Environmental Changes Recorded in Tufa from the Korana River, Croatia: Geochemical and Isotopic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Geological Setting
2.2. Sampling and Mineralogical, Isotope and Chemical Analyses
2.3. Statistical Analysis
2.4. Local Enrichment Factor Calculation
3. Results
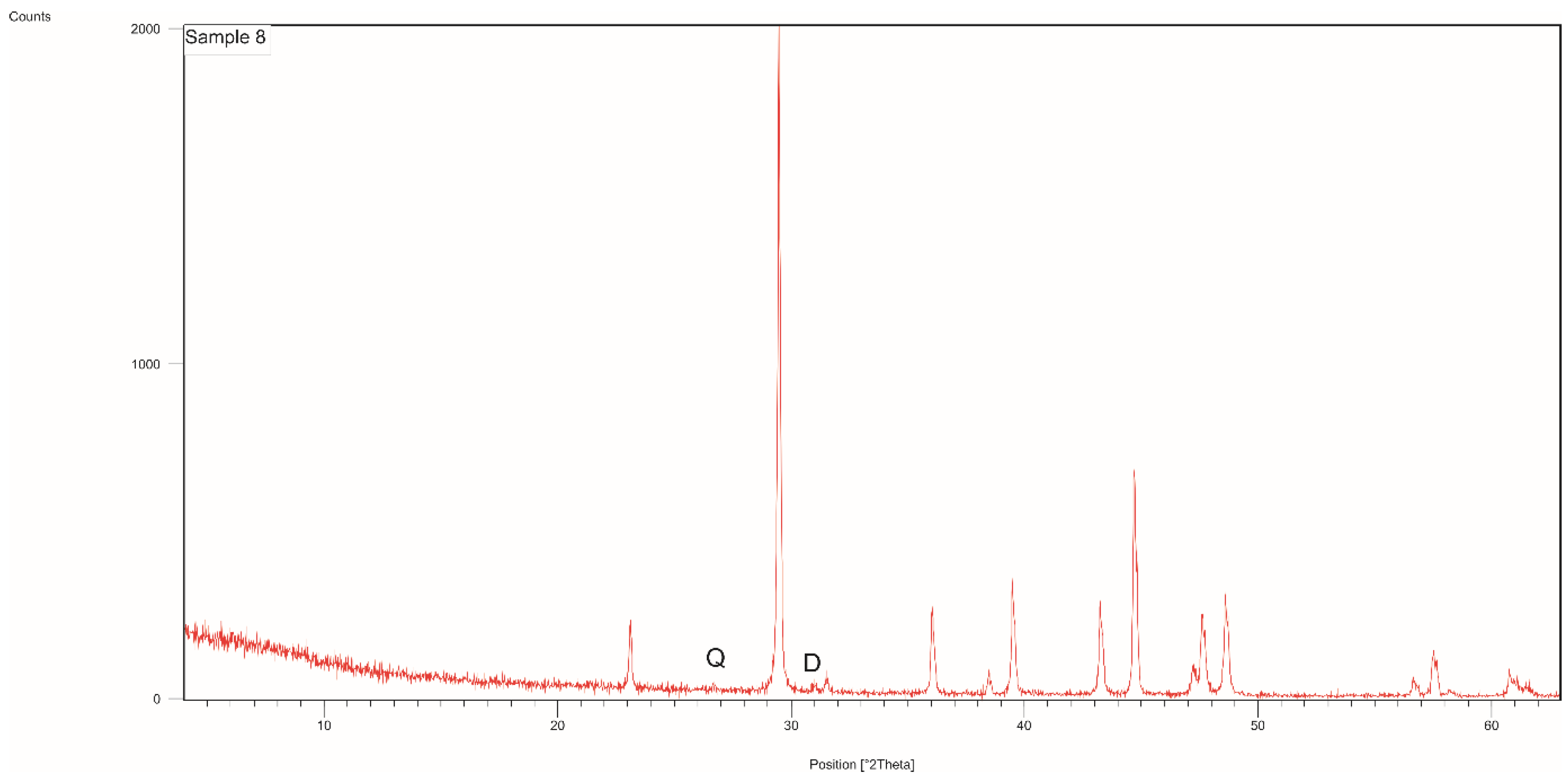
3.1. Mineralogical Analyses
3.2. Multi-Elemental, Isotope and C/N Analyses
4. Discussion
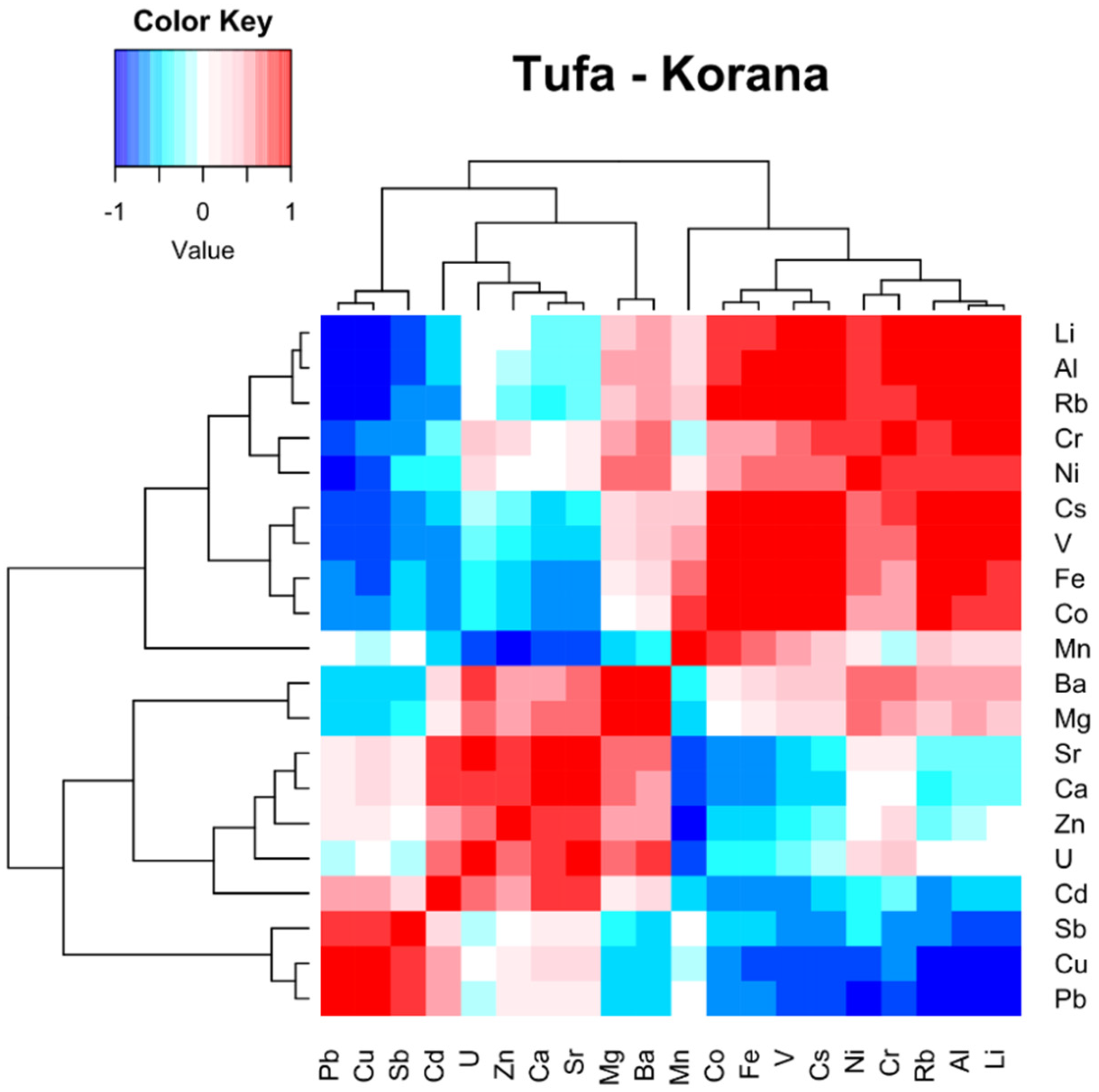
4.1. Relationship between the Elements in Tufa
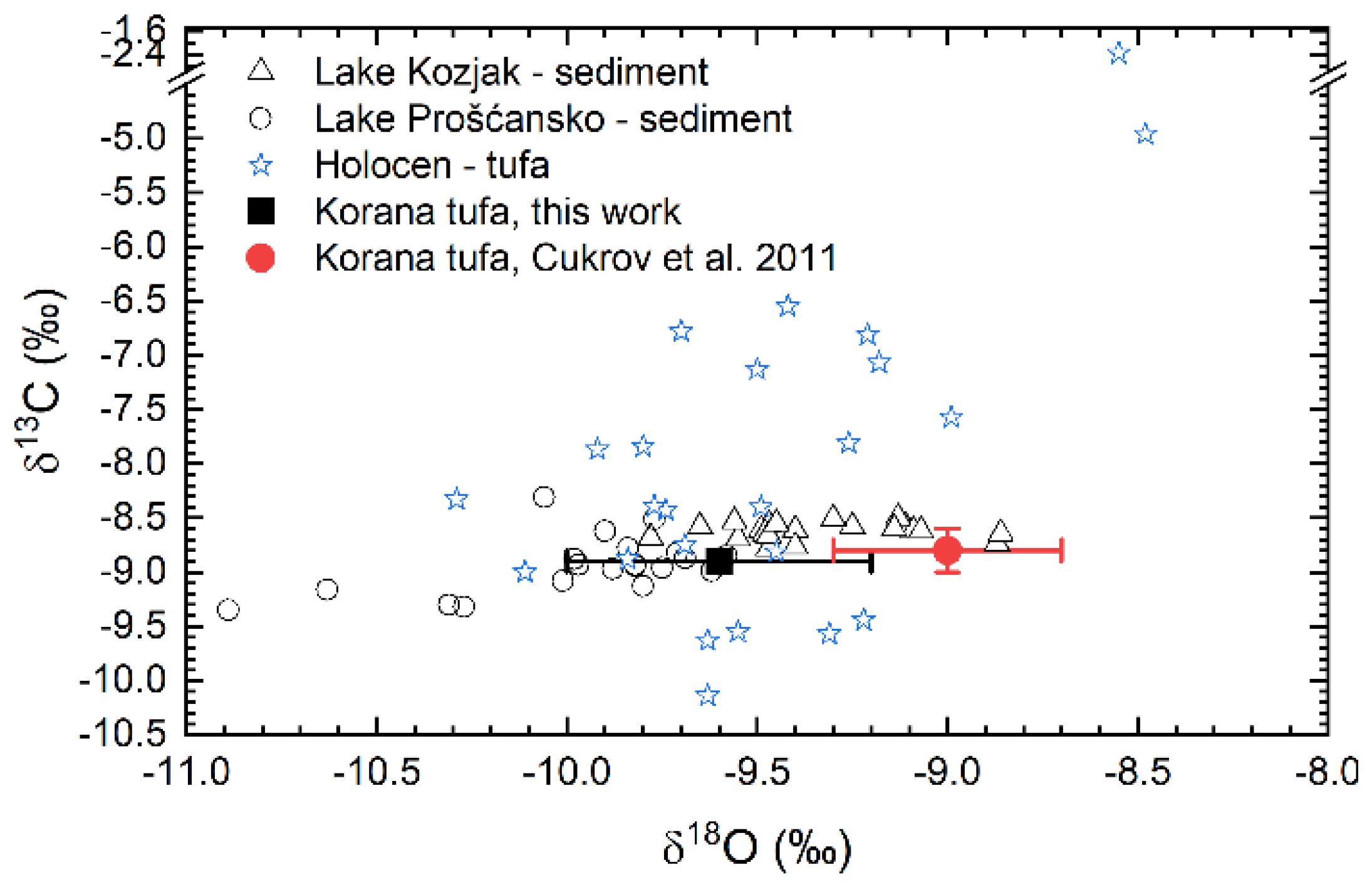
4.2. Variations of Element Concentrations and of Stable Isotope Ratios
4.2.1. Manganese Concentration as an Indicator for Decrease in Concentration of Dissolved Oxygen
4.2.2. Proxies for Water Temperature, Evaporation and Bioproductivity
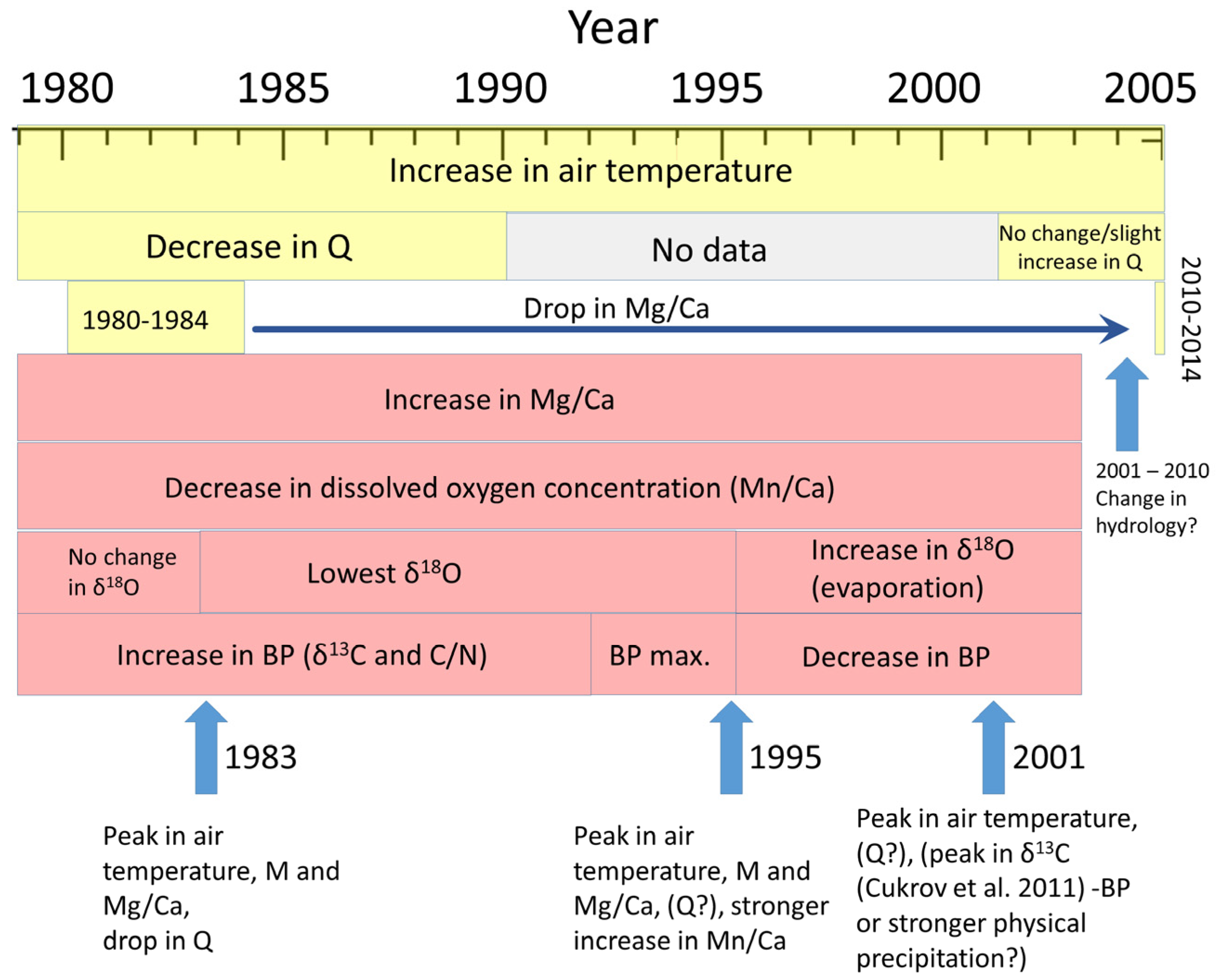
4.2.3. Synthesis of Chronological Changes through Analysis of Elemental and Stable Isotope Composition
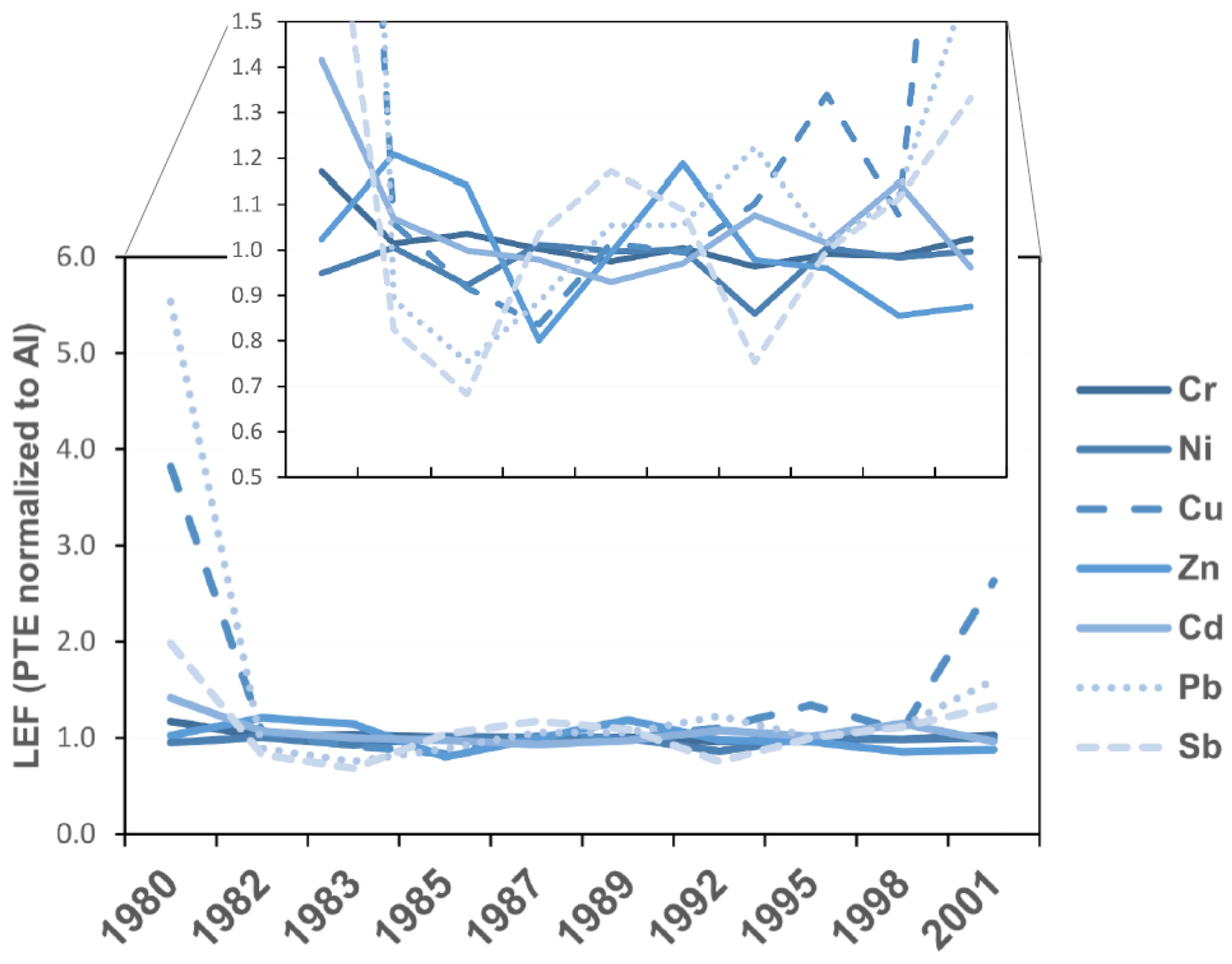
4.3. Determination of the Anthropogenic Influence
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Element Concentration (mg kg−1) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Label | Li | Rb | Cs | Mg/1000 | Ca/1000 | Sr | Ba | U | V | Cr | Mn | Fe/1000 | Co | Ni | Cu | Zn | Cd | Al/1000 | Pb | Sb |
| 5% | 8% | 5% | 6% | 5% | 9% | 10% | 7% | 5% | 5% | 4% | 3% | 5% | 7% | 7% | 6% | 7% | 7% | 6% | 7% | |
| 1 | 1.7 | 2.6 | 0.22 | 3.94 | 342 | 51.1 | 17.7 | 0.30 | 3.4 | 3.5 | 21.5 | 0.86 | 0.37 | 2.80 | 8.93 | 13.1 | 0.48 | 1.76 | 20.6 | 0.15 |
| 2 | 2.4 | 4.0 | 0.31 | 4.97 | 358 | 54.8 | 20.7 | 0.31 | 5.0 | 4.3 | 24.3 | 1.35 | 0.59 | 3.90 | 2.60 | 19.2 | 0.43 | 2.68 | 5.7 | 0.08 |
| 3 | 2.9 | 5.1 | 0.41 | 5.29 | 366 | 56.5 | 22.9 | 0.37 | 6.3 | 5.3 | 27.8 | 1.74 | 0.75 | 4.16 | 2.32 | 18.4 | 0.45 | 3.30 | 6.2 | 0.07 |
| 4 | 2.3 | 4.2 | 0.33 | 5.05 | 342 | 53.0 | 20.3 | 0.33 | 5.4 | 4.3 | 29.0 | 1.58 | 0.63 | 3.94 | 2.05 | 7.8 | 0.40 | 2.69 | 5.7 | 0.10 |
| 5 | 2.4 | 4.4 | 0.35 | 5.21 | 341 | 52.0 | 21.3 | 0.31 | 5.3 | 4.4 | 30.8 | 1.71 | 0.66 | 4.02 | 2.51 | 11.5 | 0.39 | 2.82 | 7.2 | 0.12 |
| 6 | 2.8 | 5.0 | 0.43 | 5.34 | 347 | 51.3 | 22.7 | 0.31 | 6.2 | 5.1 | 34.0 | 2.06 | 0.75 | 4.43 | 2.51 | 19.0 | 0.43 | 3.23 | 8.5 | 0.12 |
| 7 | 2.5 | 4.3 | 0.37 | 4.96 | 343 | 49.6 | 21.3 | 0.30 | 5.9 | 4.3 | 33.1 | 1.91 | 0.71 | 3.48 | 2.73 | 11.7 | 0.45 | 2.84 | 8.4 | 0.07 |
| 8 | 3.7 | 6.6 | 0.56 | 6.30 | 376 | 59.8 | 28.7 | 0.40 | 8.9 | 6.4 | 57.5 | 2.82 | 1.10 | 5.50 | 3.56 | 15.2 | 0.53 | 4.27 | 11.2 | 0.13 |
| 9 | 2.8 | 5.0 | 0.42 | 5.06 | 348 | 53.0 | 23.5 | 0.33 | 6.6 | 4.9 | 53.3 | 2.10 | 0.89 | 4.32 | 2.71 | 12.5 | 0.51 | 3.19 | 8.9 | 0.12 |
| 10 | 2.7 | 4.7 | 0.39 | 4.89 | 334 | 50.2 | 22.0 | 0.30 | 6.5 | 4.9 | 52.5 | 1.93 | 0.88 | 4.22 | 6.57 | 14.8 | 0.41 | 3.03 | 11.9 | 0.14 |
