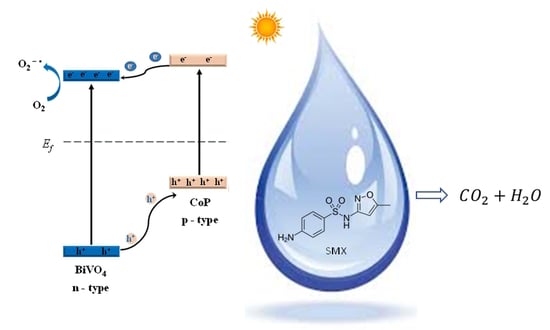
Solar Light-Induced Photocatalytic Degradation of Sulfamethoxazole by Cobalt Phosphide-Promoted Bismuth Vanadate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Matrices
2.2. Photocatalyst Preparation
2.2.1. Cobalt Phosphide (CoP)
2.2.2. Bismuth Vanadate (BiVO4)
2.2.3. Cobalt Phosphide Promoted Bismuth Vanadate (CoP/BiVO4)
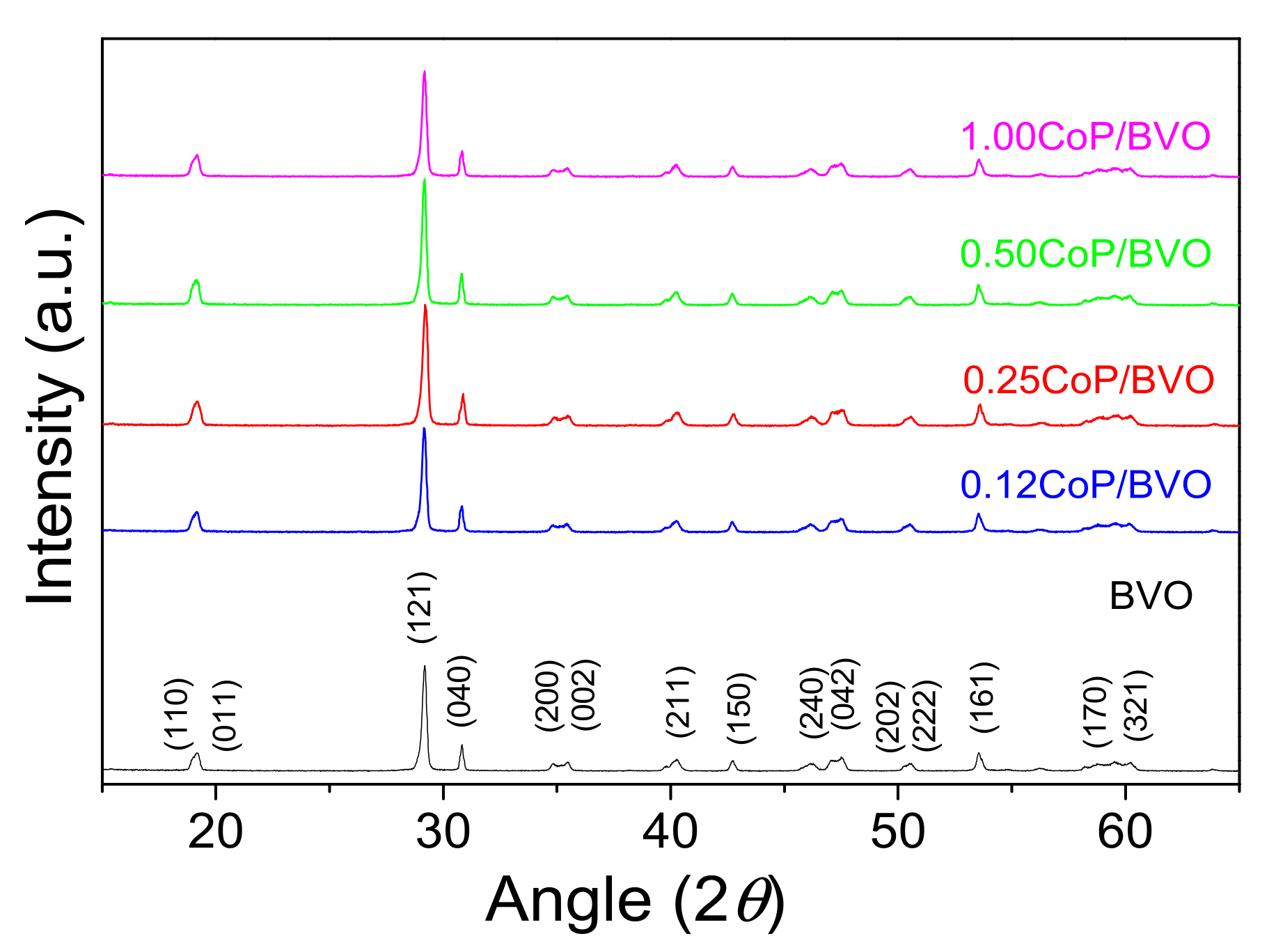
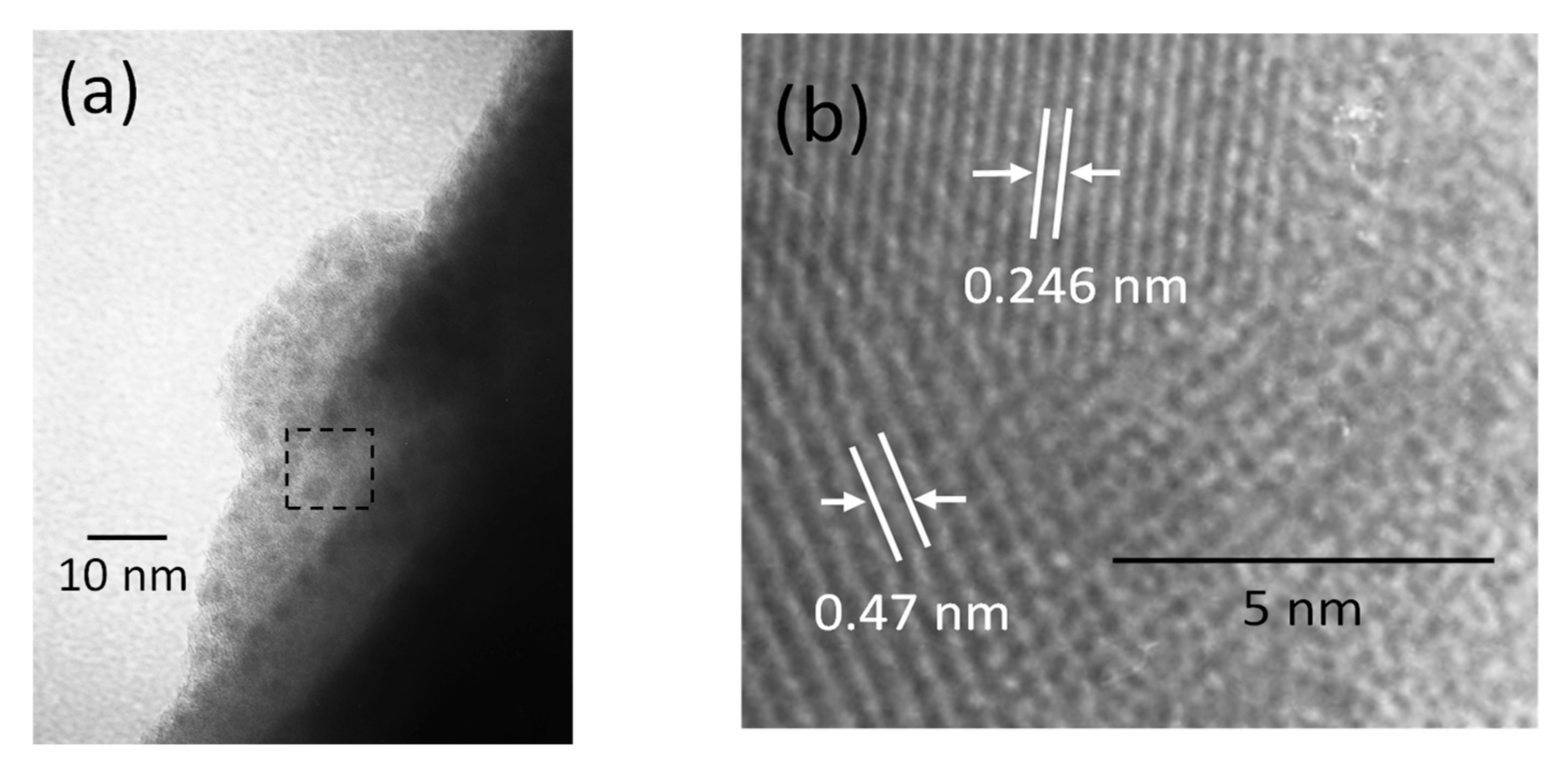
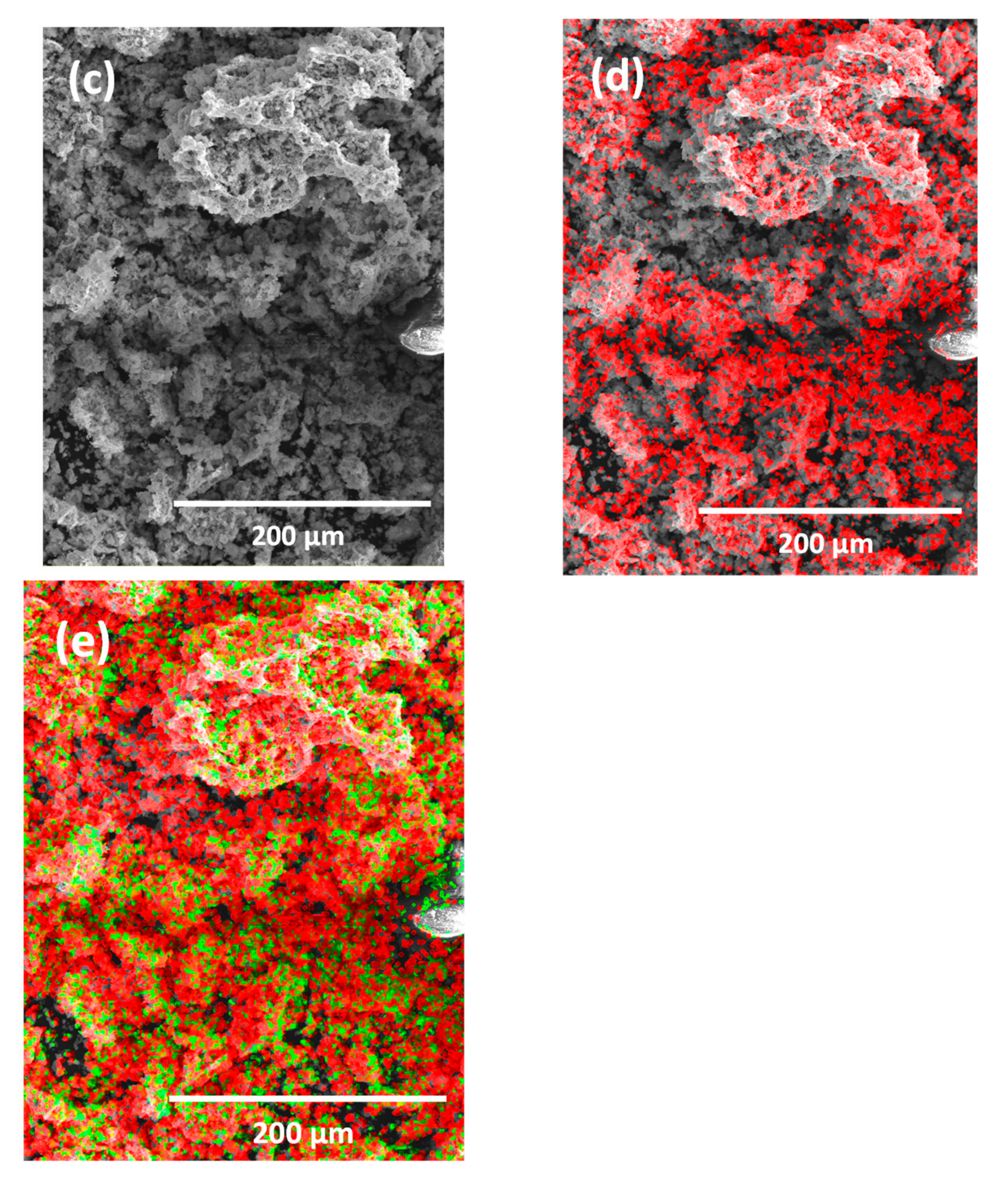
2.3. Photocatalyst Characterization
2.4. Photocatalytic Tests
2.4.1. Batch Photocatalytic Reactor
2.4.2. Pilot-Scale Demonstration of the Process
2.5. SMX Measurement
3. Results
3.1. Catalyst Characterization
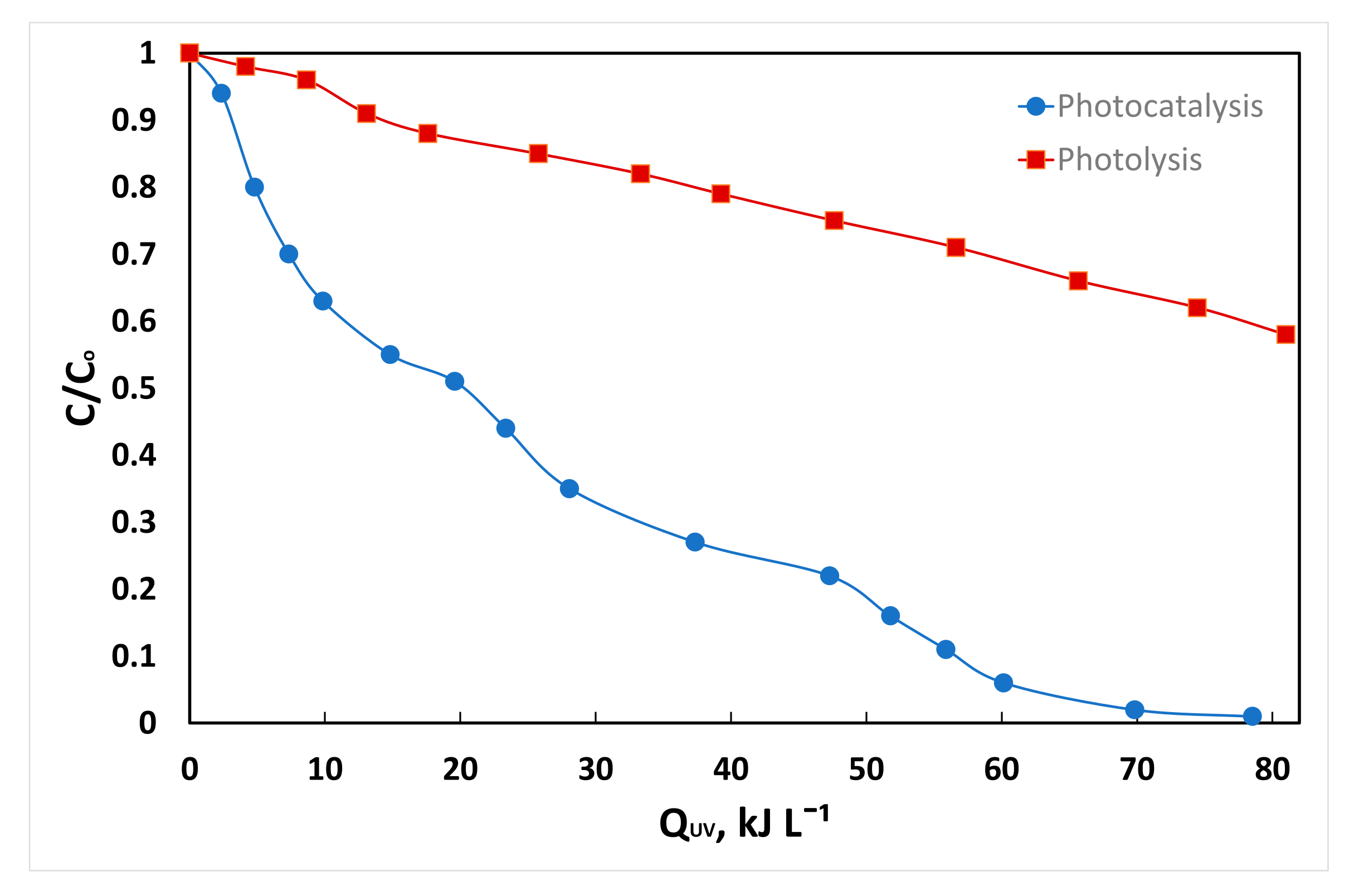
3.2. Photocatalytic Efficiency
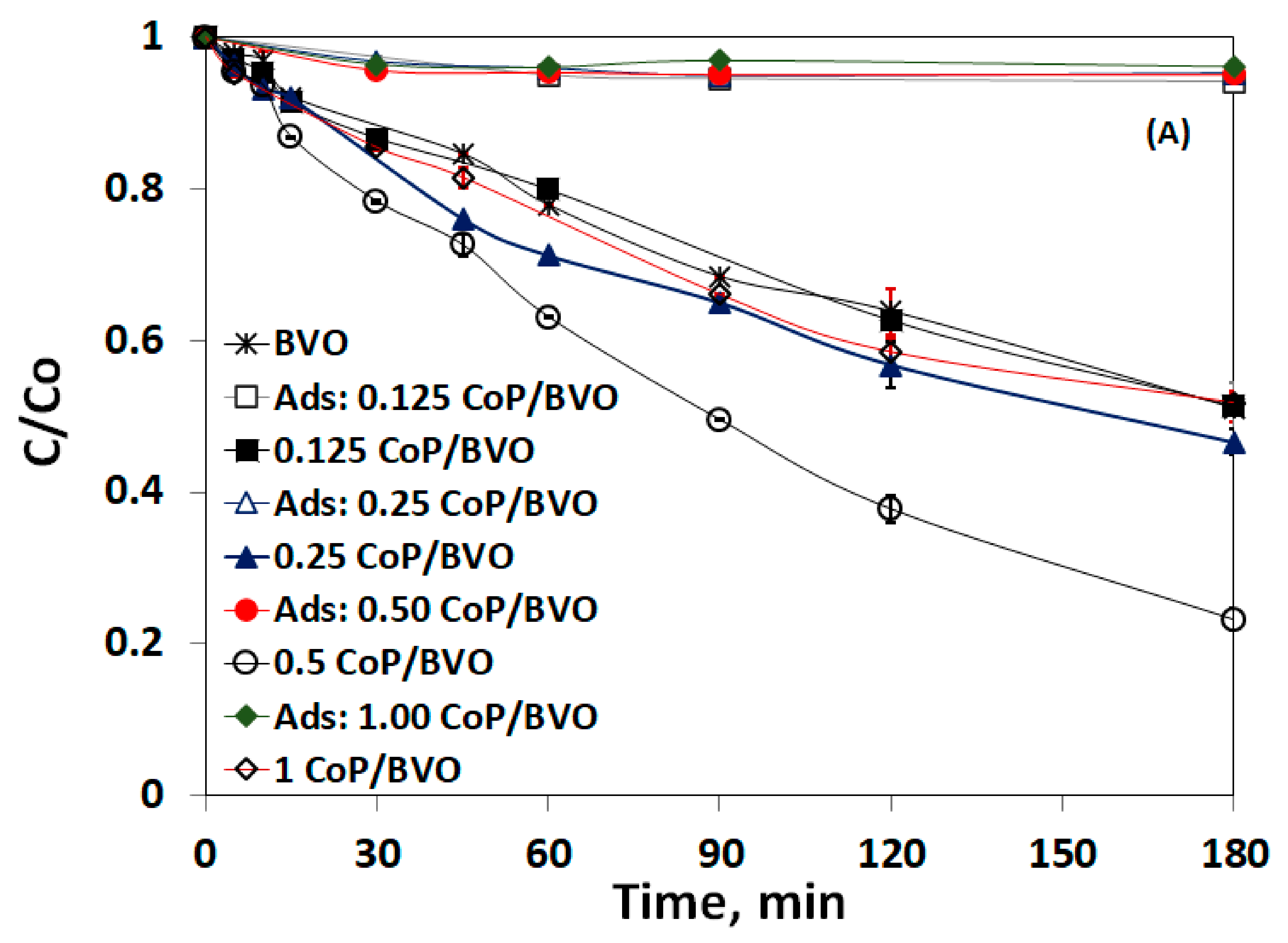
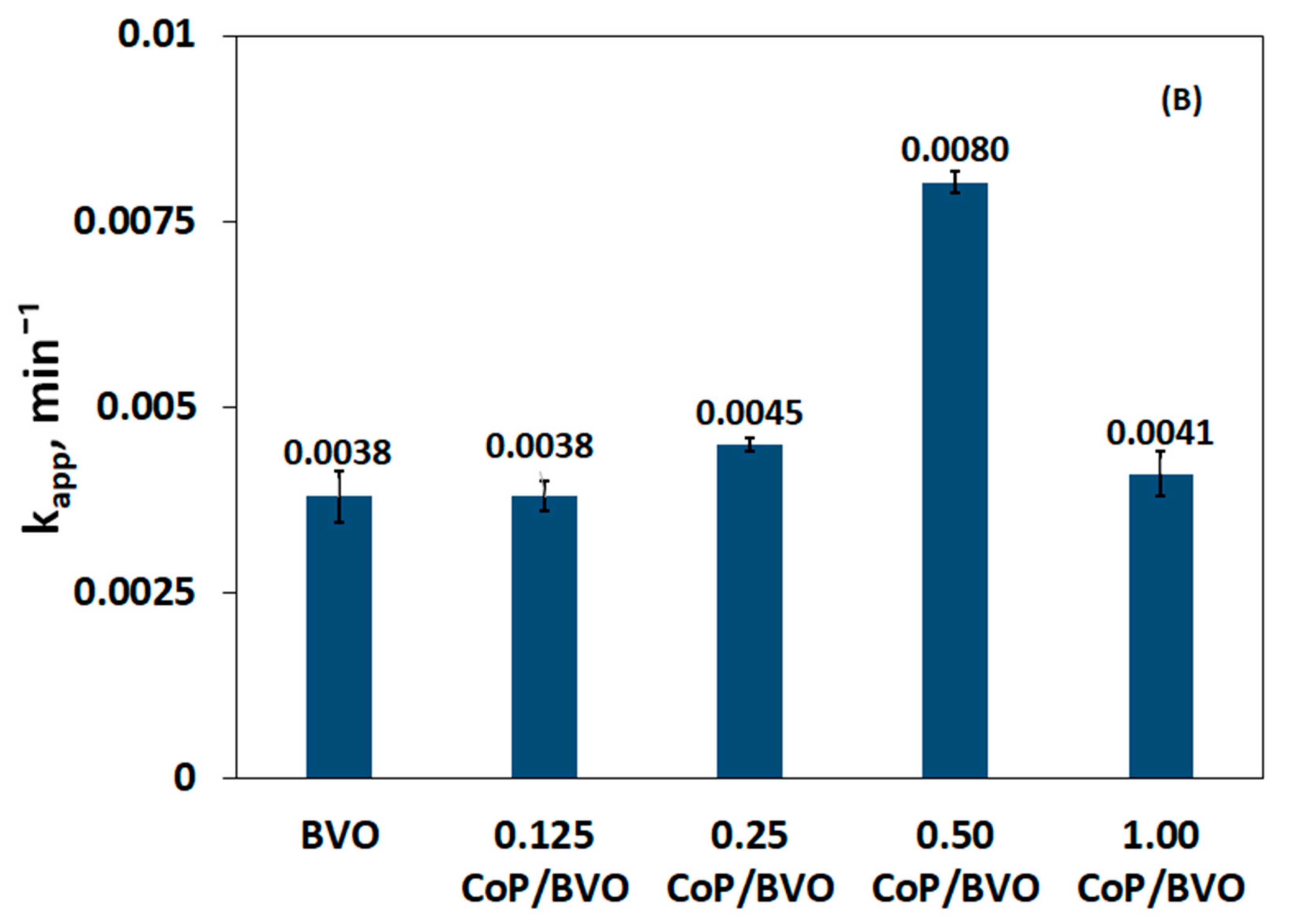
3.3. Impact of Catalyst Loading
3.4. Impact of SMX Initial Loading
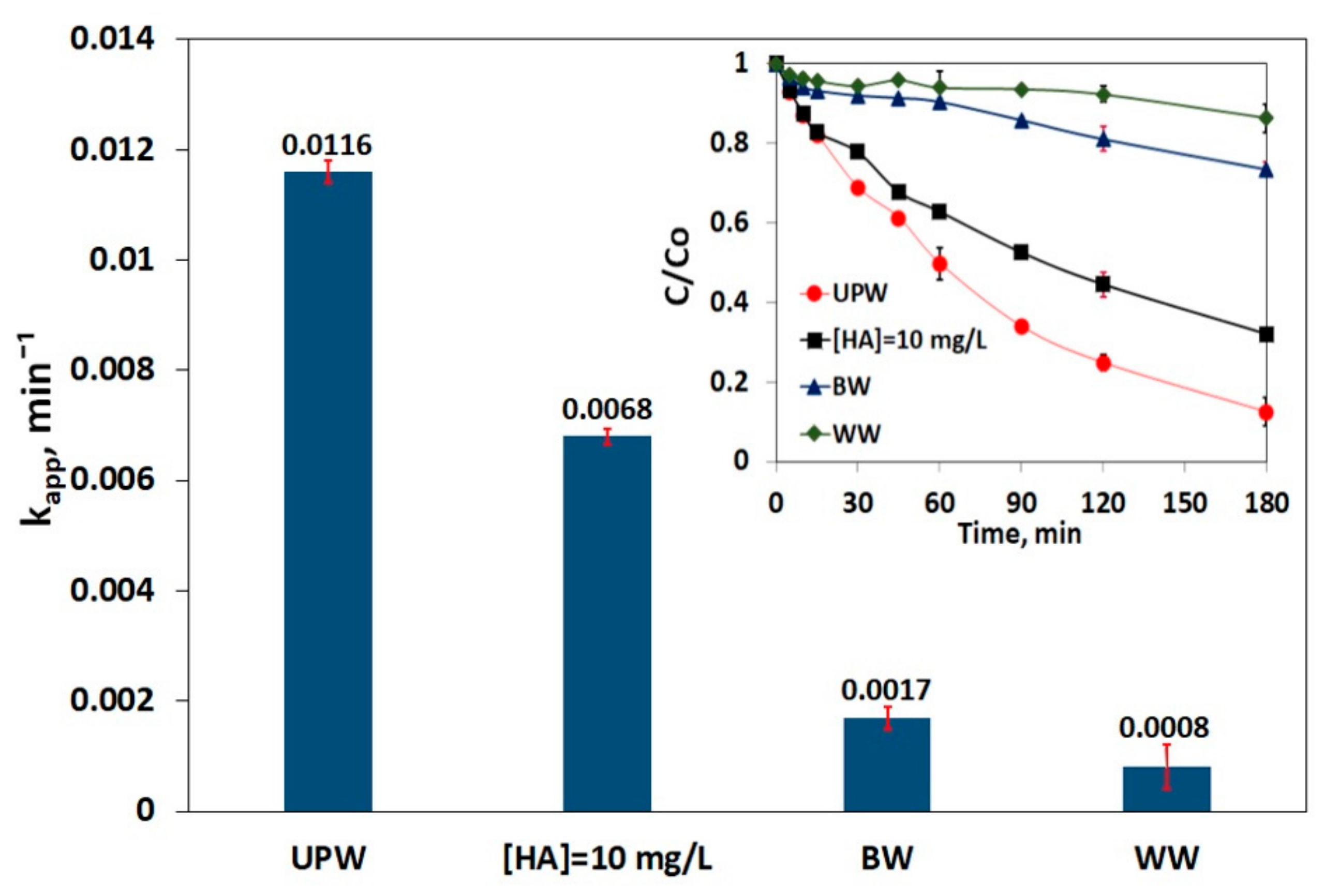
3.5. Impact of Matrix on SMX Removal
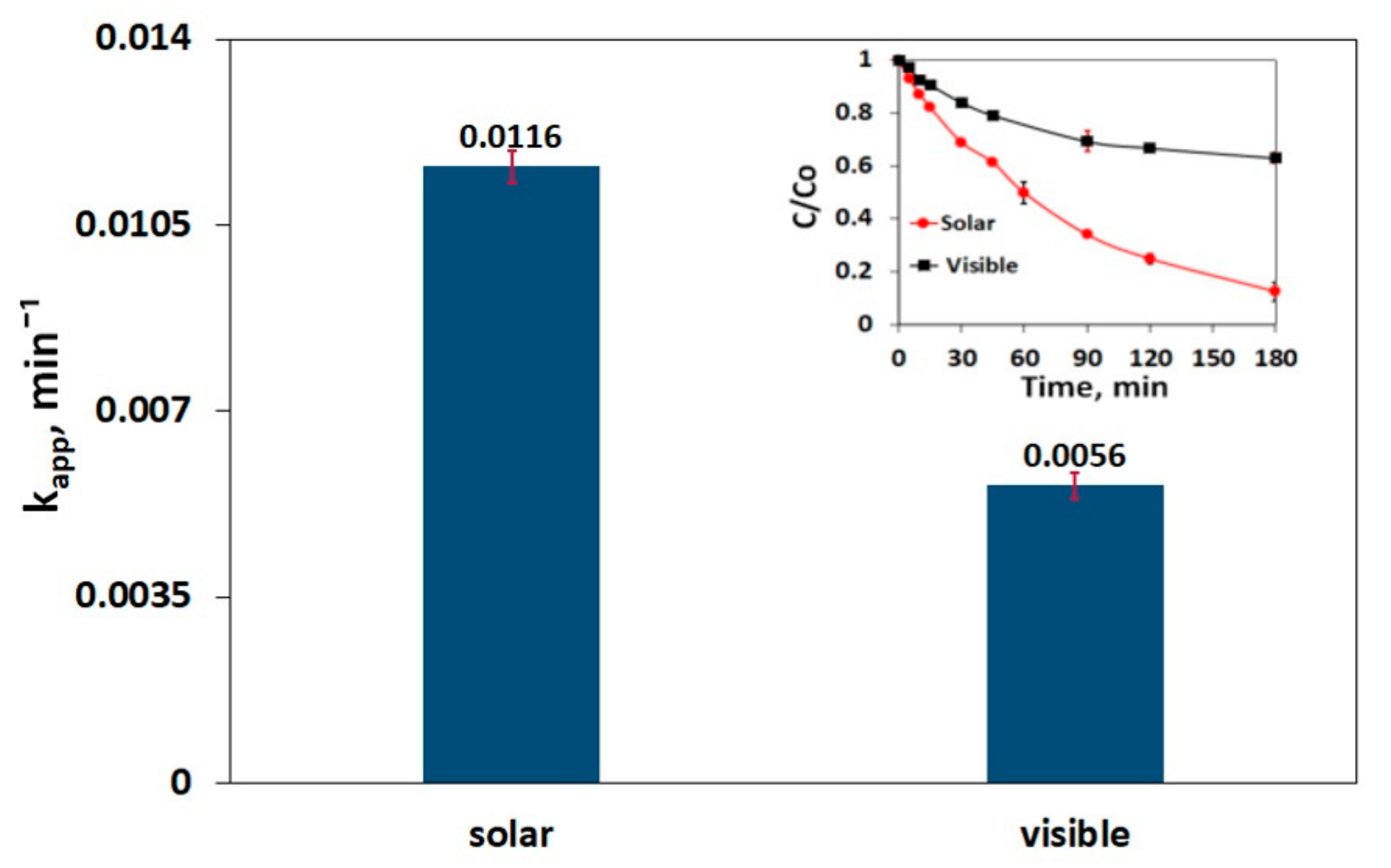
3.6. Impact of Irradiation Type on SMX Decomposition
3.7. Pharmaceuticals Degradation by Other Photocatalytic Systems
3.8. Pilot Plant Demonstration
3.9. Limitations of the Proposed System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keerthanan, S.; Jayasinghe, C.; Biswas, J.K.; Vithanage, M. Pharmaceutical and Personal Care Products (PPCPs) in the environment: Plant uptake, translocation, bioaccumulation, and human health risks. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1221–1258. [Google Scholar] [CrossRef]
