Ecological Interdependence of Pollution, Fish Parasites, and Fish in Freshwater Ecosystems of Turkey
Abstract
:1. Introduction and Background
2. Material and Methods
3. Results
3.1. Types of Pollutants
3.2. Indicator Parasites
3.3. Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, J.; Cardinale, A.G.; Allington, G.R.H.; Loreau, M. Is local biodiversity declining or not? A summary of the debate over analysis of species richness time trends. Biol. Conserv. 2018, 219, 175–183. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SCBD. Global Biodiversity Outlook 4, Secretariat of the Convention on Biological Diversity, Montreal. 2014. Available online: http://www.emdashdesign.ca (accessed on 22 September 2022).
- Perujo, N.; Van den Brink, P.J.; Segner, H.; Mantyka-Pringle, C.; Sabater, S.; Birk, S.; Bruder, A.; Romero, F.; Acuña, V. A guideline to frame stressor effects in freshwater ecosystems. Sci. Total Environ. 2021, 777, 146112. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, D.; Barinova, S.; Cianfaglione, K.; Curtean-Bănăduc, A. Editorial: Multiple freshwater stressors—Key drivers for the future of freshwater environments. Front. Environ. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a Sustainable Resource and Generator of Secondary Resources in the 21st Century: Stressors, Threats, Risks, Management and Protection Strategies, and Conservation Approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Stark, J.S. Heavy metal pollution and macrobenthic assemblages in soft sediments in two Sydney estuaries, Australia. Mar. Freshw. Res. 1998, 49, 533–540. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Olosutean, H.; Bănăduc, D. Influence of environmental variables on the structure and diversity of ephemeropteran communities: A case study of the Timiş River, Romania. Acta Zool. Bulg. 2016, 68, 215–224. [Google Scholar]
- Barletta, M.; Lima, A.R.; Costa, M.F. Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries. Sci. Total Environ. 2019, 651, 1199–1218. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Bănăduc, D. The Benthic Trophic Corner Stone Compartment in POPs Transfer from Abiotic Environment to Higher Trophic Levels—Trichoptera and Ephemeroptera Pre-Alert Indicator Role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Horak, I.; Horn, S.; Pieters, R. Agrochemicals in freshwater systems and their potential as endocrine disrupting chemicals: A South African context. Environ. Pollut. 2021, 268, 115718. [Google Scholar] [CrossRef]
- Chen, H.L.; Selvam, S.B.; Ting, K.N.; Gibbins, C.N. Microplastic pollution in freshwater systems in Southeast Asia: Contamination levels, sources, and ecological impacts. Environ. Sci. Pollut. Res. 2021, 28, 54222–54237. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, A. Data concerning the benthic communities of the Cibin River (Olt River Basin) Transylv. Transylv. Rev. Syst. Ecol. Res. 1999, 1, 99–110. [Google Scholar]
- Barinova, S.; Dyadichko, V. Zoological Water Quality Indicators for Assessment of Organic Pollution and Trophic Status of Continental Water Bodies. Transylv. Rev. Syst. Ecol. Res. 2022, 24, 65–106. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Berg, V.; Lyche, J.L.; Bănăduc, D. Bioaccumulation of persistent organic pollutants in the gonads of Barbus barbus (Linnaeus, 1758). Ecotoxicol. Environ. Saf. 2020, 201, 110852. [Google Scholar] [CrossRef] [PubMed]
- Bănăduc, D.; Marić, S.; Cianfaglione, K.; Afanasyev, S.; Somogyi, D.; Nyeste, K.; Antal, L.; Koščo, J.; Ćaleta, M.; Wanzenböck, J.; et al. Stepping Stone Wetlands, Last Sanctuaries for European Mudminnow: How Can the Human Impact, Climate Change, and Non-Native Species Drive a Fish to the Edge of Extinction? Sustainability 2022, 14, 13493. [Google Scholar] [CrossRef]
- Boeraș, I.; Curtean-Bănăduc, A.; Bănăduc, D.; Cioca, G. Anthropogenic Sewage Water Circuit as Vector for SARS-CoV-2 Viral ARN Transport and Public Health Assessment, Monitoring and Forecasting—Sibiu Metropolitan Area (Transylvania/Romania) Study Case. Int. J. Environ. Res. Public Health 2022, 19, 11725. [Google Scholar] [CrossRef]
- Bănăduc, D.; Oprean, L.; Bogdan, A. Fish Species Community Interest Management Issues in Natura 2000 Site Sighișoara-Târnava Mare (Transylvania, Romania). In Proceedings of the 18th International Economic Conference on Crisis After the Crisis—Inquiries from a National European and Global Perspective, Sibiu, Romania, 19–20 May 2011; pp. 23–27. [Google Scholar]
- Bănăduc, D.; Curtean-Bănăduc, A.; Cianfaglione, K.; Akeroyd, J.R.; Cioca, L.-I. Proposed Environmental Risk Management Elements in a Carpathian Valley Basin, within the Roşia Montană European Historical Mining Area. Int. J. Environ. Res. Public Health 2021, 18, 4565. [Google Scholar] [CrossRef]
- Simić, V.; Bănăduc, D.; Curtean-Bănăduc, A.; Petrović, A.; Veličković, T.; Stojković-Piperac, M.; Simić, S. Assessment of the ecological sustainability of river basins based on the modified the ESHIPPOfish model on the example of the Velika Morava basin (Serbia, Central Balkans). Front. Environ. Sci. 2022, 10, 1125. [Google Scholar] [CrossRef]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes: A broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Virga, G.; Arnieri, F.; Costantino, M. Differences in Growth Pattern in Two Freshwater Fish Species (Leuciscidae) during Summer Drought in North-West Italy, Transylv. Rev. Syst. Ecol. Res. 2023, 25, 55–64. [Google Scholar]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The Role of Aquatic Refuge Habitats for Fish, and Threats in the Context of Climate Change and Human Impact, during Seasonal Hydrological Drought in the Saxon Villages Area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Zare-Shahraki, M.; Ebrahimi-Dorche, E.; Bruder, A.; Flotemersch, J.; Blocksom, K.; Bănăduc, D. Fish Species Composition, Distribution and Community Structure in Relation to Environmental Variation in a Semi-Arid Mountainous River Basin, Iran. Water 2022, 14, 2226. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, E.; Fricke, R.; Sungur, S.; Eagderi, S. Endemic freshwater fishes of Turkey. FishTaxa 2018, 3, 1–39. [Google Scholar]
- Şekercioğlu, Ç.H.; Anderson, S.; Akçay, E.; Bilgin, R.; Can, Ö.E.; Semiz, G.; Tavşanoğlu, Ç.; Yokeş, M.B.; Soyumert, A.; Ipekdal, K.; et al. Turkey’s globally important biodiversity in crisis. Biol. Conserv. 2011, 144, 2752–2769. [Google Scholar] [CrossRef]
- FishBase. World Wide Web Electronic Publication. Version (08/2021). Froese, R.; Pauly, D. (Eds.) 2021. Available online: www.fishbase.org (accessed on 1 August 2021).
