Fixation Effect of Modified Bamboo Charcoal on Typical Heavy Metals in Sediment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Main Experimental Apparatus and Materials
2.2. Preparation Method of Bamboo Charcoal
2.3. Collection and Treatment Method of Sediment
2.4. Sediment Cultivation Experiment
2.5. Determination of Heavy Metal Concentration in Overlying Water and Pore Water
2.6. Determination of Total Heavy Metals in Sediment
2.7. Determination of Different Forms of Heavy Metals in Sediment through BCR Extraction Method
2.8. Determination of TCLP Leaching Toxicity Risk of Heavy Metals in Sediment
3. Results
3.1. Morphological Analysis
3.2. Concentration of Heavy Metals in Pore Water and Overlying Water
3.2.1. Concentration of Heavy Metals in Overlying Water and Pore Water Polluted by Single Cu(II)
3.2.2. Concentration of Heavy Metals in Overlying Water and Pore Water of Cu(II)–Cd(II) Mixed Polluted Sediment
3.3. Fixation Effect of Heavy Metals
3.3.1. Fixation Effect of Heavy Metals in Single Cu(II)-Contaminated Sediment
- (1)
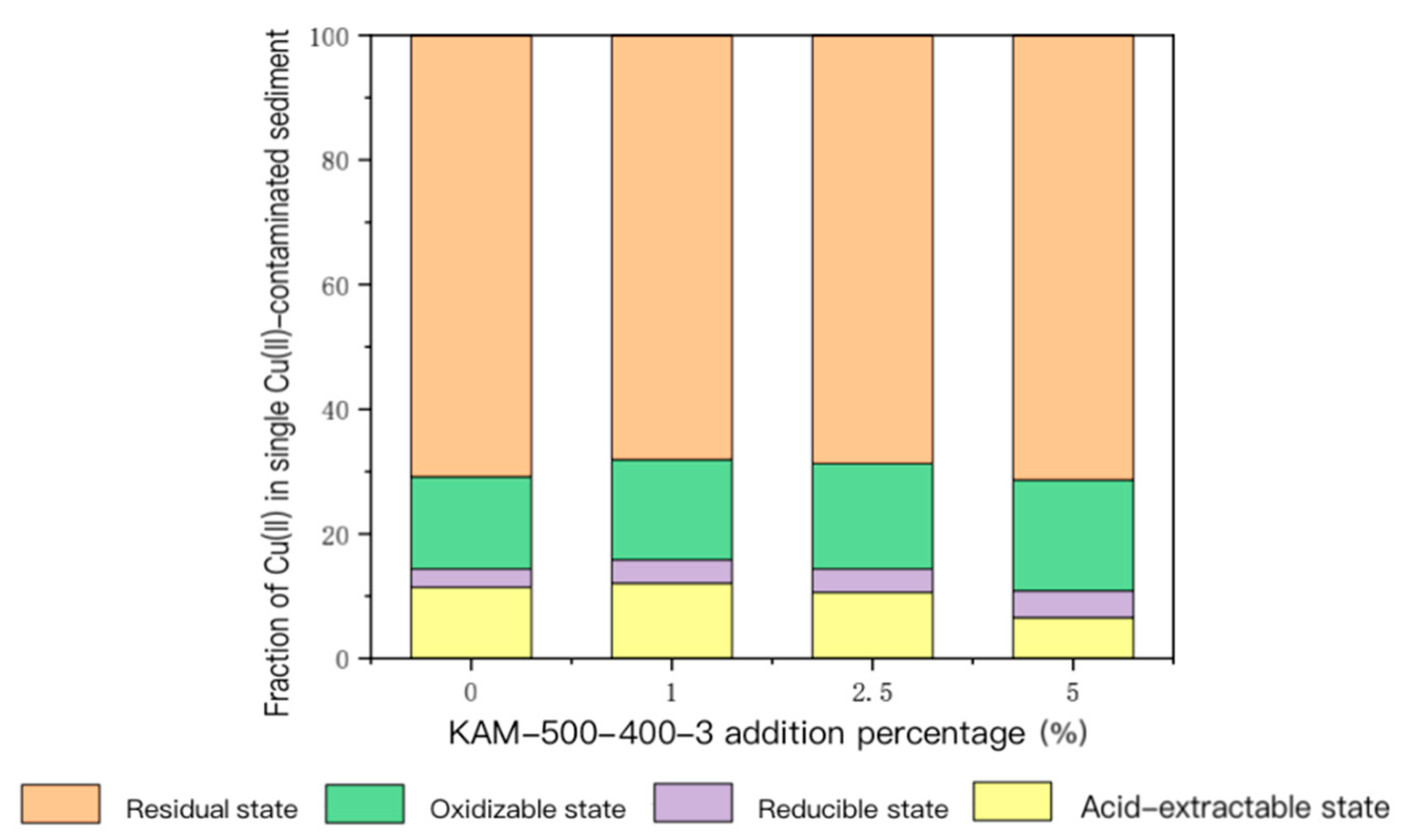
- Analysis of speciation changes of heavy metal Cu(II) in sediment
- (2)
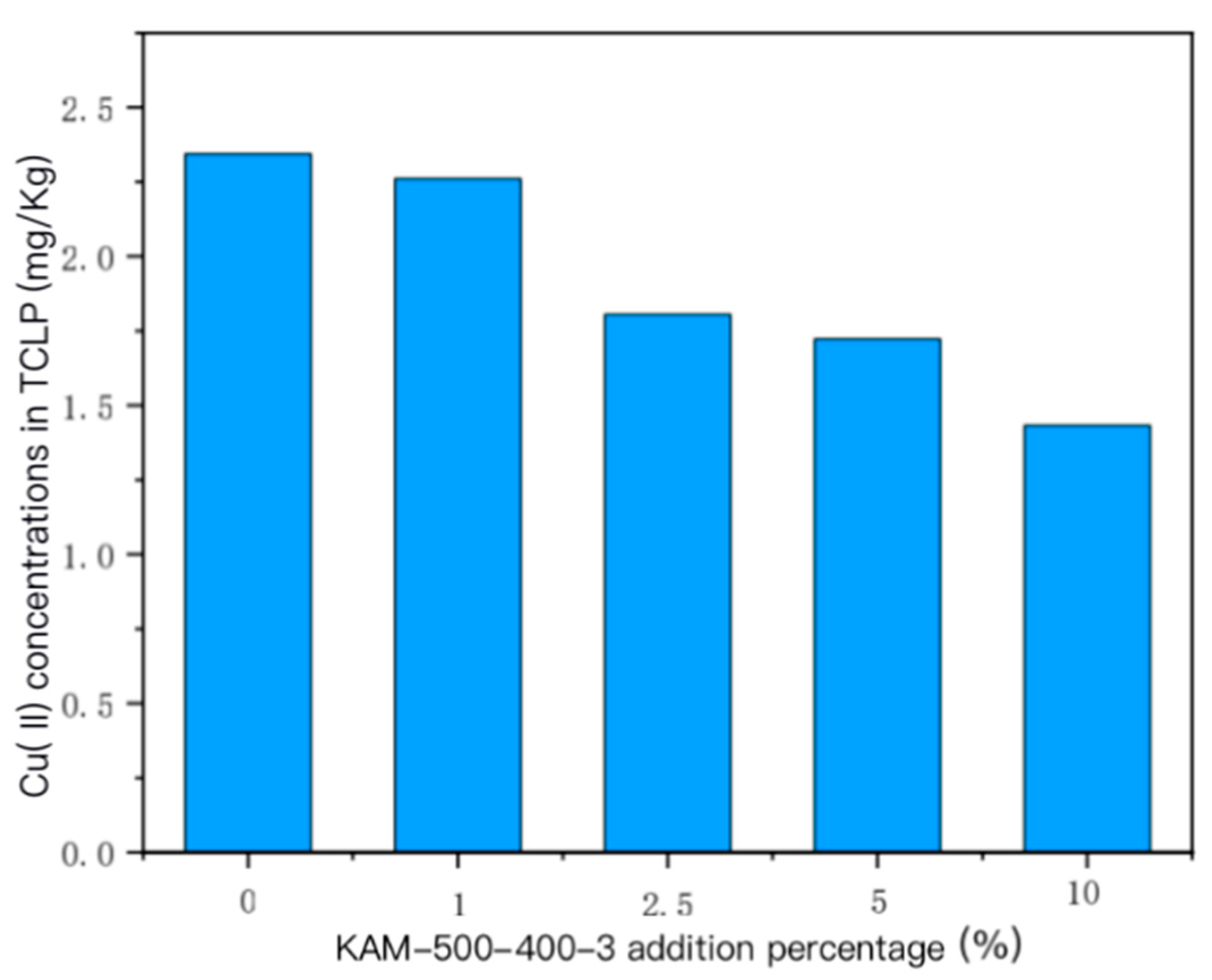
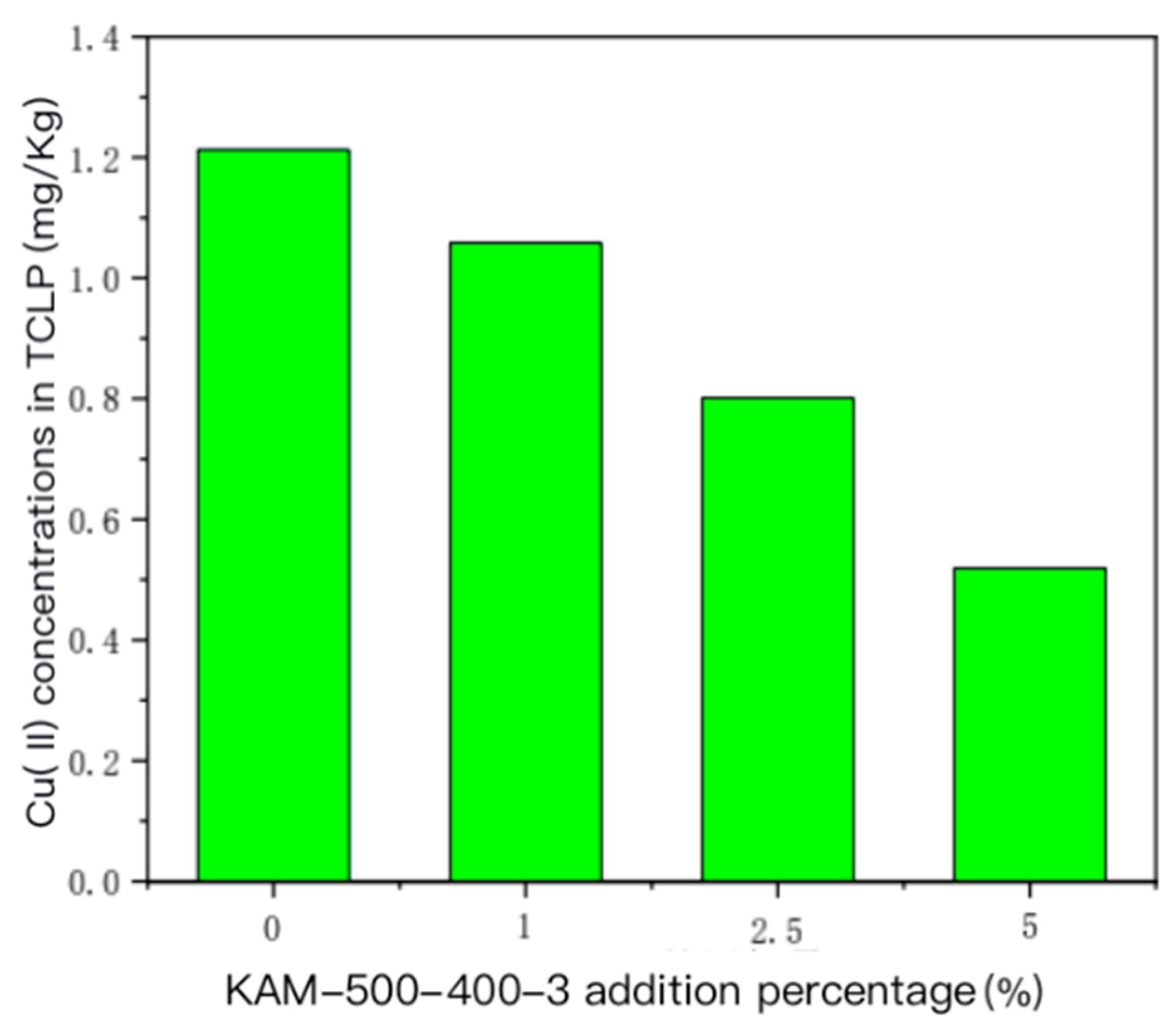
- TCLP leaching toxicity risk analysis of heavy metal Cu(II) in sediment
3.3.2. Fixation Effect of Heavy Metals in Mixed Polluted Sediment of Cu(II)–Cd(II)
- (1)
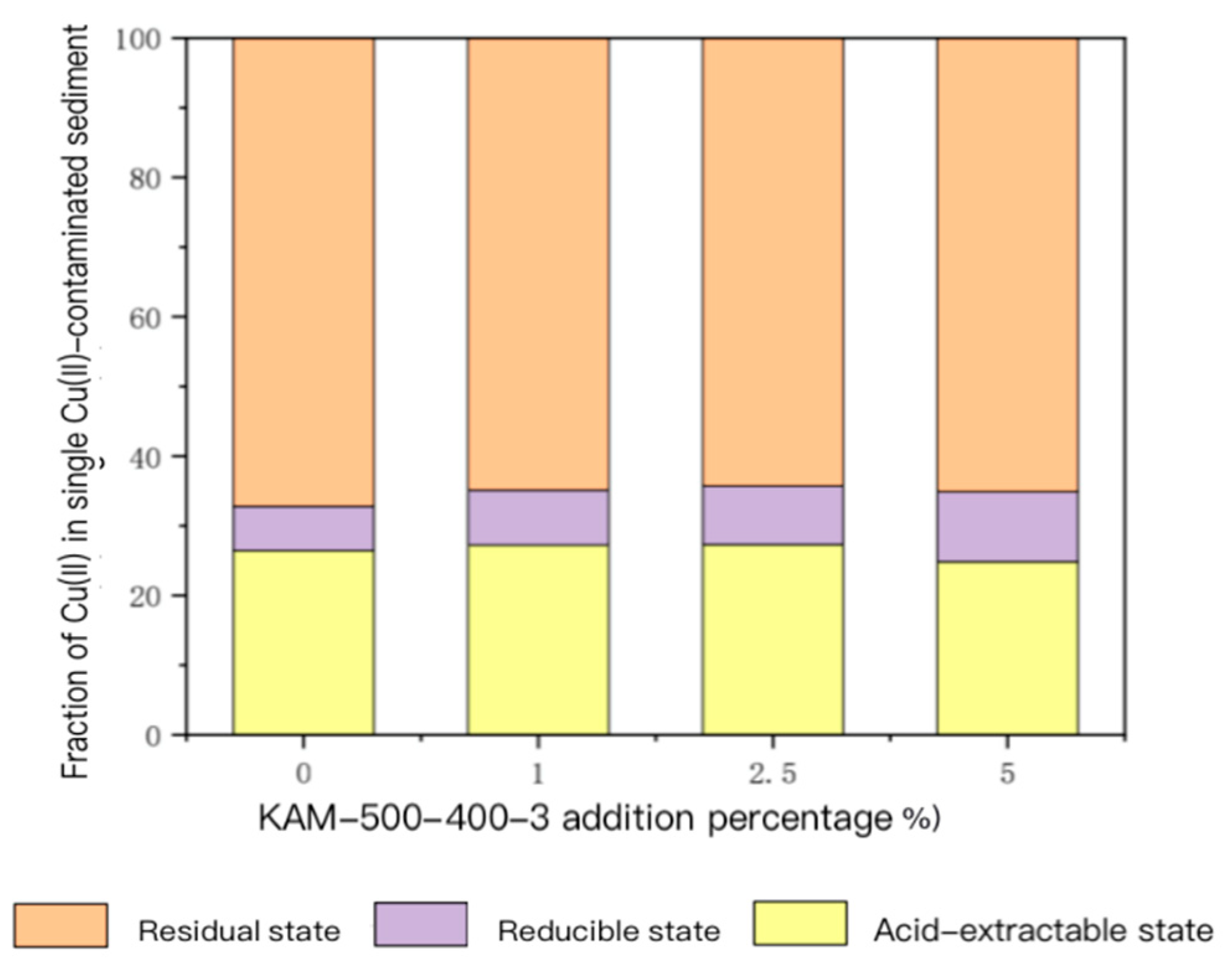
- Analysis of speciation changes in Cu(II) and Cd(II) in sediment
