Optimal Dietary Protein/Energy Ratio and Phosphorus Level on Water Quality and Output for a Hybrid Grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) Recirculating Aquaculture System
Abstract
1. Introduction
2. Materials and Methods
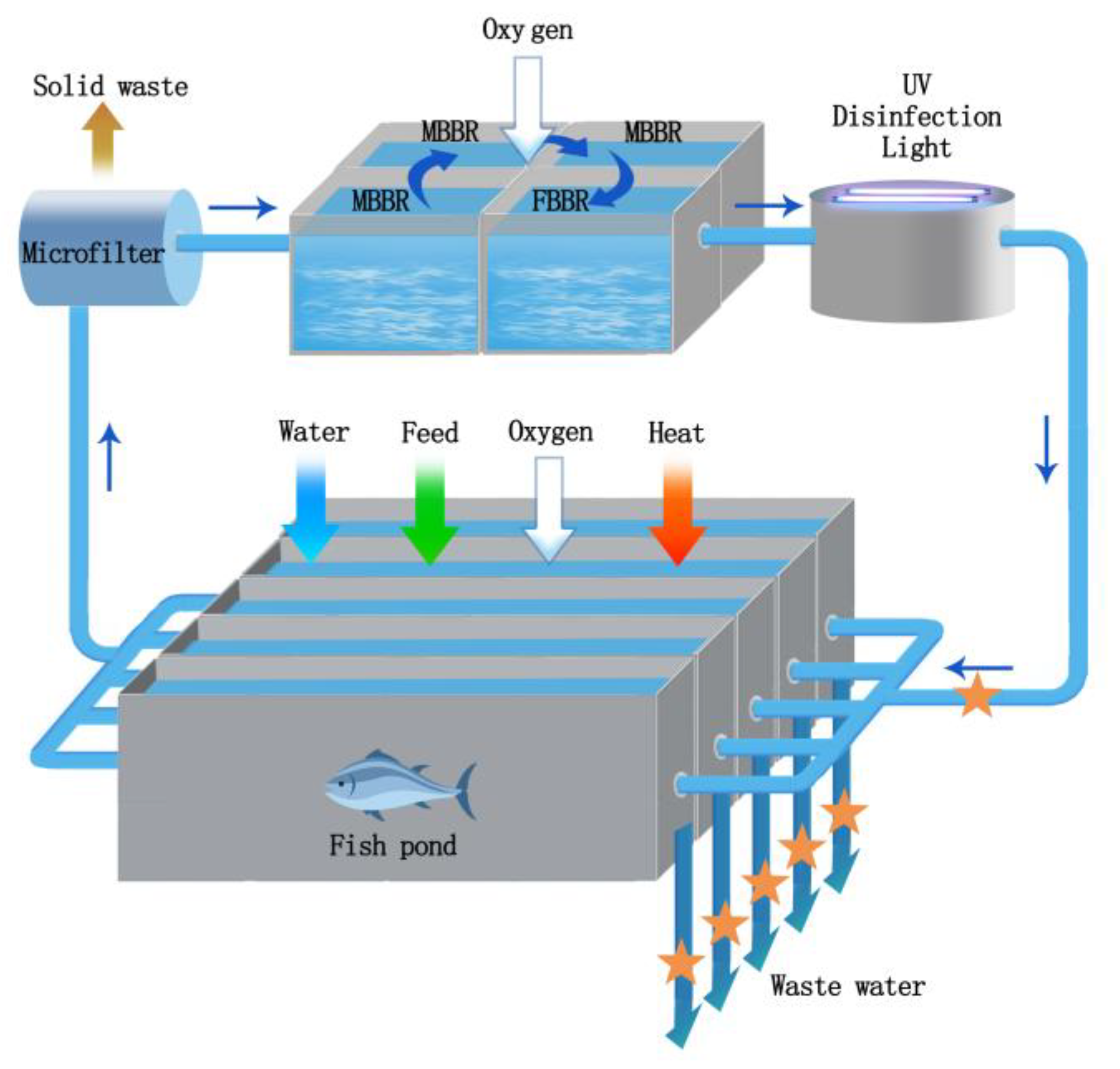
2.1. Recirculating Aquaculture System
2.2. Experimental Subject
2.3. Experimental Feeds
2.4. Experimental Design and Sample Analysis
2.5. Calculations and Statistics
2.5.1. Calculation of Growth Efficiency
2.5.2. Statistical Methods
3. Results
3.1. Growth Efficiency
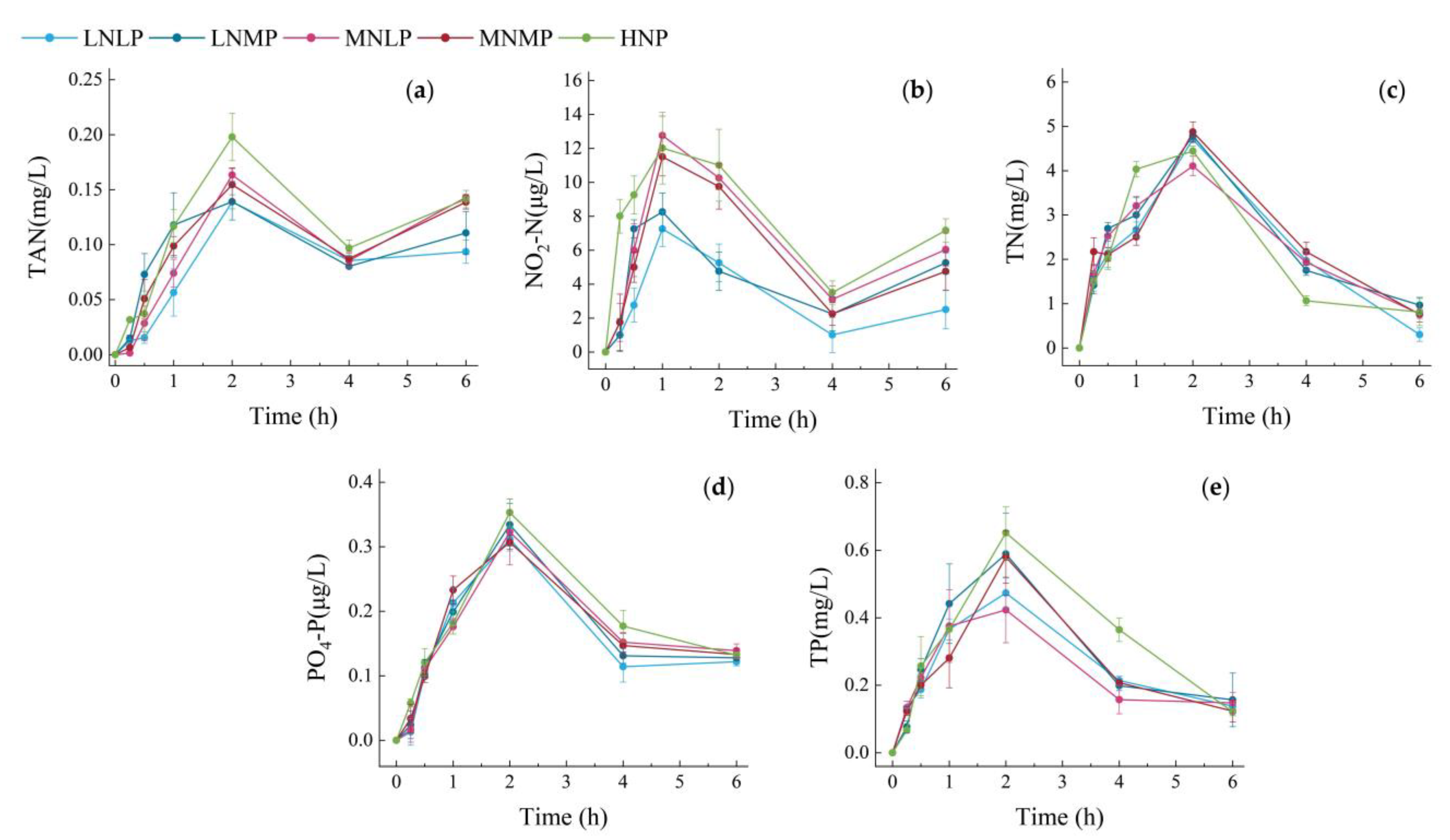
3.2. Changes in Water Nutrient Pollutants over Time within 6 h after Feeding
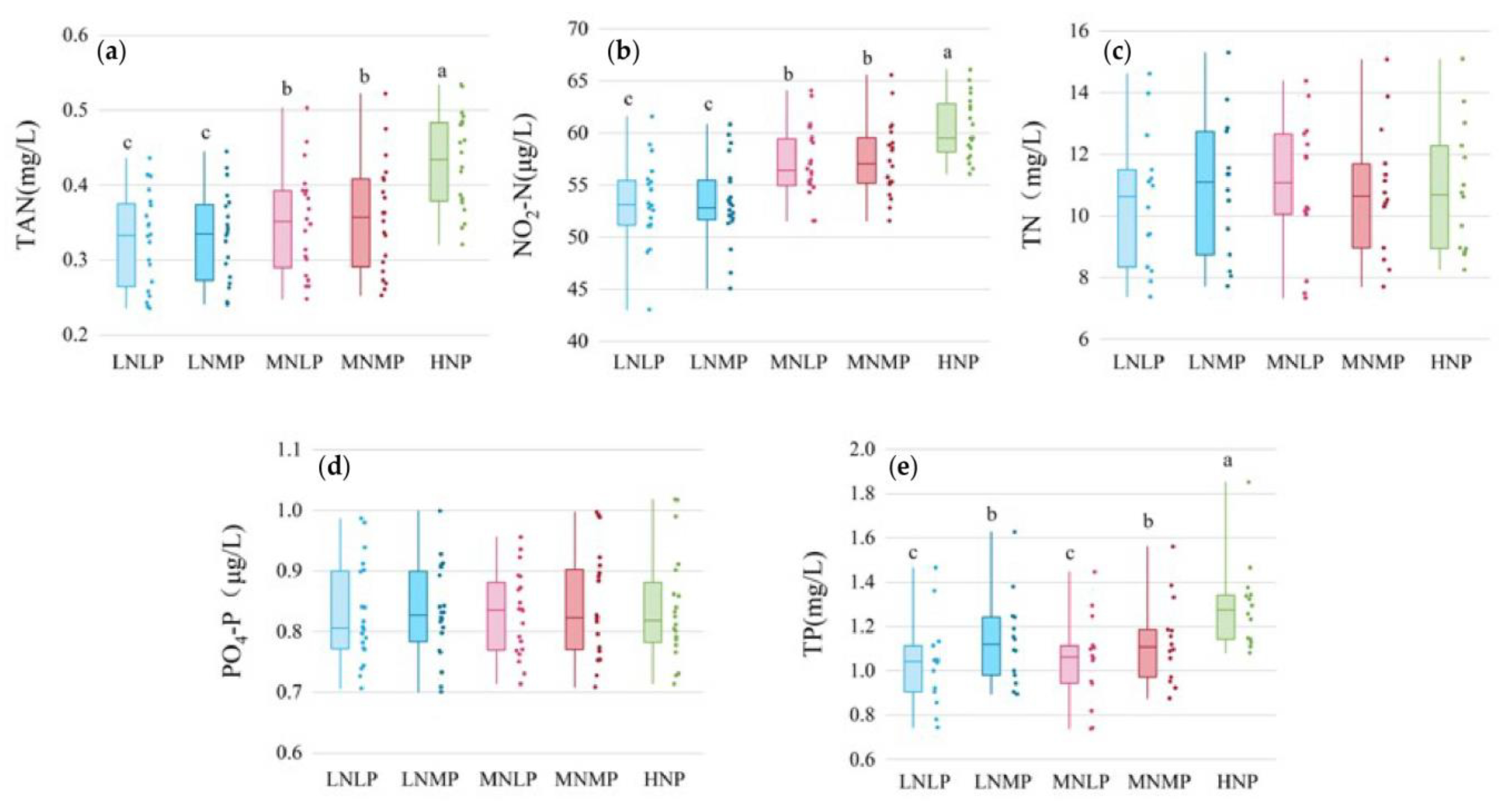
3.3. Concentrations of Nutrient Pollutants in Water during the Entire Experimental Period
4. Discussion
4.1. Growth Performance and Feed Utilization
4.2. The Change of Water Quality after Feeding
4.3. Effects of Diets with Different P/E Ratios and Phosphorus Levels on RAS Water Quality
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Rosenthal, H.; Castell, J.D.; Chiba, K.; Forster, J.R.M.; Hilge, V.; Hogendoorn, H.; Wickins, J. Flow-Through and Recirculation Systems; EIFAC; Food & Agriculture Org.: Rome, Italy, 1986; Volume 100. [Google Scholar]
- Saeki, A. Studies on fish culture in the aquarium of closed-circulating system. Its fundamental theory and standard plan. Bull. Jpn. Soc. Sci. Fish. 1958, 23, 684–695. [Google Scholar] [CrossRef]
- Liu, W.; Xu, B.; Tan, H.; Zhu, S.; Luo, G.; Shitu, A.; Wan, Y. Investigating the conversion from nitrifying to denitrifying water-treatment efficiencies of the biofloc biofilter in a recirculating aquaculture system. Aquaculture 2022, 550, 737817. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Gao, P.; Yu, D.; Yao, Z.; Gao, M.; Zhao, Y.; Jin, C.; She, Z. Thermophilic bacteria combined with alkyl polyglucose pretreated mariculture solid wastes using as denitrification carbon source for marine recirculating aquaculture wastewater treatment. Sci. Total Environ. 2021, 792, 148447. [Google Scholar] [CrossRef]
- Rubio-Rincon, F.J.; Lopez-Vazquez, C.M.; Welles, L.; van Loosdrecht, M.C.M.; Brdjanovic, D. Cooperation between Candidatus Competibacter and Candidatus Accumulibacter clade I, in denitrification and phosphate removal processes. Water Res. 2017, 120, 156–164. [Google Scholar] [CrossRef]