- Gogoi, A.; Mazumder, P.; Tyagi, V.K.; Tushara Chaminda, G.G.; An, A.K.; Kumar, M. Occurrence and fate of emerging contaminants in water environment: A review. Groundw. Sustain. Dev. 2018, 6, 169–180. [Google Scholar] [CrossRef]
- Jin, X.; Zhou, X.; Sun, P.; Lin, S.; Cao, W.; Li, Z.; Liu, W. Photocatalytic degradation of norfloxacin using N-doped TiO2: Optimization, mechanism, identification of intermediates and toxicity evaluation. Chemosphere 2019, 237, 124433. [Google Scholar] [CrossRef]
- Tran, M.L.; Fu, C.-C.; Juang, R.-S. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Tomara, T.; Frontistis, Z.; Petala, A.; Mantzavinos, D. Photocatalytic performance of Ag2O towards sulfamethoxazole degradation in environmental samples. J. Environ. Chem. Eng. 2019, 7, 103177. [Google Scholar] [CrossRef]
- Faka, V.; Griniezaki, M.; Kiriakidis, G.; Grilla, E.; Mantzavinos, D.; Mao, S.; Shen, S.; Frontistis, Z.; Binas, V. Solar light induced photocatalytic degradation of sulfamethoxazole by ZnWO4/CNNs nanocomposites. J. Photochem. Photobiol. A 2022, 432, 114108. [Google Scholar] [CrossRef]
- You, J.; Guo, Y.; Guo, R.; Liu, X. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–642. [Google Scholar] [CrossRef]
- Malathi, A.; Madhavan, J.; Ashokkumar, M.; Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Appl. Catal. A Gen. 2018, 555, 47–74. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; Obregón, S.; Colón, G. Photocatalytic activity of bismuth vanadate under UV-A and visible light irradiation: Inactivation of Escherichia coli vs oxidation of methanol. Catal. Today 2015, 240, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Kohtani, S.; Tomohiro, M.; Tokumura, K.; Nakagaki, R. Photooxidation reactions of polycyclic aromatic hydrocarbons over pure and Ag-loaded BiVO4 photocatalysts. Appl. Catal. B 2005, 58, 265–272. [Google Scholar] [CrossRef]
- Platero, F.; Caballero, A.; Colón, G. Tuning the co-catalyst loading for the optimization of thermo-photocatalytic hydrogen production over Cu/TiO2. Appl. Catal. A-Gen. 2022, 643, 118804. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Repousi, V.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photocatalytic degradation of bisphenol A over Rh/TiO2 suspensions in different water matrices. Catal. Today 2017, 284, 59–66. [Google Scholar] [CrossRef]
- Callejas, J.F.; Read, C.G.; Roske, C.W.; Lewis, N.S.; Schaak, R.E. Synthesis, Characterization, and Properties of Metal Phosphide Catalysts for the Hydrogen-Evolution Reaction. Chem. Mater. 2016, 28, 6017–6044. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, X.; Huang, X.; Sun, H.; Chen, L.; Guo, F. Fabrication of visible-light driven CoP/ZnSnO3 composite photocatalyst for high-efficient photodegradation of antibiotic pollutant. Sep. Purif. Technol. 2021, 257, 11790. [Google Scholar] [CrossRef]
- Shi, L.; Yin, J.; Liu, Y.; Liu, H.; Zhang, H.; Tang, H. Embedding Cu3P quantum dots onto BiOCl nanosheets as a 0D/2D S-scheme heterojunction for photocatalytic antibiotic degradation. Chemosphere 2022, 309, 136607. [Google Scholar] [CrossRef]
- Thiebault, T. Sulfamethoxazole/Trimethoprim ratio as a new marker in raw wastewaters: A critical review. Sci. Total Environ. 2020, 715, 136916. [Google Scholar] [CrossRef]