- (2)
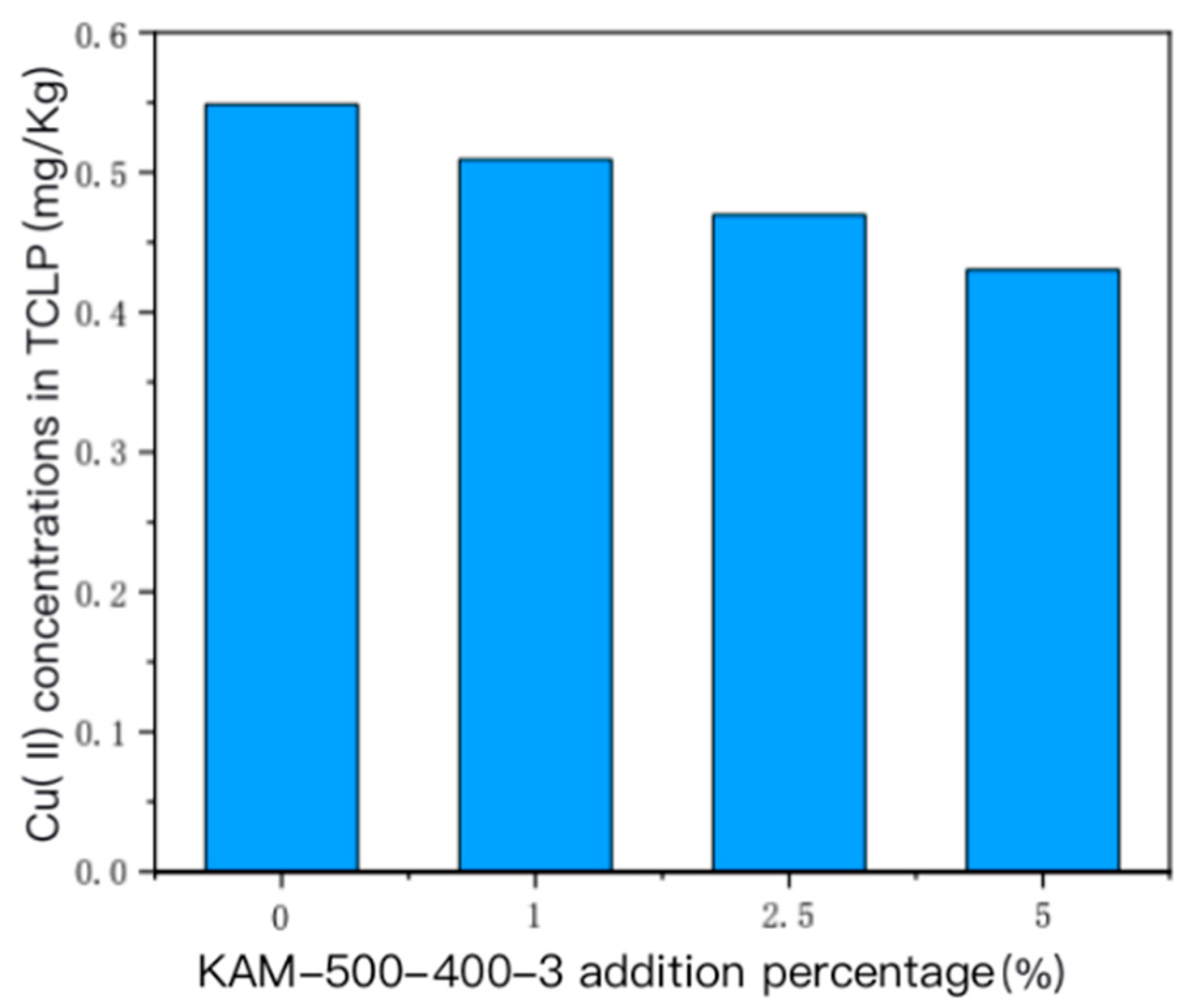
- TCLP leaching toxicity risk analysis of heavy metals Cu(II) and Cd(II) in sediment
4. Discussion
- (1)
- With the increase in KAM500-400-3 addition, the concentrations of Cu(II) in the overlying and pore water in the single Cu(II)-contaminated sediment and the mixed Cu(II)–Cd(II)-contaminated sediment were similar; that is, when a small amount was added, the impact on Cu(II) in the overlying and pore water was weak. When the addition amount gradually increased, the concentration of Cu(II) in the overlying water and pore water decreased. The Cd(II) content in the overlying and pore water of Cu(II)–Cd(II)-contaminated sediment was lower than the detection limit, so it could not be detected [19,47].
- (2)
- For the sediment polluted by single Cu(II), with the gradual increase in KAM500-400-3 addition, the content of acid-extractable Cu(II), which is highly bioavailable, gradually decreased, and its proportion in the total Cu(II) also continually decreased. The content and proportion of Cu(II) in the reducible, oxidizable, and residual states gradually increased. For sediment contaminated by Cu(II)–Cd(II), the main form of Cu(II) in the sediment was residual. With the increase in the KAM500-400-3 addition amount, the change in residual Cu(II) was not remarkable. The proportion of acid-extractable Cu(II) in the total amount of Cu(II) substantially reduced, and the proportions of reducible and oxidizable Cu(II) gradually