Degradation of Sulfoxaflor Pesticide in Aqueous Solutions Utilizing Photocatalytic Ozonation with the Simultaneous Use of Titanium Dioxide and Iron Zeolite Catalysts
Abstract
:1. Introduction
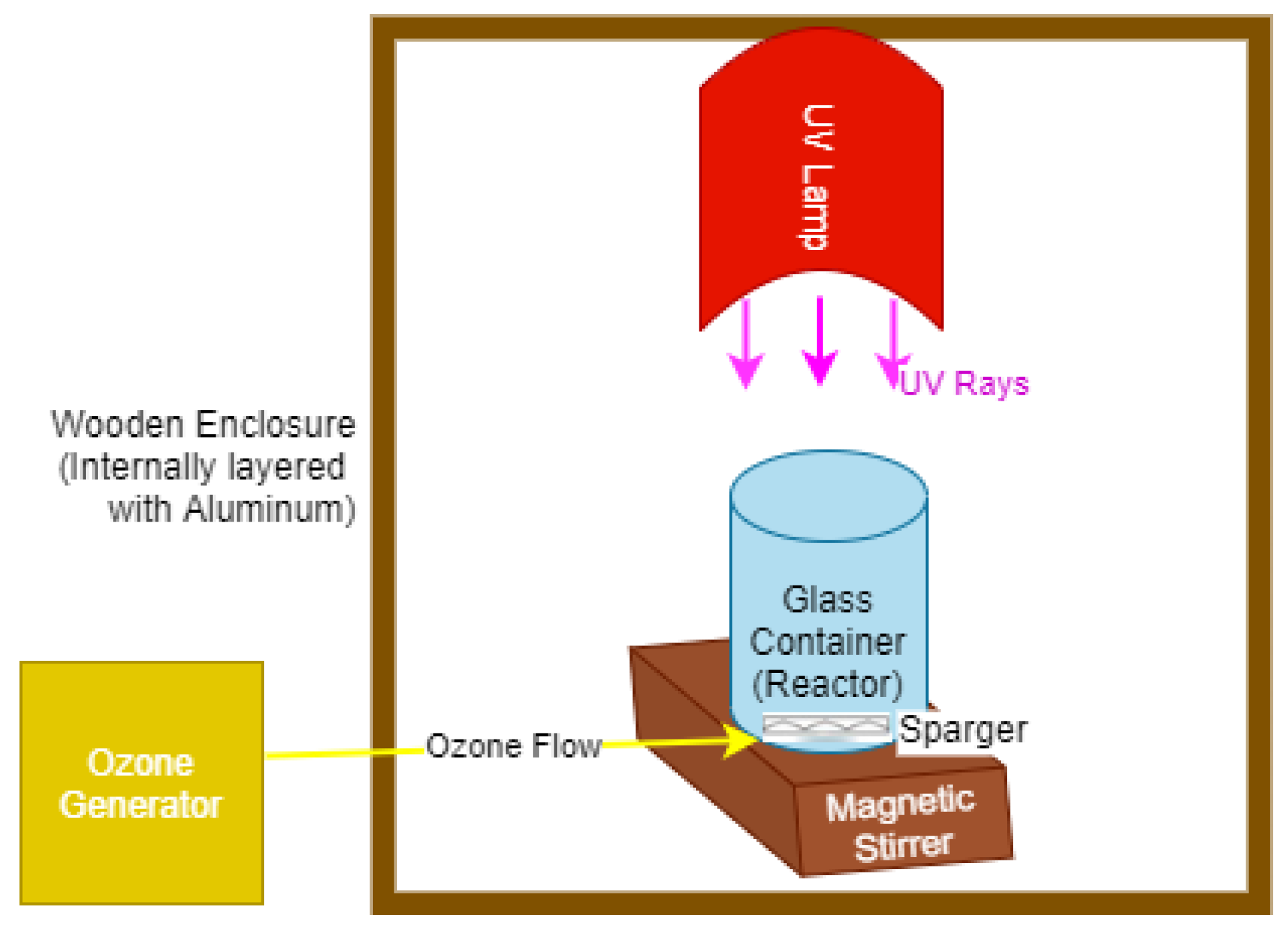
2. Materials and Methods
3. Results
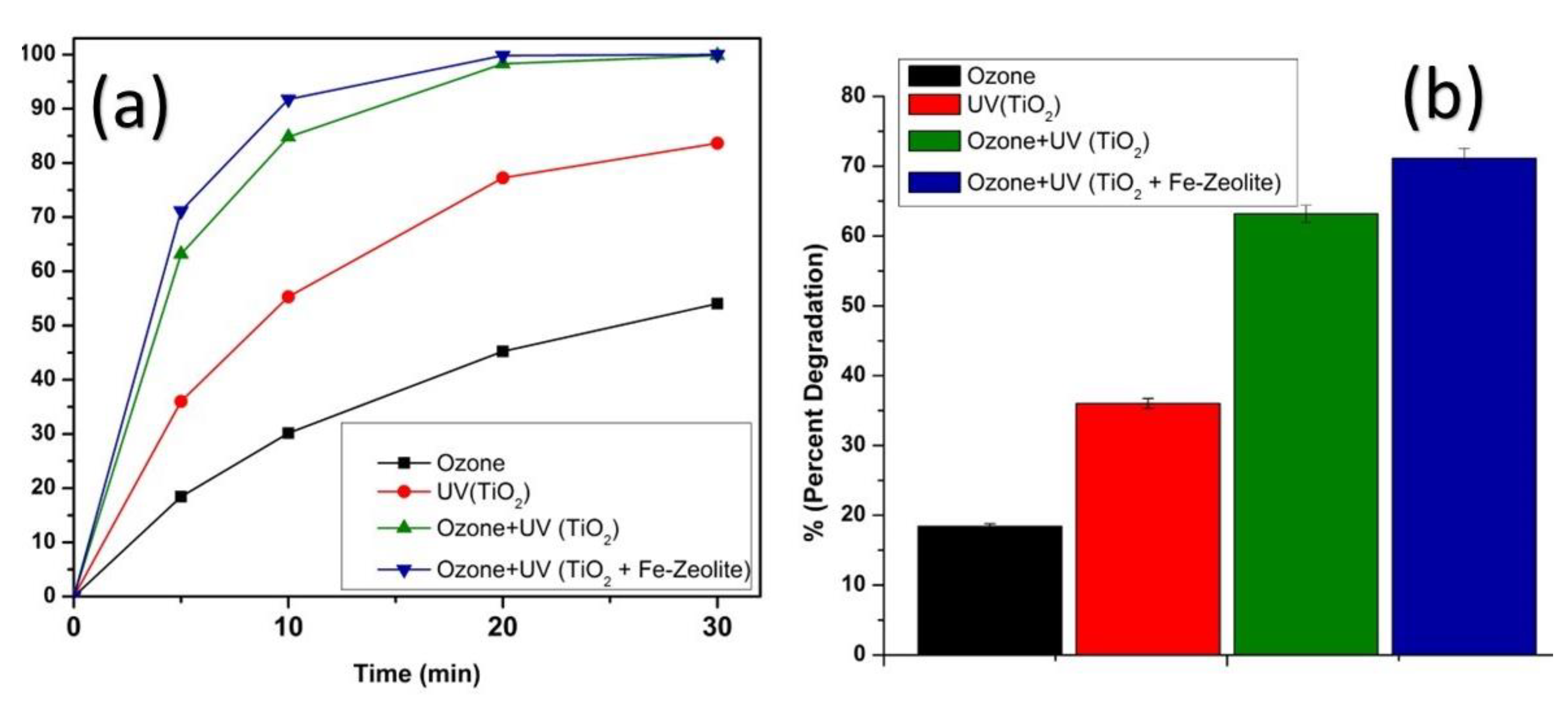
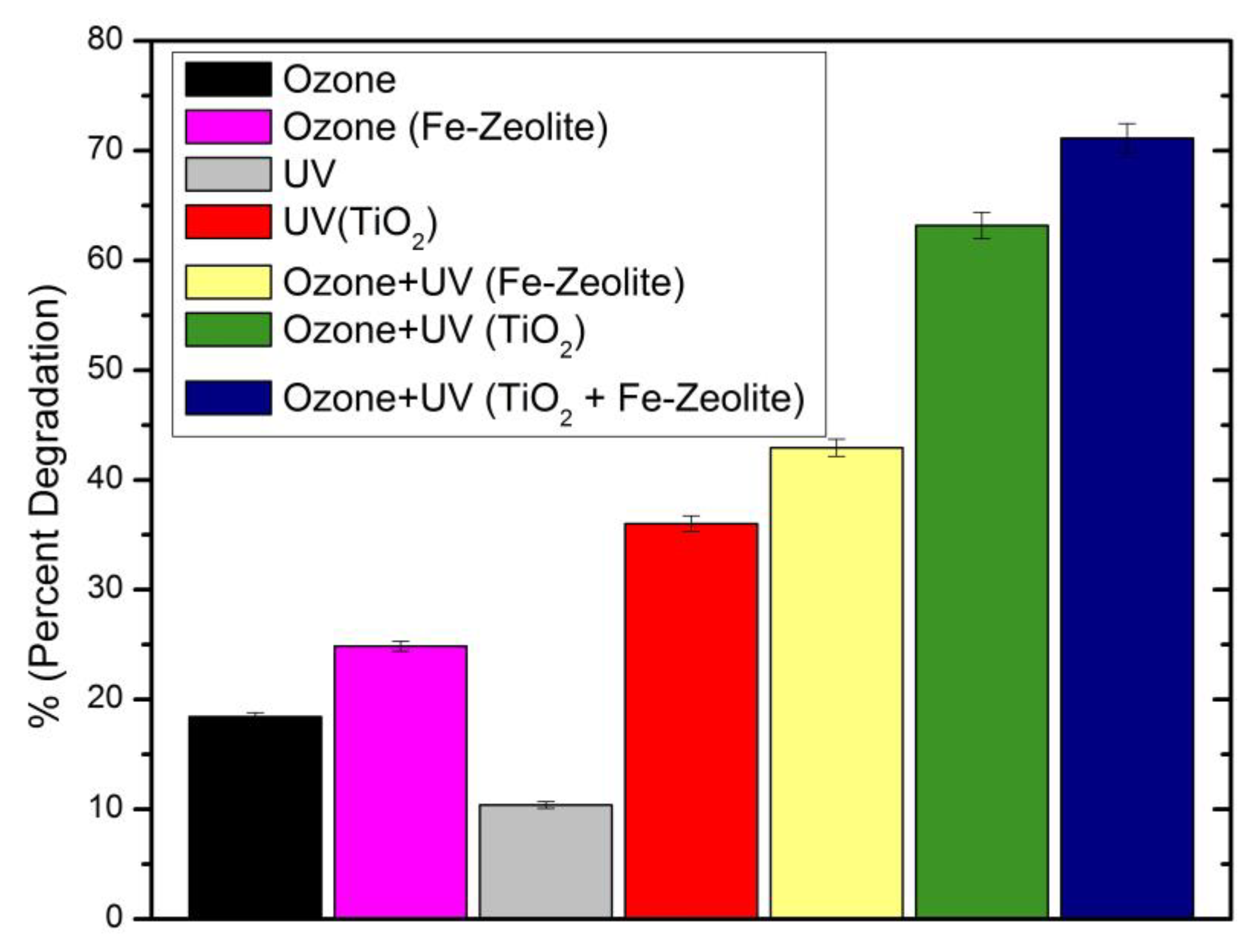
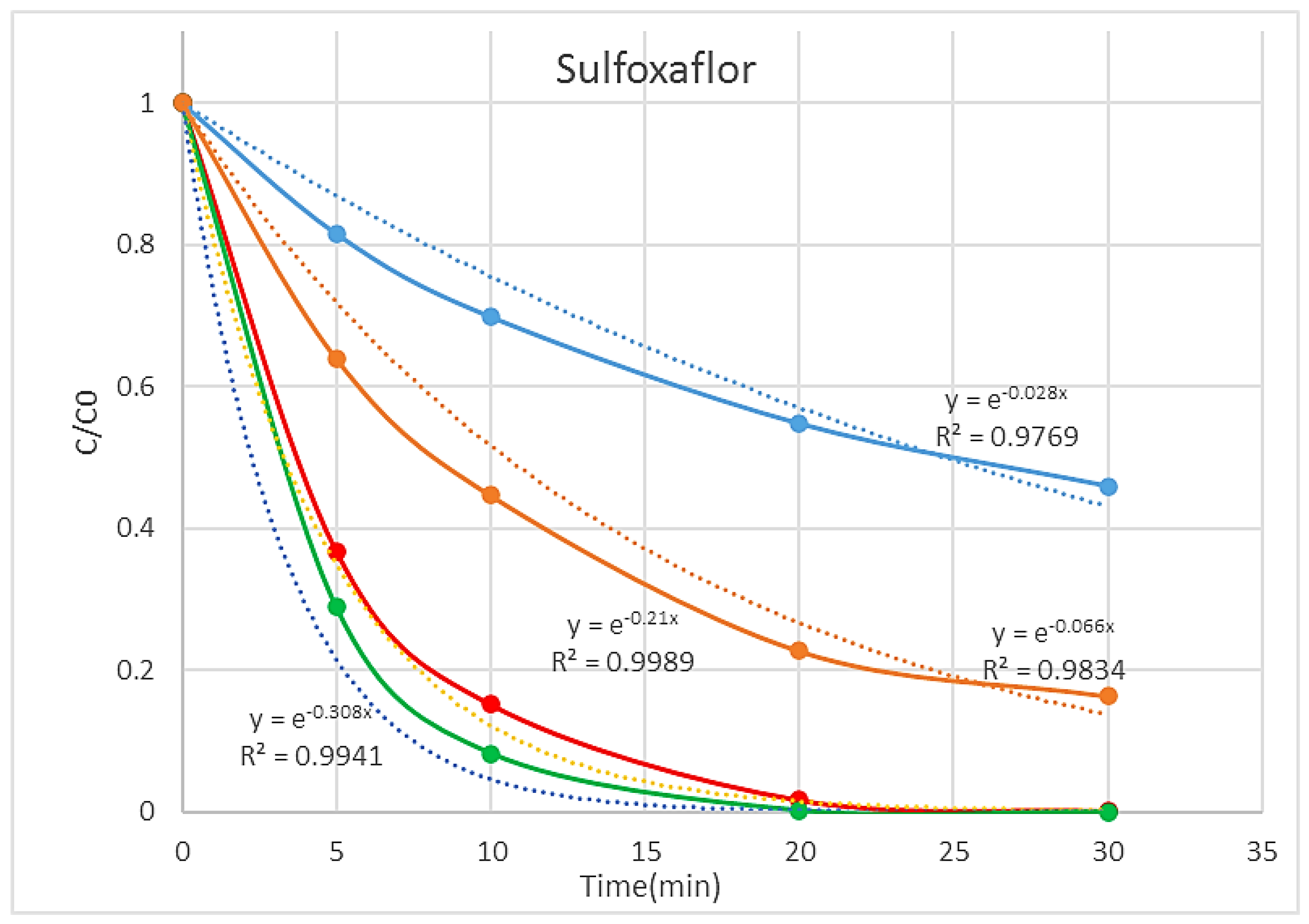
3.1. Degradation Studies
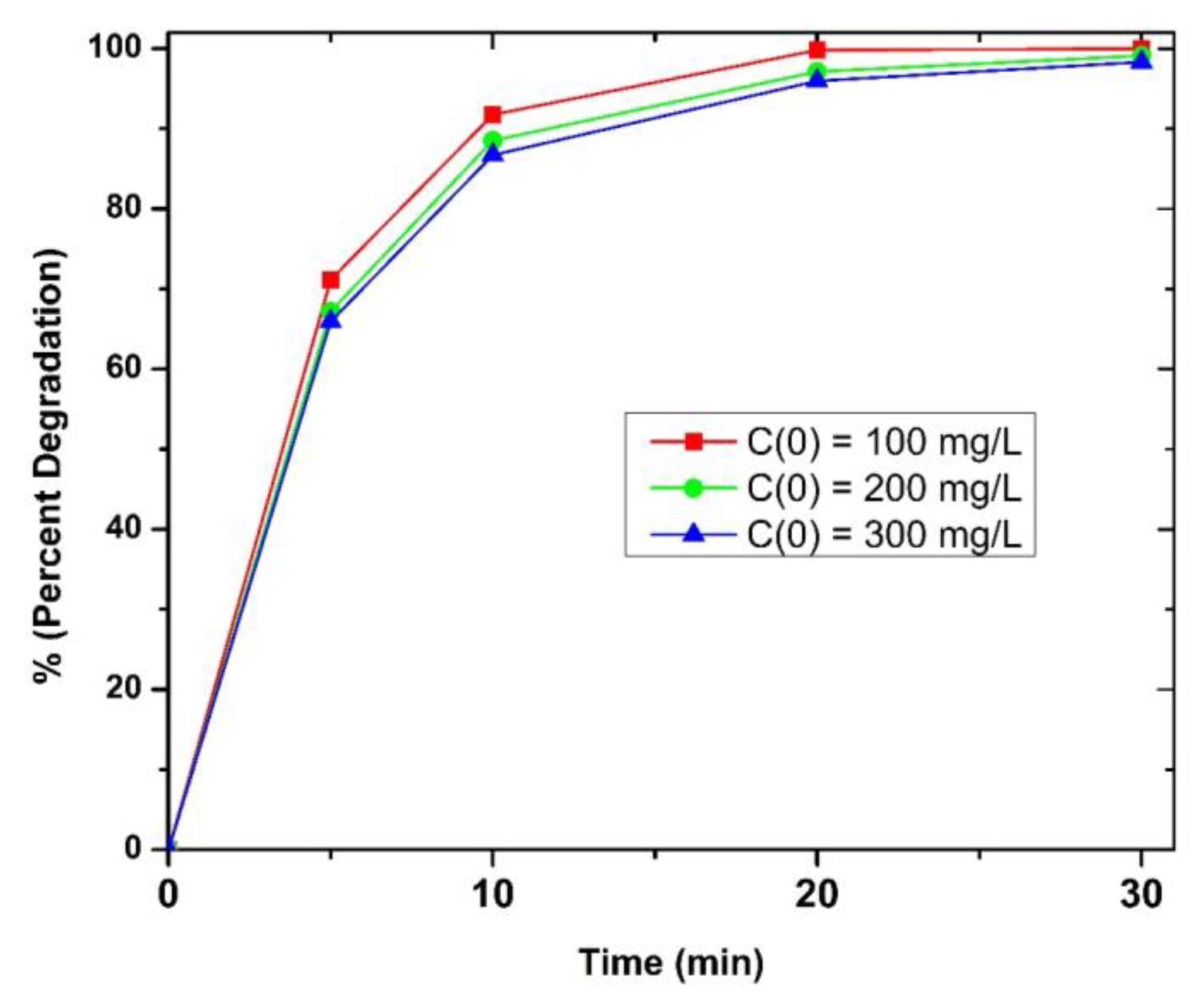
3.2. Initial Concentration
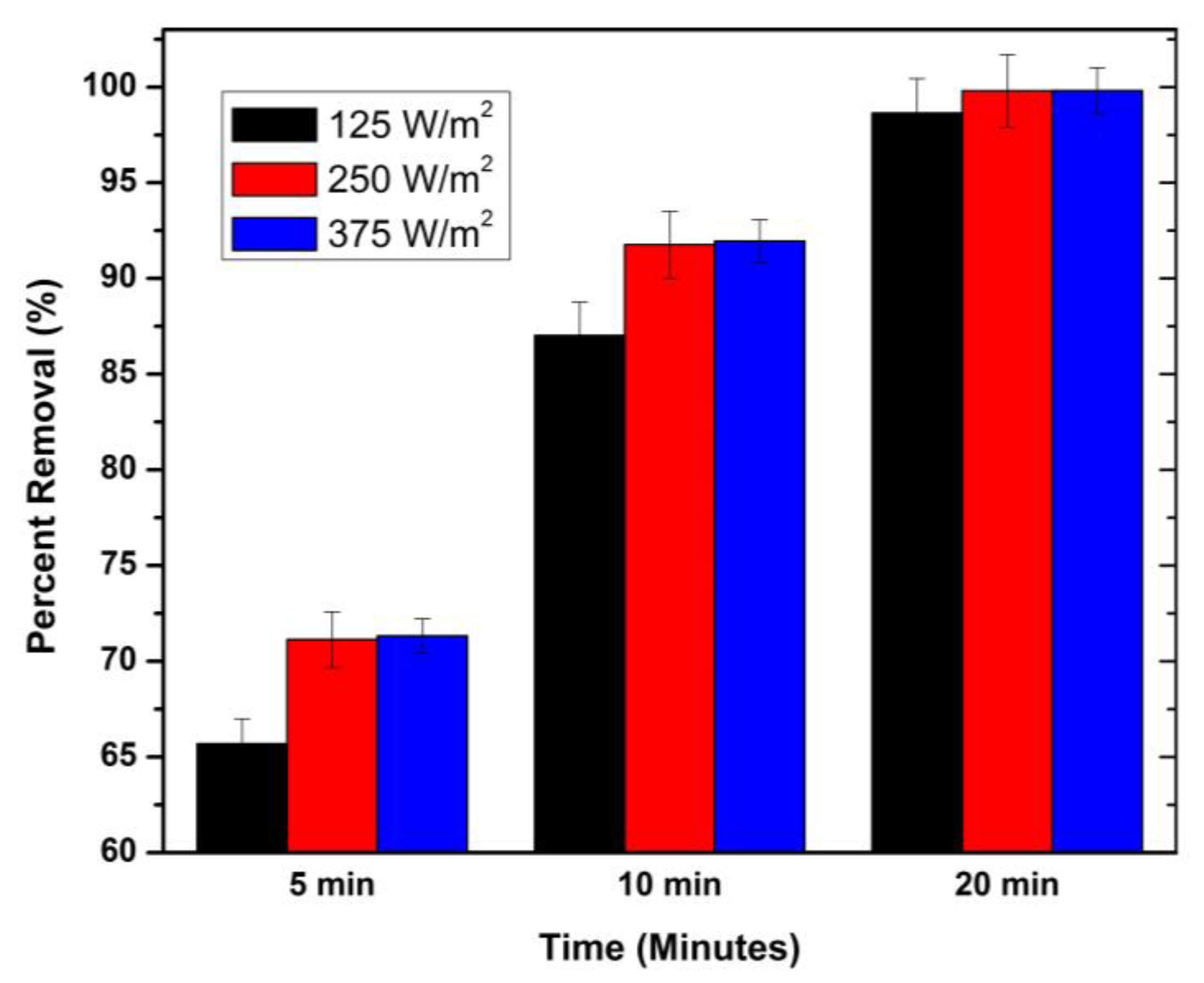
3.3. UV Intensity
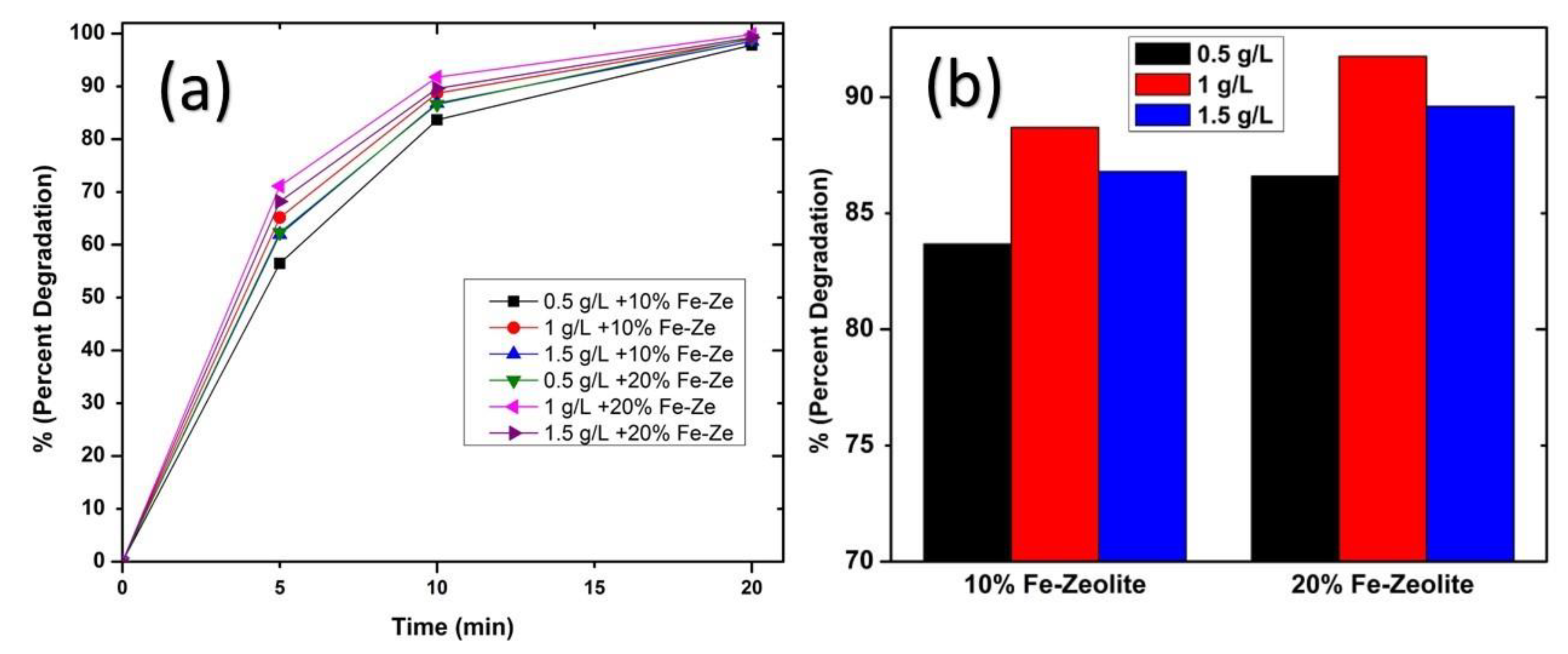
3.4. Catalyst Dose and Fe-Zeolite Percentage
3.5. Catalyst Reuse
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niknam, A.; Zare, H.K.; Hosseininasab, H.; Mostafaeipour, A.; Herrera, M. A Critical Review of Short-Term Water Demand Forecasting Tools—What Method Should I Use? Sustainability 2022, 14, 5412. [Google Scholar] [CrossRef]
- Heidari, H.; Arabi, M.; Warziniack, T. Vulnerability to Water Shortage Under Current and Future Water Supply-Demand Conditions Across U.S. River Basins. Earth’s Futur. 2021, 9, e2021EF002278. [Google Scholar] [CrossRef]
- Cui, J.; Zheng, L.; Deng, Y. Emergency water treatment with ferrate(vi) in response to natural disasters. Environ. Sci. Water Res. Technol. 2018, 4, 359–368. [Google Scholar] [CrossRef]
- Zheng, L.; Cui, J.; Deng, Y. Emergency water treatment with combined ferrate(vi) and ferric salts for disasters and disease outbreaks. Environ. Sci. Water Res. Technol. 2020, 6, 2816–2831. [Google Scholar] [CrossRef]