- Lesser, L.E.; Mora, A.; Moreau, C.; Mahlknecht, J.; Hernández-Antonio, A.; Ramírez, A.I.; Barrios-Piña, H. Survey of 218 organic contaminants in groundwater derived from the world’s largest untreated wastewater irrigation system: Mezquital Valley, Mexico. Chemosphere 2018, 198, 510–521. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Chamorro, S.; Marti, E.; Huerta, B.; Gros, M.; Sànchez-Melsió, A.; Borrego, C.M.; Barceló, D.; Balcázar, J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015, 69, 234–242. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C. Occurrence, fate and health risk assessment of 10 common antibiotics in two drinking water plants with different treatment processes. Sci. Total Environ. 2019, 674, 316–326. [Google Scholar] [CrossRef]
- Lindberg, R.H.; Östman, M.; Olofsson, U.; Grabic, R.; Fick, J. Occurrence and behaviour of 105 active pharmaceutical ingredients in sewage waters of a municipal sewer collection system. Water Res. 2014, 58, 221–229. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Antibiotics in the soil environment— degradation and their impact on microbial activity and diversity. Front. Microbiol. 2019, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Musial, J.; Mlynarczyk, D.T.; Stanisz, B.J. Photocatalytic degradation of sulfamethoxazole using TiO2-based materials—Perspectives for the development of a sustainable water treatment technology. Sci. Total Environ. 2023, 856, 159122. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Huang, X.; Chen, Z.; Sun, H.; Chen, L. Prominent co-catalytic effect of CoP nanoparticles anchored on high-crystalline g-C3N4 nanosheets for enhanced visible light photocatalytic degradation of tetracycline in wastewater. Chem. Eng. J. 2020, 395, 125118. [Google Scholar] [CrossRef]