- Içek, E.; Birecikligil, S.S.; Ronald, F. Freshwater fishes of Turkey: A revised and updated annotated checklist. Biharean Biol. 2015, 9, 141–157. [Google Scholar]
- Tarkan, A.S.; Marr, S.M.; Ekmekçi, F.G. Non-native and translocated freshwater fish species in Turkey. FiSHMED Fishes Mediterr. Environ. 2015, 3, 1–28. [Google Scholar] [CrossRef]
- Ulman, A.; Zengin, M.; Demirel, N.; Pauly, D. The Lost Fish of Turkey: A Recent History of Disappeared Species and Commercial Fishery Extinctions for the Turkish Marmara and Black Seas. Front. Mar. Sci. 2020, 7, 650. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Bănăduc, D.; Bucşa, C. Watersheds management (Transylvania/ Romania): Implications, risks, solutions, Strategies to enhance environmental security in transition countries. In NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2007; p. 225. [Google Scholar] [CrossRef]
- Costea, G.; Pusch, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A review of hydropower plants in Romania: Distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sustain. Energy Rev. 2021, 145, 111003. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Marić, S.; Gábor, G.; Didenko, A.; Planellas, S.R.; Bănăduc, D. Hucho hucho (Linnaeus, 1758): Last natural viable population in the Eastern Carpathians—Conservation elements. Turk. J. Zool. 2019, 43, 215–223. [Google Scholar] [CrossRef]
- Burcea, A.; Boeraş, I.; Mihuţ, C.-M.; Bănăduc, D.; Matei, C.; Curtean-Bănăduc, A. Adding the Mureş River Basin (Transylvania, Romania) to the List of Hotspots with High Contamination with Pharmaceuticals. Sustainability 2020, 12, 10197. [Google Scholar] [CrossRef]
- Ktener, A.; Eğribaş, E.; Başusta, N. A preliminary investigation on serious mortalities of fish in Balıklıgöl (Halil-ür Rahman Gölü, Şanlıurfa). Gazi Univ. J. Sci. 2008, 21, 9–13. [Google Scholar]
- Yabanlı, M.; Türk, N.; Tenekecioğlu, E.; Uludağ, R. A Research for Massive Fish Kills in Lake Bafa (Turkey). Sakarya Univ. J. Sci. 2011, 15, 36–40. [Google Scholar]
- Pekmezci, G.Z.; Yardimci, B.; Bolukbas, C.S.; Beyhan, Y.E.; Umur, S. Mortality Due to Heavy Infestation of Argulus foliaceus (Linnaeus, 1758) (Branchiura) in Pond-Reared Carp, Cyprinus carpio L., 1758 (Pisces). Crustaceana 2011, 84, 553–557. [Google Scholar] [CrossRef]
- MacKenzie, K. Parasites as biological tags in population studies of marine organisms: An update. Parasitology 2002, 124, S153–S163. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, K.; Hemmingsen, W. Parasites as biological tags in marine fisheries research: European Atlantic waters. Parasitology 2015, 142, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Del Monte-Luna, P.; Brook, B.W.; Zetina-Rejón, M.J.; Cruz-Escalona, V.H. The carrying capacity of ecosystems. Glob. Ecol. Biogeogr. 2004, 13, 485–495. [Google Scholar] [CrossRef]
- Iwanowicz, D.D. Overview on the effects of parasites on fish health. In Proceedings of the Third Bilateral Conference between Russia and the United States. Bridging America and Russia with Shared Perspectives on Aquatic Animal Health, USGS Organization, Leetown Science Center, USA; 2011; pp. 176–184. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=464af280b7778724425654fe3f557768b1fc0846 (accessed on 10 February 2023).
- Zeeman, M.G.; Brindley, W.A. Effects of toxic agents upon fish immune systems: A review. Immunol. Consid. Toxicol. 1981, 2, 1–60. [Google Scholar]
- Wojdani, A.; Alfred, L.J. Alterations in cell-mediated immune functions induced in mouse splenic lymphocytes by polycyclic aromatic hydrocarbons. Cancer Res. 1984, 44, 942–945. [Google Scholar] [PubMed]
- Poulin, R. Toxic pollution and parasitism in freshwater fish. Parasitol. Today 1992, 8, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.R. Biological Monitoring cf Aquatic Systems; Loeb, S.L., Spacie, S., Eds.; Kerr Center for Sustainable Agriculture (USA), 1994; pp. 357–373. Available online: https://kerrcenter.com/ (accessed on 10 February 2023).
- Sprague, J.B. Measurement of pollutant toxicity to fish I. Bioassay methods for acute toxicity. Water Res. 1969, 3, 793–821. [Google Scholar] [CrossRef]
- Khan, R.A.; Thulin, J. Influence of Pollution on Parasites of Aquatic Animals. Adv. Parasitol. 1991, 30, 201–238. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, K.; Williams, H.H.; Williams, B.; McVicar, A.H.; Siddall, R. Parasites as Indicators of Water Quality and the Potential Use of Helminth Transmission in Marine Pollution Studies. Adv. Parasitol. 1995, 35, 85–144. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, K.D. Environmental parasitology: What can parasites tell us about human impacts on the environment? Parasitol. Today 1997, 13, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Sures, B. The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: A review. Aquat. Ecol. 2001, 35, 245–255. [Google Scholar] [CrossRef]
- Gilbert, B.M.; Avenant-Oldewage, A. Parasites and pollution: The effectiveness of tiny organisms in assessing the quality of aquatic ecosystems, with a focus on Africa. Environ. Sci. Pollut. Res. Int. 2017, 24, 18742–18769. [Google Scholar] [CrossRef]
- Öktener, A. A checklist of metazoan parasites recorded in freshwater fish from Turkey. Zootaxa 2003, 394, 1–28. [Google Scholar] [CrossRef]
- Öktener, A. A checklist of parasitic helminths reported from sixty-five species of marine fish from Turkey including two new records of monogeneans. Zootaxa 2005, 1063, 33–52. [Google Scholar] [CrossRef]
- Öktener, A. Revision of Parasitic Helminths Reported in Freshwater Fish from Turkey with New Records. Transylv. Rev. Syst. Ecol. Res. 2014, 16, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Öktener, A. An Updated Checklist of Parasitic Helminths of Marine Fish from Turkey. Transylv. Rev. Syst. Ecol. Res. 2015, 16, 55–96. [Google Scholar] [CrossRef] [Green Version]
- Alaş, A.; Öktener, A.; Türker, D.Ç. Review of Parasitic Copepods Recorded in Fish from Turkey. Transylv. Rev. Syst. Ecol. Res. 2015, 17, 39–62. [Google Scholar] [CrossRef] [Green Version]
- Alaş, A.; Öktener, A. Different Parasitic Phyla of Fish from Turkey excluding Helminths and Crustacea. J. Zool. Stud. 2015, 2, 24–41. [Google Scholar]
- Tekin Özan, S.; Kır, İ. An investigation of parasites of goldfish (Carassius carassius L., 1758) in Kovada Lake. Turk. Parazitolojii Derg. 2005, 29, 200–203. [Google Scholar]
- Tekin-Özan, S.; Kir, I. Accumulation of some heavy metals in Raphidascaris acus (Bloch, 1779) and its host (Esox lucius L., 1758). Türkiye Parazitoloji Derg. 2007, 31, 327–329. [Google Scholar]
- Genc, E.; Sangun, M.K.; Dural, M.; Can, M.F.; Altunhan, C. Element concentrations in the swimbladder parasite Anguillicola crassus (nematoda) and its host the European eel, Anguilla anguilla from Asi River (Hatay-Turkey). Environ. Monit. Assess. 2008, 141, 59–65. [Google Scholar] [CrossRef] [PubMed]
- WoRMS Editorial Board. World Register of Marine Species. 2022. Available online: https://www.marinespecies.org (accessed on 3 April 2022).
- Grant, B.; Huchzermeyer, D.; Hohls, B. Manual for Fish Kill Investigations in South Africa (WRC Report No. TT 589/14); Research Commission of South Africa: Report to the Water Research Commission; 2014; 135p, ISBN 978-1-4312-0531-8. Available online: https://www.coastkzn.co.za/wp-content/uploads/2019/02/TT589-14-WEB.pdf (accessed on 22 September 2022).
- Helfrich, L.A.; Smith, S.A. Fish Kills: Their Causes and Prevention; Communications and Marketing, College of Agriculture and Life Sciences, Virginia Polytechnic Institute State University: Blacksburg, VA, USA, 2009; pp. 252–420. [Google Scholar]
- Meyer, F.P.; Barclay, L.A. (Eds.) Field Manual for the Investigation of Fish Kills; Resource Publication 177; U.S. Fish and Wildlife Service: Washington, DC, USA, 1990; 120p.