| Mean | 2.6 | 4.6 | 0.38 | 5.10 | 349 | 53.1 | 22.1 | 0.33 | 6.0 | 4.7 | 36.4 | 1.81 | 0.73 | 4.08 | 3.6 | 14.3 | 0.45 | 2.98 | 9.43 | 0.11 |
| Sample Label | δ13C | δ18O | C/Natomic |
|---|---|---|---|
| (‰) | (‰) | ||
| 1 | −8.90 | −9.49 | n.a. |
| 2 | −8.86 | −9.36 | n.a. |
| 3 | −8.80 | −9.76 | 14 |
| 4 | −8.75 | −9.99 | n.a. |
| 5 | −8.73 | −9.68 | 11 |
| 6 | −8.82 | −10.01 | n.a. |
| 7 | −8.85 | −9.90 | n.a. |
| 8 | −8.95 | −9.49 | 8 |
| 9 | −8.87 | −8.75 | n.a. |
| 10 | −8.97 | −9.12 | 10 |
| Mean | −8.85 | −9.56 | 11 |
| Sample Label | Significance of Correlation for | |||
|---|---|---|---|---|
| M/Ca vs. T | M/Al vs. T | M/Ca vs. Mg/Ca | M/Ca vs. Al/Ca | |
| Li | + | + | + | + |
| Rb | + | + | + | + |
| Cs | + | + | + | + |
| Mg | + | + | + | + |
| Sr | - | - | - | - |
| Ba | + | + | + | + |
| U | - | - | - | - |
| V | + | + | + | + |
| Cr | + | + | + | + |
| Mn | + | + | - | + |
| Fe | + | + | + | + |
| Co | + | + | + | + |
| Zn | - | - | - | - |
| Cd | - | - | - | - |
| Al | + | + | + | + |
| Pb | - | - | + | - |
| Element | R2 | Intercept | Slope |
|---|---|---|---|
| Cr | 0.96 | 0.56336 | 0.00139 |
| Ni | 0.92 | 1.19887 | 0.00100 |
| Cu | 0.70 | 2.11433 | 0.00013 |
| Zn | 0.49 | 2.12011 | 0.00322 |
| Cd | 0.65 | 0.21260 | 0.00007 |
| Pb | 0.63 | −1.41657 | 0.00292 |
| Sb | 0.50 | 0.03438 | 0.00002 |
References
- Gombert, P. Role of karstic dissolution in global carbon cycle. Glob. Planet. Chang. 2002, 33, 177–184. [Google Scholar] [CrossRef]
- Srdoč, D.; Horvatinčić, N.; Obelić, B.; Krajcar, I.; Sliepčević, A. Procesi taloženja kalcita u krškim vodama s posebnim osvrtom na Plitvička jezera (Calcite deposition processes in karst waters with special emphasis on the Plitvice Lakes, Yugoslavia). Carsus Iugosl. 1985, 11, 101–204. [Google Scholar]
- Bissett, A.; Reimer, A.; de Beer, D.; Shiraishi, F.; Arp, G. Metabolic Microenvironmental Control by Photosynthetic Biofilms under Changing Macroenvironmental Temperature and pH Conditions. Appl. Environ. Microbiol. 2008, 74, 6306–6312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Gradzinski, M. Factors controlling growth of modern tufa: Results of a field experiment. Geol. Soc. Lond. Spec. Publ. 2010, 336, 143–191. [Google Scholar] [CrossRef]
- Krajcar Bronić, I.; Barešić, J.; Sironić, A. Application of 14C method to chronology of the Croatian Dinaric karst—A case of the Plitvice Lakes. Radiocarbon 2022, 64, 805–817. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Srdoč, D.; Horvatinčić, N. Early Diagenesis of Plitvice Lakes Waterfall and Barrier Travertine Deposits. Géogr. Phys. Quat. 1994, 48, 247–255. [Google Scholar] [CrossRef] [Green Version]
- Sironić, A.; Barešić, J.; Horvatinčić, N.; Brozinčević, A.; Vurnek, M.; Kapelj, S. Changes in the geochemical parameters of karst lakes over the past three decades—The case of Plitvice Lakes, Croatia. Appl. Geochem. 2017, 78, 12–22. [Google Scholar] [CrossRef]