increased. The form of Cd(II) in the sediment was also dominated by that in the residual state, and the pattern of the changes in the various forms of Cd(II) was also similar to that of Cu(II); that is, with the increase in the amount of added KAM500-400-3, the change in the residual state of Cd(II) was not remarkable, the proportion of the acid-extractable state of Cd(II) in the total amount of Cd(II) gradually decreased, the proportion of the reducible state of Cd(II) gradually increased, and the content of the oxidizable state of Cd(II) was lower than the detection limit and could not be detected [48].
- (3)
- For the sediment polluted by single Cu(II), the concentration of the TCLP-extractable solution of Cu(II) in the sediment gradually decreased with the increase in the amount of added KAM500-400-3. For the sediment polluted by Cu(II)–Cd(II), the increase in the added amount of KAM500-400-3 reduced the extractable concentrations of Cu(II) and Cd(II) TCLP, thus effectively fixing the heavy metals in the sediment and reducing their leaching risk.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J. Research progress of source and treatment methods of heavy metals in water. Guangdong Chem. Ind. 2014, 41, 87–88. [Google Scholar]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Yi, Y.; Yang, Z.; Zhang, S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011, 159, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xiong, Y.; Wu, F.; Wang, S.; Yang, H.; Xie, W.; Xie, Y. Composition and source identification of biomarkers in surface sediments from typical freshwater lakes in China. Environ. Pollut. Control 2017, 39, 822–828. [Google Scholar]