- Burut-Archanai, S.; Eaton-Rye, J.J.; Incharoensakdi, A.; Powtongsook, S. Phosphorus removal in a closed recirculating aquaculture system using the cyanobacterium Synechocystis sp. PCC 6803 strain lacking the SphU regulator of the Pho regulon. Biochem. Eng. J. 2013, 74, 69–75. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 739399. [Google Scholar] [CrossRef]
- Luo, J.; Sun, Z.; Lu, L.; Xiong, Z.; Cui, L.; Mao, Z. Rapid expansion of coastal aquaculture ponds in Southeast Asia: Patterns, drivers and impacts. J. Environ. Manag. 2022, 315, 115100. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs. Several Opinions on Accelerating the Green Development of Aquaculture Industry. Fish. China 2019, 6, 12–13. [Google Scholar]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakarn, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S. Improving Productivity and Environmental Performance of Aquaculture; World Resources Institute: Washington, DC, USA, 2014. [Google Scholar]
- Fudge, M.; Higgins, V.; Vince, J.; Rajaguru, R. Social acceptability and the development of commercial RAS aquaculture. Aquaculture 2023, 568, 739295. [Google Scholar] [CrossRef]
- Kankainen, M.; Setälä, J.; Berrill, I.K.; Ruohonen, K.; Noble, C.; Schneider, O. How to measure the economic impacts of changes in growth, feed efficiency and survival in aquaculture. Aquac. Econ. Manag. 2012, 16, 341–364. [Google Scholar] [CrossRef]
- Gutierrez-Wing, M.T.; Malone, R.F. Biological filters in aquaculture: Trends and research directions for freshwater and marine applications. Aquac. Eng. 2006, 34, 163–171. [Google Scholar] [CrossRef]
- Yang, L.; Chou, L.S.; Shieh, W.K. Biofilter treatment of aquaculture water for reuse applications. Water Res. 2001, 35, 3097–3108. [Google Scholar] [CrossRef]
- Costigan, E.M.; Oehler, M.A.; MacRae, J.D. Phosphorus recovery from recirculating aquaculture systems: Adsorption kinetics and mechanism. J. Water Process Eng. 2022, 49, 102992. [Google Scholar] [CrossRef]
- Mortula, M.M.; Gagnon, G.A. Alum residuals as a low technology for phosphorus removal from aquaculture processing water. Aquac. Eng. 2007, 36, 233–238. [Google Scholar] [CrossRef]
- Jin, J.; Chu, Z.; Ruan, R.; Liu, W.; Chen, X.; Li, C. Phosphorus Absorption and Excretion in Hybrid Sturgeon (Huso dauricus ♀ × Acipenser schrenckii ♂) Intubated with Different Ca/P Ratios. Fishes 2022, 7, 138. [Google Scholar] [CrossRef]