- Lojen, S.; Trkov, A.; Ščančar, J.; Vazquez-Navarro, J.A.; Cukrov, N. Continuous 60-year stable isotopic and earth-alkali element records in a modern laminated tufa (Jaruga, river Krka, Croatia): Implications for climate reconstruction. Chem. Geol. 2009, 258, 242–250. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Lawrence, R. Stable isotopic variability within modern travertines. Geogr. Phys. Quat. 1994, 48, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Pentecost, A. Travertine; Springer: Berlin/Heidelberg, Germany, 2005; p. 445. [Google Scholar]
- Pedley, M. Tufas and travertines of the Mediterranean region: A testing ground for freshwater carbonate concepts and developments. Sedimentology 2009, 56, 221–246. [Google Scholar] [CrossRef]
- Lorah, M.M.; Herman, J.S. The chemical evolution of a travertine-depositing stream: Geochemical processes and mass transfer reactions. Water Resour. Res. 1988, 24, 1541–1552. [Google Scholar] [CrossRef]
- Ihlenfeld, C.; Norman, M.D.; Gagan, M.K.; Drysdale, R.N.; Maas, R.; Webb, J. Climatic significance of seasonal trace element and stable isotope variations in a modern freshwater tufa. Geochim. Cosmochim. Acta 2003, 67, 2341–2357. [Google Scholar] [CrossRef]
- Cao, J.; Xue, H.; Sigg, L. Effects of pH and Ca competition on complexation of cadmium by fulvic acids and by natural organic ligands from a river and a lake. Aquat. Geochem. 2006, 12, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Iskrenova-Tchoukova, E.; Kalinichev, A.G.; Kirkpatrick, R.J. Metal Cation Complexation with Natural Organic Matter in Aqueous Solutions: Molecular Dynamics Simulations and Potentials of Mean Force. Langmuir 2010, 26, 5909–5919. [Google Scholar] [CrossRef] [Green Version]
- Sweere, T.; van den Boorn, S.; Dickson, A.J.; Reichart, G.-J. Definition of new trace-metal proxies for the controls on organic matter enrichment in marine sediments based on Mn, Co, Mo and Cd concentrations. Chem. Geol. 2016, 441, 235–245. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Fairchild, I.J.; Borsato, A.; Frisia, S.; Cassidy, N.J.; McDermott, F.; Hawkesworth, C.J. Seasonal variations in Sr, Mg and P in modern speleothems (Grotta di Ernesto, Italy). Chem. Geol. 2001, 175, 429–448. [Google Scholar] [CrossRef]
- Wong, C.I.; Banner, J.L.; Musgrove, M. Seasonal dripwater Mg/Ca and Sr/Ca variations driven by cave ventilation: Implications for modelling of speleothem paleoclimate records. Geochim. Cosmochim. Acta 2011, 75, 3514–3529. [Google Scholar] [CrossRef]
- Bougeois, L.; de Rafélis, M.; Reichart, G.-J.; de Nooijer, L.J.; Dupont-Nivet, G. Mg/Ca in fossil oyster shells as palaeotemperature proxy, an example from the Palaeogene of Central Asia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 441, 611–626. [Google Scholar] [CrossRef] [Green Version]
