Unraveling the Influence of Water and Nitrogen Management on Quinoa (Chenopodium quinoa Willd.) Agronomic and Yield Traits
Abstract
1. Introduction
2. Materials and Methods
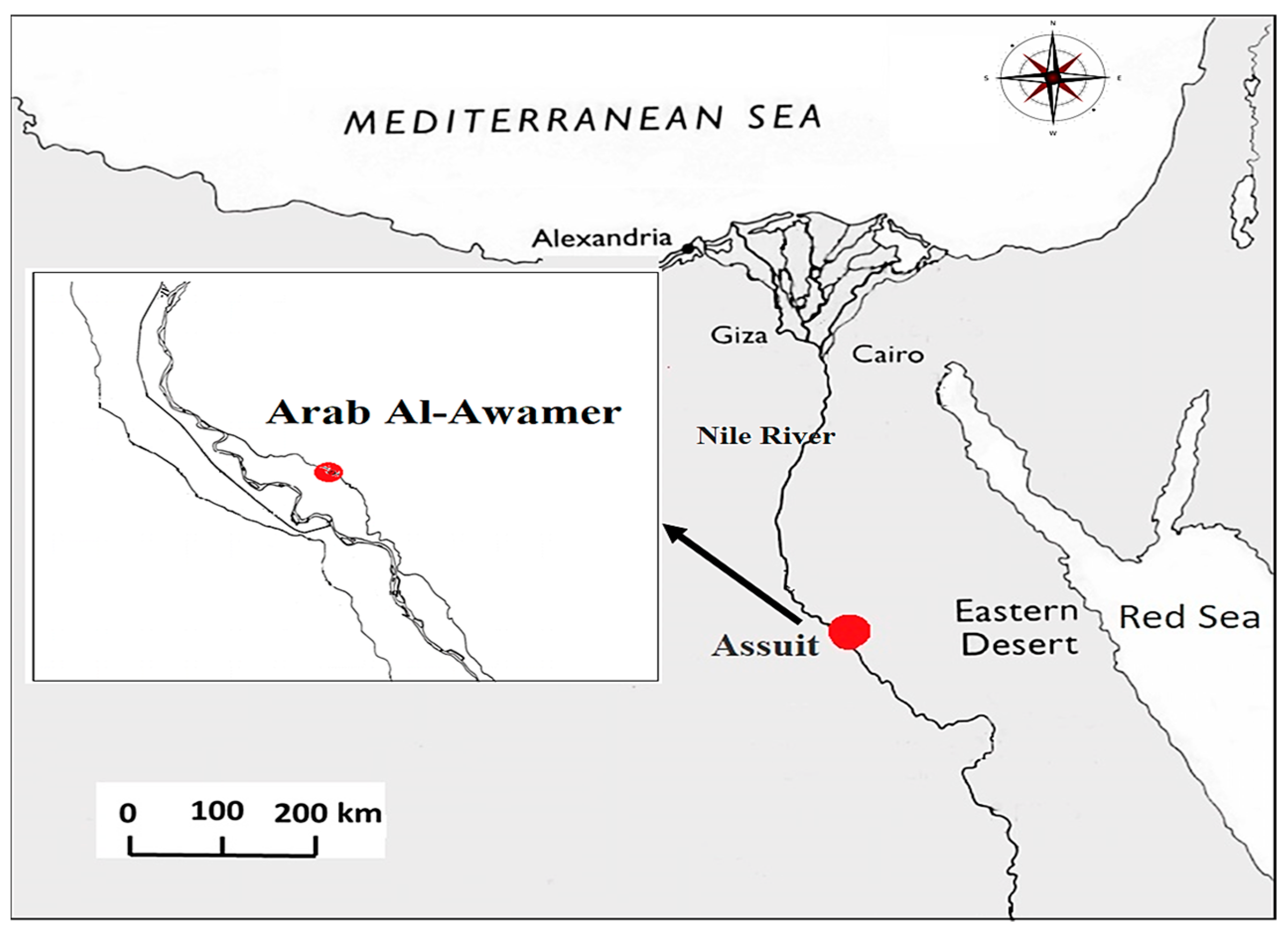
2.1. Experimental Site Description
2.2. Experimental Design and Treatments
- I.Ra = total actual irrigation water applied mm/interval.
