Physicochemical Technique in Municipal Solid Waste (MSW) Landfill Leachate Remediation: A Review
Abstract
:1. Introduction
2. Landfilling

3. Characteristics of Landfill Leachate
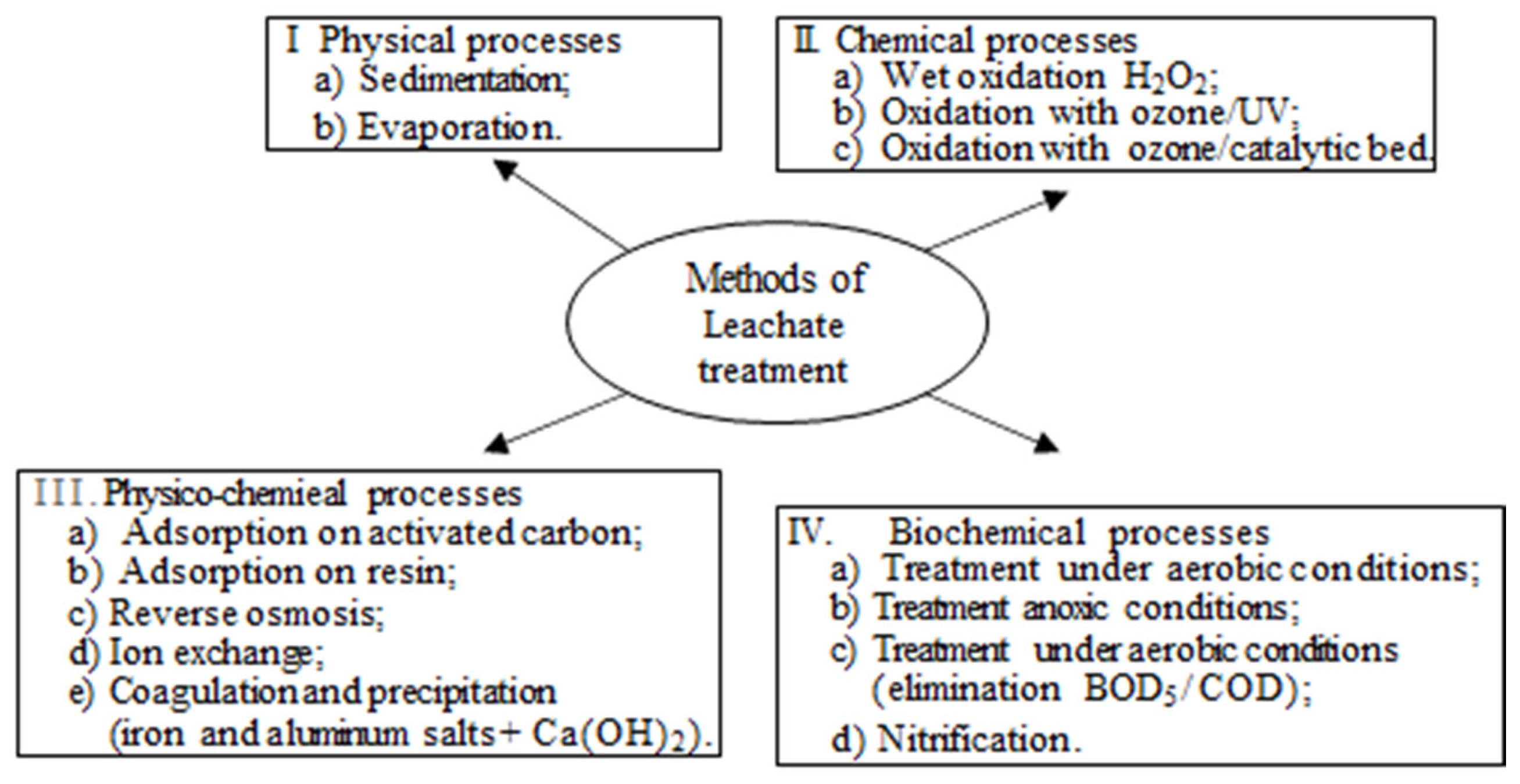
4. Landfill Leachate Treatment
5. Physicochemical Treatment for Landfill Leachate
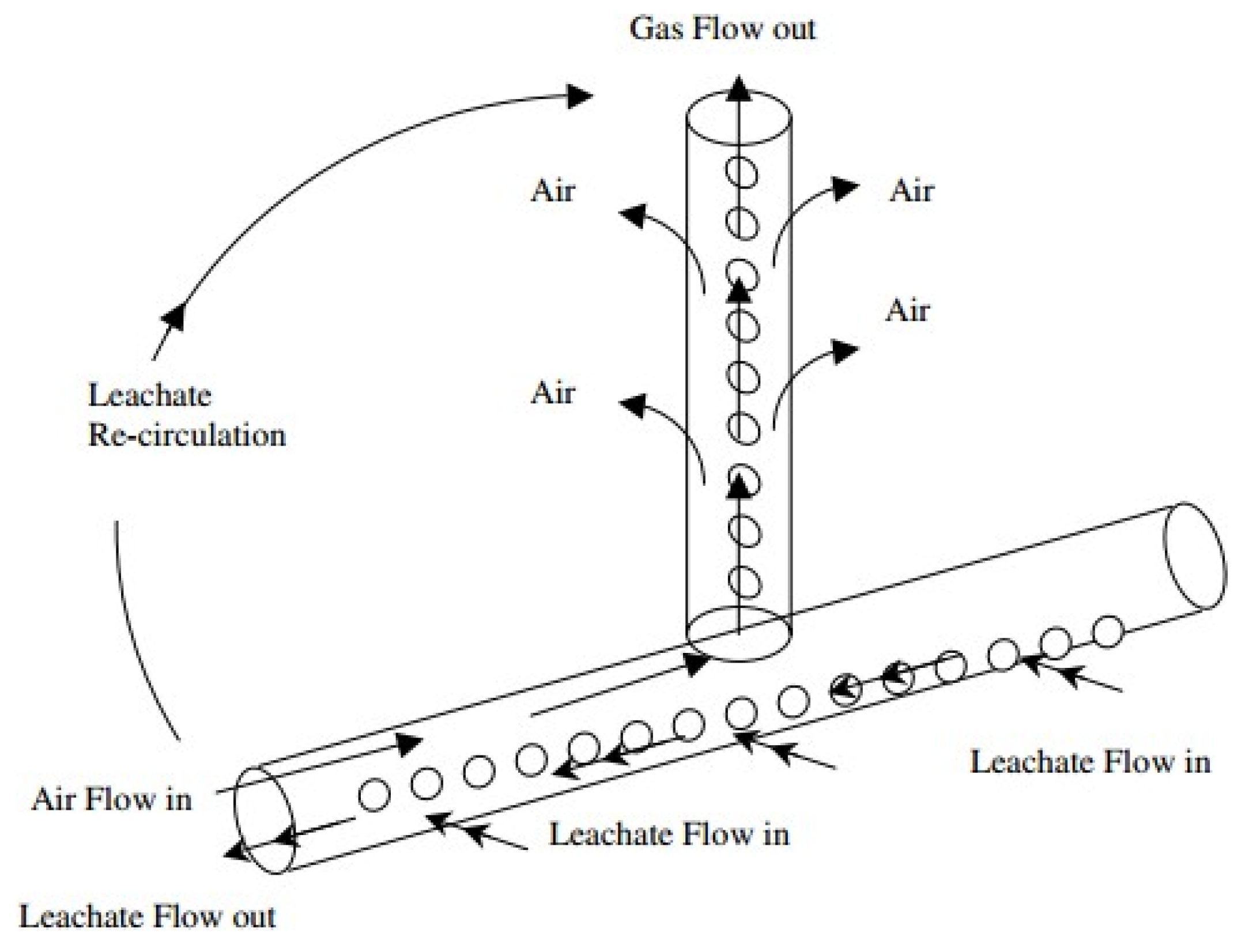
5.1. Ammoniacal Nitrogen (NH3-N) Reduction by Air Stripping
5.2. Coagulation-Flocculation Process
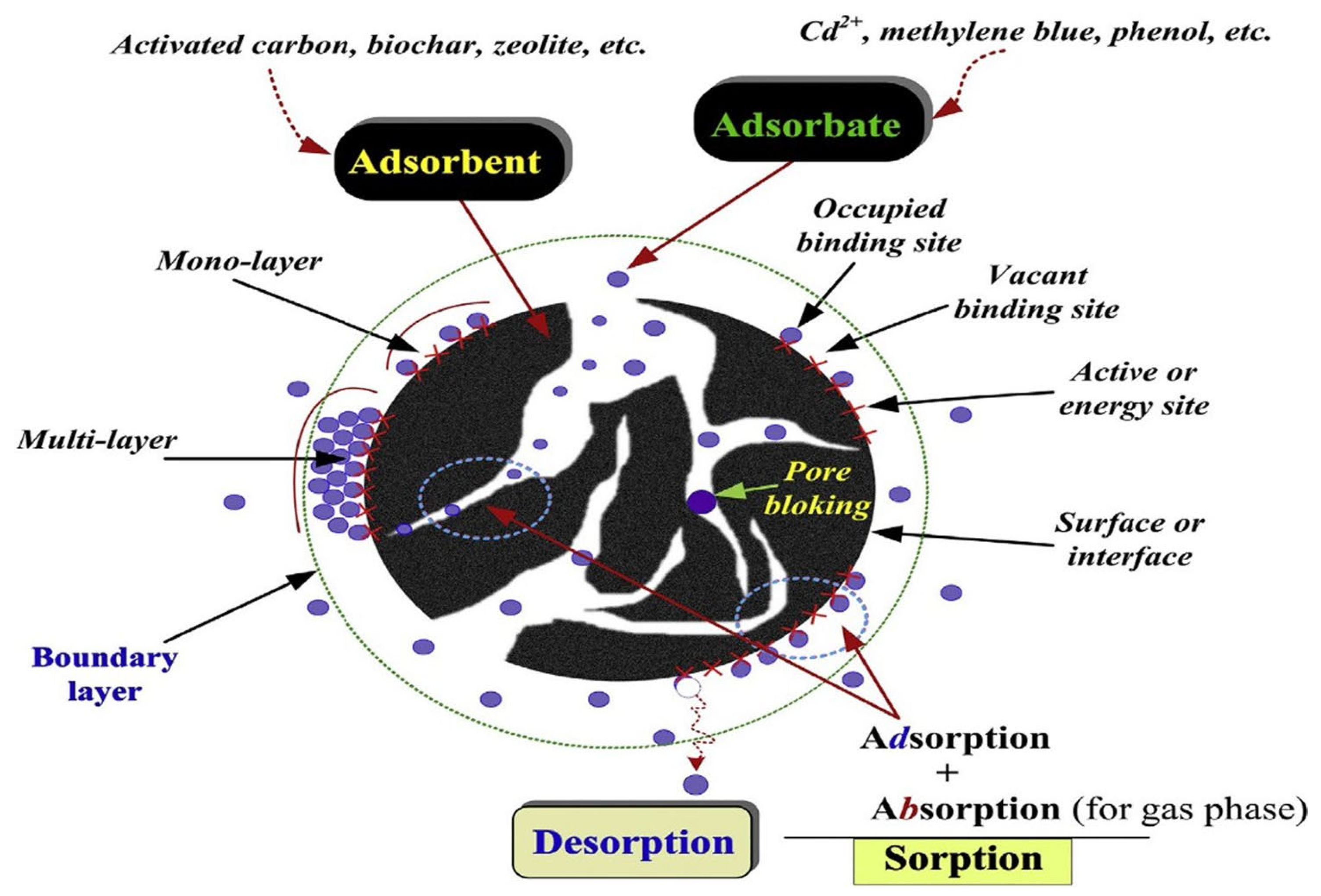
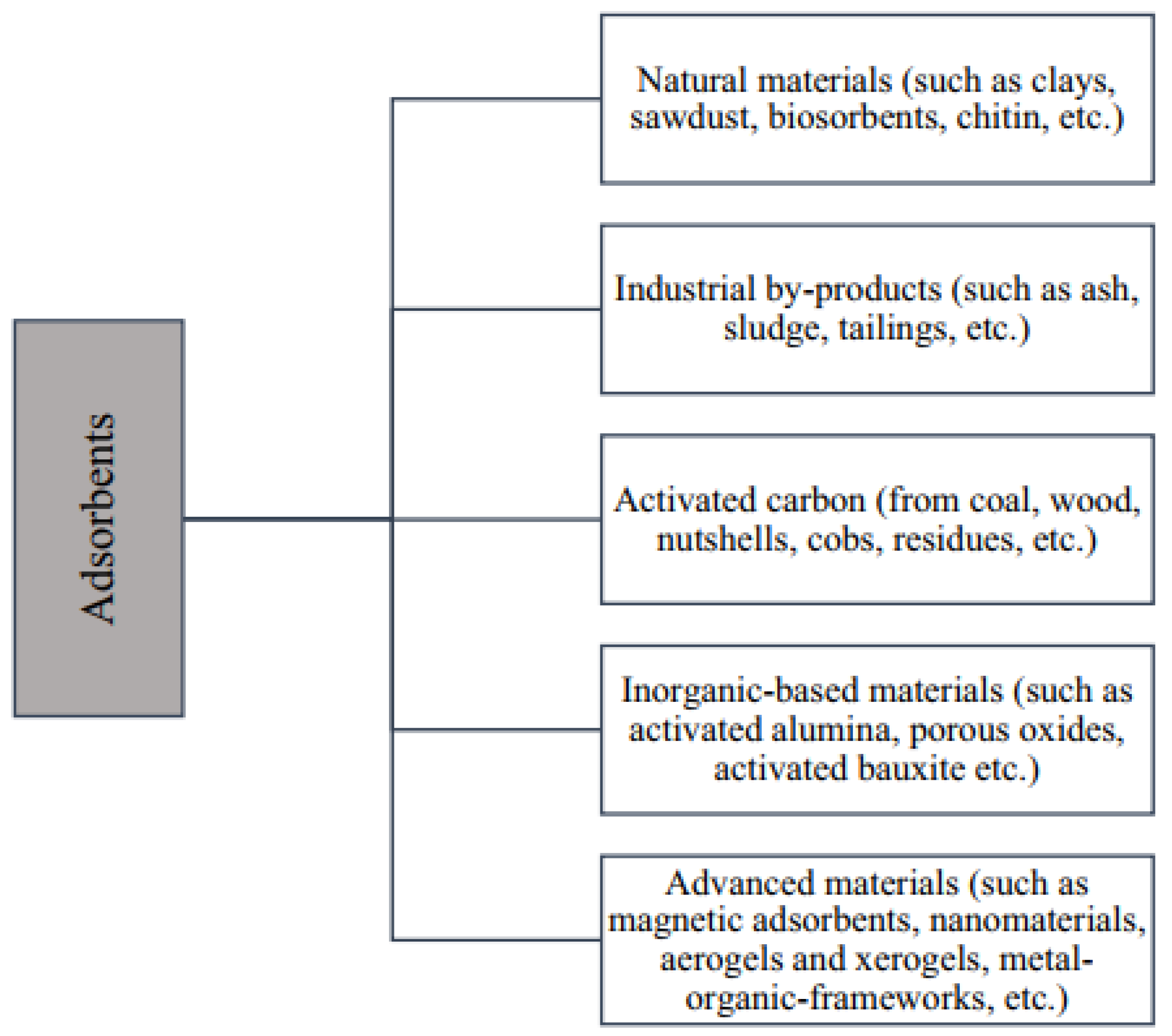
5.3. Adsorption
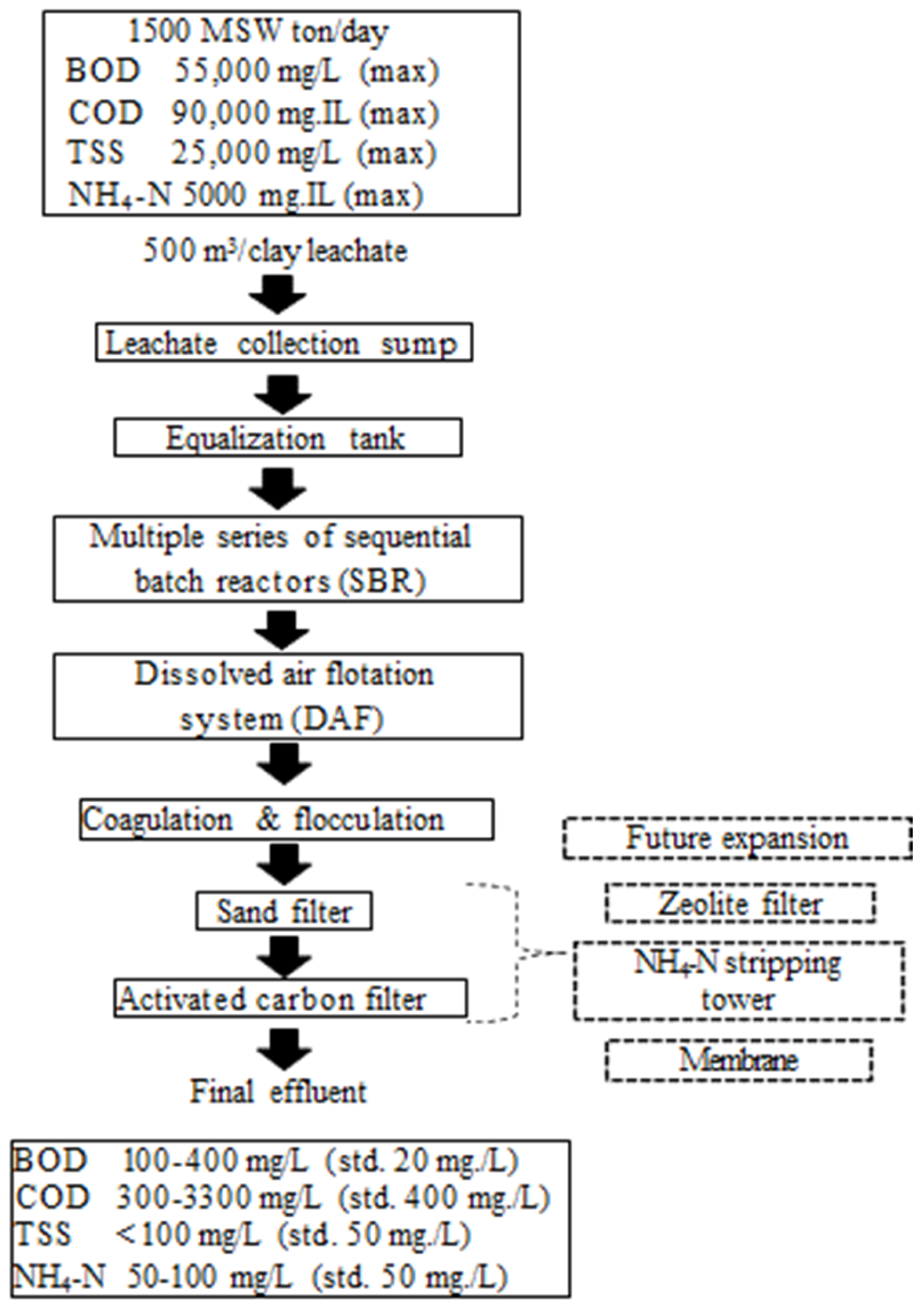
5.4. Integrated Treatment
6. Challenges
- Treating leachate is tough and demanding owing to its complex compounds, which involve large differences in its volumetric chemical