- Lowenstein, T.K.; Hönisch, B. The Use of Mg/Ca as a Seawater Temperature Proxy. The Paleontological Society Papers; Reconstructing Earth’s Deep-Time Climate; Cambridge University Press: Cambridge, UK, 2012; Volume 18, pp. 85–100. [Google Scholar] [CrossRef]
- Drysdale, R.; Couchoud, I.; Zanchetta, G.; Isola, I.; Ragattieri, E.; Hellstrom, J.; Govin, A.; Tzedakis, P.C.; Ireland, T.; Corric, E.; et al. Magnesium in subaqueous speleothems as a potential palaeotemperature proxy. Nat. Commun. 2020, 11, 5027. [Google Scholar] [CrossRef]
- McCrea, J.M. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Leng, M.J.; Marshall, J.D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quarter. Sci. Rev. 2004, 23, 811–831. [Google Scholar] [CrossRef] [Green Version]
- Zavadlav, S.; Rožič, B.; Dolenec, M.; Lojen, S. Stable isotopic and elemental characteristics of recent tufa from a karstic Krka River (south-east Slovenia): Useful environmental proxies? Sedimentology 2016, 64, 808–831. [Google Scholar] [CrossRef]
- Weber, J.N. Incorporation of strontium into reef coral skeletal carbonate. Geochim. Cosmochim. Acta 1973, 37, 2173–2190. [Google Scholar] [CrossRef]
- Smith, S.V.; Buddemeier, R.W.; Redalje, R.C.; Houck, J.E. Strontium-calcium thermometry in coral skeletons. Science 1979, 204, 404–407. [Google Scholar] [CrossRef]
- Beck, J.W.; Edwards, R.L.; Ito, E.; Taylor, F.W.; Recy, J.; Rougerie, F.; Joannot, P.; Henin, C. Sea-surface temperature from coral skeletal Strontium/Calcium ratios. Science 1992, 257, 644–647. [Google Scholar] [CrossRef]
- Corrège, T. Sea surface temperature and salinity reconstruction from coral geochemical tracers. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 408–428. [Google Scholar] [CrossRef]
- Kuffner, I.B.; Roberts, K.E.; Flannery, J.A.; Morrison, J.M.; Richey, J.N. Fidelity of the Sr/Ca proxy in recording ocean temperature in the western Atlantic coral Siderastrea Sidereal. Geochem. Geophys. Geosyst. 2017, 18, 178–188. [Google Scholar] [CrossRef]
- Saunders, P. Can Tufa Mg/Ca Ratios be Used as a Palaeoclimate Proxy? Ph.D. Thesis, University of Hull, Hull, UK, 2012; p. 220. [Google Scholar]
- Saunders, P.; Rogerson, M.; Wadhawan, J.D.; Greenway, G.; Pedley, H.M. Mg/Ca ratios in freshwater microbial carbonates: Thermodynamic, kinetic and vital effects. Geochim. Cosmochim. Acta 2014, 147, 107–118. [Google Scholar] [CrossRef]
- Terri, J.A.; Stowe, L.G. Climatic patterns and distribution of C4 grasses in North America. Oecologia 1976, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dorale, J.A.; Edwards, R.L.; Ito, E.; González, L.A. Climate and Vegetation History of the Midcontinent from 75 to 25 ka: A Speleothem Record from Crevie Cave, Missouri, USA. Science 1998, 282, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Horvatinčić, N.; Krajcar Bronić, I.; Obelić, B. Differences in the 14C age, δ13C and δ18O of Holocene tufa and speleothem in the dinaric karst. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 193, 139–157. [Google Scholar] [CrossRef]