- Tórz, A.; Burda, M.; Półgęsek, M.; Sadowski, J.; Nędzarek, A. Transformation of phosphorus in an experimental integrated multitrophic aquaculture system using the media filled beds method in plant cultivation. Aquac. Environ. Interact. 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Kong, W.; Huang, S.; Yang, Z.; Shi, F.; Feng, Y.; Khatoon, Z. Fish feed quality is a key factor in impacting aquaculture water environment: Evidence from incubator experiments. Sci. Rep. 2020, 10, 187. [Google Scholar] [CrossRef]
- Green, J.A.; Hardy, R.W. The effects of dietary protein: Energy ratio and amino acid pattern on nitrogen utilization and excretion of rainbow trout Oncorhynchus mykiss (Walbaum). J. Fish Biol. 2008, 73, 663–682. [Google Scholar] [CrossRef]
- Kaushik, S.J. Nutritional strategies for the reduction of aquaculture wastes. In FOID’ 94, Proceedings of the Third International Conference on Fisheries and Ocean Industrial Development for Productivity Enhancement of the Coastal Waters; National Fisheries University of Pusan: Pusan, Korea, 3-4 June 1994; pp. 115–132. [Google Scholar]
- Cho, C.Y.; Bureau, D.P. A review of diet formulation strategies and feeding systems to reduce excretory and feed wastes in aquaculture. Aquac. Res. 2001, 32, 349–360. [Google Scholar] [CrossRef]
- Basto-Silva, C.; Enes, P.; Oliva-Teles, A.; Capilla, E.; Guerreiro, I. Dietary protein/carbohydrate ratio and feeding frequency affect feed utilization, intermediary metabolism, and economic efficiency of gilthead seabream (Sparus aurata) juveniles. Aquaculture 2022, 554, 738182. [Google Scholar] [CrossRef]
- Thirunavukkarasar, R.; Kumar, P.; Sardar, P.; Sahu, N.P.; Harikrishna, V.; Singha, K.P.; Shamna, N.; Jacob, J.; Krishna, G. Protein-sparing effect of dietary lipid: Changes in growth, nutrient utilization, digestion and IGF-I and IGFBP-I expression of Genetically Improved Farmed Tilapia (GIFT), reared in Inland Ground Saline Water. Anim. Feed. Sci. Technol. 2022, 284, 115150. [Google Scholar] [CrossRef]
- Dalsgaard, J.; Ekmann, K.S.; Pedersen, P.B.; Verlhac, V. Effect of supplemented fungal phytase on performance and phosphorus availability by phosphorus-depleted juvenile rainbow trout (Oncorhynchus mykiss), and on the magnitude and composition of phosphorus waste output. Aquaculture 2009, 286, 105–112. [Google Scholar] [CrossRef]