- Wang, T. The Effects of Additives on Adsorption of Heavy Metals onto Surficial Sediments. Master’s Thesis, Jilin University, Nanjing, China, 2008. [Google Scholar]
- Islam, M.S.; Ahmed, M.K.; Raknuzzaman, M.; Habibullah-Al-Mamun, M.; Islam, M.K. Heavy metal pollution in surface water and sediment: A preliminary assessment of an urban river in a developing country. Ecol. Indic. 2015, 48, 282–291. [Google Scholar] [CrossRef]
- Chang, T.C.; Yen, J.H. On-site mercury-contaminated soils remediation by using thermal desorption technology. J. Hazard. Mater. 2006, 128, 208–217. [Google Scholar] [CrossRef]
- Diao, Z.; Shi, T.; Wang, S.; Huang, X.; Zhang, T.; Tang, Y.; Zhang, X.; Qiu, R. Silane-based coatings on the pyrite for remediation of acid mine drainage. Water Res. 2013, 47, 4391–4402. [Google Scholar] [CrossRef]
- Wang, F.; Bao, K.; Huang, C.; Zhao, X.; Han, W.; Yin, Z. Adsorption and pH values determine the distribution of cadmium in terrestrial and marine soils in the Nansha area, Pearl River Delta. Int. J. Environ. Res. Public Health 2022, 19, 793. [Google Scholar] [CrossRef]
- GB/T 33422–2016; Thermoplastic Elastomer—Determination of Heavy Metal Contents—Inductively Coupled Plasma Atomic Emission Spectrometric Method. China National Standardization Administration Committee: Beijing, China, 2016.