compositions. Selecting an acceptable, cost-effective, and efficient combination procedure is a demanding undertaking.
- Leachate normally varies in terms of loading due to large fluctuations in water quantity and quality. This is because it is greatly influenced by the amount of waste disposed of daily, season, and weather conditions, which make it difficult to choose and run an effective treatment method and consistent performance.
- Treatment of leachate depends on its composition. As leachate properties differ, treatment methods for leachate A might not work well for leachate B. Therefore, a treatability study is highly recommended. Experiences and performances of an existing leachate treatment plant will complement this treatability study.
- It is also not straightforward to determine an appropriate and the best combination of available technologies and how to combine them to achieve a steady operation.
- Budget restrictions in developing countries make it challenging to establish and maintain an effective treatment system.
- Treating NH3-N and total nitrogen is a challenging task. Usually, a nitrification-denitrification system or an ammonia stripping plant is required, although they are a bit costly. Zeolite filters, however, recently became a promising method as an alternative in removing NH3-N.
- In addition, some leachate treatment facilities frequently employ post-treatment steps to polish the treated effluent. Nanofiltration and reverse osmosis were employed in some sites to meet discharge limits; however, this is costly and may not be widely affordable in developing countries.
- There is a limitation of technical knowledge in underdeveloped countries on the management and operation of treatment facilities.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Department of Statistics, Malaysia (DOSM). Population & Demography. 2022. Available online: https://www.dosm.gov.my/v1/index.php?r=column/ctwoByCat&parent_id=115&menu_id=L0pheU43NWJwRWVSZklWdzQ4TlhUUT09 (accessed on 7 December 2022).
- Malaysian Investment Development Authority (MIDA). Waste to Energy for a Sustainable Future. 2021. Available online: https://www.mida.gov.my/waste-to-energy-for-a-sustainable-future/ (accessed on 7 December 2022).
