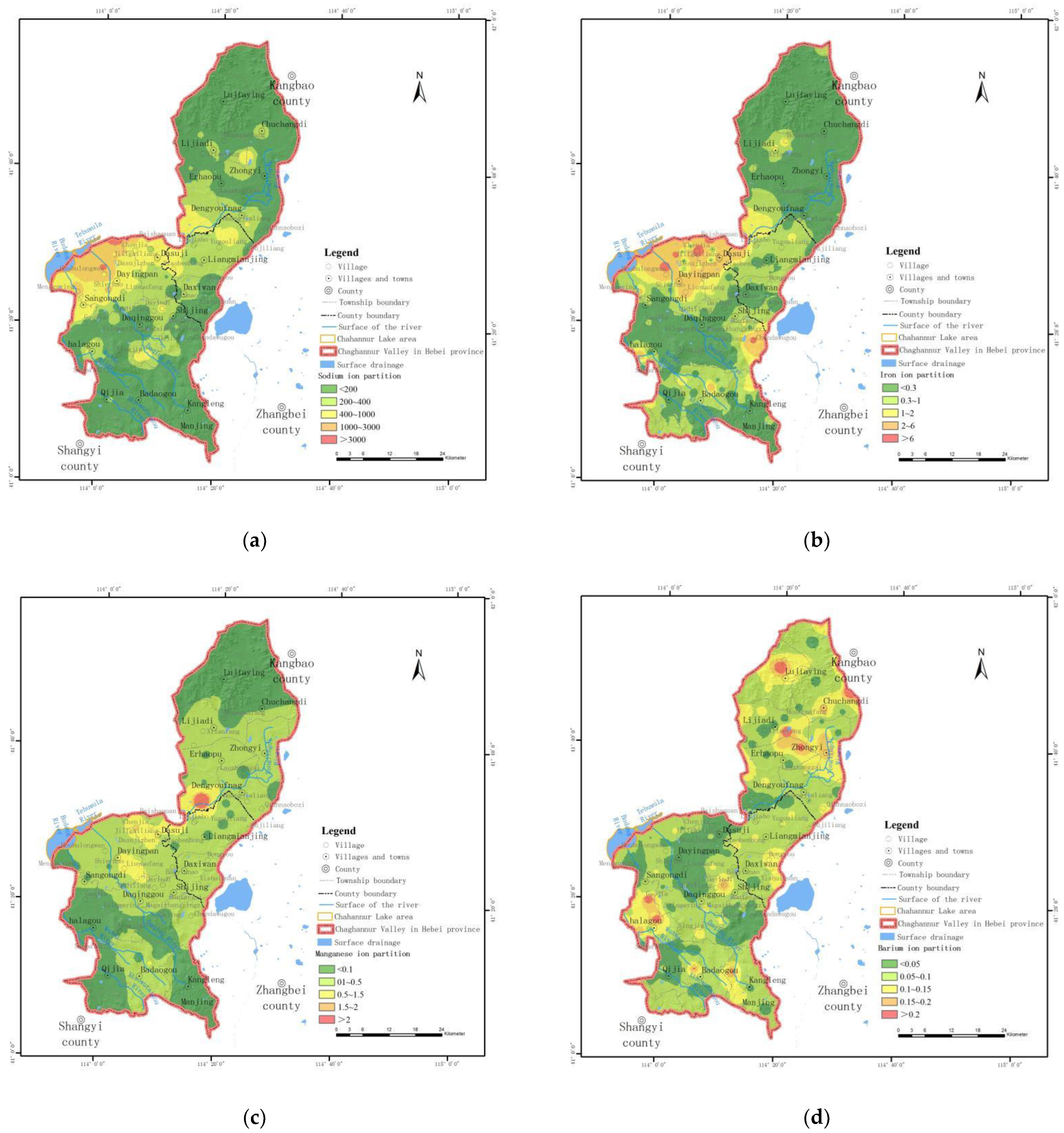
- Spatial distribution characteristics of sodium (Na+)
- Spatial distribution characteristics of iron (Fe)
- Spatial distribution characteristics of manganese (Mn)
- Spatial distribution characteristics of barium (Ba2+)
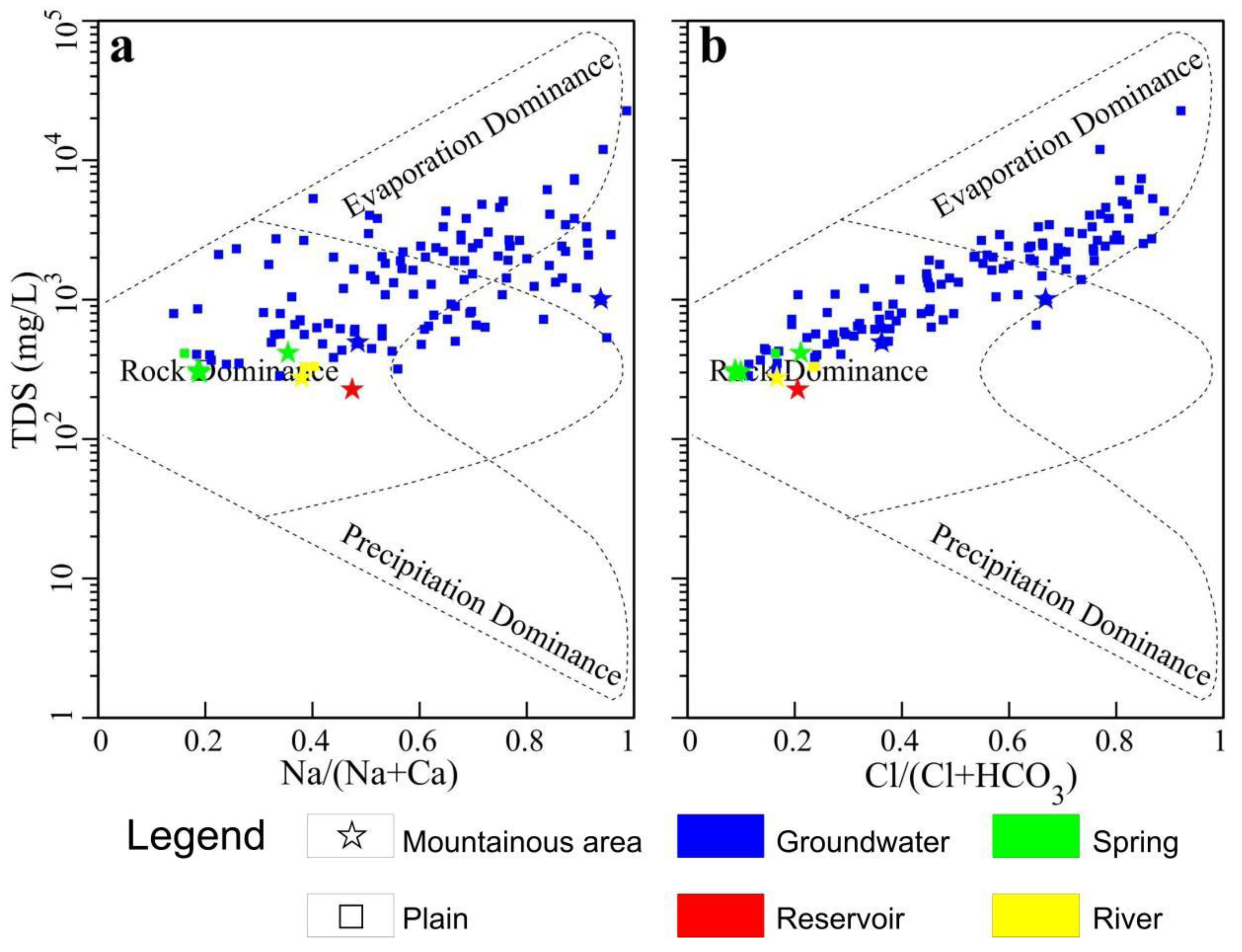
3.4. Source Determination of Groundwater Chemical Components
4. Conclusions
- (1)
- The spatial distribution law of pH, TDS, TH, Cl−, SO42−, Na+, iron, manganese, COD, barium and other indicators is described, which provides basic data support for the groundwater ecological environment research in the Chahannur Basin.