- Mackenzie, K. Parasites as Pollution Indicators in Marine Ecosystems: A Proposed Early Warning System. Mar. Pollut. Bull. 1999, 38, 955–959. [Google Scholar] [CrossRef]
- Galli, P.; Crosa, G.; Mariniello, L.; Ortis, M.; D’Amelio, S. Water quality as a determinant of the composition of fish parasite communities. Hydrobiologia 2001, 452, 173–179. [Google Scholar] [CrossRef]
- Valtonen, E.T.; Holmes, J.C.; Aronen, J.; Rautalahti, I. Parasite communities as indicators of recovery from pollution: Parasites of roach (Rutilus rutilus) and perch (Perca fluviatilis) in Central Finland. Parasitology 2003, 126, S43–S52. [Google Scholar] [CrossRef]
- Marcogliese, D.J. Parasites: Small Players with Crucial Roles in the Ecological Theater. EcoHealth 2004, 1, 151–164. [Google Scholar] [CrossRef]
- Sures, B. Environmental parasitology: Relevancy of parasites in monitoring environmental pollution. Trends Parasitol. 2004, 20, 170–177. [Google Scholar] [CrossRef]
- Sures, B.; Nachev, M.; Selbach, C.; Marcogliese, D.J. Parasite responses to pollution: What we know and where we go in ‘Environmental Parasitology’. Parasites Vectors 2017, 10, 65. [Google Scholar] [CrossRef] [Green Version]
- Gezici, F. Bölge Sınırlarının Saptanmasında Yararlanılacak Ölçütler; Türkiye’de Bölgesel Yönetim—Bir Model Önerisi; Toksöz, F., Gezici, F., Eds.; Türkiye Ekonomik ve Sosyal Etüdler Vakfı, 2014; pp. 17–30. Available online: https://www.google.ca/search?q=T%C3%BCrkiye+Ekonomik+ve+Sosyal+Et%C3%BCdler+Vakf%C4%B1&ei=E5wqZP_6Arax2roPm7CUiAc&ved=0ahUKEwi_0Ibgso3-AhW2mFYBHRsYBXEQ4dUDCA4&uact=5&oq=T%C3%BCrkiye+Ekonomik+ve+Sosyal+Et%C3%BCdler+Vakf%C4%B1&gs_lcp=Cgxnd3Mtd2l6LXNlcnAQAzIQCC4QgAQQxwEQ0QMQExDqBDIGCAAQHhATMggIABAeEA8QEzoKCAAQRxDWBBCwA0oECEEYAFDPA1jPA2DRC2gBcAF4AIABzAGIAcwBkgEDMi0xmAEAoAECoAEByAECwAEB&sclient=gws-wiz-serp (accessed on 10 February 2023).
- Anonymous. Ramsar Sites Assessment Report in Turkey; WWF-Turkey, Wildlife Conservation Foundation: Ankara, Turkey, 2008; 129p. [Google Scholar]
- Anonymous. State Hydraulic Works 2020 Annual Report; Ministry of Agriculture and Forestry, General Directorate of State Hydraulic Works: Ankara, Turkey, 2020; 159p.
- Höglund, J.; Thulin, J. Thermal effects on the seasonal dynamics of Paradiplozoon homoion (Bychowsky & Nagibina, 1959) parasitizing roach, Rutilus rutilus (L.). J. Helminthol. 1989, 63, 93–101. [Google Scholar] [CrossRef]
- Koskivaara, M.; Valtonen, E.T. Paradiplozoon homoion (Monogenea) and some other gill parasites on roach Rutilus rutilus in Finland. Aqua Fenn. 1991, 21, 137–143. [Google Scholar]
- Šebelová, Š.; Kuperman, B.; Gelnar, M. Abnormalities of the attachment clamps of representatives of the family Diplozoidae. J. Helminthol. 2002, 76, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bagge, A.M.; Valtonen, E.T. Experimental study on the influence of paper and pulp mill effluent on the gill parasite communities of roach (Rutilus rutilus). Parasitology 1996, 112, 499–508. [Google Scholar] [CrossRef]
- Karvonen, A.; Bagge, A.M.; Valtonen, E.T. Parasite assemblages of crucian carp (Carassius carassius)—Is depauperate composition explained by lack of parasite exchange, extreme environmental conditions or host unsuitability? Parasitology 2005, 131, 273–278. [Google Scholar] [CrossRef]
- Yardımcı, R.E.; Ürkü, Ç.; Yardımcı, C.H. Parasite fauna of fish in Büyükçekmece Dam Lake. Erzincan Univ. J. Sci. Technol. 2018, 11, 158–167. [Google Scholar] [CrossRef]
- Öztürk, M.O. Helminth Fauna of Fishes in Manyas (Kuş) Lake. Ph.D. Thesis, Uludag University, Bursa, Turkey, 2000. [Google Scholar]
- Öztürk, M.O. Helminth Fauna of Two Cyprinid Fish Species (Chalcalburnus chalcoides Güldenstadt, 1972, Rutilus rutilus L.) from lake Uluabat, Turkey. Hacet. J. Biol. Chem. 2005, 34, 77–91. [Google Scholar]
- Gürkan, Ü.; Tekin Özan, S. Helminth fauna of chub (Squalius cephalus L.) in Susurluk Creek (Bursa-Balıkesir). S.D.U. J. Nat. Appl. Sci. 2012, 7, 77–85. [Google Scholar]
- Özdemir, A.C. Impacts of Land Use on Water Quality in Istanbul Watersheds. Master’s Thesis, İstanbul Teknik University, İstanbul, Turkey, 2010. [Google Scholar]
- Arı, Y. Cultural Ecology of Lake Manyas: Adaptation and Change in Historical Perspective. Turk. Geogr. Rev. 2014, 40, 75–97. [Google Scholar]
- Kurtoglu, S.; Ozengin, N.; Elmaci, A.; Baskaya, H.S. Monitoring of Sediment Quality and Nutrients Dynamics of Lake Uluabat, Turkey. J. Biol. Environ. Sci. 2015, 9, 11–19. [Google Scholar]
- Selver, M.M. Helminth Fauna of Some Fish Species Catched from Kocadere Stream. Ph.D. Thesis, Uludag University, Bursa, Turkey, 2008. [Google Scholar]
- Karabiber, F.T. Parasite Fauna of Roach (Rutilus rutilus Linnaeus, 1758) in the Lake Sapanca. Master’s Thesis, Marmara University, İstanbul, Turkey, 2006. [Google Scholar]
- Soylu, E. Research Studies on the Parasite Fauna of Some Fishes in Sapanca Lake. Ph.D. Thesis, Istanbul University, Istanbul, Turkey, 1990. [Google Scholar]
- Crafford, D.; Luus-Powell, W.; Avenant-Oldewage, A. Monogenean parasites from fishes of the Vaal Dam, Gauteng Province, South Africa I. Winter survey versus summer survey comparison from Labeo capensis (Smith 1841) and Labeo umbratus (Smith, 1841) hosts. Acta Parasitol. 2014, 59, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dušek, L.; Gelnar, M.; Šebelová, Š. Biodiversity of parasites in a freshwater environment with respect to pollution: Metazoan parasites of chub (Leuciscus cephalus L.) as a model for statistical evaluation. Int. J. Parasitol. 1998, 28, 1555–1571. [Google Scholar] [CrossRef] [PubMed]
- Altuğ, G. Heavy Metal Pollution in Sapanca Lake: Sediment, Fish, Mussel; Sapanca Gölü’ne Bilimsel Açıdan Bakış; Okgerman, H., Aktuğ, G., Eds.; TÜDAV Yayınları, 2008; pp. 149–156. Available online: https://tudav.org/calismalar/yayinlar/ (accessed on 10 February 2023).