- Lin, Q.; Dong, F.; Li, C.; Cui, J. Disinfection byproduct formation from algal organic matters after ozonation or ozone combined with activated carbon treatment with subsequent chlorination. J. Environ. Sci. 2021, 104, 233–241. [Google Scholar] [CrossRef]
- Tang, W.; Pei, Y.; Zheng, H.; Zhao, Y.; Shu, L.; Zhang, H. Twenty years of China’s water pollution control: Experiences and challenges. Chemosphere 2022, 295, 133875. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Gao, P.; Cui, J.; Deng, Y. Direct regeneration of ion exchange resins with sulfate radical-based advanced oxidation for enabling a cyclic adsorption—Regeneration treatment approach to aqueous perfluorooctanoic acid (PFOA). Chem. Eng. J. 2021, 405, 126698. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A review on emerging water contaminants and the application of sustainable removal technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Ahmad, S.; Almehmadi, M.; Janjuhah, H.T.; Kontakiotis, G.; Abdulaziz, O.; Saeed, K.; Ahmad, H.; Allahyani, M.; Aljuaid, A.; Alsaiari, A.A.; et al. The Effect of Mineral Ions Present in Tap Water on Photodegradation of Organic Pollutants: Future Perspectives. Water 2023, 15, 175. [Google Scholar] [CrossRef]
- Kalyabina, V.P.; Esimbekova, E.N.; Kopylova, K.V.; Kratasyuk, V.A. Pesticides: Formulants, distribution pathways and effects on human health—A review. Toxicol. Rep. 2021, 8, 1179–1192. [Google Scholar] [CrossRef]