- ETc = crop evapotranspiration using CROPWAT model 8.0 [31].
- Lf = leaching factor 10%.
- Er = irrigation system efficiency.
| 100% ETc | 80% ETc | 60% ETc | ||||
|---|---|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | |
| December | 510.84 | 428.808 | 408.672 | 343.032 | 306.504 | 257.28 |
| January | 975 | 796.488 | 780 | 637.2 | 585 | 477.888 |
| February | 1488.528 | 1304.424 | 1190.832 | 1043.544 | 893.112 | 782.664 |
| March | 2346.912 | 2427.24 | 1877.52 | 1941.792 | 1408.152 | 1456.344 |
| April | 1125.768 | 1236.648 | 900.6 | 989.304 | 675.456 | 741.984 |
| Total | 6447.048 | 6193.608 | 5157.624 | 4954.872 | 3868.224 | 3716.16 |
2.3. Cultural and Cropping Practises
2.4. Measurement of Plant Growth Traits
2.5. Statistical Analysis
3. Results
3.1. Effect of Different Nitrogen and Water Levels on the Studied Traits
3.2. Relationships among Studied Traits as Affected by Three Water Regimes
3.3. Relationships among Studied Traits as Affected by Four Nitrogen Levels
3.4. Path Analysis under Different Water Regimes and Nitrogen Levels
3.5. Effect of Water Regimes and Nitrogen Levels on Water Productivity Levels
4. Discussion
4.1. Influence of Water Regimes and Nitrogen Levels on Quinoa’s Agronomic and Yield Traits
4.2. The Impact of Different Water Regimes on the Correlations between the Investigated Characteristics
4.3. The Impact of Different Nitrogen Levels on the Correlations between the Investigated Characteristics
4.4. Exploring Variables’ Causal Effects on Seed Yield under Different Water and Nitrogen Treatments
4.5. The Impact of Varying Water and Nitrogen Levels on the Water Efficiency of Quinoa
5. Conclusions
- Investigation into the molecular and physiological mechanisms that underlie the effects of water and nitrogen fertilizer on quinoa growth and yield.
- Development of new and improved cultivars of quinoa that are better adapted to challenging growing conditions, such as drought and low nitrogen availability.
- Investigation into the nutritional composition of quinoa under different water and nitrogen fertilizer regimes, to determine the effects on its quality as a food crop.
- Comparison of the environmental impacts of different quinoa cultivation practices, including the use of water and nitrogen fertilizers, to identify sustainable practices for the production of quinoa.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandratos, N. Countries with Rapid Population Growth and Resource Constraints: Issues of Food, Agriculture, and Development. Popul. Dev. Rev. 2005, 31, 237–258. [Google Scholar] [CrossRef]
- Tubiello, F.N.; Soussana, J.-F.; Howden, S.M. Crop and Pasture Response to Climate Change. Proc. Natl. Acad. Sci. USA 2007, 104, 19686–19690. [Google Scholar] [CrossRef] [PubMed]
- Martínez, E.A.; Fuentes, F.; Bazile, D. History of Quinoa: Its Origin, Domestication, Diversification, and Cultivation with Particular Reference to the Chilean Context. Quinoa Improv. Sustain. Prod. 2015, 19, 19–24. [Google Scholar]
- Repo-Carrasco, R.; Espinoza, C.; Jacobsen, S.-E. Nutritional Value and Use of the Andean Crops Quinoa (Chenopodium quinoa) and Kañiwa (Chenopodium pallidicaule). Food Rev. Int. 2003, 19, 179–189. [Google Scholar] [CrossRef]