- lmez, G. Determination of Environmental Objectives for Improvement of Water Quality in Surface Water Resources. Master’s Thesis, Ministry of Forestry and Water Affairs, Ankara, Turkey, 2014. [Google Scholar]
- Soylu, E.; Uzmanoğlu, S.; Özesen Çolak, S.; Soylu, M.P. Community Structure of the Parasites of the Endemic Chocolate Chub Squalius carinus Özuluğ & Freyhof, 2011 (Cyprinidae) from Işıklı Lake, Çivril, Turkey. Acta Zool. Bulg. 2017, 69, 405–409. [Google Scholar]
- Halmetoja, A.; Valtonen, E.T.; Koskenniemi, E. Perch (Perca fluviatilis L.) parasites reflect ecosystem conditions: A comparison of a natural lake and two acidic reservoirs in Finland. Int. J. Parasitol. 2000, 30, 1437–1444. [Google Scholar] [CrossRef]
- Altan, A.; Soylu, E. Composition and structure of parasite communities in white bream Blicca bjoerkna from Lake Büyük Akgöl, Sakarya-Turkey. Ege J. Fish. Aquat. Sci. 2018, 35, 199–206. [Google Scholar] [CrossRef]
- Durmaz, A. Evaluation of Heavy Metal Pollution and Sources in Shallow Lakes. Master’s Thesis, Sakarya University, Sakarya, Turkey, 2019. [Google Scholar]
- Bulut, C.; Atay, R.; Uysal, K.; Köse, E. Evaluation of Surface Water Quality in Çivril Lake. Anadolu Univ. J. Sci. Technol. C-Life Sci. Biotechnol. 2012, 2, 1–8. [Google Scholar]
- Akbulut, M.; Bat, L.; Çulha, M.; Satılmış, H. Problems of Kızılırmak Delta and solution methods. In Proceedings of the Aquatic Products Symposium, Sinop, Turkey, 20–22 September 2000; pp. 655–661. [Google Scholar]
- Jeney, Z.; Valtonen, E.T.; Jeney, G.; Jokinen, E.I. Effect of pulp and paper mill effluent (BKME) on physiological parameters of roach (Rutilus rutilus) infected by the digenean Rhipidocotyle fennica. Folia Parasitol. 2002, 49, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, M.O.; Oğuz, M.C.; Altunel, F.N. Metazoon parasites of pike (Esox lucius L.) from Uluabat Lake. Isr. J. Zool. 2000, 46, 119–130. [Google Scholar] [CrossRef]
- Baruš, V.; Šimková, A.; Prokeš, M.; Peňáz, M.; Vetešník, L. Heavy metals in two host-parasite systems: Tapeworm vs. fish. Acta Vet. Brno 2012, 81, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Dişçi, H. Determination of Endoparasites of Pike-Perch (Esox lucius L., 1758) Inhabiting Işıklı Dam Lake. Master’s Thesis, Süleyman Demirel University, Isparta, Turkey, 2002. [Google Scholar]
- Jirsa, F.; Konecny, R.; Frank, C.; Sures, B. The parasite community of the nase Chondrostoma nasus (L. 1758) from Austrian rivers. J. Helminthol. 2011, 85, 255–262. [Google Scholar] [CrossRef]
- Pilecka-Rapacz, M.; Piasecki, W.; Czerniawski, R.; Sługocki, Ł.; Krepski, T.; Domagała, J. The effect of warm discharge waters of a power plant on the occurrence of parasitic Metazoa in freshwater bream, Abramis brama (L.). Bull. Eur. Assoc. Fish Pathol. 2015, 35, 94–103. [Google Scholar]
- Akmirza, A.; Yardımcı, E.R. Fish parasites of the Sakarya River, Turkey. J. Acad. Doc. Fish. Aquac. 2014, 1, 23–29. [Google Scholar]
- Topçu, A. The Helminths of the Digestive Tract of the Carps (Cyprinus carpio) in Van Region. Ph.D. Thesis, Yuzuncu Yıl University, Van, Turkey, 1993. [Google Scholar]
- Baştürk, O. Identification of Pollution of Sakarya River by Using Geographical Information (GIS). Master’s Thesis, Sakarya University, Sakarya, Turkey, 2006. [Google Scholar]
- Eken, G.; Bozdoğan, M.; İsfendiyaroğlu, S.; Kılıç, S.; Lise, Y. (Eds.) Bendimahi Delta. Important Natural Areas of Turkey; Doğa Derneği: Ankara, Turkey, 2006; pp. 390–391. [Google Scholar]
- Anonymous. Van Province 2019 Environmental Status Report; Directorate of Environmental Management and Inspection: Pretoria, South Africa, 2021; 157p. [Google Scholar]
- Gabrashanska, M.; Nedeva, I. Content of heavy metals in the system fish-cestodes. Parassitologia 1996, 38, 58. [Google Scholar]
- Oyoo-Okoth, E.; Wim, A.; Osano, O.; Kraak, M.H.; Ngure, V.; Makwali, J.; Orina, P.S. Use of the fish endoparasite Ligula intestinalis (L., 1758) in an intermediate cyprinid host (Rastreneobola argentea) for biomonitoring heavy metal contamination in Lake Victoria, Kenya. Lakes Reserv. Res. Manag. 2010, 15, 63–73. [Google Scholar] [CrossRef]
- Keskin, N.; Arkan, F.E. Ligulosis in freshwater fishes in Turkey. Hacet. Üniv. J. Sci. Eng. 1987, 8, 57–70. [Google Scholar]
- Burgu, A.; Oğuz, T.; Körting, W.; Güralp, N. Parasites of freshwater fishes in some areas of Central Anatolia. Etlik Vet. Mikrobiyoloji Derg. 1988, 6, 143–166. [Google Scholar]
- Yılmaz, F.; Solak, K.; Alaş, A. A research on Ligula intestinalis L. from Yukarı Porsuk. In Proceedings of the XIII. Congress of National Biology, İstanbul, Turkey, 17–20 September 1996; pp. 71–79. [Google Scholar]
- Koyun, M. Helminth Fauna of Some Fish Species in Enne Dam Lake (Kütahya). Ph.D. Thesis, Uludag University, Bursa, Turkey, 2001. [Google Scholar]
- Kır, I. Investigation of Parasites and Growth of Common Carp (Cyprinus carpio l. 1758), Heckel’s Orontes Barbell (Barbus capito pectoralis Heckel, 1843) and Gibel Carp (Carassius carassius L. 1758) in Karacaören I Dam Lake. Ph.D. Thesis, Süleyman Demirel University, Isparta, Turkey, 1998. [Google Scholar]
- Arslan, M.Ö.; Yilmaz, M.; Tasçi, G.T. Infections of Ligula intestinalis on Freshwater Fish in Kars Plateau of North-Eastern Anatolia, Turkey. Türkiye Parazitolojii Derg. 2015, 39, 218–221. [Google Scholar] [CrossRef]
- Başaran, A.; Kelle, A. Distribution of Ligula intestinalis Some Freshwater Fish Living on the Devegecidi Dam Lake. Hacet. Univ. J. Fac. Sci. 1976, 26, 45–56. [Google Scholar]
- Anonymous. Ankara Province Environmental Report in 2019; Provincial Directorate of Environment and Urbanization: Ankara, Turkey, 2019; 296p. [Google Scholar]