- Syafrudin, M.; Kristanti, R.A.; Yuniarto, A.; Hadibarata, T.; Rhee, J.; Al-Onazi, W.A.; Algarni, T.S.; Almarri, A.H.; Al-Mohaimeed, A.M. Pesticides in Drinking Water—A Review. Int. J. Environ. Res. Public Health 2021, 18, 468. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kania, J.; Malina, G.; Kmiecik, E.; Wątor, K. Pesticides from the EU First and Second Watch Lists in the Water Environment. CLEAN–Soil Air Water 2019, 47, 1800376. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The Technology Horizon for Photocatalytic Water Treatment: Sunrise or Sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zhang, M.; Xu, L.; Wang, S.; Yang, T.; Wu, M.; Lu, W.; Li, Y.; Yu, D. Unraveling timescale-dependent Fe-MOFs crystal evolution for catalytic ozonation reactivity modulation. J. Hazard. Mater. 2022, 431, 128575. [Google Scholar] [CrossRef]
- Guo, D.; Liu, Y.; Ji, H.; Wang, C.-C.; Chen, B.; Shen, C.; Li, F.; Wang, Y.; Lu, P.; Liu, W. Silicate-Enhanced Heterogeneous Flow-Through Electro-Fenton System Using Iron Oxides under Nanoconfinement. Environ. Sci. Technol. 2021, 55, 4045–4053. [Google Scholar] [CrossRef]
- Lee, C.-G.; Javed, H.; Zhang, D.; Kim, J.-H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous Electrospun Fibers Embedding TiO2 for Adsorption and Photocatalytic Degradation of Water Pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N. Photocatalytic ozonation of wastewater: A review. Environ. Chem. Lett. 2020, 18, 1491–1507. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P.; Gopinath, K.P.; Lichtfouse, E. Synthesis and application of titanium dioxide photocatalysis for energy, decontamination and viral disinfection: A review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Raashid, M.; Akram, A.; Kazmi, M.; Farman, S. Removal of methylene blue dye from aqueous solutions by adsorption in combination with ozonation on iron loaded sodium zeolite: Role of adsorption. Desalin. Water Treat. 2021, 237, 302–306. [Google Scholar] [CrossRef]
- Watson, G.B.; Siebert, M.W.; Wang, N.X.; Loso, M.R.; Sparks, T.C. Sulfoxaflor—A sulfoximine insecticide: Review and analysis of mode of action, resistance and cross-resistance. Pestic. Biochem. Physiol. 2021, 178, 104924. [Google Scholar] [CrossRef]
- Azpiazu, C.; Bosch, J.; Bortolotti, L.; Medrzycki, P.; Teper, D.; Molowny-Horas, R.; Sgolastra, F. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bello, F.D.; Medana, C.; Guarino, B.; Dioni, A.; Fabbri, D.; Calza, P. Investigation of sulfoxaflor, flupyradifurone and their transformation products in plant-based food matrices. Food Control 2022, 132, 108537. [Google Scholar] [CrossRef]
- Zhou, W.; Apkarian, R.; Wang, Z.L.; Joy, D. Fundamentals of Scanning Electron Microscopy (SEM). In Scanning Microscopy for Nanotechnology; Springer: New York, NY, USA, 2006; pp. 1–40. [Google Scholar]
- Khan, I.; Sadiq, M.; Khan, I.; Saeed, K. Manganese Dioxide Nanoparticles/Activated Carbon Composite as Efficient UV and Visible-Light Photocatalyst. Environ. Sci. Pollut. Res. 2019, 26, 5140–5154. [Google Scholar] [CrossRef]