- Horvatinčić, N.; Barešić, J.; Babinka, S.; Obelić, B.; Krajcar Bronić, I.; Vreča, P.; Suckow, A. Towards a deeper understanding how carbonate isotopes (14C, 13C, 18O) reflect environmental changes: A study with recent 210Pb-dated sediments of the Plitvice Lakes, Croatia. Radiocarbon 2008, 50, 233–253. [Google Scholar] [CrossRef] [Green Version]
- Baldini, J.U.L.; McDermott, F.; Hoffmann, D.L.; Richards, D.A.; Clipson, N. Very high-frequency and seasonal cave atmosphere P-CO2 variability: Implications for stalagmite growth and oxygen isotope-based paleoclimate records. Earth Plan. Sci. Lett. 2008, 272, 118–129. [Google Scholar] [CrossRef]
- Dorale, J.A.; Wozniak, L.A.; Bettis, E.A.; Carpenter, S.J.; Mandel, R.D.; Hajic, E.R.; Lopinot, N.H.; Ray, J.H. Isotopic evidence for Younger Dryas aridity in the North American midcontinent. Geology 2010, 38, 519–522. [Google Scholar] [CrossRef]
- Horvatinčić, N.; Sironić, A.; Barešić, J.; Sondi, I.; Krajcar Bronić, I.; Borković, D. Mineralogical, organic and isotopic composition as palaeoenvironmental records in the lake sediments of two lakes, the Plitvice Lakes, Croatia. Quarter. Internat. 2018, 494, 300–313. [Google Scholar] [CrossRef] [Green Version]
- Herceg Romanić, S.; Kljaković-Gašpić, Z.; Bituh, T.; Žužul, S.; Dvoršćak, M.; Fingler, S.; Jurasović, J.; Klinčić, D.; Marović, G.; Orct, T.; et al. The impact of multiple anthropogenic contaminants on the terrestrial environment of the Plitvice Lakes National Park, Croatia. Environ. Monit. Assess. 2016, 188, 27. [Google Scholar] [CrossRef]
- Kljaković-Gašpić, Z.; Herceg Romanić, S.; Bituh, T.; Kašuba, V.; Brčić Karačonji, I.; Brajenović, N.; Franulović, I.; Jurasović, J.; Klinčić, D.; Kopjar, N.; et al. Assessment of multiple anthropogenic contaminants and their potential genotoxicity in the aquatic environment of Plitvice Lakes National Park, Croatia. Environ. Monit. Assess. 2018, 190, 694. [Google Scholar] [CrossRef]
- Dautović, J.; Fiket, Ž.; Barešić, J.; Ahel, M.; Mikac, N. Sources, Distribution and Behavior of Major and Trace Elements in a Complex Karst Lake System. Aquat. Geochem. 2014, 20, 19–38. [Google Scholar] [CrossRef]
- Vukosav, P.; Mlakar, M.; Cukrov, N.; Kwokal, Ž.; Pižeta, I.; Pavlus, N.; Špoljarić, I.; Vurnek, M.; Brozinčević, A.; Omanović, D. Heavy metal contents in water, sediment and fish in a karst aquatic ecosystem of the Plitvice Lakes National Park (Croatia). Environ. Sci. Pollut. Res. 2014, 21, 3826–3839. [Google Scholar] [CrossRef]
- Mikac, I.; Fiket, Ž.; Terzić, S.; Barešić, J.; Mikac, N.; Ahel, M. Chemical indicators of anthropogenic impacts in sediments of the pristine karst lakes. Chemopshere 2011, 84, 1140–1149. [Google Scholar] [CrossRef]