- Uğurlu, Ö. The Effects of Ankara Urban Development on the Mogan and Eymir Lakes Wetland Ecosystem. Master’s Thesis, Ankara University, Ankara, Turkey, 2020. [Google Scholar]
- Çıplakoğlu, G. A Research on the Eutrophication Sensitivity of the Surface Waters and Sakarya River Basin Sample. Master’s Thesis, İstanbul Technical University, İstanbul, Turkey, 2006. [Google Scholar]
- Öztürk, R.; Altan, T. Porsuk Creek Envirronmental Problems and Watershed Management Suggestions in Solutions to These Problems. CUNAS 2008, 17, 79–89. [Google Scholar]
- Sarıyıldız, A.; Harmancıoğlu, N.; Sılay, A.; Çetin, H.C. Trend Analysis of Water Quality Parameters of Gediz River. In Proceedings of the TMMOB İzmir City Symposium, DSI II, İzmir, Turkey, 11 August 2008; pp. 603–611. [Google Scholar]
- Köse, E.; Uysal, K. The comparison of heavy metal accumulation ratios in muscle, skin and gill of non-maturated common carp (Cyprinus carpio L., 1758). J. Sci. Tech. Dumlupınar Univ. 2008, 17, 19–26. [Google Scholar]
- Yalım, F.B.; Emre, N.; Gülle, İ.; Emre, Y.; Pak, F.; Aktaş, Ö.; Uysal, R.; Veske, E. Seasonal Change of Microbiological Pollution Level of Karacaören I Dam Lake, Burdur, Turkey. LimnoFish 2020, 6, 120–126. [Google Scholar] [CrossRef]
- Anonymous. Kars Province Environmental Report in 2018; Provincial Directorate of Environment and Urbanization: Kars, Turkey, 2018; 124p. [Google Scholar]
- Yıldız, H.B. Investigation of Change Water Quality Depending on Period and Space Using Enrichment Factor in the Upper Tigris Basin. Master’s Thesis, Hacettepe University, Ankara, Turkey, 2013. [Google Scholar]
- Shah, H.B.; Yousuf, A.R.; Chishti, M.Z.; Ahmad, F. Helminth communities of fish as ecological indicators of lake health. Parasitology 2013, 140, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Furness, D.N.; Zholobenko, V.; Hoole, D. Effect of tapeworm parasitisation on cadmium toxicity in the bioindicator copepod, Cyclops strenuus. Ecol. Indic. 2014, 37, 21–26. [Google Scholar] [CrossRef]
- Dalkıran, N.; Karacaoğlu, D.; Taş, D.; Karabayırlı, G.; Atak, S.; Arda Koşucu, T.N.; Coşkun, F.; Akay, E. Use of Factor Analysis to Evaluate the Water Quality of Mustafakemalpaşa Stream (Bursa). Acta Aquat. Turc. 2020, 16, 124–137. [Google Scholar] [CrossRef]
- Deniz, O.; Doğu, A.F. Water Pollution in the Lake Van Basin. 38. In Proceedings of the ICANAS (International Congress of Asian and North African Studies) Congress, Ankara, Turkey, 10–15 September 2007; pp. 299–308. [Google Scholar]
- Topal, M. Past and Present Status of Water Quality of Hazar Lake. J. Eng. Sci. Des. 2011, 1, 120–134. [Google Scholar]
- Küçükyılmaz, M.; Uslu, G.; Birici, N.; Örnekçi, G.N.; Yıldız, N.; Şeker, T. Examination Water Quality of Karakaya Dam Lake. Yunus Araştırma Bülteni 2017, 2, 145–155. [Google Scholar]
- Eliker, M. Hydrogeologıc Assesment of Uluova (Elazığ) by Geographıcal Informatıon Systems. Master’s Thesis, Cukurova University, Adana, Turkey, 2008. [Google Scholar]
- Aydoğdu, A.; Selver, M. An investigation of helminth fauna of the bleak (Alburnus alburnus L.) from the Mustafakemalpasa stream, Bursa, Turkey. Turk. Parazitolojii Derg. 2006, 30, 68–71. [Google Scholar]
- Aksoy, Ş. Endohelminths Research in Capoeta capoeta umbla from Hazar Lake. Master’s Thesis, Fırat University, Elazığ, Turkey, 1996. [Google Scholar]
- rün, İ.; Dörücü, M.; Yazlak, H.; Öztürk, E. A Research Study on the Helminths of Karakaya Dam Lake Fishes and Their Impacts; Project No. 15; İnönü University, Department of Research Project: Malatya, Turkey, 2003. [Google Scholar]
- Dörücü, M.; İspir, Ü. Study on endo-parasites of some fish species caught in Keban Dam Lake. Fırat Univ. J. Sci. Eng. 2005, 17, 400–404. [Google Scholar]
- Morley, N.J.; Costa, H.H.; Lewis, J.W. Effects of a Chemically Polluted Discharge on the Relationship Between Fecundity and Parasitic Infections in the Chub (Leuciscus cephalus) from a River in Southern England. Arch. Environ. Contam. Toxicol. 2010, 58, 783–792. [Google Scholar] [CrossRef]
- Tekin Özan, S. The Investigation of Heavy Metals and Parasites in Carp (Cyprinus carpio L., 1758) and Tench (Tinca tinca L., 1758) Inhabiting Beysehir Lake. Ph.D. Thesis, Süleyman Demirel University, Isparta, Turkey, 2005. [Google Scholar]
- Kır, İ.; Tekin Özan, S. Occurrence of helminths in tench (Tinca tinca L., 1758) of Kovada (Isparta) Lake, Turkey. Bull. Eur. Assoc. Fish Pathol. 2005, 25, 75–81. [Google Scholar]
- Ateş, H.; Uzer, Y. Attempts at Rural Development on the Success of Wetland Rehabilitation: Akşehir Rehabilitation Project. 38. In Proceedings of the ICANAS (International Congress of Asian and North African Studies) Congress, Istambul, Turkey, 1 August 2011; Volume 1, pp. 85–104. [Google Scholar]
- Büber, H.; Bozyurt, O. Environmental Problems of Beyşehir Lake and Basin. Int. Soc. Ment. Res. Think. J. 2020, 38, 2389–2408. [Google Scholar] [CrossRef]
- Kavak, M.; Şeker, E. Investigation of endohelminthes in fish caught in Pertek Region of Keban Dam Lake. Fırat Univ. J. Sci. Eng. 2017, 29, 33–40. [Google Scholar]
- Aydoğdu, A.; Yıldırımhan, H.S.; Altunel, F.N. The helminth fauna of Adriatic roach (Rutilus rubilio) in İznik Lake. Bull. Eur. Assoc. Fish Pathol. 2000, 20, 170–172. [Google Scholar]
- Ztürk, T.; Öter, A.; Çam, A.; Yılmaz, D.; Ünsal, G. Metazoan parasites of pike-perch, Stizostedion lucioperca, L., 1758 collected from Lower Kızılırmak Delta in Turkey. In Proceedings of the 15th International Conference on Diseases of Fish and Shellfish, Split, Croatia, 12–16 September 2011; p. 430. [Google Scholar]
- Hasançavuşoğlu, Z.; Gündoğdu, A. Investigation of Anionic Detergent Pollution in Sarıkum Lake (Sinop). TURJAF 2019, 7, 1825–1833. [Google Scholar] [CrossRef] [Green Version]
- Sures, B.; Siddall, R.; Taraschewski, H. Parasites as Accumulation Indicators of Heavy Metal Pollution. Parasitol. Today 1999, 15, 16–21. [Google Scholar] [CrossRef]