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Singh, E.; Kumar, A.; Mishra, R.; You, S.; Singh, L.; Kumar, S.; Kumar, R. Pyrolysis of waste biomass and plastics for production of biochar and its use for removal of heavy metals from aqueous solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Competitive adsorption of heavy metals onto sesame straw biochar in aqueous solutions. Chemosphere 2016, 142, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; DiClemente, C.C.; Norcross, J.C. In search of how people change: Applications to addictive behaviors. Am. Psychol. 1992, 47, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Kaczala, F.; Marques, M.; Hogland, W. Lead and vanadium removal from a real industrial wastewater by gravitational settling/sedimentation and sorption onto Pinus sylvestris sawdust. J. Bioresour. Technol. 2009, 100, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Velkova, Z.; Kirova, G.; Stoytcheva, M.; Kostadinova, S.; Todorova, K.; Gochev, V. Immobilized microbial biosorbents for heavy metals removal. J. Eng. Life Sci. 2018, 18, 871–881. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Shan, B.; Zhu, Y.; Tang, W. Remediation effectiveness of Phyllostachys pubescens biochar in reducing the bioavailability and bioaccumulation of metals in sediments. Environ. Pollut. 2018, 242, 1768–1776. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Chen, S.; Wang, R.; Xu, P.; Cheng, M.; Zhang, C.; Xue, W. Biochar facilitated the phytoremediation of cadmium contaminated sediments: Metal behavior, plant toxicity, and microbial activity. Sci. Total Environ. 2019, 666, 1126–1133. [Google Scholar] [CrossRef]
- Zou, Q.; An, W.; Wu, C.; Li, W.; Fu, A.; Xiao, R.; Chen, H.; Xue, S. Red mud-modified biochar reduces soil arsenic availability and changes bacterial composition. Environ. Chem. Lett. 2018, 16, 615–622. [Google Scholar] [CrossRef]
- Liu, S.J.; Liu, Y.G.; Tan, X.F.; Zeng, G.M.; Zhou, Y.H.; Liu, S.B.; Yin, Z.H.; Jiang, L.H.; Li, M.F.; Wen, J. The effect of several activated biochars on Cd immobilization and microbial community composition during in-situ remediation of heavy metal contaminated sediment. Chemosphere 2018, 208, 655–664. [Google Scholar] [CrossRef]