- Heidari, Z.; Alizadeh, R.; Ebadi, A.; Pelalak, R.; Oturan, N.; Oturan, M.A. Degradation of furosemide using photocatalytic ozonation in the presence of ZnO/ICLT nanocomposite particles: Experimental, modeling, optimization and mechanism evaluation. J. Mol. Liq. 2020, 319, 114193. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Sans, C.; Sun, L.; Yuan, X.; Pan, F.; Xia, D. Insights into the photocatalytic ozonation over Ag2O-ZnO@g-C3N4 composite: Cooperative structure, degradation performance, and synergistic mechanisms. J. Environ. Chem. Eng. 2022, 10, 107285. [Google Scholar] [CrossRef]
- Checa, M.; Montes, V.; Rivas, J.; Beltrán, F.J. Checking the Efficiency of a Magnetic Graphene Oxide–Titania Material for Catalytic and Photocatalytic Ozonation Reactions in Water. Catalysts 2022, 12, 1587. [Google Scholar] [CrossRef]
- Cheng, S.-W.; Li, Y.-H.; Yuan, C.-S.; Tsai, P.-Y.; Shen, H.-Z.; Hung, C.-H. An Innovative Advanced Oxidation Technology for Effective Decomposition of Formaldehyde by Combining Iron Modified Nano-TiO2 (Fe/TiO2) Photocatalytic Degradation with Ozone Oxidation. Aerosol Air Qual. Res. 2018, 18, 3220–3233. [Google Scholar] [CrossRef]
- Rao, T.N.; Prashanthi, Y.; Ahmed, F.; Kumar, S.; Arshi, N.; Reddy, G.R.; Naidu, T.M. Photocatalytic Applications of Fe–Ag Co-Doped TiO2 Nanoparticles in Removal of Flumioxazin Pesticide Residues in Water. Front. Nanotechnol. 2021, 3, 14. [Google Scholar] [CrossRef]