- Sures, B.; Dezfuli, B.S.; Krug, H.F. The intestinal parasite Pomphorhynchus laevis (Acanthocephala) interferes with the uptake and accumulation of lead (210Pb) in its fish host chub (Leuciscus cephalus). Int. J. Parasitol. 2003, 33, 1617–1622. [Google Scholar] [CrossRef]
- Marijić, V.F.; Smrzlić, I.V.; Raspor, B. Does fish reproduction and metabolic activity influence metal levels in fish intestinal parasites, acanthocephalans, during fish spawning and post-spawning period? Chemosphere 2014, 112, 449–455. [Google Scholar] [CrossRef]
- Soylu, E. Pomphorhynchus laevis (Müller, 1776) (Acanthocephala) in Barbus plebejus escherichi Steindachner 1897 of Büyükcoz Lake, Karasu (Sakarya). Anadolu Univ. J. Sci. 1991, 3, 31–37. [Google Scholar]
- Buhurcu, H.İ. An Investigation on Endoparasite Fauna of Some Fish Species (Cyprinus carpio and Alburnus nasreddini) from Lake Akşehir. Master’s Thesis, Afyon Kocatepe University, Afyon, Turkey, 2006. [Google Scholar]
- Katip, A. Water Quality Monitoring of Lake Uluabat. Ph.D. Thesis, Uludag University, Bursa, Turkey, 2010. [Google Scholar]
- Bebka, B. TR41 Environmental Report; Development Agency of Bursa: Eskişehir, Turkey, 2011; 86p. [Google Scholar]
- Tuuha, H.; Valtonen, E.T.; Taskinen, J. Ergasilid copepods as parasites of perch Perca fluviatilis and roach Rutilus rutilus in central Finland: Seasonality, maturity and environmental influence. J. Zool. 1992, 228, 405–422. [Google Scholar] [CrossRef]
- Sağlam, N. Investigation of External Parasites of Fish Caught in Keban Dam Lake. Master’s Thesis, Fırat University, Elazığ, Turkey, 1992. [Google Scholar]
- imşek, Ö. Parasite Fauna of Fish Species from Ömerli Dam Lake. Master’s Thesis, Marmara University, İstanbul, Turkey, 2013. [Google Scholar]
- Tezer, A.; Çetin, N.İ.; Onur, A.C.; Menteşe, E.Y.; Albayrak, İ.; Cengiz, E.C. Project for Development of Integrated Basin Management Plan Based on Ecosystem Services in Ömerli Basin; TR10/14/DFD/0039; Istanbul Development Agency: İstanbul, Turkey, 2015; 177p. [Google Scholar]
- Karakişi, H.; Demir, S. Metazoan Parasites of the Common Carp (Cyprinus carpio L., 1758) from Tahtalı Dam Lake (İzmir). Türkiye Parazitolojii Derg. 2012, 36, 174–177. [Google Scholar] [CrossRef]
- Koyun, M.; Ulupınar, M.; Mart, A. First record of Lernaea cyprinacea L. 1758 (Copepoda: Cyclopoida) on Cyprinion macrostomus Heckel, 1843 from Eastern Anatolia, Turkey. Biharean Biol. 2015, 9, 44–46. [Google Scholar]
- Akçimen, U.; Apaydın Yağcı, M.; Yeğen, V.; Uysal, R.; Bilgin, F.; Yağcı, A. First report of Eustrongylides excisus larvae and Lernaea cyprinacea on Eğirdir minnow (Pseudophoxinus egridiri). In Proceedings of the 13th International Symposium on Fisheries and Aquatic Sciences, Ankara, Turkey, 21–23 October 2018; p. 108. [Google Scholar]
- Tóro, R.M.; Gessner, A.A.; Furtado, N.A.; Ceccarelli, P.S.; De Albuquerque, S.; Bastos, J.K. Activity of the Pinus elliottii resin compounds against Lernaea cyprinacea in vitro. Vet. Parasitol. 2003, 118, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ay, Z.K. Determination of Alternative Land Use Policies to Prevent Pollution in the Tahtalı Dam Conservation Area; T.C. General Directorate of Forestry Aegean Forestry Research Institute Technical Report; T.C. General Directorate of Forestry Aegean Forestry Research Institute: İzmir, Turkey, 2001; Volume 4, p. 70. [Google Scholar]
- Beyhan, M.; Kaçıkoç, M. Pollution Status and Pollution Sources Modeling Study in Lake Eğirdir. Seven Colored Life to Seven Colored Lake Project; WWF-Türkiye (Doğal Hayatı Koruma Vakfı): İstanbul, Türkiye, 2013; 35p. [Google Scholar]
- Demir, A.D.; Şahin, Ü.; Demir, Y. Trend Analysis and Agricultural Perspective Availability of Water Quality Parameters at Murat River. Yuz. Yıl Univ. J. Agric. Sci. 2016, 26, 414–420. [Google Scholar]
- Pettersen, R.A.; Vøllestad, L.A.; Flodmark, L.E.; Poléo, A.B. Effects of aqueous aluminium on four fish ectoparasites. Sci. Total Environ. 2006, 369, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Oztürk, M.O. An investigation of metazoan parasites of common Carp (Cyprinus carpio L.) in Lake Eber, Afyon, Turkey. Turk. Parazitolojii Derg. 2005, 29, 204–210. [Google Scholar]
- Ktener, A.; Ali, A.H.; Gustinelli, A.; Fioravanti, M.L. New host records for fish louse, Argulus foliaceus L., 1758 (Crustacea, Branchiura) in Turkey. Ittiopatologia 2006, 3, 161–167. [Google Scholar]
- Ktener, A.; Alaş, A. A parasitological study of fish from the Atatürk Dam Lake, Turkey. Bull. Eur. Assoc. Fish Pathol. 2009, 29, 193–197. [Google Scholar]
- Akbeniz, E. Metazoan Parasites of Tench (Tinca tinca L., 1758) in the Lake Sapanca. Master’s Thesis, Marmara University, Istanbul, Turkey, 2006. [Google Scholar]

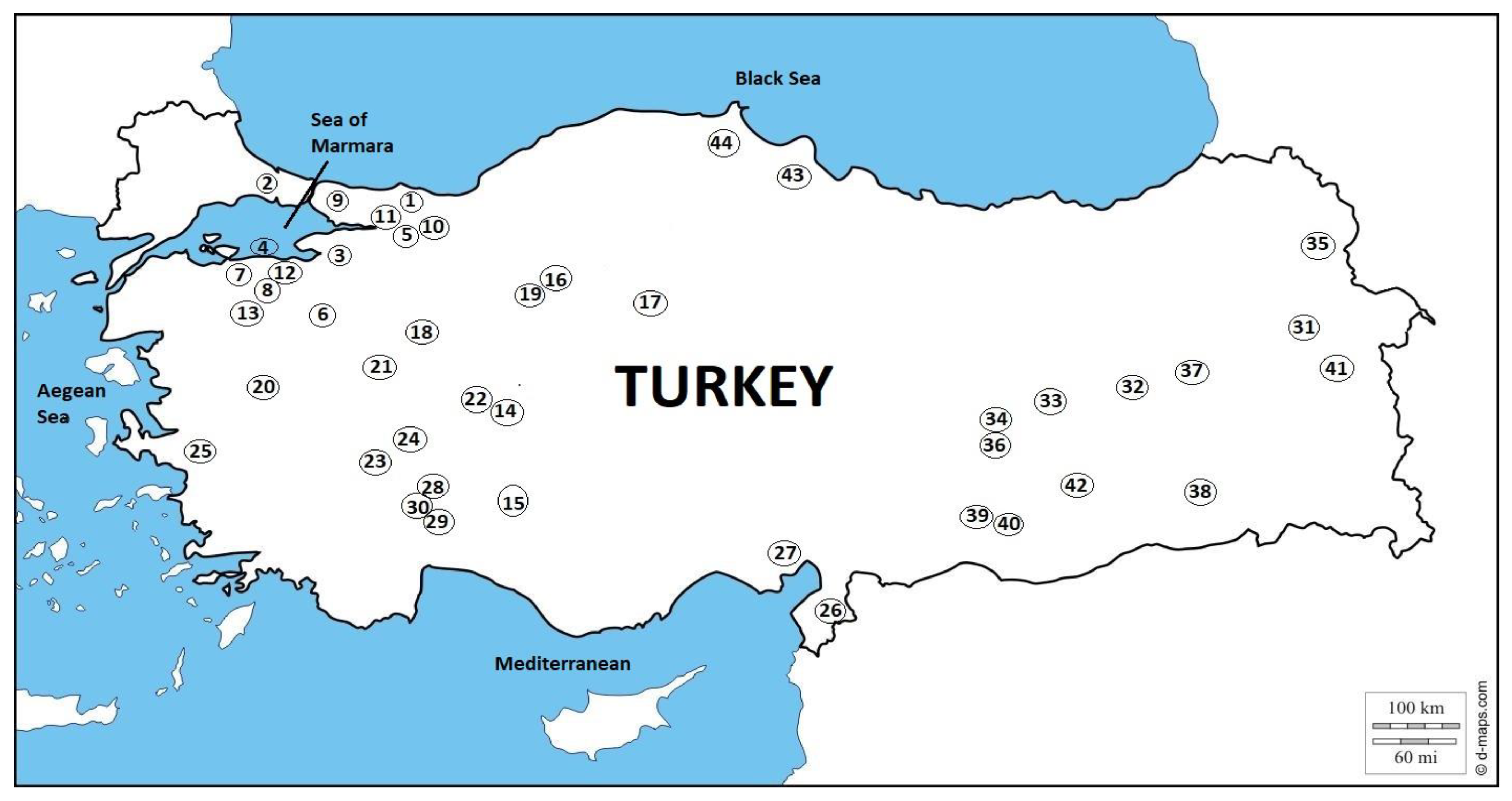
| Location | Host Fish Species: Parasite Species | Reference | Fish Deaths Date | Source | |
|---|---|---|---|---|---|
| Marmara Region | |||||
| 1 | Büyükakgöl | Blicca bjoerkna: Tylodelphys clavata, Piscicola geometra, Glochidia | Altan and Soylu (2018) | 2010 | https://www.etkihaber.com/akgolde-toplu-balik-olumleri-yasaniyor.-51904h.htm (accessed on 1 November 2022) |
| 2 | Büyükçekmece Dam Lake | Squalius cephalus: Paradiplozoon homoion Rutilus rutilus: Caryophyllaeus laticeps | Yardımcı et al. (2018) | 2021 | https://www.yenisafak.com/video-galeri/gundem/rengi-degisen-buyukcekmece-golunde-olen-baliklar-kiyiya-vurdu-2220804 (accessed on 10 November 2022) |
| 3 | İznik Lake | Rutilus frisii: Caryophyllaeus laticeps, Rutilus rubilio: Neoechinorhynchus rutili | Aydoğdu et al. (1997) Aydoğdu et al. (2000) | 2020 | https://www.tv100.com/bursanin-iznik-ilcesinde-iznik-golunu-besleyen-en-buyuk-dere-olan-cakirca-deresinde-yasanan-toplu-balik-olumleri-vatandaslari-tedirgin-etti-video-499455 (accessed on 23 November 2022) |