- Othman, A.R.; Kawamura; Senoo; Fui, F.C. Effects of different salinities on growth, feeding performance and plasma cortisol level in hybrid TGGG (tiger grouper, Epinephelus fuscoguttatus × giant grouper, Epinephelus lanceolatus) juveniles. Int. Res. J. Biol. Sci. 2015, 4, 15–20. [Google Scholar]
- Zhang, Z.; Yang, Z.; Ding, N.; Xiong, W.; Zheng, G.; Lin, Q.; Zhang, G. Effects of temperature on the survival, feeding, and growth of pearl gentian grouper (female Epinephelus fuscoguttatus × male Epinephelus lanceolatus). Fish. Sci. 2018, 84, 399–404. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, X.; Li, W.; Wu, M.; Luo, Y.; Lu, S.; Lin, H. Effects of dietary protein and lipid levels on growth, feed utilization, body and plasma biochemical compositions of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) juveniles. Aquaculture 2015, 446, 148–155. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Liu, Q.; Ye, H.; Zou, C.; Ye, C.; Wang, A.; Lin, H. Effects of dietary ginkgo biloba leaf extract on growth performance, plasma biochemical parameters, fish composition, immune responses, liver histology, and immune and apoptosis-related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ x Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish Shellfish Immunol. 2018, 72, 399–409. [Google Scholar] [CrossRef]
- Gillis, M.B.; Norris, L.C.; Heuser, G.F. Studies on the Biological Value of Inorganic Phosphates: One Figure. J. Nutr. 1954, 52, 115–125. [Google Scholar] [CrossRef]
- Saleh, N.E.; Wassef, E.A.; Kamel, M.A.; El-Haroun, E.R.; El-Tahan, R.A. Beneficial effects of soybean lecithin and vitamin C combination in fingerlings gilthead seabream (Sparus aurata) diets on; fish performance, oxidation status and genes expression responses. Aquaculture 2022, 546, 737345. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Su, N.; Wang, A.; Tan, X.; Sun, Z.; Ye, C. Effects of replacing fish meal with rendered animal protein blend on growth performance, hepatic steatosis and immune status in hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Aquaculture 2019, 511, 734203. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Cheng, K.; Xia, S.; Wang, M.; Sun, G. The feature and rule of change of growth and feed intake of Cynoglossus semilaevis and water quality in industrial culture with recirculation aquaculture system. J. Hydroecology 2009, 30, 52–59. [Google Scholar] [CrossRef]
- Puigagut, J.; Angles, H.; Chazarenc, F.; Comeau, Y. Decreasing phosphorus discharge in fish farm ponds by treating the sludge generated with sludge drying beds. Aquaculture 2011, 318, 7–14. [Google Scholar] [CrossRef]