- Bačić, N.; Mikac, N.; Lučić, M.; Sondi, I. Occurrence and distribution of technology-critical elements in recent freshwater and marine pristine lake sediments in Croatia: A case study. Arch. Environ. Contam. Toxicol. 2021, 81, 574–588. [Google Scholar] [CrossRef]
- Plitvički vjesnik, 1979, 53, p. 8. (Journal, in Croatian).
- Felja, I.; Krajcar Bronić, I.; Horvatinčić, N. A response of 14C activity in recent tufa from the Plitvice Lakes to changes of atmospheric CO2. In Abstract Book: European Society for the Isotope Research, X Isotope Workshop, Zlotniki Lubanskie, Poland, 22–26 June 2009; European Society for the Isotope Research and Laboratory of Isotope Geology and Geoecology, Institute of Geological Sciences, University of Wroclaw: Wroclaw, Poland, 2009; pp. 89–90. [Google Scholar]
- CMHS. Croatian Meteorological and Hydrological Service. 2020. Available online: https://meteo.hr/index_en.php (accessed on 25 August 2020).
- Polšak, A. Geološki aspekti zaštite Plitivičkih jezera. In Čovjek i Priroda; Nacionalni Park Plitvička Jezera: Zagreb, Croatia, 1974; pp. 23–32. [Google Scholar]
- Butko, I.; Felja, I.; Juračić, M. Geology of the Plitvice Lakes. In Croatia: STRAVAL Case Study Plitvice Lakes National Park; IUCN: Girona, Spain, 2011; pp. 25–27. [Google Scholar]
- Horvatinčić, N.; Briansó, J.; Obelić, B.; Barešić, J.; Krajcar Bronić, I. Study of Pollution of the Plitvice Lakes by Water and Sediment Analyses. Water Air Soil Poll. Focus 2006, 6, 475–485. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Chryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, F.T.; Bischoff, W.D.; Bishop, F.C.; Loijens, M.; Schoonmaker, J.; Wollast, R. Magnesian calcites: Low-temperature occurrence, solubility and solid-solution behavior. In Carbonates: Mineralogy and Chemistry; Reeder, R.J., Ed.; Mineralogical Society of America, Reviews in Mineralogy: Washington, DC, USA, 1983; Volume 11, pp. 97–144. [Google Scholar]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Mass fractions of forty-six major and trace elements, including rare earth elements, in sediment and soil reference materials used in environmental studies. Geostand. Geoanalyt. Res. 2017, 41, 123–135. [Google Scholar] [CrossRef]
- Mandić, M. Determination of Equilibrium Conditions of Carbonate Precipitation in Postojna Cave with Possible Application to Paleoclimatology. Ph.D. Thesis, University of Zagreb, Faculty of Science, Zagreb, Croatia, 2013; p. 204. [Google Scholar]
- Pawlowsky-Glahn, V.; Egozcue, J.J.; Tolosana-Delgado, R. Modeling and Analysis of Compositional Data; John Wiley & Sons: London, UK, 2015. [Google Scholar]
- Kynčlová, P.; Hron, K.; Filzmoser, P. Correlation between compositional parts based on symmetric balances. Math. Geosci. 2017, 49, 777–796. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.R-project.org/ (accessed on 15 February 2020).