| 4 | Karacabey Lagoon Lake | Esox lucius: Raphidascaris acus | Öztürk et al. (2002) | 2020 | https://www.bursadabugun.com/haber/bursa-karacabey-deki-balik-olumlerinin-arastirilmasi-istendi-1324902.html (accessed on 23 November 2022) |
| 5 | Karasu Stream | Luciobarbus escherichii: Pomphorhynchus laevis | Soylu (1991) | 2021 | https://www.hurriyet.com.tr/gundem/karasu-nehrinin-ustunu-tamamen-kapladi-yore-halkini-tedirgin-eden-balik-olumleri-41837035 (accessed on 23 November 2022) |
| 6 | Kocadere Stream | R. rutilus: Dactylogyrus crucifer, Diplostomum spathaecum | Selver (2008) | 2018 | https://kuzeyormanlari.org/2018/09/16/bursa-inegol-kocadereyi-yine-zehirlediler-baliklar-4-kez-topluca-can-verdi/ (accessed on 24 November 2022) |
| 7 | Manyas Lake | R. rutilus: Dactylogyrus crucifer, Paradiplozoon homoion, Ligula intestinalis | Öztürk (2000) | 2014 | https://www.mynet.com/manyas-golunde-balik-olumleri-180101621484 (accessed on 23 November 2022) |
| 8 | Mustafakemalpaşa Stream | Alburnus alburnus: Bothriocephalus acheilognathi | Aydoğdu and Selver (2006) | 2019 | https://www.bursadabugun.com/haber/bursa-mustafakemalpasa-daki-balik-olumleri-suruyor-1200184.html (accessed on 27 November 2022) |
| 9 | Ömerli Dam Lake | Alburnus istanbulensis: Ergasilus sieboldi | Şimşek (2013) | 2021 | https://www.denizhaber.net/omerli-barajindaki-balik-olumleri-korkuttu-haber-102459.htm (accessed on 27 November 2022) |
| 10 | Sakarya River | Abramis brama: Caryophyllaeus laticeps, Glochidia E. lucius: Raphidascaris acus | Akmırza and Yardımcı (2014) | 2021 | https://www.halk54.com/gundem/sakarya-nehri-nde-balik-olumleri-goruldu-h53220.html (accessed on 3 December 2022) |
| 11 | Sapanca Lake | B. bjoerkna: Dactylogyrus crucifer, Asymphylodora imitans, Piscicola geometra R. rutilus: Dactylogyrus crucifer, D. vistulae, Diplostomum spathaecum, Tylodelphys clavata E. lucius: Raphidascaris acus Tinca tinca: Glochidia | Soylu (1990), Karabiber (2006), Soylu (2006) | 2007 | https://www.hurriyet.com.tr/gundem/sapanca-golunde-balik-olumleri-basladi-7575460 (accessed on 15 December 2022) |
| 12 | Uluabat Lake | R. rutilus: Dactylogyrus crucifer, Paradiplozoon homoion, Caryophyllaeus laticeps E. lucius: Rhipidocotyle fennica, Raphidascaris acus, Acanthocephalus anguillae T. tinca: Piscicola geometra | Öztürk et al. (2000), Öztürk (2005), Öztürk (2002) | 2014 | https://www.olay.com.tr/yuzlerce-olu-balik-ve-kus-kiyiya-vurdu-337894 (accessed on 15 December 2022) |
| 13 | Susurluk Stream | S. cephalus: D. vistulae, Paradiplozoon megan | Gürkan and Tekin Özan (2012) | 2019 | https://www.denizhaber.net/susurluk-cayinda-toplu-balik-olumleri-yasandi-haber-91537.htm (accessed on 15 December 2022) |
| Central Anatolia Region | |||||
| 14 | Akşehir Lake | Alburnus nasreddini: Pomphorhynchus laevis | Buhurcu (2006) | 2010 | https://www.milliyet.com.tr/gundem/aksehir-golunde-kus-olumleri-1287711 (accessed on 15 December 2022) |
| 15 | Beyşehir Lake | T. tinca: Proteocephalus torulosus, Acanthocephalus anguillae | Tekin Özan (2005) | 2020 | https://www.konhaber.com/haber-beysehir_de_tedirgin_eden_toplu_balik_olumleri-1360411.html (accessed on 15 December 2022) |
| 16 | Çayırhan Stream | Alburnus orontis: Ligula intestinalis | Keskin and Erakan (1987) | 2020 | https://www.haberler.com/ankara-daki-cayirhan-golu-nde-toplu-balik-olumu-13660519-haberi/ (accessed on 17 December 2022) |
| 17 | Eymir Lake | Alburnus sp: Ligula intestinalis, Pomphorhynchus laevis | Burgu et al. (1988) | 2010 | https://www.internethaber.com/eymir-golunde-balik-olumleri-247392h.htm (accessed on 17 December 2022) |
| 18 | Porsuk Stream | A. alburnus: Ligula intestinalis | Yılmaz et al. (1996) | 2021 | https://www.gazeteduvar.com.tr/su-akisi-duran-porsuk-cayinda-baliklar-toplu-halde-oldu-haber-1529347 (accessed on 17 December 2022) |
| 19 | Sarıyar Dam Lake | Alburnus sp: Ligula intestinalis, Pomphorhynchus laevis | Burgu et al. (1988) | 2020 | https://www.cnnturk.com/turkiye/ankarada-barajdaki-balik-olumlerine-inceleme?page=4 (accessed on 17 December 2022) |
| Aegean Region | |||||
| 20 | Demirkopru Dam Lake | Capoeta capoeta: Ligula intestinalis | Keskin and Erakan (1987) | 2015 | https://www.salihlisektorgazetesi.com/14823/haber/demirkopru-baraji-nda-balik-olumleri-.aspx (accessed on 17 December 2022) |
| 21 | Enne Dam Lake | A. alburnus: Ligula intestinalis, Paraergasilus longidigitus Carassius carassius: Argulus foliaceus | Koyun (2001), Koyun et al. (2007) | 2018 | https://www.mynet.com/kutahyada-toplu-balik-olumleri-110104353108 (accessed on 10 February 2023) |
| 22 | Eber Lake | Cyprinus carpio: Argulus foliaceus | Öztürk (2005) | 2017 | https://www.sondakika.com/haber/haber-eber-golu-nde-yasanan-toplu-balik-olumleri-9293003/ (accessed on 17 December 2022) |
| 23 | Işıklı Dam Lake | Squalius carinus: Dactylogyrus vistulae, Tylodelphys clavata E. lucius: Bathybothrium rectangulum | Soylu et al. (2017), Dişçi (2002) | 2017 | https://www.denizlihaber.com/denizli/civril/iddialar-cok-vahim-isikliyi-besleyen-kufi-cayi-zirai-ilaclarla-zehirleniyor-balik-olumleri-basladi/ (accessed on 19 December 2022) |
| 24 | Örenler Dam Lake | S. cephalus: Dactylogyrus vistulae, Diplostomum spathaecum, Pomphorhynchus laevis | Kurupınar (2009) | 2015 | https://www.afyonzafer.net/sandikli/sandikli-orenler-barajindaki-baliklar-telef-oldu-h23431.html (accessed on 17 December 2022) |
| 25 | Tahtalı Dam Lake | C. carpio: Lernaea cyprinacea | Karakişi and Demir (2012) | 2012 | https://www.etkihaber.com/izmire-icme-suyu-saglayan-tahtali-barajindaki-balik-olumleri-korkuttu-139705h.htm (accessed on 17 December 2022) |