- Kanigaridou, Y.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Solakidou, M.; Konstantinou, I.; Deligiannakis, Y.; Mantzavinos, D.; Kondarides, D.I. Solar photocatalytic degradation of bisphenol A with CuOx/BiVO4: Insights into the unexpectedly favorable effect of bicarbonates. Chem. Eng. J. 2017, 318, 39–49. [Google Scholar] [CrossRef]
- Zeng, D.; Xu, W.; Ong, W.-J.; Xu, J.; Ren, H.; Chen, Y.; Zheng, H.; Peng, D.-L. Toward noble-metal-free visible-light-driven photocatalytic hydrogen evolution: Monodisperse sub-15 nm Ni2P nanoparticles anchored on porous g-C3N4 nanosheets to engineer 0D-2D heterojunction interfaces. Appl. Catal. B 2018, 221, 47–55. [Google Scholar] [CrossRef]
- Petala, A.; Tsikritzis, D.; Kollia, M.; Ladas, S.; Kennou, S.; Kondarides, D.I. Synthesis and Characterization of N-Doped TiO2 Photocatalysts with Tunable Response to Solar Radiation. Appl. Surf. Sci. 2014, 305, 281–291. [Google Scholar] [CrossRef]
- Ioannidou, E.; Ioannidi, A.; Frontistis, Z.; Antonopoulou, M.; Tselios, C.; Tsikritzis, D.; Konstantinou, I.; Kennou, S.; Kondarides, D.I.; Mantzavinos, D. Correlating the properties of hydrogenated titania to reaction kinetics and mechanism for the photocatalytic degradation of bisphenol A under solar irradiation. Appl. Catal. B 2016, 188, 65–76. [Google Scholar] [CrossRef]