- Li, C.; Zhang, B.; Luo, P.; Shi, H.; Li, L.; Gao, Y.; Wu, W.M. Performance of a pilot-scale aquaponics system using hydroponics and immobilized biofilm treatment for water quality control. J. Clean. Prod. 2019, 208, 274–284. [Google Scholar] [CrossRef]
- Khalil, H.S.; Mansour, A.T.; Goda, A.M.A.; Omar, E.A. Effect of selenium yeast supplementation on growth performance, feed utilization, lipid profile, liver and intestine histological changes, and economic benefit in meagre, Argyrosomus regius, fingerlings. Aquaculture 2019, 501, 135–143. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, X.; Luo, Y.; Wu, M.; Lu, S.; Jin, Z.; Yao, W. Optimal dietary protein level and protein to energy ratio for hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) juveniles. Aquaculture 2016, 465, 28–36. [Google Scholar] [CrossRef]
- Gómez-Montes, L.; García-Esquivel, Z.; D’Abramo, L.R.; Shimada, A.; Vásquez-Peláez, C.; Viana, M.T. Effect of dietary protein: Energy ratio on intake, growth and metabolism of juvenile green abalone Haliotis fulgens. Aquaculture 2003, 220, 769–780. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Effect of dietary protein levels on growth performance, hematological profile and biochemical composition of fingerlings rainbow trout, Oncorhynchus mykiss reared in Indian himalayan region. Aquac. Rep. 2020, 16, 100268. [Google Scholar] [CrossRef]
- Alam, M.S.; Liang, X.; Liu, L. Indirect effect of different dietary protein to energy ratio of bait fish mori diets on growth performance, body composition, nitrogen metabolism and relative AMPK & mTOR pathway gene expression of Chinese perch. Aquac. Rep. 2020, 16, 100276. [Google Scholar] [CrossRef]
- Woods, L.C., III; Yust, D.; McLeod, C.; Subramanyam, M. Effects of dietary protein: Energy ratio on weight gain, body composition, serum glucose and triglyceride levels, and liver function of striped bass. Water Sci. Technol. 1995, 31, 195–203. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Lu, K.; Wang, L.; Song, K.; Li, X.; Zhang, C.; Rahimnejad, S. Effects of dietary phosphorus level on growth, body composition, liver histology and lipid metabolism of spotted seabass (Lateolabrax maculatus) reared in freshwater. Aquac. Fish. 2022, 8, 528–537. [Google Scholar] [CrossRef]