| Mediterranean Region | |||||
| 26 | Asi River | Anguilla anguilla: Anguillicola crassus | Genç et al. (2008) | 2021 | https://www.trthaber.com/haber/guncel/asi-nehrinde-su-sicakliginin-yukselmesi-ve-kalitesinin-azalmasi-balik-olumlerine-neden-olabilir-601224.html (accessed on 17 December 2022) |
| 27 | Ceyhan River | A. anguilla: Anguillicola crassus | Genç et al. (2005) | 2021 | https://www.ntv.com.tr/galeri/turkiye/kahramanmarasta-meydana-gelen-balik-olumlerinin-nedeni-arastiriliyor,RIt7rfdox0CHT0ljQDDsKA/4_o5u0oyAUWZNHzq4mqVwA (accessed on 10 February 2023) |
| 28 | Egirdir Lake | Pseudophoxinus egridiri: Lernaea cyprinacea | Akçimen et al. (2018) | 2012 | https://www.denizhaber.net/egirdir-golunde-toplu-balik-olumleri-haber-41608.htm (accessed on 17 December 2022) |
| 29 | Karacaören I. Dam Lake | Luciobarbus pectoralis: Ligula intestinalis | Kır (1998) | 2013 | https://www.sondakika.com/haber/haber-ozel-haber-karacaoren-baraji-nda-sazan-olumleri-4798468/ (accessed on 19 December 2022) |
| 30 | Kovada Lake | C. carassius: Argulus foliaceus T. tinca: Proteocephalus torulosus | Tekin Özan and Kır (2005), Kır and Tekin Özan (2005) | 2014 | http://www.egirdirses.com/haber-valimizin-dikkatine-...-7971.html (accessed on 19 December 2022) |
| Eastern Anatolia Region | |||||
| 31 | Bendimahi Brook | C. carpio: Bothriocephalus acheilognathi, Caryophyllaeus laticeps, Neoechinorhynchus rutili | Topçu (1993) | 2021 | https://www.vansiyaseti.com/van/van-baligi-olumleri-arastiriliyor-h73155.html (accessed on 19 December 2022) |
| 32 | Fırat River | Mastacembellus mastacembelus: Diplostomum spathaecum, Glochidia | Koyun and Çelik (2020) | 2021 | https://jinepsgazetesi.com/2021/06/milyonlarca-balik-neden-oldu/ (accessed on 19 December 2022) |
| 33 | Hazar Lake | Capoeta umbla: Bothriocephalus acheilognathi | Aksoy (1996) | 2020 | https://www.gunisigigazetesi.net/elazig-guncel/baliklar-neden-oluyor-h78174.html (accessed on 19 December 2022) |
| 34 | Karakaya Dam Lake | C. umbla, Acanthobrama marmid: Bothriocephalus acheilognathi | Örün et al. (2003) | 2021 | https://www.ntv.com.tr/galeri/turkiye/karakaya-barajinda-toplu-balik-olumleri,zLDDlyH2lkOtmQZ2sUfj7g/szqvGqbDeUq8xZCOVvluJw (accessed on 19 December 2022) |
| 35 | Kars Stream | C. capoeta, Barbus plebejus: Ligula intestinalis | Arslan et al. (2015) | 2021 | https://www.trthaber.com/haber/turkiye/kars-cayinda-toplu-balik-olumleri-goruldu-600223.html (accessed on 17 December 2022) |
| 36 | Keban Dam Lake | Chondrostoma regium: Bothriocephalus acheilognathi, Ergasilus sieboldi Capoeta trutta: Lamproglena pulchella, Ergasilus sieboldi Alburnus mossulensis: Neoechinorhynchus rutili, Ergasilus briani Barbus rajanorum: Piscicola geometra | Sağlam (1992), Dörücü and İspir (2005), Kavak and Şeker (2017) | 2014 | https://www.sondakika.com/haber/haber-keban-baraj-golu-nde-balik-olumlerine-inceleme-6504258/ (accessed on 17 December 2022) |
| 37 | Murat River | Cyprinion macrostomum: Lernaea cyprinacea | Koyun et al. (2015 | 2019 | https://www.denizhaber.net/murat-nehrindeki-balik-olumleri-endiselendirdi-haber-90238.htm (accessed on 17 December 2022) |
| South Easten Anatolia Region | |||||
| 38 | Dicle River | M. mastacembelus: Glochidia | Koyun and Çelik (2020) | 2021 | https://www.gazeteduvar.com.tr/diclede-baliklar-neden-oluyor-haber-1522077 (accessed on 20 December 2022) |
| 39 | Atatürk Dam Lake | Planiliza abu, M. mastacembelus, C. carpio, Carasobarbus luteus: Argulus foliaceus | Öktener et al. (2006), Öktener and Alaş (2009) | 2019 | https://www.haberturk.com/adiyaman-da-kiyiya-vuran-baliklara-inceleme-2526158 (accessed on 20 December 2022) |
| 40 | Halil-ür Rahman Lake | C. luteus: C. umbla, Lamproglena pulchella | Öktener et al. (2008) | 2021 | https://www.hurriyet.com.tr/gundem/balikligolde-balik-olumleri-raporlastiriliyor-41753569 (accessed on 20 December 2022) |
| 41 | Zernek Dam Lake | C. carpio: Bothriocephalus acheilognathi | Topçu (1993) | 2021 | https://www.rudaw.net/turkish/kurdistan/1709202114 (accessed on 20 December 2022) |
| 42 | Devegecidi Dam Lake | A. marmid, A. mossulensis: Ligula intestinalis | Başaran and Kelle (1976) | 2021 | https://www.facebook.com/TigrisHaber/videos/diyarbak%C4%B1r-devege%C3%A7idi-baraj%C4%B1nda-toplu-bal%C4%B1k-%C3%B6l%C3%BCmleri/895500778037894/ (accessed on 20 December 2022) |
| Black Sea Region | |||||
| 43 | Bafra Fish Lakes | Sander lucioperca: Tylodelphys clavata, Bothriocephalus acheilognathi C. carpio: Ergasilus sieboldi | Öztürk et al. (2011), Öztürk et al. (2012) | 2014 | https://www.haberler.com/kizilirmak-deltasi-nda-balik-ve-sulun-olumleri-6450737-haberi/ (accessed on 20 December 2022) |
| 44 | Sarıkum Lagoon Lake | Aphanius chantrei: Neoechinorhynchus rutili, Ergasilus sieboldi | Öztürk (2005) | 2018 | https://www.ajanssinop.com/koruma-alaninda-balik-katliami-iddiasi/3877/ (accessed on 20 December 2022) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öktener, A.; Bănăduc, D. Ecological Interdependence of Pollution, Fish Parasites, and Fish in Freshwater Ecosystems of Turkey. Water 2023, 15, 1385. https://doi.org/10.3390/w15071385
Öktener A, Bănăduc D. Ecological Interdependence of Pollution, Fish Parasites, and Fish in Freshwater Ecosystems of Turkey. Water. 2023; 15(7):1385. https://doi.org/10.3390/w15071385
Chicago/Turabian StyleÖktener, Ahmet, and Doru Bănăduc. 2023. "Ecological Interdependence of Pollution, Fish Parasites, and Fish in Freshwater Ecosystems of Turkey" Water 15, no. 7: 1385. https://doi.org/10.3390/w15071385
APA StyleÖktener, A., & Bănăduc, D. (2023). Ecological Interdependence of Pollution, Fish Parasites, and Fish in Freshwater Ecosystems of Turkey. Water, 15(7), 1385. https://doi.org/10.3390/w15071385