- Liu, Q.; Sheng, Y.; Wang, W.; Li, C.; Zhao, G. Remediation and its biological responses of Cd contaminated sediments using biochar and minerals with nanoscale zero-valent iron loading. Sci. Total Environ. 2020, 713, 136650. [Google Scholar] [CrossRef]
- Sun, T.; Li, K.; Fu, Y.; Ma, W.; Xie, X.; Sun, Y. Effect of modified biochar on immobilization remediation of weakly alkaline Cd-contaminated soil and environmental quality. Acta Sci. Circumst. 2020, 40, 2571–2580. [Google Scholar]
- Xiao, Y.; Xue, Y.; Gao, F.; Mosa, A. Sorption of heavy metal ions onto crayfish shell biochar: Effect of pyrolysis temperature, pH and ionic strength. J. Taiwan Inst. Chem. Eng. 2017, 80, 114–121. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Zhang, X.; Li, Q.; Yang, W. Effect on Cr(VI) adsorption performance of acid-base modified biochar. Environ. Eng. 2020, 38, 28–34. [Google Scholar]
- Herath, A.; Layne, C.A.; Perez, F.; Hassan, E.I.B.; Pittman, C.U.; Mlsna, T.E. KOH-activated high surface area Douglas Fir biochar for adsorbing aqueous Cr(VI), Pb(II) and Cd(II). Chemosphere 2021, 269, 128409. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Akakuru, O.U.; Xu, X.; Wu, A. Research progress and mechanism of nanomaterials-mediated in-situ remediation of cadmium-contaminated soil: A critical review. J. Environ. Sci. 2021, 104, 351–364. [Google Scholar] [CrossRef]
- Bian, R.; Chen, D.; Liu, X.; Cui, L.; Li, L.; Pan, G.; Xie, D.; Zheng, J.; Zhang, X.; Zheng, J.; et al. Biochar soil amendment as a solution to prevent Cd-tainted rice from China: Results from a cross-site field experiment. Ecol. Eng. 2013, 58, 378–383. [Google Scholar] [CrossRef]
- Zhi, T.; Cheng, L.; Xu, X. Mechanism and application of algae for the removal of heavy metals in water bodies. J. Chem. Adv. 2011, 23, 1782–1794. [Google Scholar]
- Verma, S.; Kuila, A. Bioremediation of heavy metals by microbial process. J. Environ. Technol. Innov. 2019, 14, 100369. [Google Scholar] [CrossRef]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. J. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar]
