Macrozoobenthos Structure and Dynamics in a Mediterranean Hypersaline Ecosystem with Implications for Wetland Conservation
Abstract
:1. Introduction
2. Materials and Methods
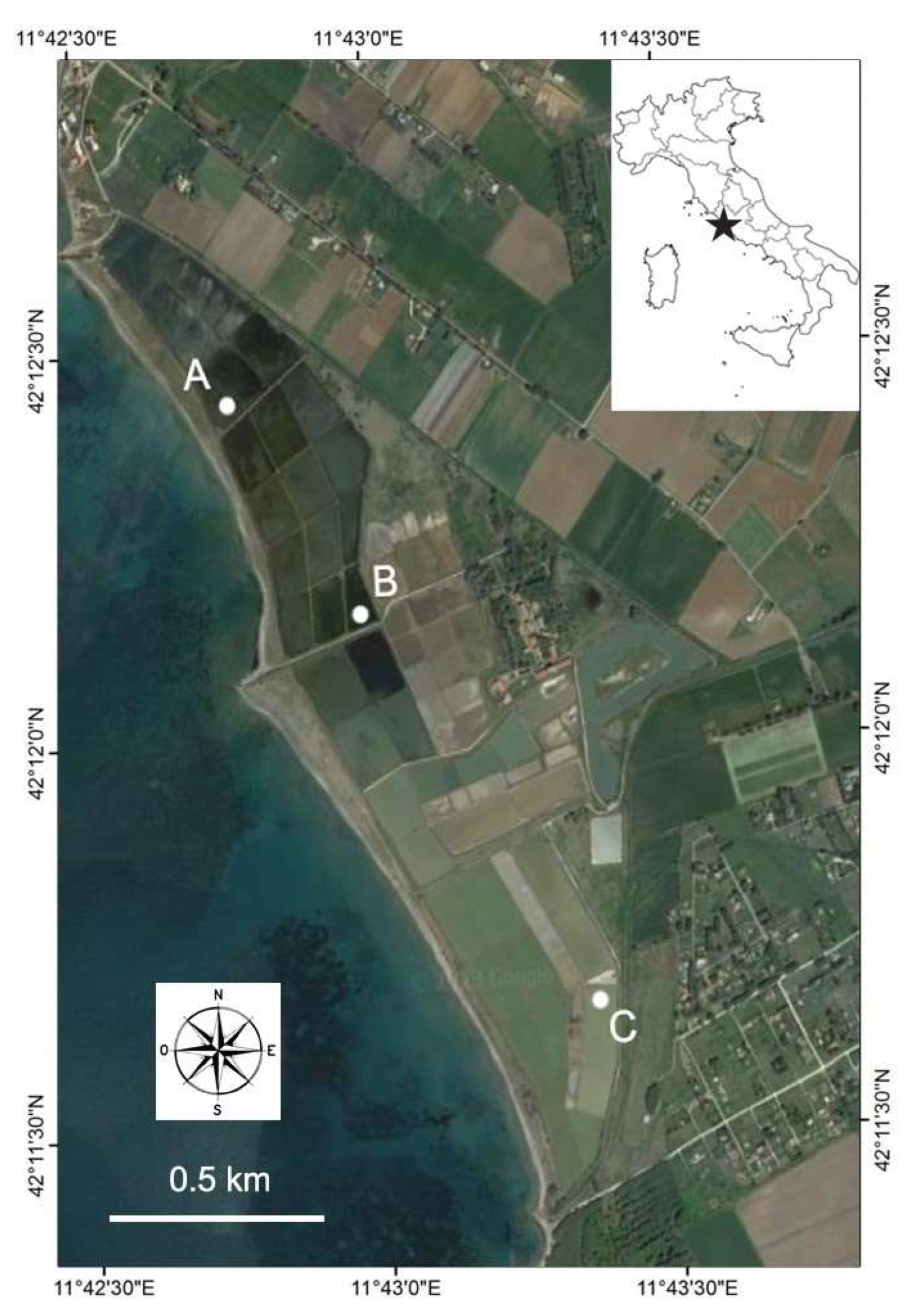
2.1. Study Area and Sampling Sites
2.2. Sampling and Laboratory Analysis
2.3. Data Analysis
3. Results
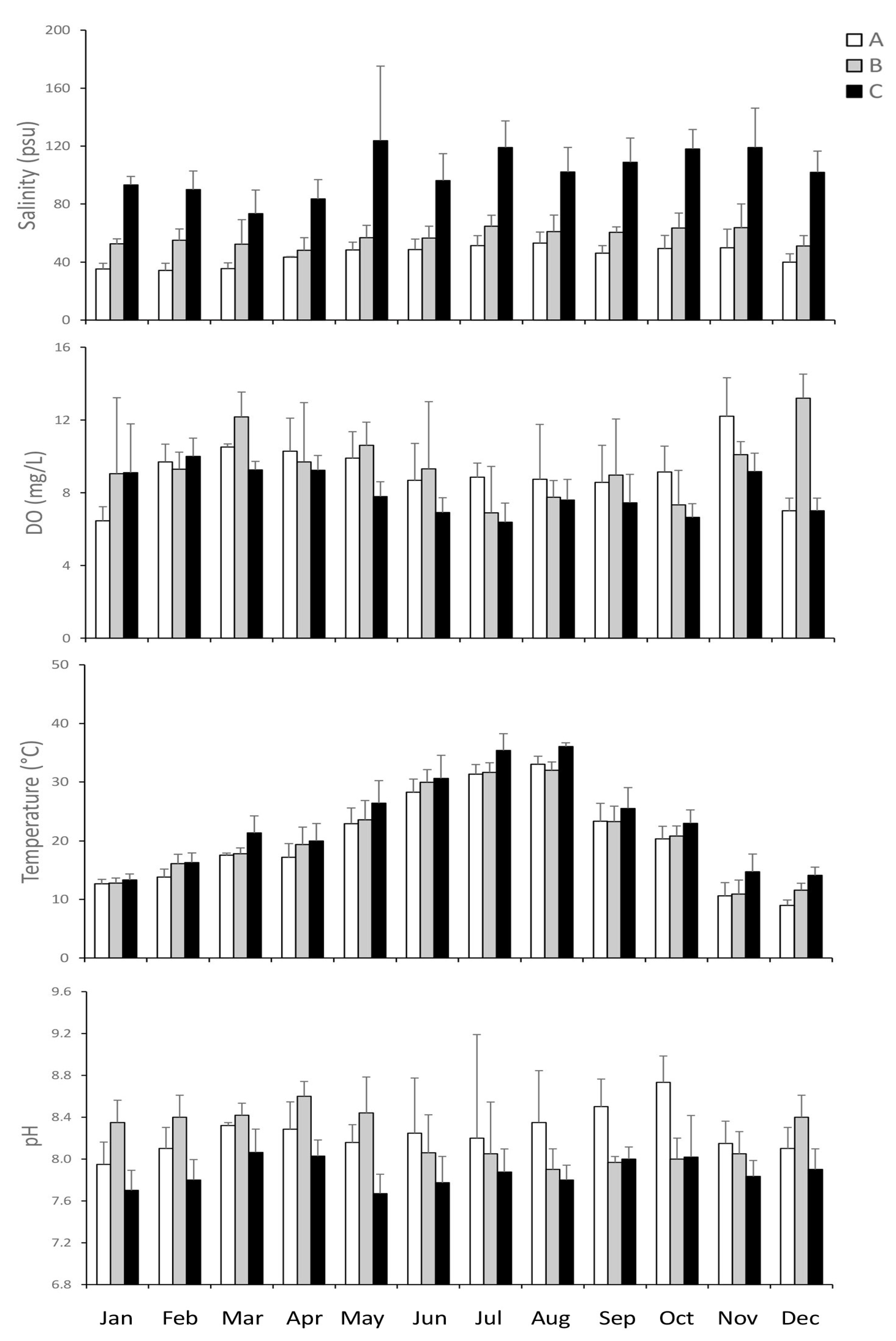
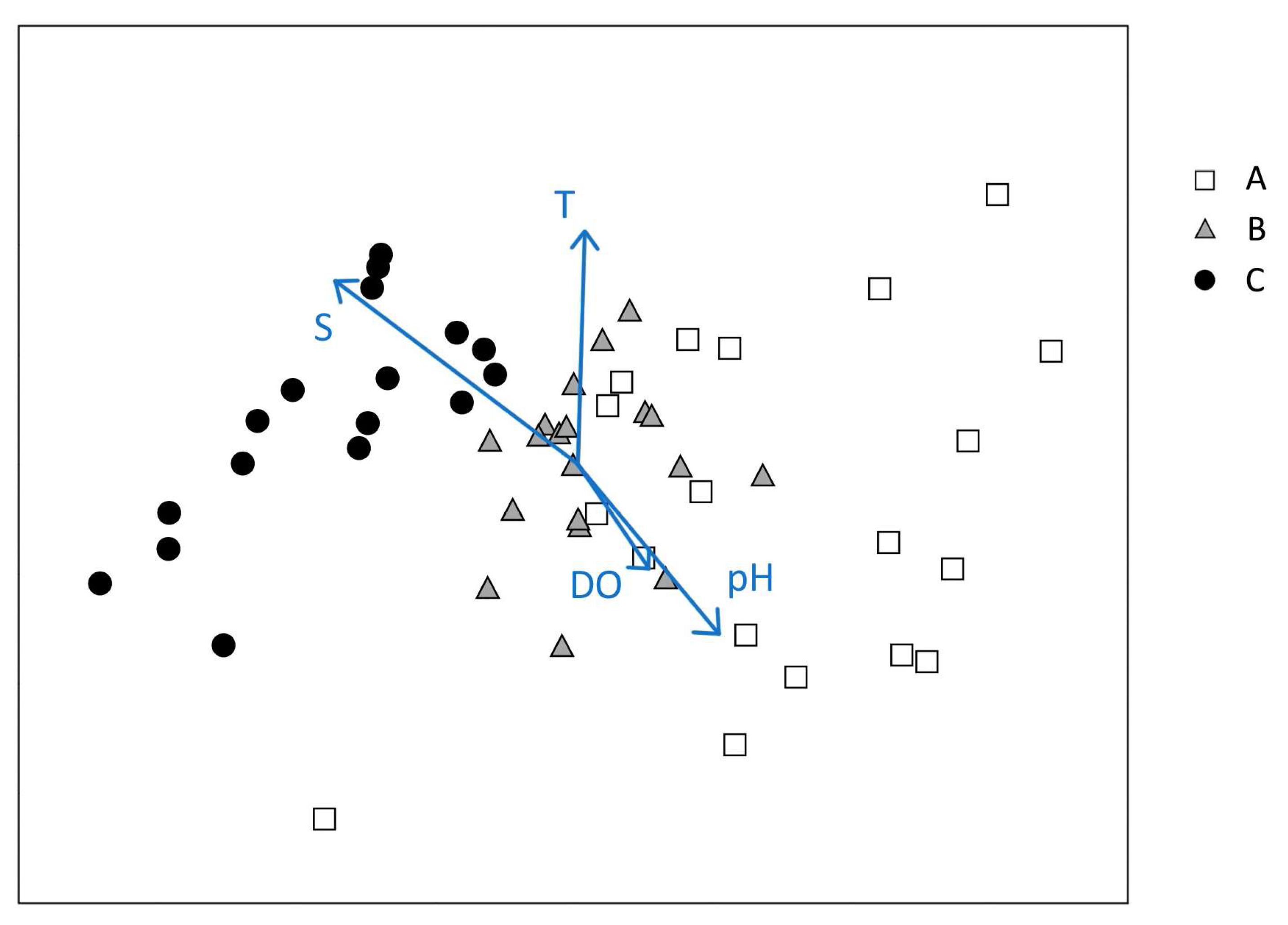
3.1. Environmental Variables
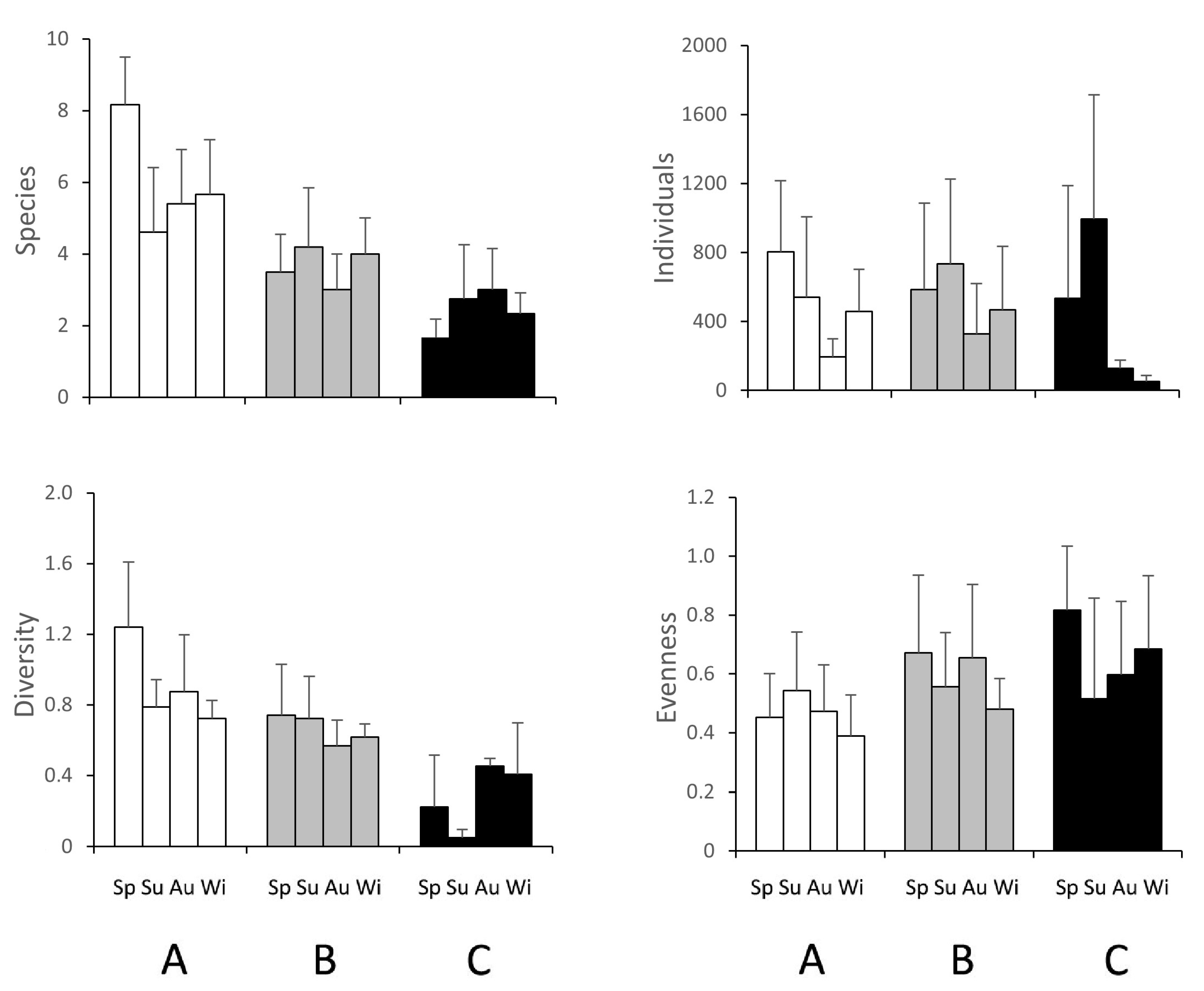
3.2. Macrozoobenthic Community
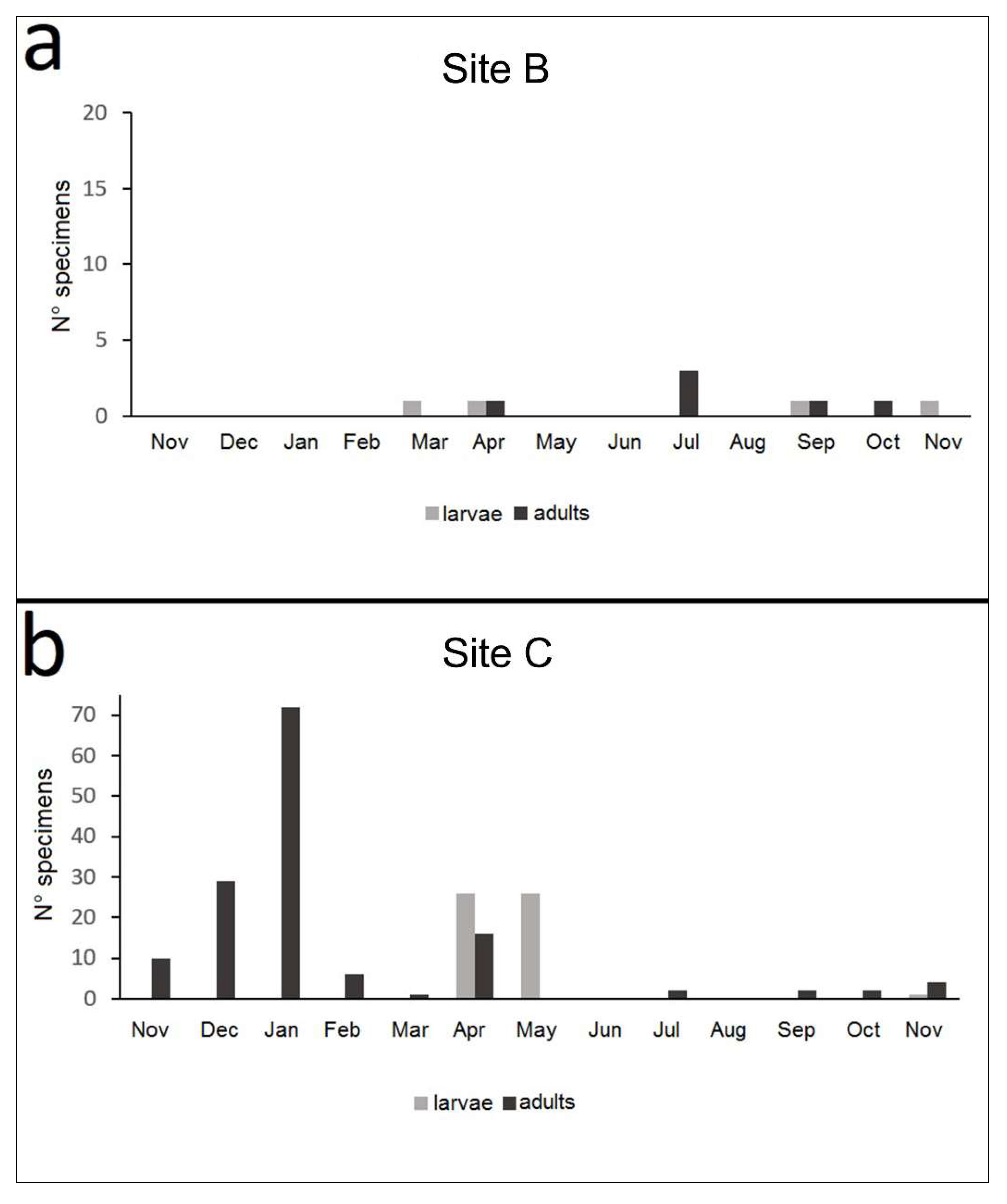
3.3. Nebrioporus Ceresyi Population
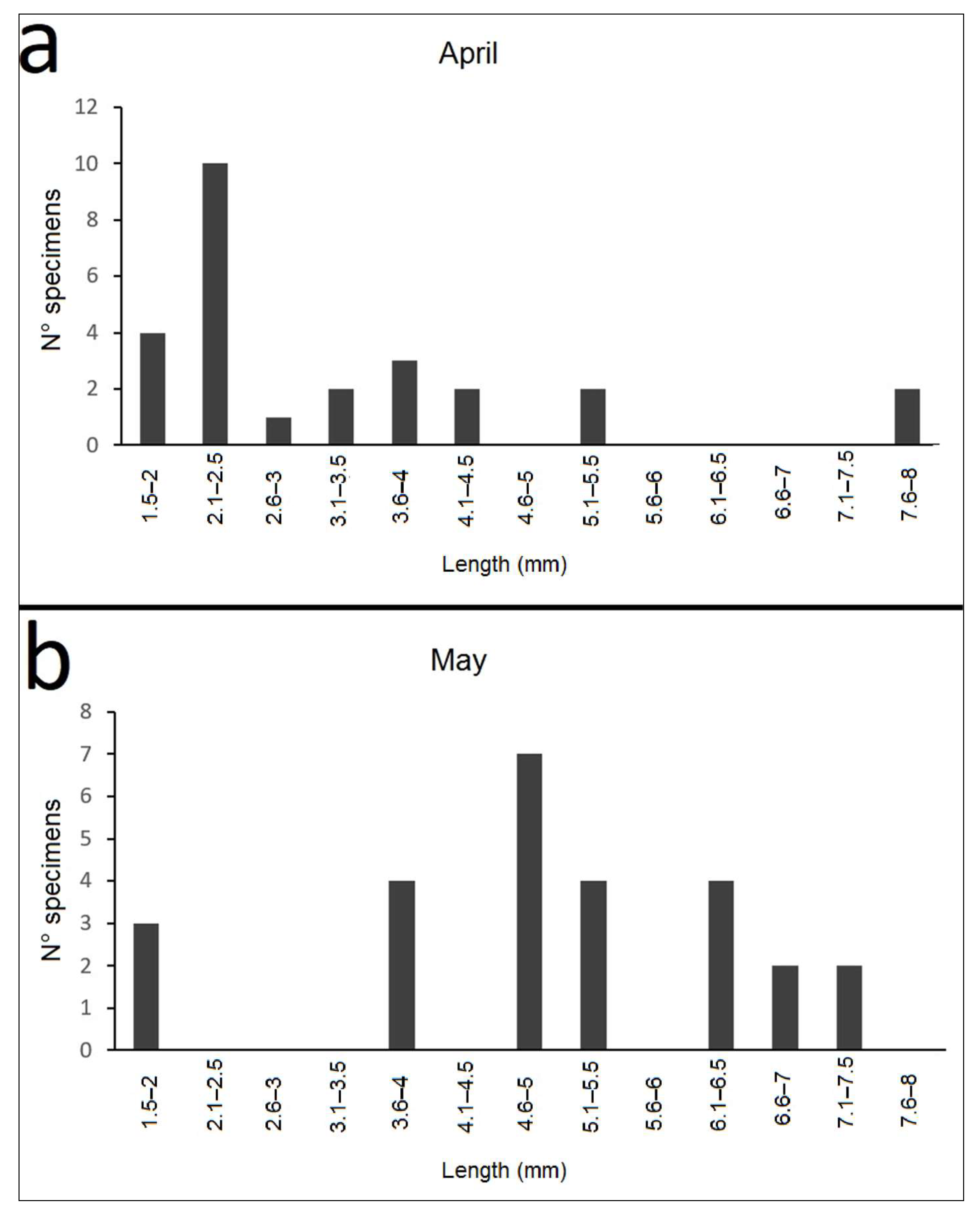
3.3.1. Brief Description of Larvae
3.3.2. Brief Description of Adults
3.3.3. Life Cycle
4. Discussion
4.1. Macrozoobenthic Community Structure and Dynamics
4.2. Nebrioporus Ceresyi Life Cycle and Distribution
4.3. Implications for Conservation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Javor, B. Hypersaline Environments: Microbiology and Biogeochemistry; Brock/Springer Series in Contemporary Bioscience; Springer: Berlin/Heidelberg, Germany, 1989; ISBN 978-3-642-74372-6. [Google Scholar]
- Perthuisot, J.-P.; Guelorget, O.; Groupe d’étude du Domaine Paralique. Le Domaine Paralique: Expressions Géologiques, Biologiques et Économiques du Confinement; Presses de l’École Normale Supérieure: Paris, France, 1983; ISBN 978-2-7288-0094-0. [Google Scholar]
- Basset, A.; Sabetta, L.; Fonnesu, A.; Mouillot, D.; Do Chi, T.; Viaroli, P.; Giordani, G.; Reizopoulou, S.; Abbiati, M.; Carrada, G.C. Typology in Mediterranean Transitional Waters: New Challenges and Perspectives. Aquat. Conserv. Mar. Freshw. Ecosyst. 2006, 16, 441–455. [Google Scholar] [CrossRef]
- Basset, A.; Pinna, M.; Sabetta, L.; Barbone, E.; Galuppo, N. Hierarchical Scaling of Biodiversity in Lagoon Ecosystems. Transit. Waters Bull. 2008, 2, 75–86. [Google Scholar] [CrossRef]