- Raashid, M.; Kazmi, M.; Ikhlaq, A.; Iqbal, T.; Sulaiman, M.; Shakeel, A. Degradation of Aqueous CONFIDOR®Pesticide by Simultaneous TiO2 Photocatalysis and Fe-Zeolite Catalytic Ozonation. Water 2021, 13, 3327. [Google Scholar] [CrossRef]
- Gomes, J.; Roccamante, M.; Contreras, S.; Medina, F.; Oller, I.; Martins, R.C. Scale-up impact over solar photocatalytic ozonation with benchmark-P25 and N-TiO2 for insecticides abatement in water. J. Environ. Chem. Eng. 2021, 9, 104915. [Google Scholar] [CrossRef]
- Baghirzade, B.S.; Yetis, U.; Dilek, F.B. Imidacloprid elimination by O3 and O3/UV: Kinetics study, matrix effect, and mechanism insight. Environ. Sci. Pollut. Res. 2021, 28, 24535–24551. [Google Scholar] [CrossRef]
- Sraw, A.; Toor, A.P.; Wanchoo, R.K. Adsorption Kinetics and Degradation Mechanism Study of Water Persistent Insecticide Quinalphos: For Heterogeneous Photocatalysis onto TiO2. Desalin. Water Treat. 2016, 57, 16831–16842. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.; Bansal, A. Photocatalytic degradation of imidacloprid using semiconductor hybrid nano-catalyst: Kinetics, surface reactions and degradation pathways. Int. J. Environ. Sci. Technol. 2020, 18, 1425–1442. [Google Scholar] [CrossRef]
- Chen, S.; Deng, J.; Deng, Y.; Gao, N. Influencing factors and kinetic studies of imidacloprid degradation by ozonation. Environ. Technol. 2019, 40, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, X.; Lv, K.; Sun, S.; Deng, K.; Du, D. Photocatalytic degradation pathway for azo dye in TiO2/UV/O3 system: Hydroxyl radical versus hole. J. Mol. Catal. A Chem. 2013, 367, 31–37. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Aguinaco, A.; García-Araya, J.F. Mechanism and kinetics of sulfamethoxazole photocatalytic ozonation in water. Water Res. 2009, 43, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Zhu, D.; Li, L.; Lan, B. Enhanced photocatalytic ozonation of organics by g-C3N4 under visible light irradiation. J. Hazard. Mater. 2014, 280, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Mecha, A.C.; Onyango, M.S.; Ochieng, A.; Fourie, C.J.; Momba, M.N. Synergistic effect of UV–vis and solar photocatalytic ozonation on the degradation of phenol in municipal wastewater: A comparative study. J. Catal. 2016, 341, 116–125. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous photodegradation of industrial dyes: An insight to different mechanisms and rate affecting parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Eskandarloo, H.; Modirshahla, N.; Shokri, M. Influence of the Chemical Structure of Organic Pollutants on Photocatalytic Activity of TiO2 Nanoparticles: Kinetic Analysis and Evaluation of Electrical Energy per Order (EEO). Dig. J. Nanomater. Bios. 2011, 6, 1887–1895. [Google Scholar]



| Titanium Dioxide | Zeolite | |
|---|---|---|
| Particle size (μm) | 0.5 | 0.40 |
| Pore size (Å) | 86 | 10 |
| Surface area (m2/g) | 54 | 61.3 |
| Process | Pel (kW) | Rate Constants (min−1) | EEO (kWh m−3 order−1) |
|---|---|---|---|
| O3 | 0.020 | 0.028 | 274.3 |