- Jelonek, P.; Neczaj, E. The use of Advanced Oxidation Processes (AOP) for the treatment of landfill leachate. In Proceedings of the 4th International Conference on Advances in Sustainable Sewage Sludge Management, Szczyrk, Poland, 3–5 December 2012. [Google Scholar]
- Saxena, V.; Kumar Padhi, S.; Kumar Dikshit, P.; Pattanaik, L. Recent developments in landfill leachate treatment: Aerobic granular reactor and its future prospects. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100689. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and treatment of landfill leachate: A review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of landfill leachate with different techniques: An overview. J. Water Reuse Desalin. 2021, 11, 66–96. [Google Scholar] [CrossRef]
- Banchon, C.; Cañas, R. Coagulation and oxidation strategies for landfill leachate wastewater. Res. Sq. 2022, 1–16. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, Y.; Wang, Z.; Jiang, H.; Ren, S.; Qiu, J. New insights into co-treatment of mature landfill leachate with municipal sewage via integrated partial nitrification, Anammox and denitratation. J. Hazard. Mater. 2021, 415, 125506. [Google Scholar] [CrossRef]
- Rahim, I.R.; Jamaluddin, A. Cost Analysis of The Fukuoka Method Landfill System in North Kolaka Regency, Southeast Sulawesi, Indonesia. In Proceedings of the Indonesia International Conference on Science, Technology and Humanity, Yogyakarta, Jakarta, 7 December 2015; pp. 15–20. [Google Scholar]
- Tashiro, T. The “Fukuoka Method”: Semi-Aerobic Landfill Technology. In Proceedings of the IRBC Conference, Metro Vancouver, BC, Canada, 20–22 September 2011; Available online: http://www.metrovancouver.org/2011IRBC/Program/IRBCDocs/IRBCFactsheet_FukuokaMethodWasteMgmt_Fukuoka.pdf (accessed on 11 September 2022).
- Theng, L.C.; Matsufuji, Y.; Mohd, N.H. Implementation of the Semi-Aerobic Landfill System (Fukuoka Method) in Developing Countries: A Malaysia Cost Analysis. Waste Manag. 2005, 25, 702–711. [Google Scholar] [CrossRef]
- Amiri, A.W.; Tsutsumi, J.I.G.; Nakamatsu, R.A. Case Study of Fukuoka Landfill Method and Environmental Impact Assessment of Solid Waste Management in Kabul City. Int. J. Techn. Res. Appl. 2016, 4, 46–51. [Google Scholar]
- Sheppard, D.A. Practical Guide to Landfill Management in Pacific Island Countries and Territories; Secretariat of the Pacific Regional Environment Programme: Apia, Samoa, 2010. [Google Scholar]
- Anqi, T.; Zhang, Z.; Suhua, H.; Xia, L. Review on landfill leachate treatment methods. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 565. [Google Scholar] [CrossRef]
- Aziz, H.A.; Alias, S.; Adlan, M.N.; Asaari, A.H.; Zahari, M.S. Colour Removal from Landfill Leachate By Coagulation And flocculation Processes. Bioresour. Technol. 2007, 98, 218–220. [Google Scholar] [CrossRef]
- Bu, L.; Wang, K.; Zhao, Q.L.; Wei, L.L.; Zhang, J.; Yang, J.C. Characterisation of Dissolved Organic Matter During Landfill Leachate Treatment by Sequencing Batch Reactor, Aeration Corrosive Cell-Fenton, And Granular Activated Carbon Inseries. J. Hazard. Mater. 2010, 179, 1096–1105. [Google Scholar] [CrossRef]
- Galeano, L.A.; Vicente, M.A.; Gil, A. Treatment of Municipal Leachate of Landfillby Fenton-Like Heterogeneous Catalytic Wet Peroxide Oxidation Using An Al/Fepillared Montmorillonite As Active Catalyst. Chem. Eng. J. 2011, 178, 146–153. [Google Scholar] [CrossRef]
- Cherni, Y.; Elleuch, L.; Messaoud, M.; Kasmi, M.; Chatti, A.; Trabelsi, I. Recent Technologies for Leachate Treatment: A Review. Euro-Mediterr. J. Environ. Integr. 2021, 6, 79. [Google Scholar] [CrossRef]
- Matsufuji, J. Technical Guideline on Sanitary Landfill; Japan International Co.: Tokyo, Japan, 1990. [Google Scholar]
- Costa, A.M.; Alfaia, R.G.D.S.M.; Campos, J.C. Landfill Leachate Treatment in Brazil—An Overview. J. Environ. Manag. 2019, 232, 110–116. [Google Scholar] [CrossRef]
- Aziz, H.A.; Ramli, S.F. Recent development in sanitary landfilling and landfill leachate treatment in Malaysia. Int. J. Environ. Eng. 2018, 9, 201. [Google Scholar] [CrossRef]
- Taoufik, M.; Elmoubarki, R.; Moufti, A.; Elhalil, A.; Farnane, M.; Machrouhi, A. Treatment of Landfill Leachate by Coagulation-Flocculation with FeCl3: Process Optimisation Using Box—Behnken Design. J. Mater. Environ. Sci. 2018, 9, 2458–2467. [Google Scholar]
- Lippi, M.; Gaudie Ley, M.B.R.; Mendez, G.P.; Felix Cardoso Junior, R.A. State of Art of Landfill Leachate Treatment: Literature Review and Critical Evaluation. Ciência Nat. 2018, 40, 78. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.; Gami, J. Landfill Leachate Technologies: A Review. Glob. Res. Dev. J. Eng. 2019, 54–58. [Google Scholar]
- Rathnayake, W.A.P.P.; Herath, G.B.B. A Review of Leachate Treatment Techniques. In Proceedings of the 9th International Conference on Sustainable Built Environment, Kandy, Sri Lanka, 13–15 December 2018; pp. 97–106. Available online: https://www.researchgate.net/publication/329915923 (accessed on 11 September 2022).