- Gravina, M.F.; Cabiddu, S.; Como, S.; Floris, A.; Padedda, B.M.; Pusceddu, A.; Magni, P. Disentangling Heterogeneity and Commonalities in Nanotidal Mediterranean Lagoons through Environmental Features and Macrozoobenthic Assemblages. Estuar. Coast. Shelf Sci. 2020, 237, 106688. [Google Scholar] [CrossRef]
- Barghini, P.; Silvi, S.; Aquilanti, A.; Marcelli, M.; Fenice, M. Bacteria from Marine Salterns as a Model of Microorganisms Adapted to High Environmental Variations. J. Environ. Prot. Ecol. 2014, 15, 897–906. [Google Scholar]
- Oren, A. The Ecology of Dunaliella in High-Salt Environments. J. Biol. Res. 2014, 21, 23. [Google Scholar] [CrossRef] [Green Version]
- Elloumi, J.; Carrias, J.-F.; Ayadi, H.; Sime-Ngando, T.; Bouaïn, A. Communities Structure of the Planktonic Halophiles in the Solar Saltern of Sfax, Tunisia. Estuar. Coast. Shelf Sci. 2009, 81, 19–26. [Google Scholar] [CrossRef]
- Franciscolo, M.E. Coleoptera. Haliplidae, Hygrobiidae, Gyrinidae, Dytiscidae. In Fauna d’Italia; Edizioni Calderini: Bologna, Italy, 1979; 804p. [Google Scholar]
- Toledo, M. Revision in Part of the Genus Nebrioporus Régimbart, 1906, with Emphasis on the N. laeviventris-Group (Coleoptera: Dytiscidae). Zootaxa 2009, 2040, 1–111. [Google Scholar] [CrossRef]
- Cimmaruta, R.; Blasi, S.; Angeletti, D.; Nascetti, G. The Recent History of the Tarquinia Salterns Offers the Opportunity to Investigate Parallel Changes at the Habitat and Biodiversity Levels. Transit. Waters Bull. 2011, 4, 53–59. [Google Scholar] [CrossRef]
- Sangiorgio, F.; Pinna, M.; Gravili, C. Macroinvertebrati bentonici. In Nuovi Approcci Metodologici per la Classificazione dello Stato di Qualità Degli Ecosistemi Acquatici di Transizione; Basset, A., Sangiorgio, F., Sabetta, L., Eds.; ISPRA, Roma—Università del Salento: Lecce, Italy, 2009; pp. 64–97. [Google Scholar]