- Gomes, A.I.; Silva, T.F.C.V.; Duarte, M.A.; Boaventura, R.A.R.; Vilar, V.J.P. Cost-effective solar collector to promote photo-Fenton reactions: A case study on the treatment of urban mature leachate. J. Clean. Prod. 2018, 199, 369–382. [Google Scholar] [CrossRef]
- Ioannidi, A.; Petala, A.; Frontistis, Z. Copper phosphide promoted BiVO4 photocatalysts for the degradation of sulfamethoxazole in aqueous media. J. Environ. Chem. Eng. 2020, 8, 104340. [Google Scholar] [CrossRef]
- Gkika, C.; Petala, A.; Frontistis, Z.; Bampos, G.; Hela, D.; Konstantinou, I.; Mantzavinos, D. Heterogeneous activation of persulfate by lanthanum strontium cobaltite for sulfamethoxazole degradation. Catal. Today 2021, 361, 130–138. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernandez-Garcia, M.; Colon, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Fan, H.; Wang, D.; Wang, L.; Li, H.; Wang, P.; Jiang, T.; Xie, T. Hydrothermal synthesis and photoelectric properties of BiVO4 with different morphologies: An efficient visible-light photocatalyst. Appl. Surf. Sci. 2011, 257, 7758–7762. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective Preparation of Monoclinic and Tetragonal BiVO4 with Scheelite Structure and Their Photocatalytic Properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Min, S.; Wang, F.; Jin, Z.; Xu, J. Cu2O nanoparticles decorated BiVO4 as an effective visible-light-driven p-n heterojunction photocatalyst for methylene blue degradation. Superlattices Microstruct. 2014, 74, 294–307. [Google Scholar] [CrossRef]