- Khôi, T.T.; Thủy, T.T.T.; Nga, N.T.; Huy, N.N.; Thủy, N.T. Air Stripping for Ammonia Removal from Landfill Leachate in Vietnam: Effect of Operation Parameters. TNU J. Sci. Technol. 2021, 226, 73–81. [Google Scholar] [CrossRef]
- Leite, V.D.; Paredes, J.M.R.; de Sousa, T.A.T.; Lopes, W.S.; de Sousa, J.T. Ammoniacal Nitrogen Stripping from Landfill Leachate at Open Horizontal Flow Reactors. Water Environ. Res. 2018, 90, 387–394. [Google Scholar] [CrossRef]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Achak, M.; Elayadi, F.; Boumya, W. Chemical Coagulation/Flocculation Processes for Removal of Phenolic Compounds from Olive Mill Wastewater: A Comprehensive Review. Am. J. Appl. Sci. 2019, 16, 59–91. [Google Scholar] [CrossRef] [Green Version]
- Mohd-Salleh, S.N.A.; Mohd-Zin, N.S.; Othman, N. A Review of Wastewater Treatment Using Natural Material and Its Potential As Aid And Composite Coagulant. Sains Malays. 2019, 48, 155–164. [Google Scholar] [CrossRef]
- Djeffal, K.; Bouranene, S.; Fievet, P.; Déon, S.; Gheid, A. Treatment of Controlled Discharge Leachate by Coagulation-Flocculation: Influence Of Operational Conditions. Sep. Sci. Technol. 2021, 56, 168–183. [Google Scholar] [CrossRef]
- Mohd-Salleh, S.N.A.; Mohd-Zin, N.S.; Othman, N.; Mohd-Amdan, N.S.; Mohd-Shahli, F. Dosage and pH Optimisation on Stabilised Landfill Leachate via Coagulation-Flocculation Process. MATEC Web Conf. 2018, 250, 06007. [Google Scholar] [CrossRef] [Green Version]
- Aziz, H.A.; Noor, A.F.M.; Keat, Y.W.; Alazaiza, M.Y.D.; Hamid, A.A. Heat Activated Zeolite for the Reduction of Ammoniacal Nitrogen, Colour, and COD in Landfill Leachate. Int. J. Environ. Res. 2020, 14, 463–478. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A. Synergy of Adsorption and Advanced Oxidation Processes in Recalcitrant Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 1125–1142. [Google Scholar] [CrossRef]
- Abuabdou, S.M.A.; Teng, O.W.; Bashir, M.J.K.; Aun, N.C.; Sethupathi, S. Adsorptive Treatment of Stabilised Landfill Leachate Using Activated Palm Oil Fuel Ash (POFA). In Proceedings of the Conference: International Symposium on Green and Sustainable Technology (ISGST2019) Universiti Tunku Abdul Rahman, Kampar, Malaysia, 23–26 April 2019. [Google Scholar] [CrossRef]
- Shehzad, A.; Bashir, M.J.K.; Sethupathi, S.; Lim, J.W. An Overview of Heavily Polluted Landfill Leachate Treatment Using Food Waste as an Alternative and Renewable Source of Activated Carbon. Process Saf. Environ. Prot. 2015, 98, 309–318. [Google Scholar] [CrossRef]
- Torretta, V.; Ferronato, N.; Katsoyiannis, I.A.; Tolkou, A.K.; Airoldi, M. Novel and Conventional Technologies for Landfill Leachates Treatment: A Review. Sustainability 2017, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and Non-Conventional Adsorbents for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Reshadi, M.A.M.; Bazargan, A.; McKay, G. A Review of the Application of Adsorbents for Landfill Leachate Treatment: Focus on Magnetic Adsorption. Sci. Total Environ. 2020, 731, 138863. [Google Scholar] [CrossRef]
- Kasmuri, N.; Sabri, S.N.M.; Wahid, M.A.; Rahman, Z.A.; Abdullah, M.M.; Anur, M.Z.K. Using Zeolite in the Ion Exchange Treatment to Remove Ammonia-Nitrogen, Manganese and Cadmium. AIP Conf. Proc. 2018, 2031, 020004. [Google Scholar] [CrossRef]
- Rohers, F.; Dalsasso, R.L.; Nadaleti, W.C.; Matias, M.S.; de Castilhos, A.B., Jr. Physical–chemical Pre-Treatment of Sanitary Landfill Raw Leachate by Direct Ascending Filtration. Chemosphere 2021, 285, 131362. [Google Scholar] [CrossRef] [PubMed]
- Augusto, P.A.; Castelo-Grande, T.; Merchan, L.; Estevez, A.M.; Quintero, X.; Barbosa, D. Landfill Leachate Treatment by Sorption in Magnetic Particles: Preliminary Study. Sci. Total Environ. 2019, 648, 636–668. [Google Scholar] [CrossRef]
- Mosanefi, S.; Alavi, N.; Eslami, A.; Saadani, M.; Ghavami, A. Ammonium Removal from Landfill Fresh Leachate Using Zeolite as Adsorbent. J. Mater. Cycles Waste Manag. 2021, 23, 1383–1393. [Google Scholar] [CrossRef]
- Ai, J.; Wu, X.; Wang, Y.; Zhang, D.; Zhang, H. Treatment of Landfill Leachate with Combined Biological and Chemical Processes: Changes in the Dissolved Organic Matter and Functional Groups. Environ. Technol. Innov. 2019, 40, 2225–2231. [Google Scholar] [CrossRef]