- Anderson, M.J. A New Method for Non-Parametric Multivariate Analysis of Variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis; Primer-E Ltd: Plymouth, UK, 2014; 14p. [Google Scholar]
- Semprucci, F.; Gravina, M.F.; Magni, P. Meiofaunal Dynamics and Heterogeneity along Salinity and Trphic Gradients in a Mediterranean Transitional System. Water 2019, 11, 1488. [Google Scholar] [CrossRef] [Green Version]
- Foti, A.; Fenzi, G.A.; Di Pippo, F.; Gravina, M.F.; Magni, P. Testing the Saprobity Hypothesis in a Mediterranean Lagoon: Effects of Confinement and Organic Enrichment on Benthic Communities. Mar. Environ. Res. 2014, 99, 85–94. [Google Scholar] [CrossRef]
- Galuppo, N.; Maci, S.; Pinna, M.; Basset, A. Habitat Types and Distribution of Benthic Macroinvertebrates in a Transitional Water Ecosystem: Alimini Grande (Puglia, Italy). Transit. Waters Bull. 2007, 1, 9–19. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in Estuarine Ecology—A Review of the Remane Diagram with a Suggested Revised Model for Estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Zapparoli, M.; De Mattheis, E.; Vigna Taglianti, A. Invertebrati terrestri e dulcacquicoli della Riserva Naturale Saline di Tarquinia e delle aree adiacenti. In La Riserva Naturale Statale “Saline di Tarquinia”; Colletti, L., Ed.; Corpo Forestale dello Stato, Ufficio Territoriale della Biodiversità di Roma: Rome, Italy, 2014; pp. 159–171. [Google Scholar]
- Angelini, F. Catalogo topografico Hydroadephaga dei Coleoptera. Haliplidae, Hygrobiidae, Gyrinidae, Dytiscidae d’Italia. Mem. Soc. Entomol. Ital. 1984, 41, 45–126. [Google Scholar]
- Nardi, G.; Maltzeff, P. Gli Idroadefagi della Tenuta Presidenziale di Castelporziano (Coleoptera, Gyrinidae, Haliplidae, Noteridae, Hygrobiidae, Dytiscidae). Boll. Ass. Romana Entomol. 2001, 56, 175–232. [Google Scholar]