- Matys Grygar, T.; Popelka, J. Revisiting geochemical methods of distinguishing natural concentrations and pollution by risk elements in fluvial sediments. J. Geochem. Explor. 2016, 170, 39–57. [Google Scholar] [CrossRef]
- Kelly, S.D.; Newville, M.G.; Cheng, L.; Kemner, K.M.; Sutton, S.R.; Fenter, P.; Sturchio, N.C.; Spötl, C. Uranyl Incorporation in Natural Calcite. Environmen. Sci. Tech. 2003, 37, 1284–1287. [Google Scholar] [CrossRef]
- Pavlović, G.; Prohić, E.; Miko, S.; Tibljaš, D. Geochemical and petrographic evidence of meteoric diagenesis in tufa deposits in Northern Dalmatia (Zrmanja and Krupa rivers, Croatia). Facies 2002, 46, 27–34. [Google Scholar] [CrossRef]
- Claes, H.; Huysmans, M.; Soete, J.; Dirix, K.; Vassilieva, E.; Erthal, M.M.; Vandewijngaerde, W.; Hamaekers, H.; Aratman, C.; Özkul, M.; et al. Elemental geochemistry to complement stable isotope data of fossil travertine: Importance of digestion method and statistics. Sediment. Geol. 2019, 386, 118–131. [Google Scholar] [CrossRef]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the Elements in Some Major Units of the Earth’s Crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Pósfai, M.; Nyirő-Kósa, I.; Rostási, Á.; Bereczk-Tompa, É.; Cora, I.; Koblar, M.; Kovács, A. Mg-calcite formation in a freshwater environment (Lake Balaton): Nucleation, growth, structure and composition. Eur. Microsc. Congr. Proc. 2016, 1176–1177. [Google Scholar]
- Stoffers, P. Recent carbonate sedimentation in the lakes of Plitvice (Yug.). Neues Jahrb. Min. Mh. 1975, 9, 412–418. [Google Scholar]
- Teboul, P.A.; Durlet, C.; Gaucher, E.C.; Virgone, A.; Girard, J.P.; Curie, J.; Lopez, B.; Camoin, G.F. Origins of elements building travertine and tufa: New perspectives provided by isotopic and geochemical tracers. Sediment. Geol. 2016, 334, 97–114. [Google Scholar] [CrossRef]
- Cukrov, N.; Kwokal, Ž.; Lojen, S.; Mikac, N. Raspodjela metala i stabilnih izotopa O i C u laminarnoj sedri s Plitvičkih jezera (Distributions of metals and stable isotope (O & C) ratios in laminar tufa from the Plitvice lakes). In Proceedings, Plitvička Jezera; Šutić, B., Ed.; Hrvatska znanstvena bibliografija: Zagreb, Croatia, 2011; pp. 288–294. ISBN 978-953-96146-4-3. (In Croatian) [Google Scholar]
- Barešić, J.; Horvatinčić, N.; Roller-Lutz, Z. Spatial and seasonal variations in the stable C isotope composition of dissolved inorganic carbon and physico-chemical water parameters in the Plitvice Lakes system. Isot. Environ. Health Stud. 2011, 47, 316–329. [Google Scholar] [CrossRef]
- Srdoč, D.; Krajcar Bronić, I.; Horvatinčić, N.; Obelić, B. Increase of dissolved inorganic carbon along a river course. Radiocarbon 1986, 28, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Sironić, A.; Krajcar Bronić, I.; Horvatinčić, N.; Barešić, J.; Borković, D.; Vurnek, M.; Lovrenčić Mikelić, I. Carbon isotopes in dissolved inorganic carbon as tracers of carbon sources in karst waters of the Plitvice Lakes, Croatia. In Stable Isotope Studies of the Water Cycle and Terrestrial Environments; Bojar, A.V., Pelc, A., Lecuyer, C., Eds.; Geological Society of London Special Publications 507; Geological Society: London, UK, 2020. [Google Scholar] [CrossRef]
- Krajcar Bronić, I.; Barešić, J.; Sironić, A.; Lovrenčić Mikelić, I.; Borković, D.; Horvatinčić, N.; Kovač, Z. Isotope Composition of Precipitation, Groundwater, and Surface and Lake Waters from the Plitvice Lakes, Croatia. Water 2020, 12, 2414. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of Earth’s upper crust, natural cycles of elements, natural resources. In Elements and Their Compounds in the Environment; Merien, E., Anke, M., Inhat, M., Stoeppler, M., Eds.; Wiley-VHC Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2004; Volume 1, pp. 3–16. [Google Scholar]
- Calvert, S.E.; Pedersen, T.F. Sedimentary geochemistry of manganese: Implications for the environment of formation of manganiferous black shales. Econ. Geol. 1996, 91, 36–47. [Google Scholar] [CrossRef]
- Koretsky, C.M.; Haas, J.R.; Miller, D.; Ndenga, N.T. Seasonal variations in pore water and sediment geochemistry of littoral lake sediments (Asylum Lake, MI, USA). Geochem. Trans. 2006, 7, 11. [Google Scholar] [CrossRef] [Green Version]