| UV + TiO2 | 0.023 | 0.066 | 133.8 |
| O3 + UV + TiO2 (TiO2 only) | 0.038 | 0.21 | 69.5 |
| O3 + UV + TiO2 + Fe-Zeolite | 0.038 | 0.34 | 42.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raashid, M.; Kazmi, M.; Ikhlaq, A.; Iqbal, T.; Sulaiman, M.; Zafar, A.M.; Aly Hassan, A. Degradation of Sulfoxaflor Pesticide in Aqueous Solutions Utilizing Photocatalytic Ozonation with the Simultaneous Use of Titanium Dioxide and Iron Zeolite Catalysts. Water 2023, 15, 1283. https://doi.org/10.3390/w15071283
Raashid M, Kazmi M, Ikhlaq A, Iqbal T, Sulaiman M, Zafar AM, Aly Hassan A. Degradation of Sulfoxaflor Pesticide in Aqueous Solutions Utilizing Photocatalytic Ozonation with the Simultaneous Use of Titanium Dioxide and Iron Zeolite Catalysts. Water. 2023; 15(7):1283. https://doi.org/10.3390/w15071283
Chicago/Turabian StyleRaashid, Muhammad, Mohsin Kazmi, Amir Ikhlaq, Tanveer Iqbal, Muhammad Sulaiman, Abdul Mannan Zafar, and Ashraf Aly Hassan. 2023. "Degradation of Sulfoxaflor Pesticide in Aqueous Solutions Utilizing Photocatalytic Ozonation with the Simultaneous Use of Titanium Dioxide and Iron Zeolite Catalysts" Water 15, no. 7: 1283. https://doi.org/10.3390/w15071283
APA StyleRaashid, M., Kazmi, M., Ikhlaq, A., Iqbal, T., Sulaiman, M., Zafar, A. M., & Aly Hassan, A. (2023). Degradation of Sulfoxaflor Pesticide in Aqueous Solutions Utilizing Photocatalytic Ozonation with the Simultaneous Use of Titanium Dioxide and Iron Zeolite Catalysts. Water, 15(7), 1283. https://doi.org/10.3390/w15071283