- Di Giulio, A.; Nardi, G. Description of the pupa of Nebrioporus ceresyi (Aubé) (Coleoptera: Dytiscidae). Aquat. Insects 2006, 28, 269–275. [Google Scholar] [CrossRef]
- Latella, L.; Ruffo, S.; Stoch, F. The project CKmap (Checklist and distribution of the Italian fauna) methods and informatical techniques. Mem. Mus. Civ. St. Nat. Verona 2007, 17, 15–19. [Google Scholar]
- Bologna, M.A.; Bonato, L.; Cianferoni, F.; Minelli, A.; Oliverio, M.; Stoch, F.; Zapparoli, M. Towards the new Checklist of the Italian fauna. Biogeogr.-J. Integr. Biogeogr. 2022, 37, 1–6. [Google Scholar] [CrossRef]
- Luigioni, P. I coleotteri d’Italia. Catalogo Sinonimico—Topografico -Bibliografico. Mem. Pontif. Accad. Sci. I Nuovi Lincei 1929, 13, 1160. [Google Scholar]
- Porta, A. Fauna Coleopterorum Italica. Supplementum II; Stabilimento Tipografico Piacentino: Piacenza, Italy, 1949; 386p. [Google Scholar]
- Angelini, F. Hydroadephaga inediti per Puglie e Lucania (Coleoptera. Haliplidae, Hygrobiidae, Gyrinidae, Dytiscidae). Boll. Soc. Entomol. Ital. 1972, 104, 179–194. [Google Scholar]
- Angelini, F. Hydroadephaga nuovi per Calabria e Sila. Boll. Soc. Entomol. Ital. 1973, 105, 7–12. [Google Scholar]
- Fery, H.; Fresneda, J.; Millán, A. Bemerkungen zur Nebrioporus ceresyi-Gruppe sowie Beschreibung von Nebrioporus schoedli n. sp. (Coleoptera: Dytiscidae). Entomol. Z. 1996, 106, 306–328. [Google Scholar]
- Ragusa, E. Coleotteri nuovi o poco conosciuti della Sicilia. Nat. Sicil. 1882, 1, 248–251. [Google Scholar]
- Ragusa, E. Catalogo ragionato dei Coleotteri di Sicilia (1° parte). Nat. Sicil. 1887, 6, 221–228. [Google Scholar]
- Ragusa, E. Catalogo dei Coleotteri di Sicilia. Nat. Sicil. 1891, 10, 1–32. [Google Scholar]