- Barešić, J. Application of Isotopic and Geochemical Methods in Monitoring of Global and Local Changes in Ecological System of Plitvice Lakes. Ph.D. Thesis, University of Zagreb, Zagreb, Croatia, 2009; p. 163. [Google Scholar]
- Cruse, A.M.; Lyons, T.W. Trace metal records of regional paleoenvironmental variability in Pennsylvanian (Upper Carboniferous) black shales. Chem. Geol. 2004, 206, 319–345. [Google Scholar] [CrossRef]
- Herndon, E.M.; Havig, J.R.; Singer, D.M.; McCormick, M.L.; Kump, L.R. Manganese and iron geochemistry in sediments underlying the redox-stratified Fayetteville Green Lake. Geochim. Cosmochim. Acta 2018, 231, 50–63. [Google Scholar] [CrossRef]
- Horton, T.W.; Defliese, W.F.; Tripati, A.K.; Oze, C. Evaporation induced 18O and 13C enrichment in lake systems: A global perspective on hydrologic balance effects. Quater. Sci. Rev. 2016, 131, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Talbot, M.R. A review of the palaeohydrological interpretation of carbon and oxygen isotopic ratios in primary lacustrine carbonates. Chem. Geol. Isot. Geosci. Sect. 1990, 80, 261–279. [Google Scholar] [CrossRef]
- Radišić, M.; Rubinić, J.; Ružić, I.; Brozinčević, A. Hydrological System of the Plitvice Lakes—Trends and Changes in Water Levels, Inflows, and Losses. Hydrology 2021, 8, 174. [Google Scholar] [CrossRef]
- Seidell, A. Solubilities of Inorganic and Metal Organic Compounds, 3rd ed.; D. Van Nostrand Company: New York, NY, USA, 1940; Volume 1. [Google Scholar]
- Rambeau, C.M.C.; Baize, D.; Saby, N.P.A.; Matera, V.; Adatte, T.; Foellmi, K.B. High Cadmium concentrations in Jurassic limestone as the cause for elevated cadmium levels in deriving soils: A case study in Lower Burgundy, France. Environ. Earth. Sci. 2010, 61, 1573–1585. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Chen, J.; Cai, H.; Wei, J.; Yuan, S. Coprecipitation of metal ions into calcite: An estimation of partition coefficients based on field investigation. Acta Geochim 2021, 40, 67–77. [Google Scholar] [CrossRef]
- Kovačević, T. (Ed.) Plitvice Lakes National Park Management Plan; Plitvice Lakes National Park Public Institution: Plitvice, Croatia, 2018; pp. 1–356. [Google Scholar]
- Chen, J.B.; Gaillardet, J.; Bouchez, J.; Louvat, P.; Wang, Y.N. Anthropophile elements in river sediments: Overview from the Seine River, France. Geochem. Geophys. Geosyst. 2014, 15, 4526–4546. [Google Scholar] [CrossRef] [Green Version]
- Czarnecki, S.; Düring, R.A. Influence of long-term mineral fertilization on metal contents and properties of soil samples taken from different locations in Hesse, Germany. Soil 2015, 1, 23–33. [Google Scholar] [CrossRef] [Green Version]




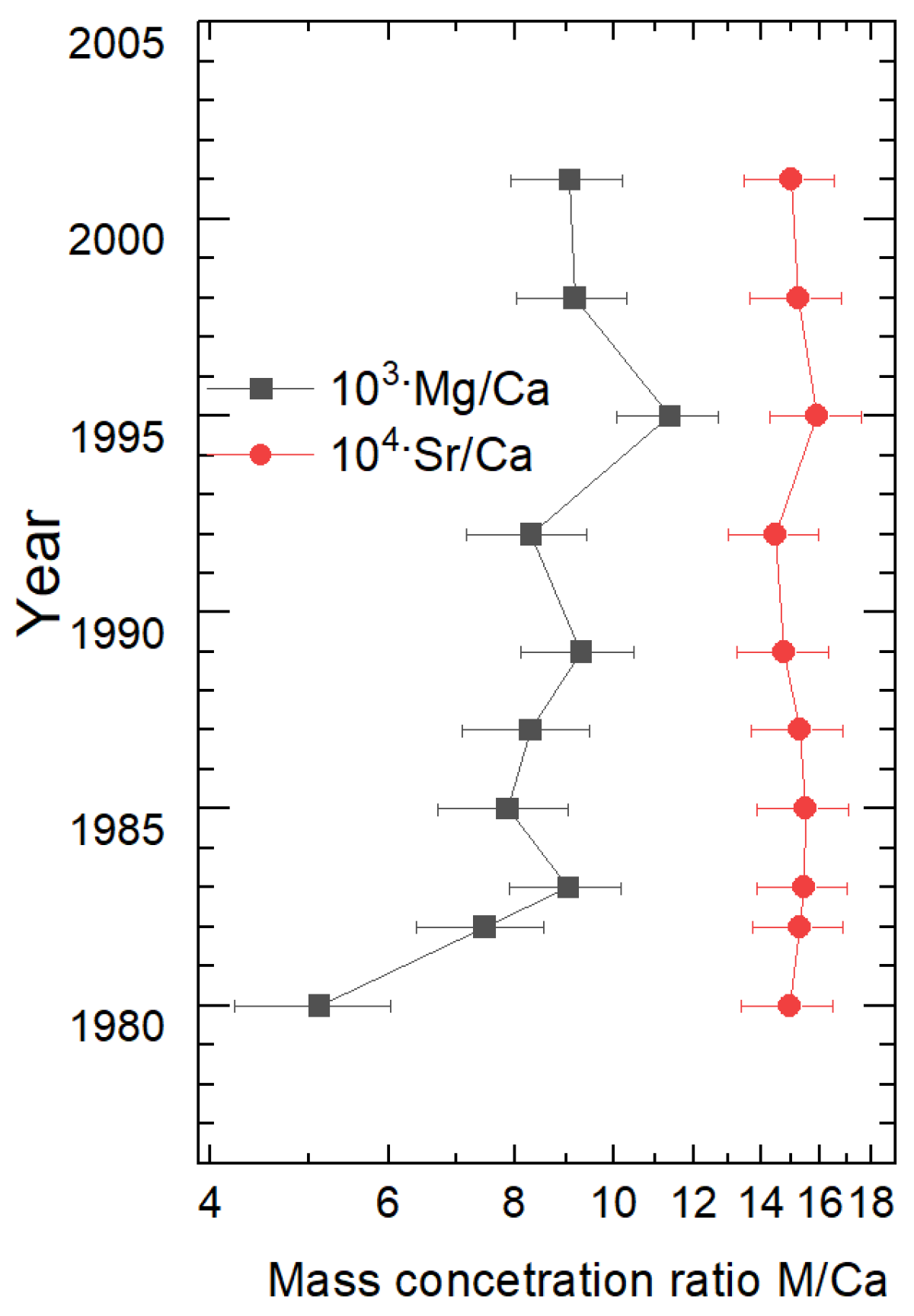
| Sample Label | Distance from the Beginning of the Growth (cm) | Assigned Year |
|---|---|---|
| 1 | 0–1.5 | 1980 ± 1 |
| 2 | 1.5–2.8 | 1982 ± 1 |
| 3 | 2.8–4.0 | 1983 ± 1 |
| 4 | 4.0–5.3 | 1985 ± 1 |
| 5 | 5.3–6.8 | 1987 ± 1 |
| 6 | 6.8–9.3 | 1989 ± 1.5 |
| 7 | 9.3–11.5 | 1992 ± 1.5 |
| 8 | 11.5–13.7 | 1995 ± 1.5 |
| 9 | 13.7–16.0 | 1998 ± 1.5 |
| 10 | 16.0–19.0 | 2001 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sironić, A.; Lučić, M.; Felja, I.; Tibljaš, D. Environmental Changes Recorded in Tufa from the Korana River, Croatia: Geochemical and Isotopic Approach. Water 2023, 15, 1269. https://doi.org/10.3390/w15071269
Sironić A, Lučić M, Felja I, Tibljaš D. Environmental Changes Recorded in Tufa from the Korana River, Croatia: Geochemical and Isotopic Approach. Water. 2023; 15(7):1269. https://doi.org/10.3390/w15071269
Chicago/Turabian StyleSironić, Andreja, Mavro Lučić, Igor Felja, and Darko Tibljaš. 2023. "Environmental Changes Recorded in Tufa from the Korana River, Croatia: Geochemical and Isotopic Approach" Water 15, no. 7: 1269. https://doi.org/10.3390/w15071269
APA StyleSironić, A., Lučić, M., Felja, I., & Tibljaš, D. (2023). Environmental Changes Recorded in Tufa from the Korana River, Croatia: Geochemical and Isotopic Approach. Water, 15(7), 1269. https://doi.org/10.3390/w15071269