- Nath, A.; Debnath, A.A. Short Review on Landfill Leachate Treatment Technologies. Mater. Today Proc. 2022, 67, 1290–1297. [Google Scholar] [CrossRef]
- Yenis Septiariva, I.; Padmi, T.; Damanhuri, E.; Helmy, Q. A Study on Municipal Leachate Treatment through a Combination of Biological Processes and Ozonation. MATEC Web Conf. 2019, 276, 06030. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, P.; Selamat, M.R.; Aziz, H.A.; Smith, C. Removal of COD and Ammonia Nitrogen by a Sawdust/Bentonite-Augmented SBR Process. J. Clean Energy Technol. 2019, 1, 125–140. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, M.S.; da Silva, L.F.; Barbosa, A.D.; Romualdo, L.L.; Sadoyama, G.; Andrade, L.S. Landfill Leachate Treatment by Combining Coagulation and Advanced Electrochemical Oxidation Techniques. ChemElectroChem 2019, 6, 1427–1433. [Google Scholar] [CrossRef]
- el Mrabet, I.; Benzina, M.; Zaitan, H. Treatment of Landfill Leachate from Fez City by Combined Fenton and Adsorption Processes Using Moroccan Bentonite Clay. Desalin. Water Treat. 2021, 225, 402–412. [Google Scholar] [CrossRef]


| Type of Landfill Leachate | |||||
|---|---|---|---|---|---|
| No. | Parameter | Unit | Young (<5 Years) | Intermediate (5–10 Years) | Stabilised (>10 Years) |
| 1 | pH | <6.5 | 6.5–7.5 | >7.5 | |
| 2 | COD | mg/L | >10,000 | 4000–10,000 | <4000 |
| 3 | BOD5/COD | 0.5–1.0 | 0.1–0.5 | <0.1 | |
| 4 | Organic compound | 80% VFA a | 5–30% VFA a + HFA b HFA b | HFA b | |
| 5 | NH3-N | mg/L | <400 | NA c | >400 |
| 6 | TOC/COD | <0.3 | 0.3–0.5 | >0.5 | |
| 7 | Kjeldahl nitrogen | g/L | 0.1–0.2 | NA c | NA c |
| 8 | Heavy metals | mg/L | Low to medium | Low | Low |
| 9 | Biodegradability | Important | Medium | Low |
| Leachate Treatment | Landfill Age (Years) | ||
|---|---|---|---|
| Young (<5) | Intermediate (5–10) | Mature (>10) | |
| Co-treatment with domestic wastewater | Good | Fair | Poor |
| Recycling | Good | Fair | Poor |
| Aerobic process (suspended growth) | Good | Fair | Poor |
| Aerobic process (fixed film) | Good | Fair | Poor |
| Anaerobic process (suspended growth) | Good | Fair | Poor |
| Anaerobic process (fixed film) | Good | Fair | Poor |
| Natural evaporation | Good | Good | Good |
| Coagulation/flocculation | Poor | Fair | Fair |
| Chemical precipitation | Poor | Fair | Poor |
| Carbon adsorption | Poor | Fair | Good |
| Oxidation | Poor | Fair | Fair |
| Air stripping | Poor | Fair | Fair |
| Ion exchange | Good | Good | Good |
| Microfiltration | Poor | - | - |
| Ultrafiltration | Poor | - | - |
| Nanofiltration | Good | Good | Good |
| Reverse osmosis | Good | Good | Good |
| Coagulation Flocculation | |||||
|---|---|---|---|---|---|
| Parameter (Removals) | Turbidity (90%) NH3–N (46.7%) COD (53.9%) | TP (47%) TOC (15%) NH3–N (20%) TN (4%) | COD (61.9%) Colour (98.8%) SS (99.5%) | Organic Matter (22.57) | |
| Electrocoagulation (EC) | |||||
| Parameter (Removals) | (with Al electrodes) COD (70%) TN (24%) Colour (56%) Turbidity (60%) | (with Fe electrodes) COD (68%) TN (15%) Colour (28%) Turbidity (16%) | COD (60%) NH3–N (37%) Colour (94%) Turbidity (88%) SS (89%) | heavy metals Cr (51%) As (59%) Cd (71%) Zn (72%) Ba ((95%) Pb (>99%) | |
| Adsorption | |||||
| Parameter (Removals) | COD (77.3%) Colour (82.5%) | COD (93.6%) NH3–N (84.8%) | Colour (100%) COD (∼80%) NH3+-N (100%) | COD (36%) NH3–N (99%) Cl (18%) | COD (51.0%) NH3–N (32.8%) Cl (66.0%) Br (81.0%) Cu (97.1%) |
| Treatment Method | Leachate Parameters | |||||
|---|---|---|---|---|---|---|
| BOD | COD | SS | NH3-N | Colour | Heavy Metals | |
| Activated Sludge Process | ▲ | ● | Ø | Ø | Ø | Ø |
| Contact Aeration Process | ▲ | ● | Ø | Ø | Ø | Ø |
| Rotary Biodisk Conductor Process | ▲ | ● | Ø | Ø | Ø | Ø |
| Biological Trickling Process | ▲ | ● | ▲ | Ø | Ø | Ø |
| Biological Nitrogen | ▲ | ● | Ø | ▲ | Ø | Ø |
| Flocculation-Sedimentation | ● | ▲ | ▲ | Ø | ▲ | ● |
| Sand filtration | Ø | Ø | ▲ | × | ● | × |
| Activated Carbon (Adsorption) | ▲ | ▲ | ● | Ø | ▲ | ● |
| Chemical Oxidation | × | ● | × | × | ▲ | × |
| AOP | Removal Efficiency | AOP | Removal Efficiency | ||
|---|---|---|---|---|---|
| Parameters | Removal (%) | Parameters | Removal (%) | ||
| Fenton | TOC COD | 68.9 69.6 | Ozone (O3) | Colour COD Ammonia | 100 88 79 |
| COD | 88.6 | COD Colour | 70 100 | ||
| COD | 70 | COD Colour | 16.5 40.5 | ||