- Focarile, A. Ricerche entomologiche nell’arcipelago delle Eolie e nell’isola di Ustica (Sicilia). La coleotterofauna dello stagno salmastro a Punta Lingua nell’Isola di Salina. Mem. Soc. Entomol. Ital. 1972, 51, 19–37. [Google Scholar]
- Detter, K. Adephagan water beetles of Elba Island (Tuscany) (Coleoptera Haliplidae, Dytiscidae, Noteridae, Gyrinidae). Mem. Soc. Entomol. Ital. 2006, 85, 85–122. [Google Scholar] [CrossRef] [Green Version]
- Baccetti, N.; Dall’Antonia, P.; Magagnoli, P.; Melega, L.; Serra, L.; Soldatini, C.; Zenatello, M. Risultati dei Censimenti Degli Uccelli Acquatici Svernanti in Italia: Distribuzione, Stima e Trend delle Popolazioni nel 1991–2000; ISPRA: Rome, Italy, 2002; 240p. [Google Scholar]
- Gravina, M.F.; Colozza, N.; D’Ambrosio, L.; Giorgi, M.; Martinoli, M.; Talarico, L.; Tancioni, L. Monitoraggio Dell’habitat “Lagune Costiere” Di Interesse Comunitario Prioritario Presente Nella Riserva Naturale Statale “Saline di Tarquinia”. Relazione Finale. Giugno 2016; Corpo Forestale dello Stato, Ufficio Territoriale per la Biodiversità di Roma: Rome, Italy, 2016. [Google Scholar]
- Gravina, M.F.; Bontempi, D.; D’Ambrosio, L.; Giorgi, M.; Talarico, L.; Ventura, D. Monitoraggio Dell’habitat “Lagune Costiere” Di Interesse Comunitario Prioritario Presente Nella Riserva Naturale Statale “Saline di Tarquinia”. Relazione Finale. Dicembre 2017; Raggruppamento Carabinieri Biodiversità—Reparto Biodiversità di Roma—Nucleo Carabinieri Biodiversità di Saline di Tarquinia: Roma, Italy, 2017. [Google Scholar]
- Gravina, M.F.; Giorgi, M.; Puthod, P. Monitoraggio Dell’habitat “Lagune Costiere” di Interesse Comunitario Prioritario Presente Nella Riserva Naturale Statale “Saline di Tarquinia”. Relazione Finale. Giugno 2021; Raggruppamento Carabinieri Biodiversità—Reparto Biodiversità di Roma—Nucleo Carabinieri Biodiversità di Saline di Tarquinia: Roma, Italy, 2021. [Google Scholar]