- Bastidas, E.G.; Roura, R.; Rizzolo, D.A.D.; Massanés, T.; Gomis, R. Quinoa (Chenopodium quinoa Willd.), from Nutritional Value to Potential Health Benefits: An Integrative Review. J. Nutr. Food Sci. 2016, 6, 3. [Google Scholar]
- James, L.E.A. Quinoa (Chenopodium quinoa Willd.): Composition, Chemistry, Nutritional, and Functional Properties. Adv. Food Nutr. Res. 2009, 58, 1–31. [Google Scholar]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A. Quinoa Biodiversity and Sustainability for Food Security under Climate Change. A Review. Agron. Sustain. Dev. 2014, 34, 349–359. [Google Scholar] [CrossRef]
- Lin, P.-H.; Chao, Y.-Y. Different Drought-Tolerant Mechanisms in Quinoa (Chenopodium quinoa Willd.) and Djulis (Chenopodium formosanum Koidz.) Based on Physiological Analysis. Plants 2021, 10, 2279. [Google Scholar] [CrossRef]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of Salinity on Physiological, Biochemical and Photostabilizing Attributes of Two Genotypes of Quinoa (Chenopodium quinoa Willd.) Exposed to Arsenic Stress. Ecotoxicol. Environ. Saf. 2020, 187, 109814. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Amjad, M.; Saqib, M.; Murtaza, B.; Asif Naeem, M.; Shabbir, A.; Murtaza, G. Soil Sodicity Is More Detrimental than Salinity for Quinoa (Chenopodium quinoa Willd.): A Multivariate Comparison of Physiological, Biochemical and Nutritional Quality Attributes. J. Agron. Crop Sci. 2021, 207, 59–73. [Google Scholar] [CrossRef]
- Naz, H.; Akram, N.A.; Ashraf, M.; Hefft, D.I.; Jan, B.L. Leaf Extract of Neem (Azadirachta indica) Alleviates Adverse Effects of Drought in Quinoa (Chenopodium quinoa Willd.) Plants through Alterations in Biochemical Attributes and Antioxidants. Saudi J. Biol. Sci. 2022, 29, 1367–1374. [Google Scholar] [CrossRef]
- Yaqoob, H.; Akram, N.A.; Iftikhar, S.; Ashraf, M.; Khalid, N.; Sadiq, M.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Seed Pretreatment and Foliar Application of Proline Regulate Morphological, Physio-Biochemical Processes and Activity of Antioxidant Enzymes in Plants of Two Cultivars of Quinoa (Chenopodium quinoa Willd.). Plants 2019, 8, 588. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of High Temperature on Pollen Morphology, Plant Growth and Seed Yield in Quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2019, 205, 33–45. [Google Scholar] [CrossRef]
- González, J.A.; Gallardo, M.; Hilal, M.B.; Rosa, M.D.; Prado, F.E. Physiological Responses of Quinoa (Chenopodium quinoa) to Drought and Waterlogging Stresses: Dry Matter Partitioning. Bot. Stud. 2009, 50, 35–42. [Google Scholar]
- Kaul, H.-P.; Kruse, M.; Aufhammer, W. Yield and Nitrogen Utilization Efficiency of the Pseudocereals Amaranth, Quinoa, and Buckwheat under Differing Nitrogen Fertilization. Eur. J. Agron. 2005, 22, 95–100. [Google Scholar]
- Razzaghi, F.; Plauborg, F.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effect of Nitrogen and Water Availability of Three Soil Types on Yield, Radiation Use Efficiency and Evapotranspiration in Field-Grown Quinoa. Agric. Water Manag. 2012, 109, 20–29. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Jørgensen, I.; Stølen, O. Cultivation of Quinoa (Chenopodium quinoa) under Temperate Climatic Conditions in Denmark. J. Agric. Sci. 1994, 122, 47–52. [Google Scholar] [CrossRef]