- Sharma, S.; Tiwari, S.; Hasan, A.; Saxena, V.; Pandey, L.M. Recent advances in conventional and contemporary methods for remediation of heavy metal-contaminated soils. 3 Biotech 2018, 8, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. J. Soil Sediment Contam. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Song, J. Heavy Metal Pollution Characteristics of Shandong Peninsula River Sediment and Landfill Disposal Risk Assessment; University of Chinese Academy of Sciences (Yantai Institute of Coastal Zone, Chinese Academy of Sciences): Beijing, China, 2019. [Google Scholar]
- Zhang, M.; Gao, B.; Varnoosfaderani, S.; Hebard, A.; Yao, Y.; Inyang, M. Preparation and characterization of a novel magnetic biochar for arsenic removal. J. Bioresour. Technol. 2013, 130, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X.; Fu, K. Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: A fixed-bed column study. J. Bioresour. Technol. 2012, 113, 114–120. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; He, H.; Zhang, J.; Liu, J.; Wang, D.; Huang, L.; Tu, Z. Preparation, performances and mechanisms of Co@AC composite for herbicide atrazine removal in water. Water 2021, 13, 240. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y.; Dundar, M. Adsorption of chromium(VI) on pomace one anoliveoil industry waste: Batch and column studies. J. Hazard. Mater. 2006, 138, 142–151. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, Y.; Zhang, P.; Zhao, Q.; An, L. Adsorption of Pb and Cd in water by a steel slag fixation bed. J. Environ. Eng. 2016, 34, 25–30. [Google Scholar]
- Lu, X.; Zhong, L.; Meng, F. Study on the dynamic adsorption characteristics of Cr (VI) in simulated wastewater from peanut shell. J. Water Resour. Prot. 2013, 29, 87–90. [Google Scholar]
- Liu, D.; Yang, X.; Zhang, L.; Tang, Y.; He, H.; Liang, M.; Tu, Z.; Zhu, H. Immobilization of biomass materials for removal of refractory organic pollutants from wastewater. Int. J. Environ. Res. Public Health 2022, 19, 13830. [Google Scholar] [CrossRef]
- Quan, S. Characteristics of Soil Heavy Metal Pollution and Its Heat Treatment in e-Waste Pickling Area; Graduate School of Chinese Academy of Sciences (Guangzhou Institute of Geochemistry): Guangzhou, China, 2015. [Google Scholar]