- Zerunian, S. Pesci delle acque interne d’Italia. In Quaderni di Conservazione della Natura; Ministero dell’Ambiente—Istituto Nazionale Fauna Selvatica: Bologna, Italy, 2004; Volume 20. [Google Scholar]

| Variable | Factors | Total Sum of Squares | Df | Mean Square | Pseudo-F | p |
|---|---|---|---|---|---|---|
| Sites | 2.26 | 2 | 1.13 | 142.31 | 0.0001 | |
| Seasons | 0.11 | 3 | 0.036 | 4.56 | 0.0041 | |
| Salinity | Interaction | 0.13 | 6 | 0.022 | 2.73 | 0.014 |
| Residual | 0.62 | 78 | 0.0079 | |||
| Total | 3.11 | 89 | ||||
| Sites | 57.37 | 2 | 28.69 | 7.85 | 0.0005 | |
| Seasons | 27.41 | 3 | 9.14 | 2.50 | 0.061 | |
| Dissolved oxygen | Interaction | 20.65 | 6 | 3.44 | 0.94 | 0.47 |
| Residual | 321.64 | 88 | 3.65 | |||
| Total | 427.07 | 99 | ||||
| Sites | 0.094 | 2 | 0.047 | 3.40 | 0.036 | |
| Seasons | 1.39 | 3 | 0.46 | 33.4 | 0.0001 | |
| Temperature | Interaction | 0.019 | 6 | 0.0032 | 0.23 | 0.98 |
| Residual | 1.22 | 88 | 0.0139 | |||
| Total | 2.72 | 99 | ||||
| Sites | 0.014 | 2 | 0.0071 | 21.9 | 0.0001 | |
| Seasons | 0.00045 | 3 | 0.00015 | 0.46 | 0.71 | |
| pH | Interaction | 0.0046 | 6 | 0.00076 | 2.35 | 0.035 |
| Residual | 0.029 | 88 | 0.00032 | |||
| Total | 0.048 | 99 |
| A vs. B | |||||
|---|---|---|---|---|---|
| Taxa | Av. Dissim. | Contrib. % | Cum. % | Mean ab. A | Mean ab. B |
| Gammarus aequicauda | 9.65 | 20.04 | 20.04 | 1.40 | 0.21 |
| Chironomidae | 7.63 | 15.85 | 35.89 | 1.50 | 2.06 |
| Hydrobia acuta | 6.05 | 12.56 | 48.45 | 1.88 | 2.20 |
| Cerastoderma glaucum | 5.37 | 11.14 | 59.60 | 0.40 | 0.60 |
| Idotea chelipes | 3.87 | 8.04 | 67.64 | 0.62 | 0.016 |
| Gammaridae juv. | 2.73 | 5.68 | 73.32 | 0.38 | 0.10 |
| Monocorophium insidiosum | 2.63 | 5.47 | 78.79 | 0.44 | 0 |
| Nemertea | 2.33 | 4.84 | 83.64 | 0.34 | 0.025 |
| Cerithium vulgatum | 2.08 | 4.31 | 87.95 | 0.24 | 0 |