- Iqbal, S.M.A.B.S.; Afzal, I. Evaluating the Response of Nitrogen Application on Growth Development and Yield of Quinoa Genotypes. Int. J. Agric. Biol. 2014, 16, 886–892. [Google Scholar]
- Alandia, G.; Jacobsen, S.; Kyvsgaard, N.C.; Condori, B.; Liu, F. Nitrogen Sustains Seed Yield of Quinoa under Intermediate Drought. J. Agron. Crop Sci. 2016, 202, 281–291. [Google Scholar] [CrossRef]
- Barber, S.A. Soil Nutrient Bioavailability: A Mechanistic Approach; John Wiley & Sons: Hoboken, NJ, USA, 1995; ISBN 0471587478. [Google Scholar]
- Liu, C.; Rubæk, G.H.; Liu, F.; Andersen, M.N. Effect of Partial Root Zone Drying and Deficit Irrigation on Nitrogen and Phosphorus Uptake in Potato. Agric. Water Manag. 2015, 159, 66–76. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Saifullah; Ahmad, A. Water Stress and Nitrogen Management Effects on Gas Exchange, Water Relations, and Water Use Efficiency in Wheat. J. Plant. Nutr. 2011, 34, 1867–1882. [Google Scholar] [CrossRef]
- El-Sorady, G.A.; El-Banna, A.A.A.; Abdelghany, A.M.; Salama, E.A.A.; Ali, H.M.; Siddiqui, M.H.; Hayatu, N.G.; Paszt, L.S.; Lamlom, S.F. Response of Bread Wheat Cultivars Inoculated with Azotobacter Species under Different Nitrogen Application Rates. Sustainability 2022, 14, 8394. [Google Scholar] [CrossRef]
- Morgan, J.A. The Effects of N Nutrition on the Water Relations and Gas Exchange Characteristics of Wheat (Triticum aestivum L.). Plant Physiol. 1986, 80, 52–58. [Google Scholar] [CrossRef]
- Nielsen, D.C.; Halvorson, A.D. Nitrogen Fertility Influence on Water Stress and Yield of Winter Wheat. Agron. J. 1991, 83, 1065–1070. [Google Scholar] [CrossRef]
- Alvar-Beltrán, J.; Saturnin, C.; Dao, A.; Dalla Marta, A.; Sanou, J.; Orlandini, S. Effect of drought and nitrogen fertilisation on quinoa (Chenopodium quinoa Willd.) under field conditions in Burkina Faso. Ital. J. Agrometeorol. 2019, 1, 33–43. [Google Scholar]
- Klute, A. Water Retention: Laboratory Methods. Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1986; Volume 5, pp. 635–662. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Pentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; Volume 498, pp. 151–154. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56. FAO Rome 1998, 300, D05109. [Google Scholar]
- James, L.G. Principles of Farm Irrigation Systems Design; John Wiley and Sons Limited: Hoboken, NJ, USA, 1988; ISBN 047183954X. [Google Scholar]
- Clarke, D.; Smith, M.; El-Askari, K. CropWat for Windows: User Guide; IHE: Oak Brook, IL, USA, 2001. [Google Scholar]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 99–114. [Google Scholar] [CrossRef]
- El-Shamy, M.A.; Alshaal, T.; Mohamed, H.H.; Rady, A.M.S.; Hafez, E.M.; Alsohim, A.S.; Abd El-Moneim, D. Quinoa Response to Application of Phosphogypsum and Plant Growth-Promoting Rhizobacteria under Water Stress Associated with Salt-Affected Soil. Plants 2022, 11, 872. [Google Scholar] [CrossRef]