- (2)
- The plasma concentrations of TDS, TH, Cl, Na and COD are high in the Chahannur Lake area and its surrounding areas, which are strongly influenced by human activities.
- (3)
- The hydrochemical type of groundwater in the basin is mainly bicarbonate-type water, but there is less chloride-type water and sulfate-type water in the central and northeastern mountains of the basin. The bicarbonate-type water is mainly affected by the natural conditions of rock weathering, while the chloride-type water and sulfate-type water in the central and northeast are mainly affected by human influence and evaporation and concentration.
- (4)
- Na+ in the basin groundwater is mainly derived from the dissolution of atmospheric precipitation and rock salt, Ca2+ and Mg2+ ions are mainly derived from the dissolution of carbonate minerals, and silicate minerals are less dissolved. The concentrations of Ca2+ and Mg2+ in groundwater in and around the lake area were increased because of anthropogenic influences and high ion exchange intensity in the basin.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, M.A.; Yasar, A.; Bashir, M.A.; Siyal, S.H.; Najam, T.; Javed, M.S.; Ahmad, K.; Hussain, S.; Anjum, S.; Hussain, E.; et al. Quality assessment of the noncarbonated-bottled drinking water: Comparison of their treatment techniques. Int. J. Environ. Anal. Chem. 2022, 102, 8195–8206. [Google Scholar] [CrossRef]
- Tian, X.; Gong, Z.; Fu, L.; You, D.; Li, F.; Wang, Y.; Chen, Z.; Zhou, Y. Determination of Groundwater Recharge Mechanism Based on Environmental Isotopes in Chahannur Basin. Water 2023, 15, 180. [Google Scholar] [CrossRef]
- Cao, G.; Qin, J. Save Chahanul. Xinhua Daily Telegraph, 2 November 2021. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, J.; Nie, H.; Xiao, C. Chahannur: Ecological dilemma and conservation and restoration of typical shrinking lakes. Earth 2021, 52–57. [Google Scholar]
- Zhong, L.; Deng, J.; Song, Z.; Ding, P. Northwest ecological geology survey research progress and prospect. J. Northwest Geol. 2022, 3, 108–119. [Google Scholar]
- Si, L.T. Study on the relationship between soil salinization and buried depth of groundwater level in Chahannur Watershed. Hebei Univ. Geosci. 2022. Available online: https://cdmd.cnki.com.cn/Article/CDMD-10077-1023418608.htm (accessed on 19 March 2023).
- Chen, X. Khan was 4, lake area, and influence factors analysis. Qinghai Norm. Univ. 2021. Available online: https://d.wanfangdata.com.cn/thesis/D02425940 (accessed on 19 March 2023).
- Wang, Y.-X.; Xing, S.; Ding, N. Khan was 4, lake area and its response to climate change analysis. J. Environ. Eng. 2022, 40, 47–68. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, M.; Xia, J.; Gao, Y. Causes of water surface shrinkage of Bashang Chahannur Lake and its restoration measures. J. Minzu Univ. China (Nat. Sci. Ed.) 2021, 30, 53–59. [Google Scholar]
- Chen, M.; Li, Y.; Lei, X.; Li, N.; Gao, Q.; Wang, C. Khan was 4, underground water level, watershed space-time evolution characteristics and driving factors in the quantitative evaluation. J. Arid Zone Resour. Environ. 2022, 4, 105–111. [Google Scholar]
- Chen, P.; Zhang, B.; Ma, R.; Shi, J.; Si, L.; Wu, J.; Zhao, L. Risk of ecosystem degradation based on health and resilience: A case study of Chahannur Basin on Bashang Plateau. China Geol. 1–21 [2023-03-21]. Available online: http://kns.cnki.net/kcms/detail/11.1167.P.20230227.1227.002.html (accessed on 19 March 2023).