| Nebrioporus ceresyi | 0.99 | 2.06 | 90.01 | 0 | 0.13 |
| Perinereis cultrifera | 0.96 | 2.00 | 92.01 | 0.14 | 0.032 |
| Corophiidae juv. | 0.88 | 1.83 | 93.84 | 0.16 | 0 |
| Nereididae juv. | 0.74 | 1.53 | 95.37 | 0.11 | 0.016 |
| Monocorophium sextonae | 0.72 | 1.49 | 96.87 | 0.13 | 0 |
| Melita palmata | 0.68 | 1.41 | 98.28 | 0.068 | 0.016 |
| Neodexiospira pseudocorrugata | 0.42 | 0.86 | 99.14 | 0.053 | 0 |
| Victorella pavida | 0.41 | 0.86 | 100 | 0 | 0.061 |
| Ephydra bivittata | 0 | 0 | 100 | 0 | 0 |
| A vs. C | |||||
| Taxa | Av. Dissim. | Contrib. % | Cum. % | Mean ab. A | Mean ab. C |
| Hydrobia acuta | 15.43 | 22.30 | 22.30 | 1.88 | 0.37 |
| Gammarus aequicauda | 11.88 | 17.17 | 39.47 | 1.40 | 0 |
| Chironomidae | 10.66 | 15.40 | 54.87 | 1.50 | 2.17 |
| Nebrioporus ceresyi | 6.46 | 9.33 | 64.21 | 0 | 0.64 |
| Idotea chelipes | 4.45 | 6.42 | 70.63 | 0.62 | 0 |
| Cerastoderma glaucum | 3.73 | 5.39 | 76.02 | 0.40 | 0.028 |
| Monocorophium insidiosum | 3.02 | 4.36 | 80.39 | 0.44 | 0 |
| Nemertea | 2.65 | 3.83 | 84.21 | 0.34 | 0 |
| Gammaridae juv. | 2.53 | 3.66 | 87.87 | 0.38 | 0 |
| Cerithium vulgatum | 2.52 | 3.64 | 91.51 | 0.24 | 0 |
| Ephydra bivittata | 1.16 | 1.68 | 93.19 | 0 | 0.13 |
| Corophiidae | 1.00 | 1.44 | 94.63 | 0.16 | 0 |
| Perinereis cultrifera | 0.94 | 1.36 | 95.99 | 0.14 | 0 |
| Monocorophium sextonae | 0.82 | 1.18 | 97.17 | 0.13 | 0 |
| Nereididae juv. | 0.76 | 1.10 | 98.26 | 0.11 | 0 |
| Melita palmata | 0.71 | 1.02 | 99.28 | 0.068 | 0 |
| Neodexiospira pseudocorrugata | 0.72 | 0.72 | 100 | 0.053 | 0 |
| Victorella pavida | 0 | 0 | 100 | 0 | 0 |
| B vs. C | |||||
| Taxa | Av. Dissim. | Contrib. % | Cum. % | Mean ab. B | Mean ab. C |
| Hydrobia acuta | 21.42 | 42.73 | 42.73 | 2.20 | 0.37 |
| Chironomidae | 9.23 | 18.42 | 61.15 | 2.06 | 2.17 |
| Nebrioporus ceresyi | 7.35 | 14.67 | 75.82 | 0.13 | 0.64 |