- Pulvento, C.; Sellami, M.H.; De Mastro, G.; Calandrelli, D.; Lavini, A. Quinoa Vikinga Response to Salt and Drought Stress under Field Conditions in Italy. Environ. Sci. Proc. 2022, 16, 5. [Google Scholar]
- Sun, W.; Wei, J.; Wu, G.; Xu, H.; Chen, Y.; Yao, M.; Zhan, J.; Yan, J.; Wu, N.; Chen, H. CqZF-HD14 Enhances Drought Tolerance in Quinoa Seedlings through Interaction with CqHIPP34 and CqNAC79. Plant Sci. 2022, 323, 111406. [Google Scholar] [CrossRef]
- Balbaa, M.G.; Osman, H.T.; Kandil, E.E.; Javed, T.; Lamlom, S.F.; Ali, H.M.; Kalaji, H.M.; Wróbel, J.; Telesiñski, A.; Brysiewicz, A. Determination of Morpho-Physiological and Yield Traits of Maize Inbred Lines (Zea mays L.) under Optimal and Drought Stress Conditions. Front. Plant Sci. 2022, 13, 959203. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Liu, Z.; Li, J.; Xu, J.; Wu, H.; Xu, Z. Spatiotemporal Patterns of Maize Drought Stress and Their Effects on Biomass in the Northeast and North China Plain from 2000 to 2019. Agric. For. Meteorol. 2022, 315, 108821. [Google Scholar] [CrossRef]
- Ouyang, W.; Chen, L.; Ma, J.; Liu, X.; Chen, H.; Yang, H.; Guo, W.; Shan, Z.; Yang, Z.; Chen, S. Identification of Quantitative Trait Locus and Candidate Genes for Drought Tolerance in a Soybean Recombinant Inbred Line Population. Int. J. Mol. Sci. 2022, 23, 10828. [Google Scholar] [CrossRef] [PubMed]
- Anda, A.; Simon, B.; Soós, G.; Teixeira da Silva, J.A.; Menyhárt, L. Water Stress Modifies Canopy Light Environment and Qualitative and Quantitative Yield Components in Two Soybean Varieties. Irrig. Sci. 2021, 39, 549–566. [Google Scholar] [CrossRef]
- Li, J.; Yao, X.; Yao, Y.; An, L.; Feng, Z.; Wu, K. Genome-Wide Association Mapping of Hulless Barely Phenotypes in Drought Environment. Front. Plant Sci 2022, 13, 924892. [Google Scholar] [CrossRef] [PubMed]
- Feiziasl, V.; Jafarzadeh, J.; Sadeghzadeh, B.; Shalmani, M.A.M. Water Deficit Index to Evaluate Water Stress Status and Drought Tolerance of Rainfed Barley Genotypes in Cold Semi-Arid Area of Iran. Agric. Water Manag. 2022, 262, 107395. [Google Scholar] [CrossRef]
- Sattar, A.; Wang, X.; Ul-Allah, S.; Sher, A.; Ijaz, M.; Irfan, M.; Abbas, T.; Hussain, S.; Nawaz, F.; Al-Hashimi, A. Foliar Application of Zinc Improves Morpho-Physiological and Antioxidant Defense Mechanisms, and Agronomic Grain Biofortification of Wheat (Triticum aestivum L.) under Water Stress. Saudi J. Biol. Sci. 2022, 29, 1699–1706. [Google Scholar] [CrossRef]
- Kamara, M.M.; Rehan, M.; Mohamed, A.M.; El Mantawy, R.F.; Kheir, A.M.S.; Abd El-Moneim, D.; Safhi, F.A.; ALshamrani, S.M.; Hafez, E.M.; Behiry, S.I. Genetic Potential and Inheritance Patterns of Physiological, Agronomic and Quality Traits in Bread Wheat under Normal and Water Deficit Conditions. Plants 2022, 11, 952. [Google Scholar] [CrossRef]
- Manikanta, C.L.N.; Beena, R.; Rejeth, R. Root Anatomical Traits Influence Water Stress Tolerance in Rice (Oryza sativa L.). J. Crop Sci. Biotechnol. 2022, 25, 421–436. [Google Scholar] [CrossRef]
- Suleiman, S.O.; Habila, D.G.; Mamadou, F.; Abolanle, B.M.; Olatunbosun, A.N. Grain Yield and Leaf Gas Exchange in Upland NERICA Rice under Repeated Cycles of Water Deficit at Reproductive Growth Stage. Agric. Water Manag. 2022, 264, 107507. [Google Scholar] [CrossRef]