- Luo, Z.; Tan, X.Y.; Liu, X.; Wang, W.M. Dietary total phosphorus requirement of juvenile yellow catfish Pelteobagrus fulvidraco. Aquac. Int. 2010, 18, 897–908. [Google Scholar] [CrossRef]
- Yang, S.; Lin, T.; Liu, F.; Liou, C. Influence of dietary phosphorus levels on growth, metabolic response and body composition of juvenile silver perch (Bidyanus bidyanus). Aquaculture 2006, 253, 592–601. [Google Scholar] [CrossRef]
- Wilson, Z.; Carter, C.G.; Purser, G.J. Nitrogen budgets for juvenile big-bellied seahorse Hippocampus abdominalis fed Artemia, mysids or pelleted feeds. Aquaculture 2006, 255, 233–241. [Google Scholar] [CrossRef]
- Preena, P.G.; Rejish Kumar, V.J.; Singh, I.S.B. Nitrification and denitrification in recirculating aquaculture systems: The processes and players. Rev. Aquac. 2021, 13, 2053–2075. [Google Scholar] [CrossRef]
- Sahariah, B.P.; Chakraborty, S. Kinetic analysis of phenol, thiocyanate and ammonia-nitrogen removals in an anaerobic-anoxic-aerobic moving bed bioreactor system. J. Hazard. Mater. 2011, 190, 260–267. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Wu, J.; Wang, J. Nitrogen removal in recirculating aquaculture water with high dissolved oxygen conditions using the simultaneous partial nitrification, anammox and denitrification system. Bioresour. Technol. 2020, 305, 123037. [Google Scholar] [CrossRef]
- Krumins, V.; Ebeling, J.; Wheaton, F. Part-day ozonation for nitrogen and organic carbon control in recirculating aquaculture systems. Aquac. Eng. 2001, 24, 231–241. [Google Scholar] [CrossRef]
- Van Rijn, J.; Tal, Y.; Schreier, H.J. Denitrification in recirculating systems: Theory and applications. Aquac. Eng. 2006, 34, 364–376. [Google Scholar] [CrossRef]
- Yoram, B.; Eddie, C.; Iliya, G.; Michael, K.; van Rijn, J. Phosphorus removal in a marine prototype, recirculating aquaculture system. Aquaculture 2003, 220, 313–326. [Google Scholar] [CrossRef]
- Wang, S.; Carter, C.G.; Fitzgibbon, Q.P.; Codabaccus, B.M.; Smith, G.G. Effect of dietary protein on energy metabolism including protein synthesis in the spiny lobster Sagmariasus verreauxi. Sci. Rep. 2021, 11, 11814. [Google Scholar] [CrossRef] [PubMed]
- Gauquelin, F.; Cuzon, G.; Gaxiola, G.; Rosas, C.; Arena, L.; Bureau, D.P.; Cochard, J.C. Effect of dietary protein level on growth and energy utilization by Litopenaeus stylirostris under laboratory conditions. Aquaculture 2007, 271, 439–448. [Google Scholar] [CrossRef]