- Yang, C.; Gao, G.; Guo, Z.; Song, L.; Chi, J.; Gan, S. Two-step hydrothermal synthesis of novel hierarchical Co3O4/Bi2O2CO3 p-n heterojunction composite photocatalyst with enhanced visible light photocatalytic activity. Appl. Surf. Sci. 2017, 400, 365–374. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Cui, Z.D.; Zhu, S.L.; Li, Z.Y.; Yang, X.J.; Chen, Y.J.; Ma, J.M. Design of a highly sensitive ethanol sensor using a nano-coaxial p-Co3O4/n-TiO2 heterojunction synthesized at low temperature. Nanoscale 2013, 5, 10916–10926. [Google Scholar] [CrossRef]
- Long, M.; Cai, W.; Cai, J.; Zhou, B.; Chai, X.; Wu, Y. Efficient photocatalytic degradation of phenol over Co3O4/BiVO4 composite under visible light irradiation. J. Phys. Chem. B 2006, 110, 20211–20216. [Google Scholar] [CrossRef]
- Ribeiro, A.R.L.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of water matrix on the removal of micropollutants by advanced oxidation technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Postigo, C.; Sirtori, C.; Oller, I.; Malato, S.; Maldonado, M.I.; López de Alda, M.; Barceló, D. Solar transformation and photocatalytic treatment of cocaine in water: Kinetics, characterization of major intermediate products and toxicity evaluation. Appl. Catal. B 2011, 104, 37–48. [Google Scholar] [CrossRef]
- Cho, Y.; Choi, W. Visible light-induced reactions of humic acids on TiO2. J. Photochem. Photobiol. A Chem. 2002, 148, 129–135. [Google Scholar] [CrossRef]