- Thanapornpoonpong, S. Effect of Nitrogen Fertilizer on Nitrogen Assimilation and Seed Quality of Amaranth (Amaranthus spp.) and Quinoa (Chenopodium quinoa Willd.); Niedersächsische Staats-und Universitätsbibliothek: Göttingen, Germany, 2004. [Google Scholar]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.-E. The Combined Effect of Deficit Irrigation by Treated Wastewater and Organic Amendment on Quinoa (Chenopodium quinoa Willd.) Productivity. Desalination Water Treat. 2014, 52, 2208–2213. [Google Scholar] [CrossRef]
- Miranda Casas, R. Adubacao Organica Em Condicoes de Irrigacao Suplementar e Seu Efeito Na Productividad Da Quinua (Chenopodium quinoa) No Planalto Da Bolivia. Master’s Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2014. [Google Scholar]
- Al-Naggar, A.M.M.; Abd El-Salam, R.M.; Badran, A.E.E.; El-Moghazi, M.M.A. Drought Tolerance of Five Quinoa (Chenopodium quinoa Willd.) Genotypes and Its Association with Other Traits under Moderate and Severe Drought Stress. Asian J. Adv. Agric. Res. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Ali, M.A.; Ghazy, A.I.; Alotaibi, K.D.; Ibrahim, O.M.; Al-Doss, A.A. Nitrogen Efficiency Indexes Association with Nitrogen Recovery, Utilization, and Use Efficiency in Spring Barley at Various Nitrogen Application Rates. Agron. J. 2022, 114, 2290–2309. [Google Scholar] [CrossRef]
- Spehar, C.R.; de Barros Santos, R.L. Agronomic Performance of Quinoa Selected in the Brazilian Savannah. Pesqui Agropecu Bras. 2005, 40, 609–612. [Google Scholar] [CrossRef]
- Mignone, C.M.; Bertero, H.D. Identificación Del Período Crítico de Determinación Del Rendimiento En Quínoas de Nivel Del Mar. In Proceedings of the Congreso Internacional de la Quinua, Iquiqu, Chile, 23–26 October 2007; pp. 23–26. [Google Scholar]
- Bhargava, A.; Shukla, S.; Rajan, S.; Ohri, D. Genetic Diversity for Morphological and Quality Traits in Quinoa (Chenopodium quinoa Willd.) Germplasm. Genet. Resour. Crop Evol. 2007, 54, 167–173. [Google Scholar] [CrossRef]
- Rojas, W. Multivariate Analysis of Genetic Diversity of Bolivian Quinoa Germplasm. Food Rev. Int. 2003, 19, 9–23. [Google Scholar] [CrossRef]
- Talebnejad, R.; Sepaskhah, A.R. Effect of Deficit Irrigation and Different Saline Groundwater Depths on Yield and Water Productivity of Quinoa. Agric. Water Manag. 2015, 159, 225–238. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Wang, X.; Iqbal, S.; Hafeez, M.B.; Khan, S.; Raza, A.; Iqbal, J.; Maqbool, M.M.; Fiaz, S.; Qazi, M.A. Effect of Water Stress on Grain Yield and Physiological Characters of Quinoa Genotypes. Agronomy 2021, 11, 1934. [Google Scholar] [CrossRef]
- Sicher, R.C.; Bunce, J.A. Growth, Photosynthesis, Nitrogen Partitioning and Responses to CO2 Enrichment in a Barley Mutant Lacking NADH-dependent Nitrate Reductase Activity. Physiol. Plant. 2008, 134, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimikia, M.; Jami Moeini, M.; Marvi, H.; Hasheminejhad, Y.; Ghasemzadeh Ganjehie, M. Agro-Physiological Response of Quinoa (Chenopodium quinoa Willd.) to the Nitrogen Application Rate and Split Application Method. J. Soil Sci. Plant Nutr. 2021, 21, 3437–3450. [Google Scholar] [CrossRef]