| COD UV254 Colour | 58.70 85.69 88.30 | Humic Acid Fulvic Acid | 88 83.3 | ||
| COD | 97.8 3 | COD | 43 | ||
| COD BOD5 | 48 30 | COD TOC BOD5 | 65 62 36 | ||
| COD TOC | 97.83 74.24 | COD UV254 | 46 51 | ||
| total organic carbon, total inorganic carbon total nitrogen, colour | 88.7 100 96.5 98.2 | Colour UV | ~90 ~70 | ||
| TiO2 Photocatalysis | COD Colour | 58 36 | Electro-oxidation | COD TOC | 68 40.6 |
| COD TOC | 67 82.5 | COD | 80 | ||
| COD | 84 | TOC Ammonium nitrogen | 40 99 | ||
| Ferrosonication (FS) | COD BOD5 | 46 33 | W-doped TiO2 | COD | 46 |
| Heterogeneous catalytic ozonation (O3/TiO2) | COD NTU BOD5 | 24 94 98 | Heterogeneous catalytic ozonation (O3/ZnO) | COD NTU BOD5 | 33 95 98 |
| Physicochemical | Advantages | Disadvantages | Observations |
|---|---|---|---|
| Coagulation and Flocculation | Effective at removing suspended particles, humic acids, heavy metals, and organic matter. | Owing to the expense of inputs and the handling of the created chemical sludge, the system’s functioning requires very high coagulant concentrations, making it economically impracticable to implement this technology on a large scale. | For some membrane systems, this technology serves as a pre-treatment. Some membrane systems appear to use this technique as pre-treatment. |
| PACT (Powdered Activated Carbon Treatment) | Removes some poisons, chlorine, phenols, ammoniacal nitrogen, colour, odour, and taste. Safeguards the process against BOD and organic toxin shock loads by stabilising it. It is simple to use, operate, and maintain and has inexpensive installation costs. Pre-treatment technology is used with several membrane systems. | High operating expenses with on-site regenerating or coal deployment, as well as outputs with high potential pollutants | Aeration, biological oxidation, and physical adsorption happen at the same time as coal is supplied directly to the reactor. |
| Advanced Chemical Oxidation | It divides these high molecular weight molecules, which increases their treatability by making them more receptive to microorganisms in biological reactors and partially eliminates recalcitrant organic material and refractory chemicals. | Due to the complexity of the operation and the high cost of operation, such as energy and the value of the inputs necessary in significant doses, a competent technical operator is required. | The most common oxidative technology is ozonisation. |
| Evaporation | Up to 95% reduction in leachate volume. | Polluting gases are released, and it costs a lot of energy-60 kg of gasoline are required to burn 1 m3 of leachate. An output of dry sludge equal to around 5% of the entire volume is produced. | The option that is most frequently used is the landfill’s own biogas being captured and burned. |
| Combination Treatment Category | Removal Efficiency | Combination Treatment Category | Removal Efficiency | ||
|---|---|---|---|---|---|
| Parameters | Removal (%) | Parameters | Removal (%) | ||
| Advanced oxidation process/coagulation/adsorption | COD As Fe P | 94 87 96 86 | Bioreactor/coagulation | Colour COD Ammonia TSS | 85.8 84.8 94.2 91.8 |
| Advanced oxidation process/adsorption | Ammonia COD Colour HA (ABS254) | 94.5 95.1 95.0 97.9 | Bioreactor/membrane | COD Fe Zn | 95 71 74 |
| Advanced oxidation process/adsorption (ion-exchange) | Ammonia Nitrite Nitrate Colour Turbidity COD | 90 100 98 98 98 74 | Advanced oxidation process/coagulation | COD Colour HA (UV254) | 68 97 83 |
| Electrodissolution/advanced oxidation process/chemical flocculation | COD Colour Turbidity | 85 96 76 | COD HA | 90.2 93.7 | |
| COD | 91 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, H.A.; Ramli, S.F.; Hung, Y.-T. Physicochemical Technique in Municipal Solid Waste (MSW) Landfill Leachate Remediation: A Review. Water 2023, 15, 1249. https://doi.org/10.3390/w15061249
Aziz HA, Ramli SF, Hung Y-T. Physicochemical Technique in Municipal Solid Waste (MSW) Landfill Leachate Remediation: A Review. Water. 2023; 15(6):1249. https://doi.org/10.3390/w15061249
Chicago/Turabian StyleAziz, Hamidi Abdul, Siti Fatihah Ramli, and Yung-Tse Hung. 2023. "Physicochemical Technique in Municipal Solid Waste (MSW) Landfill Leachate Remediation: A Review" Water 15, no. 6: 1249. https://doi.org/10.3390/w15061249