- Apollaro, C.; Tripodi, V.; Vespasiano, G.; De Rosa, R.; Dotsika, E.; Fuoco, I.; Critelli, S.; Muto, F. Chemical, isotopic and geotectonic relations of the warm and cold waters of the Galatro and Antonimina thermal areas, southern Calabria, Italy. Mar. Pet. Geol. 2019, 109, 469–483. [Google Scholar] [CrossRef]
- Fuoco, I.; De Rosa, R.; Barca, D.; Figoli, A.; Gabriele, B.; Apollaro, C. Arsenic polluted waters: Application of geochemical modelling as a tool to understand the release and fate of the pollutant in crystalline aquifers. J. Environ. Manag. 2022, 301, 113796. [Google Scholar] [CrossRef] [PubMed]
- Fuoco, I.; Marini, L.; De Rosa, R.; Figoli, A.; Gabriele, B.; Apollaro, C. Use of reaction path modelling to investigate the evolution of water chemistry in shallow to deep crystalline aquifers with a special focus on fluoride. Sci. Total Environ. 2022, 830, 154566. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, F.K.; Nazzal, Y.; Jafri, M.K.; Naeem, M.; Ahmed, I. Reverse ion exchange as a major process controlling the groundwater chemistry in an arid environment: A case study from northwestern Saudi Arabia. Environ. Monit. Assess. 2015, 187, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Gao, Z.; An, Y. Hydrochemical and isotopic characteristics of groundwater in the Jiuquan East Basin, China. Arab. J. Geosci. 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, W.; Zhu, G. Hydrochemical characteristics and ion sources of river water in the upstream of the Shiyang River, China. Environ. Earth Sci. 2021, 80, 1–16. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Li, F. Hydrochemical characteristics and temporal variations of geothermal water quality in Tangtou, Shandong, China. Water 2019, 11, 1643. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, M.; Gao, Z. Hydrochemical characteristics and water quality assessment of groundwater in the Yishu River Basin. Acta Geophys. 2020, 68, 877–889. [Google Scholar] [CrossRef]
- Yang, F.; Liu, S.; Jia, C. Hydrochemical characteristics and functions of groundwater in southern Laizhou Bay based on the multivariate statistical analysis approach. Estuar. Coast. Shelf Sci. 2021, 250, 107153. [Google Scholar] [CrossRef]
- Elbarbary, S.; Zaher, M.A.; El-Shahat, A.; Deep, M.A.; Khedher, K.M. Hydrochemical and isotopic characteristics of thermal groundwater at Farafra Oasis, Western Desert, Egypt. Geochem. Explor. Environ. Anal. 2020, 20, 408–424. [Google Scholar] [CrossRef]
- Carol, E.; Kruse, E.; Maspla, J. Hydrochemicai and isotopical evidence of groundwater salinization processes on the coastal plain of samborom -bon Bay, Argentina. J. Hydrol. 2009, 365, 335–345. [Google Scholar] [CrossRef]
- Shukla, S.; Saxena, A.; Khan, R.; Li, P. Spatial analysis of groundwater quality and human health risk assessment in parts of raebareli district, india. Environ. Earth Sci. 2021, 80, 1–17. [Google Scholar] [CrossRef]
- Nsabimana, A.; PLi, P.; He, S.; He, X.D.; KhorshedAlam, S.M.; Fida, M. Health risk of the shallow groundwater and its suitability for drinking purpose in Tongchuan, China. Water 2021, 13, 3256. [Google Scholar] [CrossRef]
- Patience, M.T.; Elumalai, V.; Rajmohan, N.; Li, P. Occurrence and distribution of nutrients and trace metals in groundwater in an intensively irrigated region, luvuvhu catchment, south africa. Environ. Earth Sci. 2021, 80, 1–15. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, M.X.; Xia, J.X.; Gao, Y.Q. Causes of water surface shrinkage in Chahannur Lake on the dam and its recovery countermeasures. J. Minzu Univ. China 2021, 30, 53–59. [Google Scholar]