- Gursoy, M. Effects of Different Nitrogen Doses on Yield and Quality Components in Some Two-Rowed Barley (Hordeum vulgare L.) Lines and Cultivars. J. New World Sci. 2011, 6, 114–123. [Google Scholar]
- Talebi, R.; Fayyaz, F.; Naji, A.M. Genetic Variation and Interrelationships of Agronomic Characteristics in Durum Wheat under Two Constructing Water Regimes. Braz. Arch. Biol. Technol. 2010, 53, 785–791. [Google Scholar] [CrossRef]
- Almadini, A.M.; Badran, A.E.; Algosaibi, A.M. Evaluation of Efficiency and Response of Quinoa Plant to Nitrogen Fertilization Levels. Middle East. J. Appl. Sci. 2019, 9, 839–849. [Google Scholar]
- Warburton, M.L.; Xianchun, X.; Crossa, J.; Franco, J.; Melchinger, A.E.; Frisch, M.; Bohn, M.; Hoisington, D. Genetic Characterization of CIMMYT Inbred Maize Lines and Open Pollinated Populations Using Large Scale Fingerprinting Methods. Crop Sci. 2002, 42, 1832–1840. [Google Scholar] [CrossRef]
- Bhargava, A.; Shukla, S.; Ohri, D. Genetic Variability and Interrelationship among Various Morphological and Quality Traits in Quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2007, 101, 104–116. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Li, X.; Li, M.; Zhang, D.; Hao, Z.; Weng, J.; Xu, Y.; Bai, L.; Zhang, S. Low-Nitrogen Stress Tolerance and Nitrogen Agronomic Efficiency among Maize Inbreds: Comparison of Multiple Indices and Evaluation of Genetic Variation. Euphytica 2011, 180, 281–290. [Google Scholar] [CrossRef]
- Coque, M.; Gallais, A. Genomic Regions Involved in Response to Grain Yield Selection at High and Low Nitrogen Fertilization in Maize. Theor. Appl. Genet. 2006, 112, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Pospišil, A.; Pospišil, M.; Varga, B.; Svečnjak, Z. Grain Yield and Protein Concentration of Two Amaranth Species (Amaranthus spp.) as Influenced by the Nitrogen Fertilization. Eur. J. Agron. 2006, 25, 250–253. [Google Scholar] [CrossRef]





| Month | Sunshine Hours (h) | Wind Speed (km/h) | Relative Humidity (%) | Min. Temp. (°C) | Max. Temp. (°C) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | |
| December | 9 | 9 | 15.4 | 14.3 | 57.7 | 53.6 | 8.1 | 9.4 | 21.5 | 23.6 |
| January | 8.9 | 8.9 | 16.5 | 13.5 | 59.6 | 58.9 | 5.5 | 7.1 | 18.5 | 21.4 |
| February | 9.7 | 9.7 | 17.7 | 15.9 | 54.7 | 57.4 | 7.4 | 7.3 | 21.4 | 21.6 |
| March | 9.9 | 9.9 | 19.3 | 18.6 | 44.2 | 43.4 | 10.8 | 11.3 | 26.2 | 27.1 |
| April | 10.3 | 10.3 | 18.8 | 17.1 | 38 | 34.4 | 14.7 | 15.1 | 30.6 | 32 |
| Chemical Properties | |||||||||
| pH (1:1) | EC dS/m (1:1) | Soluble cations (meq/L) | Soluble anions (meq/L) | Available phosphorus (ppm) | Total nitrogen (%) | ||||
| Ca++ | Mg++ | Na+ | K+ | CO3− + HCO3− | Cl− | ||||
| 8.31 | 0.45 | 1.69 | 1.25 | 0.38 | 0.78 | 1.99 | 1.64 | 7.08 | 0.015 |
| Physical properties | |||||||||
| Particle size distribution (%) | Texture class | Moisture content (Volumetric %) | O.M (%) | CaCO3 (%) | Bulk density | ||||
| Sand | Silt | Clay | S. P. | F.C. | W.P. | ||||
| 91.1 | 5.7 | 3.2 | Sandy | 23.0 | 10.9 | 4.5 | 0.42 | 29.80 | 1.60 |
| 100% Kc | 80% Kc | 60% Kc | ||||
|---|---|---|---|---|---|---|
| 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | 2020/2021 | 2021/2022 | |