| Cerastoderma glaucum | 6.07 | 12.11 | 87.94 | 0.60 | 0.028 |
| Gammarus aequicauda | 1.99 | 3.97 | 91.91 | 0.21 | 0 |
| Ephydra bivittata | 1.37 | 2.73 | 94.64 | 0 | 0.13 |
| Gammaridae juv. | 0.99 | 1.98 | 96.62 | 0.10 | 0 |
| Victorella pavida | 0.56 | 1.12 | 97.75 | 0.061 | 0 |
| Perinereis cultrifera | 0.37 | 0.73 | 98.48 | 0.032 | 0 |
| Nemertea | 0.26 | 0.51 | 98.99 | 0.025 | 0 |
| Melita palmata | 0.21 | 0.42 | 99.41 | 0.016 | 0 |
| Idotea chelipes | 0.15 | 0.31 | 99.71 | 0.016 | 0 |
| Nereididae juv. | 0.14 | 0.29 | 100 | 0.016 | 0 |
| Neodexiospira pseudocorrugata | 0 | 0 | 100 | 0 | 0 |
| Monocorophium sextonae | 0 | 0 | 100 | 0 | 0 |
| Monocorophium insidiosum | 0 | 0 | 100 | 0 | 0 |
| Corophiidae juv. | 0 | 0 | 100 | 0 | 0 |
| Cerithium vulgatum | 0 | 0 | 100 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonifazi, A.; Galli, S.; Gravina, M.F.; Ventura, D. Macrozoobenthos Structure and Dynamics in a Mediterranean Hypersaline Ecosystem with Implications for Wetland Conservation. Water 2023, 15, 1411. https://doi.org/10.3390/w15071411
Bonifazi A, Galli S, Gravina MF, Ventura D. Macrozoobenthos Structure and Dynamics in a Mediterranean Hypersaline Ecosystem with Implications for Wetland Conservation. Water. 2023; 15(7):1411. https://doi.org/10.3390/w15071411
Chicago/Turabian StyleBonifazi, Andrea, Simone Galli, Maria Flavia Gravina, and Daniele Ventura. 2023. "Macrozoobenthos Structure and Dynamics in a Mediterranean Hypersaline Ecosystem with Implications for Wetland Conservation" Water 15, no. 7: 1411. https://doi.org/10.3390/w15071411
APA StyleBonifazi, A., Galli, S., Gravina, M. F., & Ventura, D. (2023). Macrozoobenthos Structure and Dynamics in a Mediterranean Hypersaline Ecosystem with Implications for Wetland Conservation. Water, 15(7), 1411. https://doi.org/10.3390/w15071411