- Aly, A.A. Hydrochemical characteristics of egypt western desert oases groundwater. Arab. J. Geosci. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Chen, X.L.; Wang, Y.F.; Zhang, H.F.; Liu, F.G.; Shen, Y.J. Study on extraction method of irrigated arable land in Chahannur Basin based on ESTARFMNDVI. Chin. J. Eco-Agric. 2021, 29, 1105–1116. [Google Scholar]
- Liu, X.; Xiang, W.; Ma, X.J.; Fan, Y.L.; Si, B.C. Chemical characteristics and influencing factors of shallow groundwater in the central loess plateau. Chin. J. Environ. Sci. 2021, 41, 5201–5209. [Google Scholar]
- Cui, Y.H.; Wang, J.; Liu, Y.C.; Hao, T.; Gao, X. Surface-groundwater water chemistry and genesis analysis of Shengjinhu River-Lake confluence. Environ. Sci. 2021, 42, 3223–3231. [Google Scholar]
- Liu, X.; Xiang, W.; Si, B.C. Chemical characteristics and control factors of shallow groundwater in the Weihe and Jing River Basins. Environ. Sci. 2021, 42, 2817–2825. [Google Scholar]
- Song, C.; Ma, B.; Liang, X.; Wang, G.Z.; Mo, C.L.; Yi, C.Y. Groundwater hydrological characteristics and recharge sources in the mountainous plains of manas river Basin. Arid. Land Resour. Environ. 2021, 35, 160–168. [Google Scholar]
- Guo, Z.Y.; Shi, X.; Li, C.; Zhao, S.N.; Sun, B.; Lu, J.; Han, Z. Study on hydrogen-oxygen isotopes and water chemistry characteristics of spring water in the Ursun River Basin. Arid. Zone Res. 2020, 37, 1406–1415. [Google Scholar]
- Li, J.; Zou, S.Z.; Zhao, Y.; Zhao, R.K.; Dang, Z.W.; Pan, M.Q.; Zhu, D.N.; Zhou, C.S. Analysis of main ions and genesis of groundwater in Huixian karst wetland. Environ. Sci. 2021, 42, 1750–1760. [Google Scholar]
- Wang, L.; Dong, Y.; Xu, Z.; Qiao, X. Hydrochemical and isotopic characteristics of groundwater in the northeastern tennger desert, northern china. Hydrogeol. J. 2017, 13, 1–17. [Google Scholar] [CrossRef]
- Mthembu, P.P.; Elumalai, V.; Li, P.; Uthandi, S.; Rajmohan, N.; Chidambaram, S. Integration of heavy metal pollution indices and health risk assessment of groundwater in semi-arid coastal aquifers, south africa. Expo. Health 2022, 14, 487–502. [Google Scholar] [CrossRef]
- Rui, D.; Pla, B.; Lei, W.; Xha, B.; Lei, Z. Hydrochemical characteristics, hydrochemical processes and recharge sources of the geothermal systems in lanzhou city, northwestern china. Urban Clim. 2022, 43, 101152. [Google Scholar]
- Li, P.; Wang, D.; Li, W.; Liu, L. Sustainable water resources development and management in large river Basins: An introduction. Environ. Earth Sci. 2022, 81, 179. [Google Scholar] [CrossRef]
- Guo, Y.; Li, P.; He, X.; Wang, L. Groundwater quality in and around a landfill in northwest china: Characteristic pollutant identification, health risk assessment, and controlling factor analysis. Expo. Health 2022, 10, 885–901. [Google Scholar] [CrossRef]
- Song, H.; Pla, B.; Fsa, B.; Dan, W.; Xra, B. Identification and apportionment of shallow groundwater nitrate pollution in Weining Plain, northwest China, using hydrochemical indices, nitrate stable isotopes, and the new Bayesian stable isotope mixing model (MixSIAR). Environ. Pollut. 2022, 298, 118852. [Google Scholar]