- Petala, A.; Arvaniti, O.S.; Travlou, G.; Mantzavinos, D.; Frontistis, Z. Solar light induced photocatalytic removal of sulfamethoxazole from water and wastewater using BiOCl photocatalyst. J. Environ. Sci. Health A 2021, 56, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Mousavi, S.A.; Eskandari, P.; Amarloo, E.; Farghelitiyan, J.; Mohammadi, S. Comparative Study on Photocatalytic Performance of TiO2 Doped with Different Amino Acids in Degradation of Antibiotics. Water 2023, 15, 535. [Google Scholar] [CrossRef]
- Goh, J.W.; Xiong, Y.; Wu, W.; Huang, Z.; Ong, S.L.; Hu, J.Y. Degradation of Carbamazepine by HF-Free-Synthesized MIL-101(Cr)@Anatase TiO2 Composite under UV-A Irradiation: Degradation Mechanism, Wastewater Matrix Effect, and Degradation Pathway. Water 2022, 14, 3964. [Google Scholar] [CrossRef]
- Levakov, I.; Shahar, Y.; Rytwo, G. Carbamazepine Removal by Clay-Based Materials Using Adsorption and Photodegradation. Water 2022, 14, 2047. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidi, A.A.; Zappa, J.; Petala, A.; Souliotis, M.; Mantzavinos, D.; Frontistis, Z. Solar Light-Induced Photocatalytic Degradation of Sulfamethoxazole by Cobalt Phosphide-Promoted Bismuth Vanadate. Water 2023, 15, 1370. https://doi.org/10.3390/w15071370
Ioannidi AA, Zappa J, Petala A, Souliotis M, Mantzavinos D, Frontistis Z. Solar Light-Induced Photocatalytic Degradation of Sulfamethoxazole by Cobalt Phosphide-Promoted Bismuth Vanadate. Water. 2023; 15(7):1370. https://doi.org/10.3390/w15071370
Chicago/Turabian StyleIoannidi, Alexandra A., Joanne Zappa, Athanasia Petala, Manolis Souliotis, Dionissios Mantzavinos, and Zacharias Frontistis. 2023. "Solar Light-Induced Photocatalytic Degradation of Sulfamethoxazole by Cobalt Phosphide-Promoted Bismuth Vanadate" Water 15, no. 7: 1370. https://doi.org/10.3390/w15071370