- Peres, H.; Oliva-Teles, A. Effect of dietary protein and lipid level on metabolic utilization of diets by European sea bass (Dicentrarchus labrax) juveniles. Fish Physiol. Biochem. 2001, 25, 269. [Google Scholar] [CrossRef]
- Yadata, G.W.; Ji, K.; Liang, H.; Ren, M.; Ge, X.; Yang, Q. Effects of dietary protein levels with various stocking density on growth performance, whole body composition, plasma parameters, nitrogen emission and gene expression related to TOR signaling of juvenile blunt snout bream (Megalobrama ambylcephala). Aquaculture 2020, 519, 734730. [Google Scholar] [CrossRef]
- Ramli, N.M.; Verreth, J.A.J.; Yusoff, F.M.; Nurulhuda, K.; Nagao, N.; Verdegem, M.C. Integration of algae to improve nitrogenous waste management in recirculating aquaculture systems: A review. Front. Bioeng. Biotechnol. 2020, 8, 1004. [Google Scholar] [CrossRef]
- GB 11607-89; Water Quality Standard for Fisheries. China National Standardization Management Committee: Beijing, China, 1990.
- Xu, H.; Zhang, X.; Wei, Y.; Sun, B.; Jia, L.; Liang, M. Effects of dietary phosphorus level and stocking density on tiger puffer Takifugu rubripes: Growth performance, body composition, lipid metabolism, deposition of phosphorus and calcium, serum biochemical parameters, and phosphorus excretion. Aquaculture 2020, 529, 735709. [Google Scholar] [CrossRef]
- Jaxion-Harm, J. Effects of dietary phospholipids on early stage Atlantic Salmon (Salmo salar) performance: A comparison among phospholipid sources. Aquaculture 2021, 544, 737055. [Google Scholar] [CrossRef]
- Sugiura, S.H.; Hardy, R.W. Environmentally-friendly feeds. In Encyclopedia of Aquaculture; Stickney, R.R., Ed.; John Wiley & Sons, Inc.: New York, NJ, USA, 2000; pp. 299–310. [Google Scholar]
| Feed | |||||
|---|---|---|---|---|---|
| LNLP | LNMP | MNLP | MNMP | HNP | |
| Main component | |||||
| Fish meal | √ | √ | √ | √ | √ |
| Shrimp meal | √ | √ | √ | ||
| Squid meal | √ | √ | |||
| Soybean meal | √ | √ | √ | ||
| Fish oil | √ | √ | √ | √ | √ |
| Flour | √ | √ | √ | ||
| Minerals | √ | √ | √ | √ | √ |
| Vitamins | √ | √ | √ | √ | √ |
| Proximate Composition | |||||
| Crude Protein % | 49.77 | 50.31 | 53.12 | 52.99 | 58.40 |
| Crude Fat % | 12.82 | 12.71 | 9.5 | 9.49 | 9.52 |
| Total Phosphorus % | 1.48 | 1.98 | 1.51 | 2.02 | 2.43 |
| Available Phosphorus % | 0.96 | 1.54 | 0.98 | 1.58 | 1.97 |
| Crude Fiber % | 0.91 | 0.59 | 1.52 | 1.90 | 0.88 |
| Moisture % | 6.82 | 6.81 | 6.57 | 6.81 | 6.36 |