| December | 0.57 | 0.40 | 0.46 | 0.32 | 0.37 | 0.26 |
| January | 0.76 | 0.59 | 0.61 | 0.47 | 0.50 | 0.38 |
| February | 0.93 | 0.91 | 0.74 | 0.73 | 0.61 | 0.60 |
| March | 0.92 | 0.90 | 0.73 | 0.72 | 0.60 | 0.59 |
| April | 0.35 | 0.39 | 0.28 | 0.31 | 0.23 | 0.25 |
| Average | 0.71 | 0.64 | 0.56 | 0.51 | 0.46 | 0.42 |
| Source of Variance | Plant Height (cm) | Panicle Length (cm) | Dry Weight (g) | Seed Weight (g) | Seed Yield (t/ha) | Total Yield (t/ha) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020/21 | 2021/22 | 2020/21 | 2021/22 | 2021/22 | 2020/21 | 2021/22 | 2020/21 | 2021/22 | 2020/21 | 2021/22 | 2020/21 | |
| Water regimes (WR) | 1193.50 *** | 1306.10 *** | 777.29 *** | 782.43 *** | 1100.79 *** | 919.34 *** | 385.66 *** | 285.78 *** | 86,918.52 *** | 93,395.54 *** | 1,102,920.08 *** | 920,875.63 *** |
| Nitrogen level (NL) | 902.64 *** | 1024.65 *** | 575.05 *** | 673.50 *** | 44.81 *** | 82.93 *** | 90.31 *** | 55.29 *** | 129,969.23 *** | 154,889.98 *** | 589,359.65 *** | 786,252.82 *** |
| WR × NL | 55.75 *** | 45.35 *** | 77.19 *** | 76.88 *** | 28.33 *** | 41.18 *** | 14.02 *** | 21.68 *** | 11,597.06 *** | 12,345.87 *** | 22,062.73 *** | 110,091.25 *** |
| CV | 19.71 | 20.00 | 30.94 | 30.70 | 32.00 | 33.50 | 33.80 | 35.20 | 35.80 | 36.40 | 35.20 | 36.80 |
| SD | 12.46 | 13.08 | 10.40 | 10.79 | 8.47 | 8.21 | 5.72 | 4.98 | 135.33 | 144.74 | 344.14 | 384.42 |
| SE | 2.10 | 2.18 | 1.73 | 1.80 | 1.41 | 1.37 | 0.95 | 0.83 | 22.60 | 24.12 | 57.35 | 64.10 |
| Water Regime | Nitrogen Level | Water Productivity |
|---|---|---|
| 100% ETo | 75 kgN ha−1 | 0.31 |
| 100% ETo | 150 kgN ha−1 | 0.43 |
| 100% ETo | 225 kgN ha−1 | 0.64 |
| 80% ETo | 75 kgN ha−1 | 0.35 |
| 80% ETo | 150 kgN ha−1 | 0.57 |
| 80% ETo | 225 kgN ha−1 | 0.68 |
| 80% ETo | 300 kgN ha−1 | 0.71 |
| 60% ETo | 75 kgN ha−1 | 0.37 |
| 60% ETo | 150 kgN ha−1 | 0.46 |
| 60% ETo | 225 kgN ha−1 | 0.53 |
| 60% ETo | 300 kgN ha−1 | 0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AbdElgalil, M.A.S.; Hefzy, M.; Sas-Paszt, L.; Ali, H.M.; Lamlom, S.F.; Abdelghany, A.M. Unraveling the Influence of Water and Nitrogen Management on Quinoa (Chenopodium quinoa Willd.) Agronomic and Yield Traits. Water 2023, 15, 1296. https://doi.org/10.3390/w15071296
AbdElgalil MAS, Hefzy M, Sas-Paszt L, Ali HM, Lamlom SF, Abdelghany AM. Unraveling the Influence of Water and Nitrogen Management on Quinoa (Chenopodium quinoa Willd.) Agronomic and Yield Traits. Water. 2023; 15(7):1296. https://doi.org/10.3390/w15071296
Chicago/Turabian StyleAbdElgalil, Mostafa AbdElaal Sayed, Mohamed Hefzy, Lidia Sas-Paszt, Hayssam M. Ali, Sobhi F. Lamlom, and Ahmed M. Abdelghany. 2023. "Unraveling the Influence of Water and Nitrogen Management on Quinoa (Chenopodium quinoa Willd.) Agronomic and Yield Traits" Water 15, no. 7: 1296. https://doi.org/10.3390/w15071296
APA StyleAbdElgalil, M. A. S., Hefzy, M., Sas-Paszt, L., Ali, H. M., Lamlom, S. F., & Abdelghany, A. M. (2023). Unraveling the Influence of Water and Nitrogen Management on Quinoa (Chenopodium quinoa Willd.) Agronomic and Yield Traits. Water, 15(7), 1296. https://doi.org/10.3390/w15071296