- Khan, R.; Saxena, A.; Shukla, S.; Goel, P.; Bhattacharya, P.; Li, P. Appraisal of water quality and ecological sensitivity with reference to riverfront development along the river gomti, india. Appl. Water Sci. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; He, X. Remediation of cadmium contaminated soil by modified gangue material: Characterization, performance and mechanisms. Chemosphere 2021, 290, 133347. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhang, Y.; Zha, W.; Jin, D.W.; Liu, J.; Wang, Q.M. Analysis of groundwater chemistry and its formation in Yimin Mining Area. Environ. Chem. 2021, 40, 1480–1489. [Google Scholar]
- Ma, A.; Liu, H.; Mao, S.J.; Zhu, Z.C.; Li, M.J. Distribution characteristics of dissolved manganese in the lateral interaction zone of river water and groundwater in the lower reaches of the Han River. Earth Sci. 2021, 47, 729–741. [Google Scholar]
- Lei, M.; Zhou, J.; Liang, X.; Zhou, Y.; Zeng, Y.; Sun, Y. Hydrochemical characteristics of pore water and origin of soda water in the middle part of northern Tianshan Mountains, Xinjiang. Earth Sci. 2022, 47, 674–688. [Google Scholar]
- Kong, X.L.; Yang, Y.H.; Cao, B.; Wang, Y.X.; Pei, H.W.; Shen, Y.J. Chemical characteristics and genesis analysis of surface water-groundwater water in the upper reaches of Yongding River. Environ. Sci. 2021, 42, 4202–4210. [Google Scholar]
- Zhang, L.; Li, P.; He, X. Interactions between surface water and groundwater in selected tributaries of the wei river (china) revealed by hydrochemistry and stable isotopes. Hum. Ecol. Risk Assess. 2021, 28, 1–21. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Liu, L. Source identification and potential ecological risk assessment of heavy metals in the topsoil of the weining plain (northwest china). Expo. Health 2021, 14, 281–294. [Google Scholar] [CrossRef]
- Qza, B.; Pla, B.; Qla, B.; Xra, B.; Song, H. Groundwater contamination risk assessment using a modified DRATICL model and pollution loading: A case study in Guanzhong Basin, China. Chemosphere 2021, 291, 132695. [Google Scholar]
| Index | I | II | III | IV | V |
|---|---|---|---|---|---|
| pH | 6.5–8.5 | 5.5–6.5; 8.5–9.0 | <5.5 or > 9.0 | ||
| TH (mg/L) | ≤150 | ≤300 | ≤450 | ≤650 | >650 |
| TDS (mg/L) | ≤300 | ≤500 | ≤1000 | ≤2000 | >2000 |
| COD (mg/L) | ≤1.0 | ≤2.0 | ≤3.0 | ≤10.0 | >10.0 |
| Na (mg/L) | ≤100 | ≤150 | ≤200 | ≤400 | >400 |
| CI− (mg/L) | ≤50 | ≤150 | ≤250 | ≤350 | >350 |
| SO42− (mg/L) | ≤50 | ≤150 | ≤250 | ≤350 | >350 |
| Fe (mg/L) | ≤0.1 | ≤0.2 | ≤0.3 | ≤2.0 | >2.0 |
| Mn (mg/L) | ≤0.05 | ≤0.05 | ≤0.1 | ≤1.5 | >1.5 |
| Ba (mg/L) | ≤0.01 | ≤0.10 | ≤0.7 | ≤4.00 | >4.00 |
| Component | TDS | pH | Potassium | Sodium | Boron |
|---|---|---|---|---|---|
| Lining coefficient | 1.74 | 0.02 | 6.39 | 4.69 | 2.32 |
| Component | bicarbonate | chloride | Magnesium | manganese | Oxygen consumption |
| Lining coefficient | 0.59 | 4.79 | 1.03 | 15.13 | 1.17 |
| Component | Total hardness | iron | Calcium | ||
| Lining coefficient | 0.60 | 12.37 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Z.; Tian, X.; Fu, L.; Niu, H.; Xia, Z.; Ma, Z.; Chen, J.; Zhou, Y. Chemical Characteristics and Controlling Factorsof Groundwater in Chahannur Basin. Water 2023, 15, 1524. https://doi.org/10.3390/w15081524
Gong Z, Tian X, Fu L, Niu H, Xia Z, Ma Z, Chen J, Zhou Y. Chemical Characteristics and Controlling Factorsof Groundwater in Chahannur Basin. Water. 2023; 15(8):1524. https://doi.org/10.3390/w15081524
Chicago/Turabian StyleGong, Zhiqiang, Xizhao Tian, Lulu Fu, Haobo Niu, Zongze Xia, Zhiyuan Ma, Jian Chen, and Yahong Zhou. 2023. "Chemical Characteristics and Controlling Factorsof Groundwater in Chahannur Basin" Water 15, no. 8: 1524. https://doi.org/10.3390/w15081524