| Crude Ash % | 10.42 | 12.57 | 12.80 | 15.92 | 16.31 |
| Metabolic Energy MJ/kg | 15.00 | 15.04 | 14.56 | 14.48 | 14.06 |
| P/E g/MJ | 31.97 | 32.11 | 36.26 | 36.53 | 41.54 |
| Feed cost yuan/kg | 13.50 | 17.00 | 15.5 | 18.00 | 29.00 |
| Groups | Survival % | GR % | IW g | FW g | FCR | WGR % | SGR % d−1 | FCWG yuan kg−1 |
|---|---|---|---|---|---|---|---|---|
| LNLP | 98.15 ± 1.32 | 98.22 ± 0.81 | 105.13 ± 20.04 | 165.87 ± 36.26 d | 1.17 ± 0.03 a | 57.78 ± 1.34 c | 0.81 ± 0.02 c | 16.07 ± 0.35 c |
| LNMP | 98.83 ± 0.71 | 97.23 ± 0.92 | 112.40 ± 20.16 | 180.80 ± 45.01 c | 1.02 ± 0.04 b | 60.93 ± 3.98 c | 0.85 ± 0.04 c | 17.91 ± 0.70 b |
| MNLP | 98.81 ± 0.60 | 96.71 ± 1.08 | 110.70 ± 18.88 | 194.03 ± 30.50 b | 0.81 ± 0.02 c | 77.00 ± 4.24 b | 1.02 ± 0.04 b | 13.00 ± 0.32 e |
| MNMP | 98.31 ± 0.84 | 97.85 ± 0.76 | 109.30 ± 18.03 | 197.23 ± 36.38 b | 0.80 ± 0.01 c | 80.46 ± 1.59 b | 1.05 ± 0.02 b | 14.65 ± 0.24 d |
| HNP | 99.09 ± 0.92 | 97.58 ± 0.39 | 105.20 ± 15.08 | 207.53 ± 20.38 a | 0.70 ± 0.02 d | 97.28 ± 1.44 a | 1.21 ± 0.01 a | 20.92 ± 0.60 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Yu, H.; Cui, H.; Xue, Z.; Bai, Y.; Qu, K.; Hu, H.; Cui, Z. Optimal Dietary Protein/Energy Ratio and Phosphorus Level on Water Quality and Output for a Hybrid Grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) Recirculating Aquaculture System. Water 2023, 15, 1261. https://doi.org/10.3390/w15071261
Fan X, Yu H, Cui H, Xue Z, Bai Y, Qu K, Hu H, Cui Z. Optimal Dietary Protein/Energy Ratio and Phosphorus Level on Water Quality and Output for a Hybrid Grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) Recirculating Aquaculture System. Water. 2023; 15(7):1261. https://doi.org/10.3390/w15071261
Chicago/Turabian StyleFan, Xiangyu, Hong Yu, Hongwu Cui, Zhiyong Xue, Ying Bai, Keming Qu, Haiyan Hu, and Zhengguo Cui. 2023. "Optimal Dietary Protein/Energy Ratio and Phosphorus Level on Water Quality and Output for a Hybrid Grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) Recirculating Aquaculture System" Water 15, no. 7: 1261. https://doi.org/10.3390/w15071261
APA StyleFan, X., Yu, H., Cui, H., Xue, Z., Bai, Y., Qu, K., Hu, H., & Cui, Z. (2023). Optimal Dietary Protein/Energy Ratio and Phosphorus Level on Water Quality and Output for a Hybrid Grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) Recirculating Aquaculture System. Water, 15(7), 1261. https://doi.org/10.3390/w15071261