- Huang, A.; Yang, D.; Yang, S. Research progress in remediation of soil heavy metal pollution by modified biochar. J. Chem. Prog. 2020, 39, 5266–5274. [Google Scholar]
- Lu, X.; Wu, J.; Zheng, Y. Ppassivation of cadmium in soil by walnut biochar. J. Environ. Eng. 2020, 38, 196–202. [Google Scholar]
- Tang, J.; Zhu, W.; Kookana, R.; Katayama, A. Characteristics of biochar and its application in remediation of contaminated soil. J. Biosci. Bioeng. 2013, 116, 653659. [Google Scholar] [CrossRef] [PubMed]
- Que, W.; Zhou, Y.H.; Liu, Y.; Wen, J.; Tan, X.F.; Liu, S.J.; Jiang, L.H. Appraising the effect of in-situ remediation of heavy metal contaminated sediment by biochar and activated carbon on Cu immobilization and microbial community. J. Ecol. Eng. 2019, 127, 519–526. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L. Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ. Pollut. 2010, 158, 2282–2287. [Google Scholar] [CrossRef]
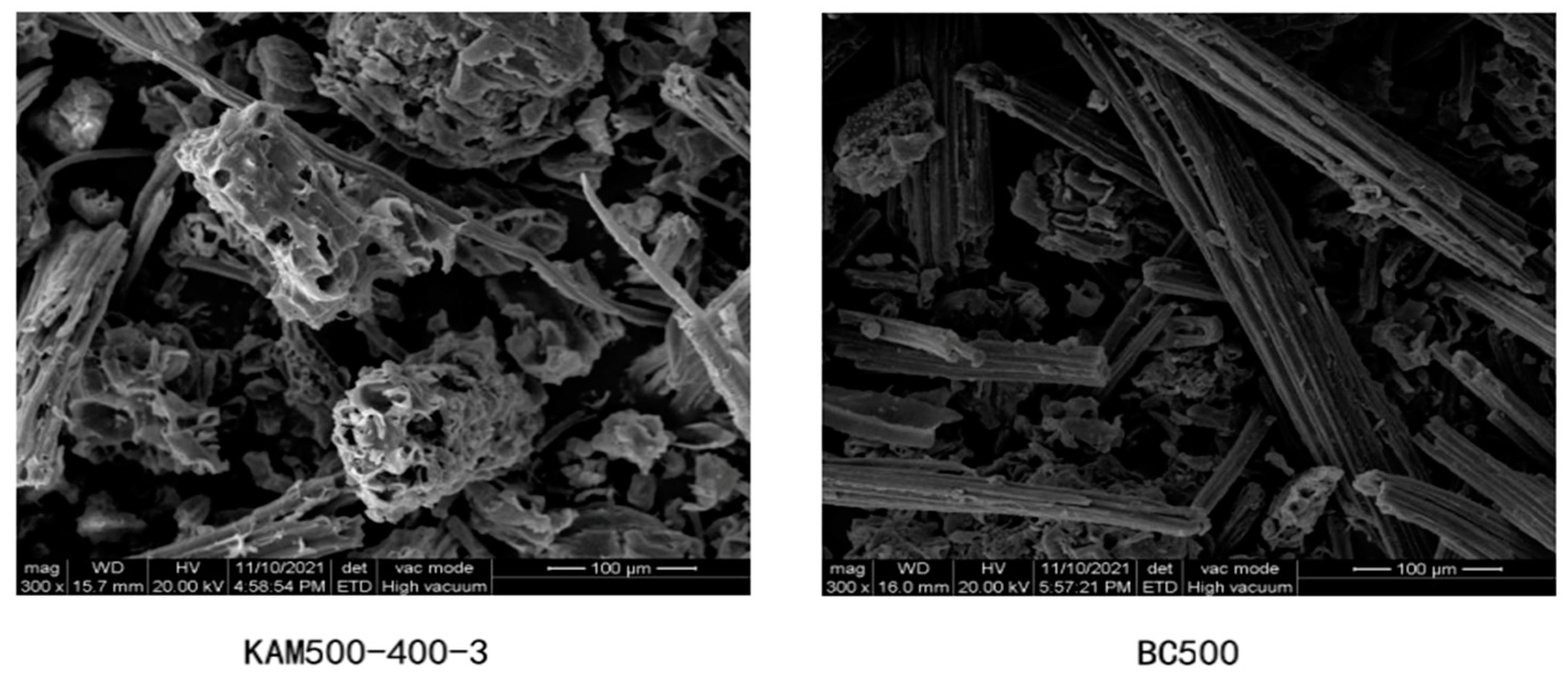
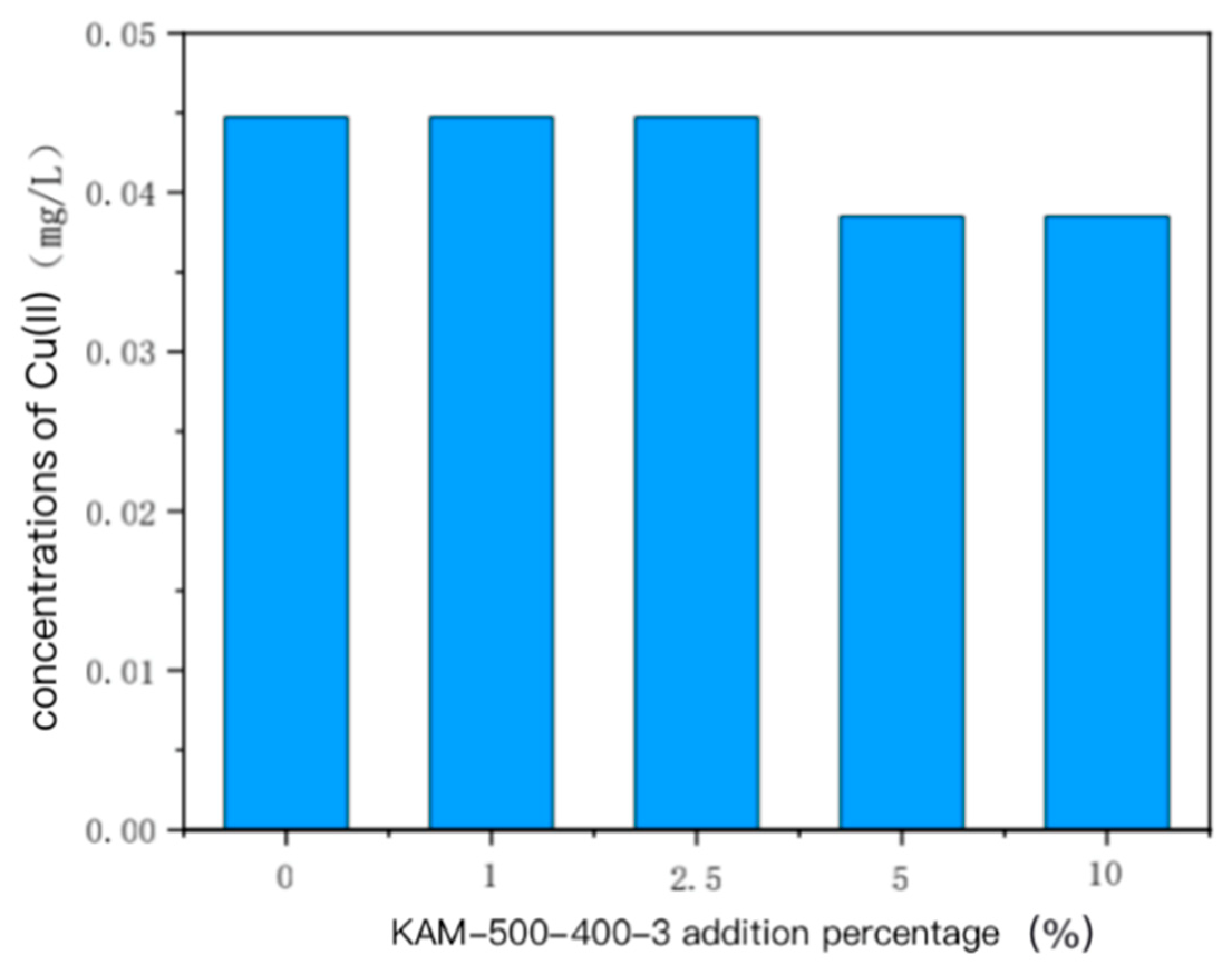
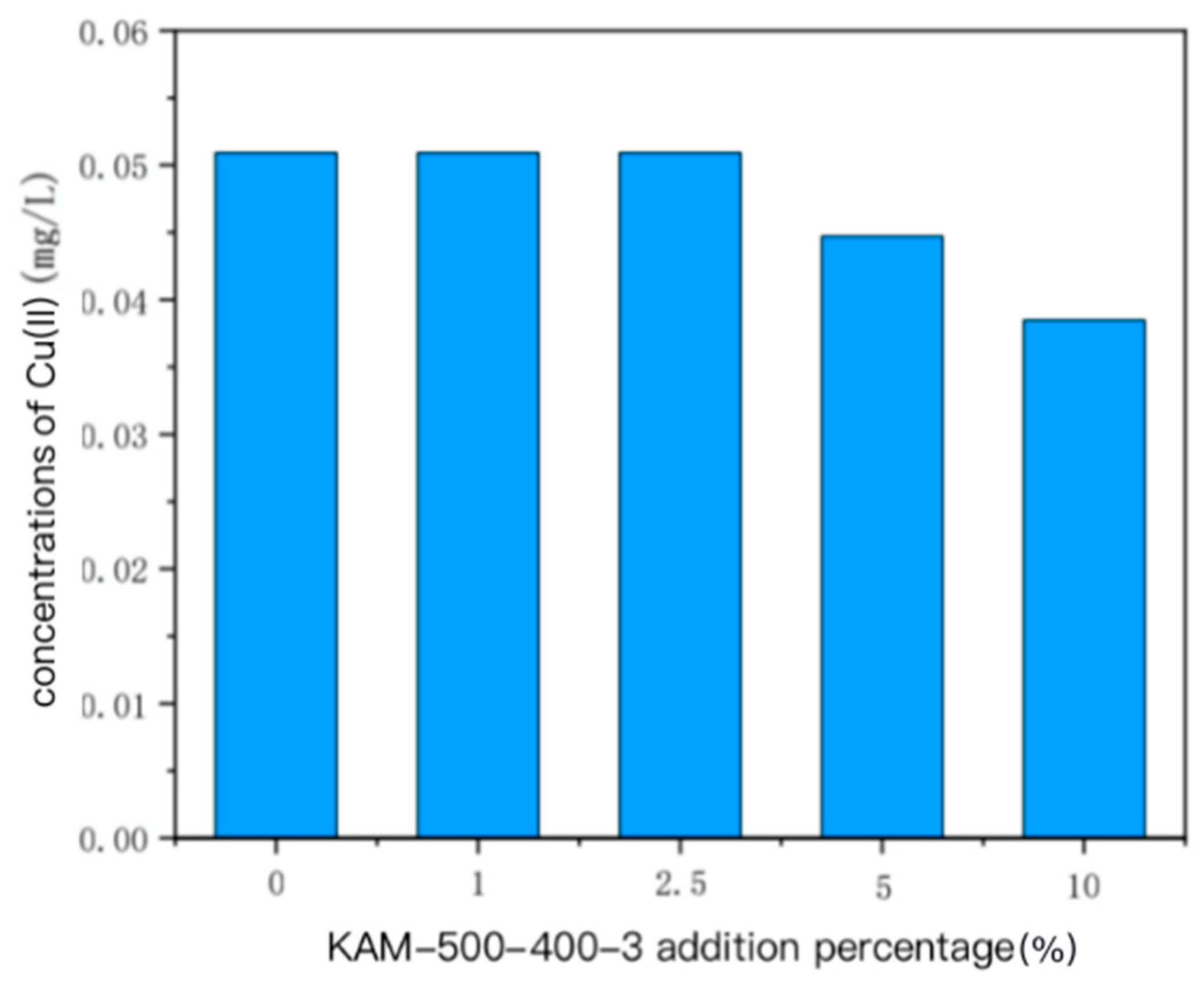
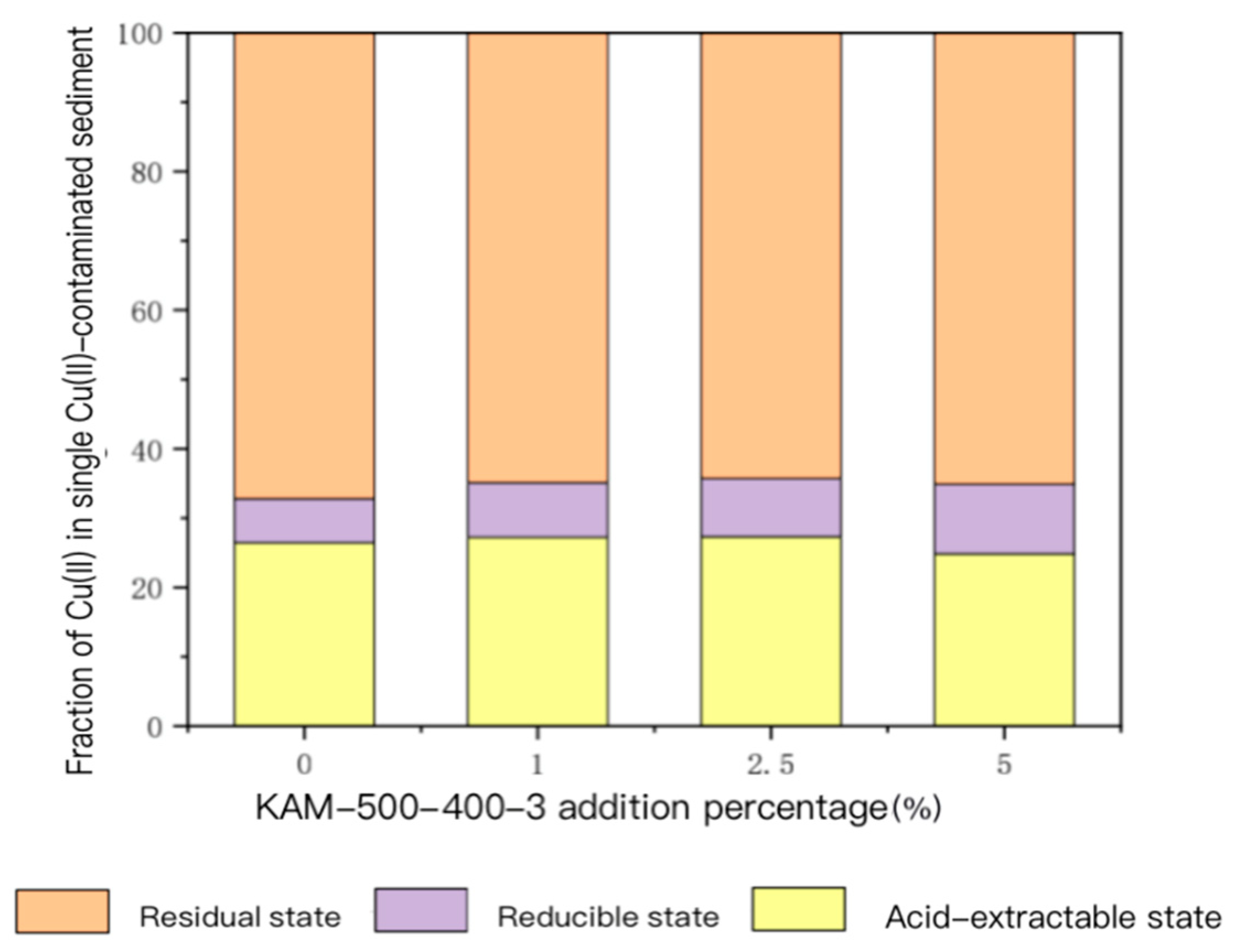
| KAM-500-400-3 Addition Percentage (%) | ||||
|---|---|---|---|---|
| 0 | 1 | 2.5 | 5 | |
| Cu(II) concentrations in overlying water | 0.01 | 0.01 | — | — |
| Cu(II) in pore water | 0.01 | 0.01 | — | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, H.; Lin, S. Fixation Effect of Modified Bamboo Charcoal on Typical Heavy Metals in Sediment. Water 2023, 15, 1230. https://doi.org/10.3390/w15061230
Wang Y, Li H, Lin S. Fixation Effect of Modified Bamboo Charcoal on Typical Heavy Metals in Sediment. Water. 2023; 15(6):1230. https://doi.org/10.3390/w15061230
Chicago/Turabian StyleWang, Yizhuo, He Li, and Shaohua Lin. 2023. "Fixation Effect of Modified Bamboo Charcoal on Typical Heavy Metals in Sediment" Water 15, no. 6: 1230. https://doi.org/10.3390/w15061230
APA StyleWang, Y., Li, H., & Lin, S. (2023). Fixation Effect of Modified Bamboo Charcoal on Typical Heavy Metals in Sediment. Water, 15(6), 1230. https://doi.org/10.3390/w15061230






